Abstract
The AAA+ ATPase p97 has a critical function in the cytoplasmic degradation of proteins misfolded in the endoplasmic reticulum through a mechanism known as ER-associated degradation (ERAD). During this process, p97 binds polyubiquitinated ERAD substrates and couples ATP hydrolysis to their dislocation from the ER as a prerequisite to destruction by the proteasome. The ubiquitin signals important for this process are not fully understood. Here we report that p97 interacts with lysine 11 (K11) and K48-linked ubiquitin polymers, but not those containing K63 linkages. Disruption of p97 through siRNA-mediated depletion, dominant negative over-expression, or chemical inhibition results in the accumulation of K11 and K48 ubiquitin chains predominantly at the ER membrane, and is associated with ER stress induction. We show that a catalytically inactive deubiquitinating enzyme and p97 cofactor YOD1 enhances the accumulation of K11- and K48-linked polyubiquitin in the cytoplasm, at the ER membrane, and bound to p97. In addition to general effects on p97-associated ubiquitin polymers, the ERAD substrate CD3δ is modified with both K11- and K48-ubiquitin chains prior to p97-dependent dislocation. Collectively, our data are consistent with a major role for p97 in the recognition of K11 and K48 polyubiquitinated proteins prior to their degradation by the proteasome.
Keywords: p97, ubiquitin, proteasome, protein quality control, endoplasmic reticulum-associated degradation, deubiquitinating enzyme
INTRODUCTION
The ubiquitin proteasome system (UPS) regulates cellular protein homeostasis by conditionally and irreversibly removing proteins in response to a variety of stimuli [1]. In addition to controlling the stability of regulatory proteins such as transcription factors and cell cycle coordinators, a major function of the UPS is to clear damaged or misfolded proteins as part of the cellular protein quality control machinery. In this context, p97/valosin-containing protein (VCP, or Cdc48 in Saccharomyces cerevisiae) functions as an intermediary between ubiquitin modification and degradation by the proteasome [2]. P97 has also been implicated in diverse cellular processes such as membrane reassembly, DNA repair, autophagosome maturation, and mitophagy [3].
The best understood role for p97 in the UPS is in the clearance of damaged or misfolded proteins through endoplasmic reticulum-associated degradation (ERAD) (reviewed in [4]). After translocation into the ER lumen, a protein either folds into its native conformation or is transported back into the cytosol through a process known as “retro-translocation” or “dislocation”. The emergence of the dislocating protein into the cytosol coincides with its modification with polyubiquitin. This serves as a recruitment signal for p97, which through its AAA+ ATPase activity and associated cofactors catalyzes the protein’s extraction from the ER membrane [5–8]. The ubiquitinated protein is then targeted for destruction by the proteasome.
Modulating ERAD has emerged as a promising anti-cancer strategy and the p97 complex has received attention as a potential therapeutic target [9]. Alterations in ER homeostasis result in the accumulation of ERAD substrates, leading to ER stress and induction of the unfolded protein response (UPR). The UPR normally promotes cell survival by alleviating ER stress [10]. However, persistent ER stress activates apoptotic signaling pathways, ultimately leading to cell death. Cancer cells are particularly sensitive to this mechanism, presumably due to increases in protein synthesis which correspondingly increase the burden on protein quality control mechanisms such as ERAD [10].
The anti-cancer effects of the FDA-approved proteasome inhibitor bortezomib (Velcade®) are proposed to occur predominantly through ER stress induction [11]. Another molecule, Eeyarestatin I (EerI), induces ER stress and cell death by blocking protein dislocation from the ER [12, 13]. Membrane-associated p97 is one of its molecular targets and EerI has been proposed to induce structural changes in p97 that alters its association with ERAD cofactors [14]. These cofactors have a variety of functions including targeting p97 to the ER membrane (e.g. UBXD8, UBXD2, VIMP, GP78), ubiquitin recognition (e.g. Npl4/Ufd1), and activities that modulate substrate ubiquitination (e.g. YOD1, UBE4B ) (reviewed in [4]).
In general, the polyubiquitin chain attached to a protein is not a static signal, but is dynamically regulated in length and through ubiquitin chain topology [15]. Different ubiquitin-ubiquitin linkages form structurally distinct chains that are recognized or “decoded” by ubiquitin-binding proteins, which couple the modification to its biological consequence [16]. K48-linked polyubiquitin targets modified proteins for degradation by the proteasome [17], and has also been implicated in the dislocation of ERAD substrates [18]. On the other hand, K63-linked polyubiquitin does not appear to function in degradation by the proteasoe and instead mediates protein-protein interactions required for signaling (reviewed in [19]). Other ubiquitin linkages have less well-defined roles, but have been identified in vivo, and likely have important cellular functions [20].
K11 ubiquitin linkages have recently been implicated in diverse cellular pathways (reviewed in [21]). A proteomic study found that K11 chains accumulate with ER stress in budding yeast, indicating a role in maintaining ER homeostasis [22]. In human cells, K11-linked ubiquitin mediates the degradation of several cell cycle regulators by the proteasome [23]. Thus, although structurally distinct [24, 25], both K11 and K48 polymers support the targeting of proteins to the proteasome. A proteomic analysis of ubiquitin-binding proteins associated with p97 found enrichment of K11 ubiquitin linkages [26], supportive of roles in p97-dependent protein processing. In this study, we used ubiquitin linkage-specific antibodies to evaluate changes in ubiquitin polymer steady state abundance due to modulating the p97 complex and chemically inhibiting known steps in ERAD. Based on our data, we propose that recognition of K11 and K48 polyubiquitin by p97 at the ER membrane represents a major function of the enzyme in maintaining cellular protein homeostasis and is critical for the dislocation of ERAD substrates prior to their degradation by the proteasome.
EXPERIMENTAL
Cell lines
HEK293F cells were grown in suspension in Freestyle Expression Medium (Invitrogen). Sf9 cells were cultured in SFM II Sf9 media (Invitrogen). HiFive cells were cultured in HiFive Expression Medium supplemented with L- glutamine to 20 mM (Invitrogen).
Baculoviruses
High titer stocks of recombinant baculovirus were generated as per manufacturer’s instructions (Invitrogen). For anti-FLAG immunoprecipitations, HiFive cells were infected in 6-well plate format (106 cells per well) with 30 μl high titer baculovirus stock for 42 hours before processing.
Constructs
YOD1 and p97 were amplified by PCR from the MegaMan Human Transcriptome library (Agilent) and cloned into pcDNA3.1+ or pFastBac1. The CD3δ open reading frame was generously provided by Dr. Ze’ev Ronai and cloned into pcDNA3.1+. Site-directed mutagenesis was performed using Quikchange (Agilent). N-terminal FLAG and C-terminal hexahistidine tags were encoded in the oligonucleotides. All constructs were verified by sequencing.
DNA transfections, siRNA transfections, and drug treatments
293F cells were plated in 50% Freestyle expression medium, 50% DMEM supplemented with 10% FBS the day prior to transfection. DNA transfections used Freestyle Max Expression Reagent (Invitrogen) and siRNA transfections used Lipofectamine 2000 (Invitrogen), and both were incubated for 48 hours. Where indicated, cells were treated with tunicamycin (1 μM, Sigma), Brefeldin A (3.6 μM, Sigma), bortezomib (3μM, LC Laboratories), or Eeyarestatin-I (10 μM, ChemBridge) for 8 hours.
Antibodies
Anti-K48 (clone Apu2.07) and -K63 (clone Apu3) ubiquitin and GAPDH (MAB374) antibodies were purchased from Millipore. The anti-K11 ubiquitin antibody (clone 2A3/2E6) was a kind gift from V. Dixit (Genentech) and has been previously described [25]. α-Tubulin, Calnexin (SPA-860), p97 (sc-57492) and Grp78 (sc13968), and hexahistidine-tag (A00186) antibodies were purchased from Zymed, Enzo Life Sciences, Santa Cruz Biotechnology, and Genscript, respectively.
Immunoblotting
To maintain the specificity of ubiquitin linkage-specific antibodies, we followed previously published protocols [25]. Proteins were resolved by SDS-PAGE in reducing sample buffer, and wet-transferred at 25V for 1 hour to nitrocellulose. Membranes were blocked in 2% non-fat dried milk in TBS-T (0.02% Tween-20) for 1 hour, followed by incubation with primary antibodies (anti-K11 polyubiquitin clone 2A3/2E6 (3 μg/ml), anti-K48 polyubiquitin clone Apu2.07 (1:2000), anti-K63 polyubiquitin clone Apu3 (1:2000)) for 1 hour at room temperature. Membranes were washed three times in TBS-T, followed by incubation with secondary antibodies for 30 minutes at room temperature. Anti-K11 was detected with anti-human IgG IRDye800 (Rockland), and anti-K48/63 with anti-rabbit DyLight 680 (Thermo Scientific), and analyzed on a Licor Odyssey infrared imaging system. Appropriate antigen specificity under these conditions was verified with equimolar amounts of purified diubiquitin molecules (K11, K48, and K63).
Immunoprecipitation
For analysis of CD3δ ubiquitination, transfected 293F cells were collected by centrifugation (1,000 x g for 5 min) and resuspended in RIPA lysis buffer (50 mM HEPES pH 7.6, 150 mM NaCl, 1% Triton X-100, 1 mM EDTA, 0.1% (w/v) SDS and 1% (w/v) deoxycholate, 1 mM N-ethylmaleimide) [27]. Samples were incubated on ice for 30 minutes, clarified by centrifugation (20,000 x g, 10 min, 4°C) and incubated with 20 μl of anti-FLAG Sepharose (Sigma) for 2 hours at 4°C. Beads were washed with Triton X-100 Wash Buffer (50 mM HEPES pH 7.6, 150 mM NaCl, 1% Triton X-100, 1 mM EDTA) and eluted by boiling in sample buffer. For analysis of protein interactions (co-immunoprecipitation), cells were lysed in Triton X-100 Lysis Buffer (25 mM HEPES pH 7.6, 300 mM NaCl, 0.2% Triton X-100, 2 mM EDTA, 5 mM MgCl2, and 1 mM DTT), incubated on ice for 20 minutes, clarified by centrifugation (20,000 x g, 10 min, 4°C) and incubated with 20 μl of anti-FLAG Sepharose for 2 hours at 4°C. After washing with lysis buffer, purifying proteins were eluted by boiling in sample buffer.
Subcellular fractionation
239F cells were resuspended in fractionation buffer (250 mM sucrose, 25 mM HEPES pH 7.6, 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, and 1 mM DTT), and lysed by drawing through a 25G needle 20 times. An aliquot sample was taken of this whole cell extract. Lysates were centrifuged at 9,000 x g for 10 min to pellet unbroken cells and nuclei. The supernatant was fractionated into microsome and cytosol fractions by centrifugation at 100,000 x g for 1 hour at 4°C. The pellet (microsomes) was resuspended in fractionation buffer containing 0.2% Triton X-100. For endogenous p97 immunopreciptiation from cytosol and microsome fractions, NaCl and Triton X-100 were adjusted (150 mM and 0.2%, respectively), and incubated with a p97 monoclonal antibody (2 μg) for 1 hour at 4°C, followed by 25 μl of Protein A/G UltraLink resin (Pierce) for 1 hour at 4°C. Complexes were washed with fractionation buffer containing 150 mM NaCl, 0.2% Triton X-100, and eluted by boiling in sample buffer.
RESULTS
p97 depletion and dominant negative over-expression increase K11 and K48 ubiquitin linkages at the ER
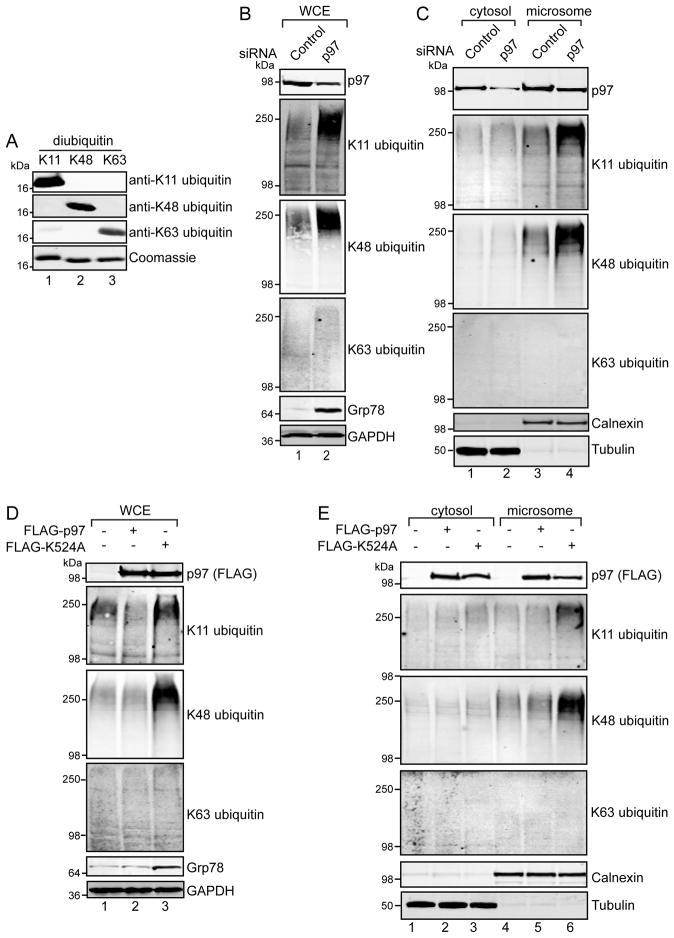
We first tested the specificity of K11, K48, and K63 ubiquitin linkage specific antibodies on purified K11, K48, and K63 linked diubiquitin using published detection methods to maintain proper epitope recognition by immunoblotting, while minimizing non-specific cross-reactivity [25]. Our analyses did not find significant cross-reactivity for any of these antibodies (Figure 1A), suggesting they predominantly recognize their cognate epitopes.
FIGURE 1. p97 depletion and p97 K524A over-expression increase K11 and K48 ubiquitin conjugates at the ER.
(A) K11, K48, or K63 linked diubiquitin (0.5 μg each) were resolved by SDS-PAGE and immunoblotted (see Experimental for details). In parallel, a gel was stained with Coomassie blue to confirm equal loading of diubiquitin in each lane. (B) 293 cells were transfected with non-targeting control or p97 siRNAs. Forty hours post-transfection, cells were lysed in fractionation buffer by mechanical shearing. Samples were taken to represent the whole cell extract (WCE), which were examined by immunoblotting with the indicated antibodies. (C) Unbroken cells and nuclei from (B) were pelleted by low-speed centrifugation, and the post-nuclear fraction further separated into cytosol and microsomal fractions by high-speed centrifugation. Equal amounts of each fraction were analyzed by immunoblotting. The ER chaperone calnexin was used as a marker for the microsome fraction, and α-tubulin was used as a marker for the cytosol. (D–E) 293 cells were transfected with FLAG-tagged p97 or an ATPase deficient form of p97 containing the K524A mutation. Forty hours post-transfection, whole-cell (D) or cytosol and microsomal (E) extracts were prepared and immunoblotted.
To assess the effects of p97 on the steady state levels of polyubiquitin in cells, we depleted p97 in 293 cells using a targeting siRNA. P97 depletion resulted in a marked induction of Grp78, an ER-resident chaperone and marker of ER stress [28], which was accompanied by an increase in the steady state levels of K11 and K48 ubiquitin linkages in whole cell extracts, with K63 ubiquitin linkages only increasing slightly (Figure 1B). Given the important functions of cytosolic and ER-membrane associated p97 in ERAD [4], we fractionated extracts from p97-depleted cells to examine the subcellular partitioning of these ubiquitin polymers. Interestingly, the steady state levels of both K11- and K48-, but not K63-linkages, increased at the ER membrane upon p97 depletion, with no discernible effect in the cytosol (Figure 1C).
As a complementary approach, we tested the effects of transient expression of p97 harboring an ATPase mutation (K524A). This variant of p97 exerts a dominant negative effect on ERAD as it binds but does not release substrates destined for degradation by the 26S proteasome [29, 30]. Similar to p97 depletion, expression of K524A induced Grp78 and increased K11 and K48 ubiquitin linkages in whole cell extracts, but had little, if any, effect on K63 linkages (Figure 1D). In addition, K524A increased K11 and K48, but not K63 ubiquitin linkages in the microsome fraction (Figure 1E, compare lane 6 to 4 and 5), with no obvious effect on cytosolic ubiquitin conjugates (compare lane 3 to 1 and 2). These observations reflect the major function of p97 at the ER membrane in ERAD substrate dislocation [4]. They additionally suggest that p97 associates with both K11 and K48 ubiquitin polymers, presumably once they are attached to proteins on the cytoplasmic side of the ER membrane.
EerI and bortezomib cause the accumulation of K11- and K48-linked ubiquitin on p97
EerI is a cell permeable molecule reported to inhibit several activities at the ER membrane including protein translocation from the cytoplasm into the ER lumen through Sec61 [31], dislocation from the ER to the cytosol through p97 [14], and the deubiquitination of ERAD substrates in a p97-dependent manner [32]. We reasoned that experiments testing EerI and the proteasome inhibitor bortezomib would allow for ER membrane and proteasome dependent effects on ubiquitinated proteins to be evaluated and compared to those of genetically modulating p97 described above.
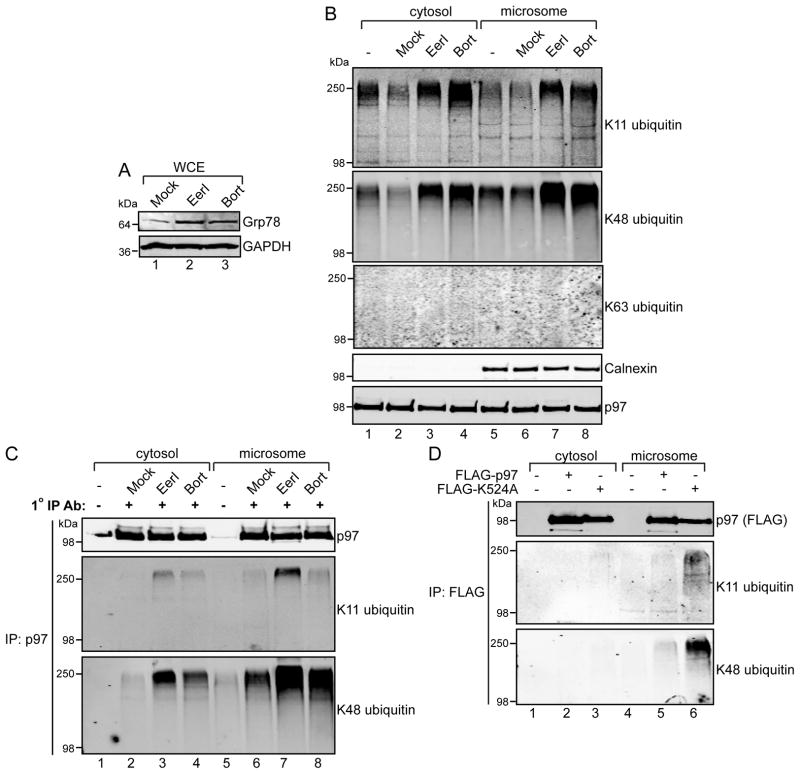
Upon examination of extracts from cells treated with these inhibitors, we found that Grp78 accumulated with both EerI and bortezomib treatment (Figure 2A). In contrast with p97 depletion or inhibition by K524A over-expression, treatment with EerI and bortezomib led to the increase of K11 and K48 ubiquitin linkages in both the cytoplasm and microsome fractions, while those for K63 were unaffected (Figure 2B). While bortezomib reflects inhibition of the general cellular functions of the proteasome, the effects of EerI on cytoplasmic ubiquitin conjugates are somewhat unexpected in light of its reported ER membrane targeting [14]. We hypothesized that this effect could be due to the molecule inhibiting both ER membrane-associated and cytosolic p97.
FIGURE 2. EerI and bortezomib cause the accumulation of K11 and K48 polyubiquitin on p97.
(A) 293 cells were treated with EerI (10 μM) or bortezomib (Bort, 3 μM) for 8 hours, and whole-cell extracts prepared as in Figure 1B and analyzed by immunoblot. (B) Cell extracts were further fractionated into cytosol and microsomes. (C) Cytosol and microsome fractions from (B) were incubated with a p97 monoclonal antibody and captured with Protein A/G resin. Elutates were analyzed by immunoblotting with the indicated antibodies. (D) FLAG-tagged proteins were recovered from cytosol or microsomal fractions with anti-FLAG Sepharose, and elutates analyzed by immunoblotting.
To explore this possibility, we immunoprecipitated endogenous p97 from cytosol and microsome fractions to directly examine p97-associated ubiquitin (Figure 2C). Although both treatments resulted in an increase in K11- and K48-linked ubiquitin associated with p97 in both fractions, the levels of both were higher in the microsome preparations. Consistent with these observations, the ubiquitin linkages were also enriched with dominant negative p97 K524A at the ER membrane (Figure 2D). Thus, in spite of distinct molecular targets, bortezomib and EerI cause similar increases in K11 and K48 polyubiquitin associated with p97. As this occurs mostly at the ER membrane, these signals may reflect the major function of p97 in ubiquitin-dependent protein homeostasis by the proteasome and are not necessarily indicative of a specific ERAD role.
ER stressors upstream of p97 do not promote K11 and K48 ubiquitin conjugate accumulation
To test our hypothesis, we sought to distinguish between the functions of p97 in ERAD and general ubiquitin-protein homeostasis. We reasoned that if the EerI-dependent changes in K11- and K48-linkages associated with p97 are directly caused by ER stress induction, other molecules that activate this response should have similar effects.
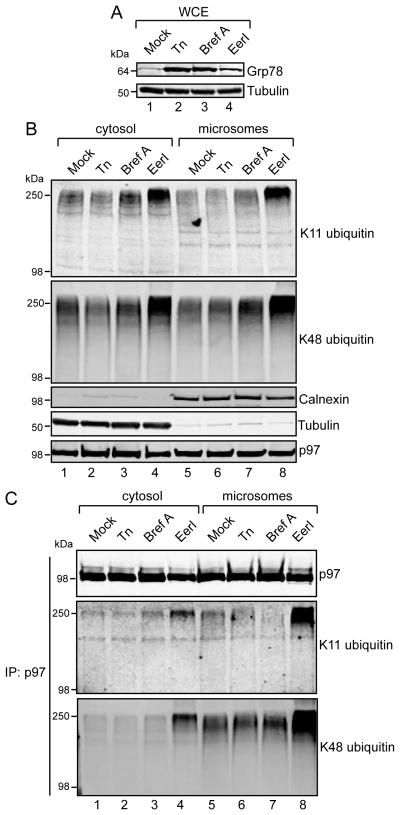
We treated cells with tunicamycin (Tn) and Brefeldin A (Bref A), two agents that promote protein misfolding in the ER lumen, leading to ER stress [33]. Importantly, these ER stressors function upstream of p97, and would therefore not be expected to directly interfere with p97-mediated substrate dislocation. Tn and Bref A potently induced ER stress as judged by Grp78 induction (Figure 3A). In comparison to EerI, Tn and BrefA did not cause increases in K11- or K48-linked ubiquitin conjugates in either the cytosol or microsome fractions (Figure 3B). Furthermore, these treatments did not affect the abundance of these linkages purifying with immunoprecipitated p97 (Figure 3C). Collectively, these experiments suggest that ER stress is not sufficient to increase K11 and K48 ubiquitin linkages at the ER membrane or associated with p97. Moreover, the p97-dependent clearance of ERAD substrates, as judged by steady state levels of K11 and K48 polyubiquitin, is not perturbed when ER stress is induced upstream of p97.
FIGURE 3. ER stressors upstream of p97 do not promote K11 and K48 polyubiquitin accumulation.
(A, B) 293 cells were treated with tunicamycin (Tn, 1 μM), Brefeldin A (Bref A, 3.6 μM), or EerI (10 μM) for 8 hours and whole cell extracts prepared (A) and fractionated into cytosol and microsomes (B) prior to analysis by immunoblotting with the indicated antibodies. (C) p97 was immunoprecipitated from the fractions prepared in (B) with p97 monoclonal antibodies and elutates were analyzed by immunoblotting.
A catalytically inactive p97-associated deubiquitinase causes K11 and K48 polyubiquitin accumulation on p97
In addition to its ATPase activity, p97 requires associated cofactors that participate in ERAD substrate clearance [4]. Deubiquitinating enzymes (also known as deubiquitinases), in particular, have been proposed to facilitate dislocation by editing the length of the ubiquitin chain which promotes ER membrane extraction and protein unfolding during ERAD through an unclear mechanism [34–37]. In support of this model, over-expression of catalytically inactive YOD1 (referred to as C160S) has been shown to block the ubiquitin-dependent degradation of model ERAD substrates [35].
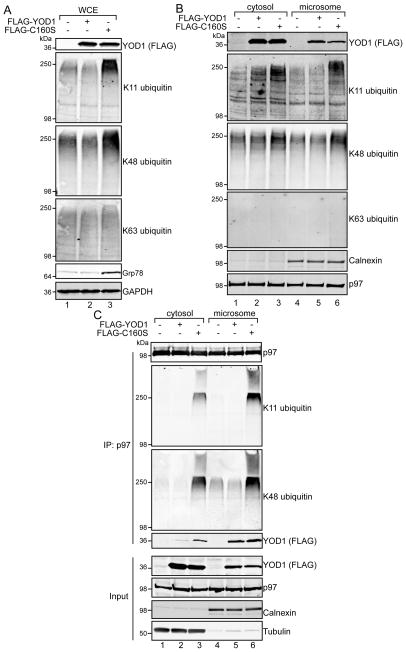
We examined the effects of C160S on p97-associated ubiquitin. Over-expression of FLAG-tagged C160S induced ER stress and caused the accumulation of K11 and K48 polyubiquitin in whole cell extracts (Figure 4A), without affecting K63 polyubiquitin, similar to our earlier observations with p97 loss-of-function experiments (Figure 1B, 1D, and 2A). Subcellular fractionation experiments revealed cytosolic and ER pools of YOD1 (Figure 4B). However, only C160S over-expression resulted in the accumulation of K11 and K48 polyubiquitin in the cytosol and at the ER membrane (compare lane 1 to 3, and 4 to 6). The ubiquitin conjugates observed at the ER membrane likely represent stalled dislocation substrates [35]. The increase in the cytosol could reflect non-ERAD functions of YOD1, especially since only a fraction of YOD1 appeared to be associated with the ER membrane.
FIGURE 4. Catalytically inactive YOD1 causes accumulation of K11 and K48 polyubiquitin on p97.
(A, B) 293 cells were transfected with FLAG-tagged YOD1 or the catalytically inactive variant, C160S. Whole cell (A), and cytosol and microsome extracts (B) were prepared and analyzed by immunoblotting with the indicated antibodies. (C) Endogenous p97 was immunoprecipitated from the cytosol or microsome fractions prepared from cells transfected with FLAG-YOD1 or FLAG-C160S. Elutates were analyzed by immunoblotting. The input represents 5% of that used for immunoprecipitation.
To investigate whether C160S also increases polyubiquitin associated with p97, endogenous p97 was immunoprecipitated from cytosol or microsome fractions transiently expressing YOD1 or C160S. Interestingly, greater amounts of YOD1 and C160S co-immunoprecipitated with p97 from the microsome fraction compared to the cytosol fraction, suggesting they preferentially interact with the membrane-associated pool of p97 (Figure 4C, compare lane 2 to 5, and lane 3 to 6). However, both cytosolic and microsomal p97 had increased co-purification of K11 and K48 polyubiquitin only with C160S expression. These data are consistent with our EerI experiments, suggesting that C160S also inhibits p97 both at the ER membrane and in the cytoplasm.
The effects of YOD1-C160S are through p97
Although YOD1 has been shown to interact with p97 through its UBX-like (UBX-L) domain [35], over-expression of YOD1 or C160S could uncouple its p97-dependent functions, leading to p97-independent changes in cellular polyubiquitin pools. If correct, this would indirectly contribute to the accumulation of polyubiquitin associated with p97 upon C160S co-expression. Potentially consistent with this is the observation that higher levels of YOD1 and C160S were observed in the cytosol (Figure 4B) while more of these proteins associated with p97 purified from microsomes (Figure 4C).
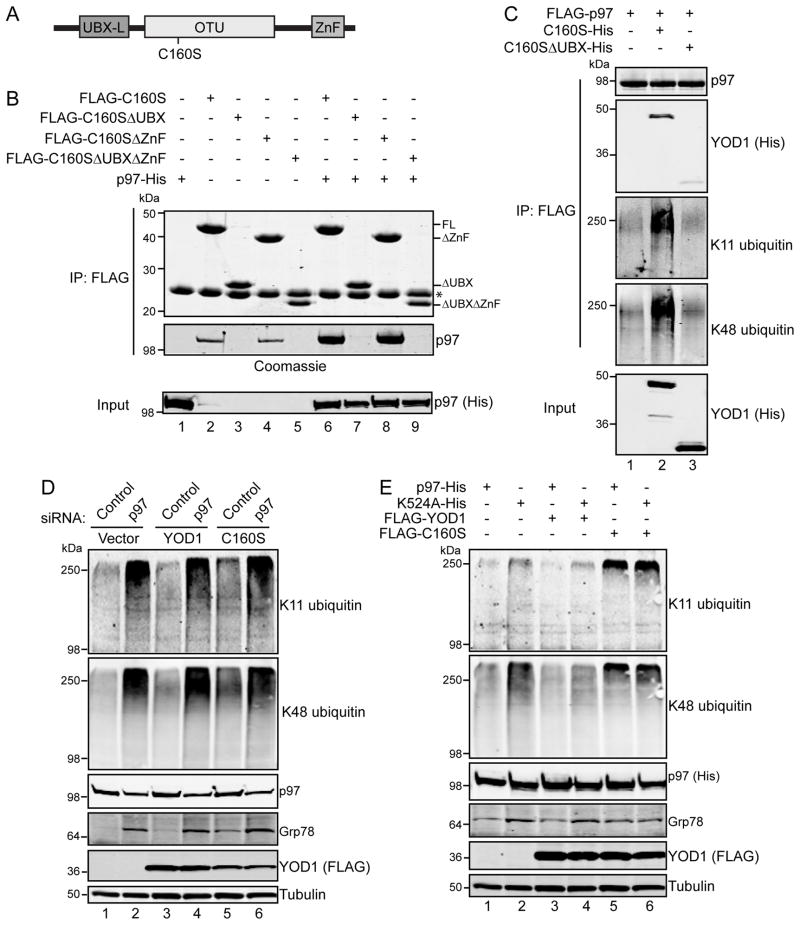
To address this, we first investigated the binding determinants for the p97-C160S interaction. In addition to its N-terminal UBX-L domain, YOD1 consists of a catalytic ovarian tumor (OTU) domain and a C-terminal zinc finger domain (ZnF) (Figure 5A). Similar to Ernst et al, we found that removal of the UBX-L domain significantly impaired expression of the protein in 293 cells (data not shown). To circumvent this issue and allow for more direct comparisons, we co-infected insect cells with baculoviruses expressing FLAG-tagged C160S derivatives and His-tagged p97, and immunoprecipitated C160S from cell extracts (Figure 5B). As expected, p97 in this purified, reconstituted system interacts with the C160S UBX-L domain (compare lane 6 to 7 and 9). Moreover, we detected an interaction between C160S and endogenous insect cell p97 with a similar UBX-L domain requirement albeit at levels lower than baculovirus-expressed p97 (compare lane 2 to 3 and 5).
FIGURE 5. The dominant negative effects of YOD1-C160S on ubiquitin levels and Grp78 are through p97.
(A) YOD1 domain organization. UBX-L, ubiquitin regulatory X-like; OTU, ovarian tumor; ZnF, C2H2 zinc finger. (B) FLAG-tagged YOD1-C160S or variants lacking the UBX-like and ZnF domains were expressed in insect cells from recombinant baculoviruses, with or without co-expressed His-tagged p97. Forty-eight hours after infection, FLAG-tagged proteins were immunoprecipitated from cell extracts with anti-FLAG affinity resin. Elutates were resolved by SDS-PAGE and proteins visualized by Coomassie staining. A fraction of the input (5%) used for immunoprecipitation was analyzed by immunoblotting with anti-His antibodies to assess expression of recombinant p97. Note that FLAG-YOD1 and FLAG-YOD1ΔZnF without p97-His co-infection co-immunoprecipitate endogenous insect cell p97 (lanes 2 and 4). (C) FLAG-tagged p97 was expressed in baculovirus infected insect cells with catalytically inactive YOD1 (C160S-His) or a variant lacking the UBX-like domain (C160SΔUBX-His). FLAG-p97 was immunoprecipitated and eluates analyzed by immunoblotting. (D) 293 cells were transfected with FLAG-YOD1 or FLAG-C160S together with non-targeting control or p97 siRNAs. Whole-cell extracts were analyzed by immunoblot. (E) 293 cells were transfected with His-tagged p97 or the ATPase deficient mutant, K524A, in combination with FLAG-YOD1 or FLAG-C160S. Whole-cell extracts were examined by immunoblotting with the indicated antibodies.
We next evaluated the ubiquitin conjugates associated with FLAG-tagged p97 immunoprecipitated from cells co-infected with His-tagged C160S or C160S lacking the UBX-L domain (Figure 5C). Whereas a basal level of K11 and K48 polyubiquitin co-purified with p97 in the absence of C160S expression, both increased with the association between C160S and p97 (compare lane 1 to 2), confirming our earlier observation (Figure 4C). Importantly, this effect is directly attributable to the interaction between p97 and C160S as deletion of the UBX-L domain did not increase ubiquitin conjugates associated with p97 over p97 alone (compare lane 1 to 3).
To further investigate the relationship between p97 and YOD1, we performed experiments in which YOD1 or C160S were tested with p97 depletion (Figure 5D) or K524A over-expression in 293 cells (Figure 5E). Although C160S expression is sufficient to induce Grp78 and increase the steady state levels of K11 and K48 ubiquitin conjugates (compare lane 5 to 1 and 3 in Figure 5D and 5E), its co-expression did not further increase these levels over p97 depletion or dominant negative over-expression alone (compare lane 6 to 2).
Taken together, these observations suggest that the effects of C160S on ubiquitin conjugates are predominantly through p97-associated functions and the increases caused by C160S are through directly inhibiting p97 rather than non-specifically affecting ubiquitin-dependent protein homeostasis mechanisms.
p97 depletion and inhibition promote the accumulation of ubiquitinated CD3δ at the ER
As our observations thus far have found bulk changes in polyubiquitin, we next wanted to directly assess the ubiquitination of a specific protein. We turned our attention to the model ERAD substrate CD3δ, a type I transmembrane protein. In the absence of its other T-cell receptor subunits, CD3δ is dislocated from the ER membrane into the cytoplasm for ubiquitin-dependent degradation by the proteasome [38].
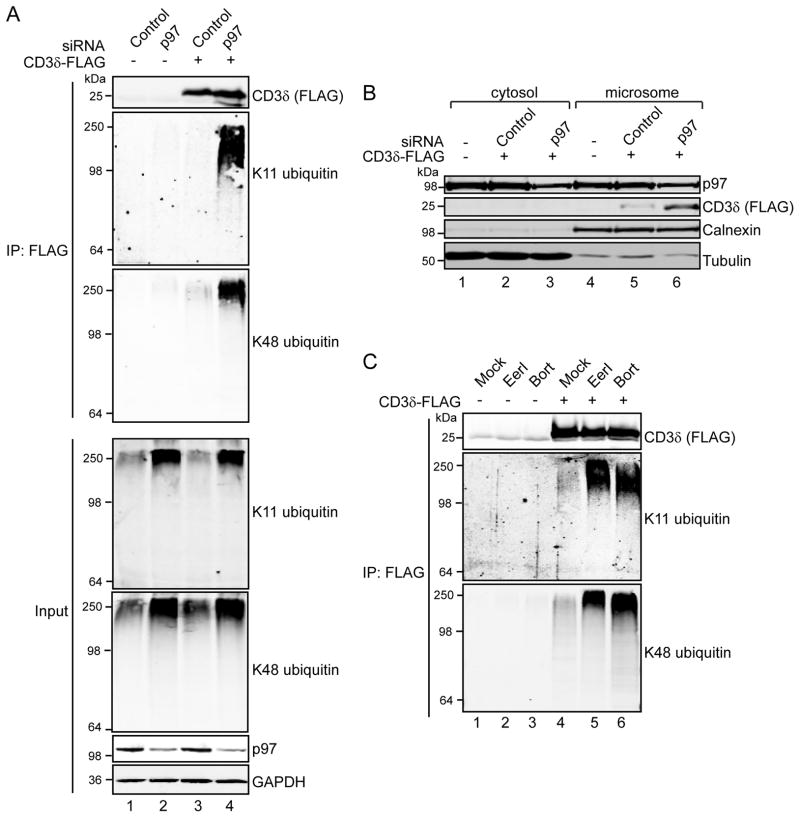
To study the effects of p97 depletion on CD3δ ubiquitination, we expressed CD3δ with a C-terminal FLAG tag and depleted p97 levels using a targeting siRNA. Proteins were solubilized in RIPA buffer containing the ionic detergents 0.1% (w/v) SDS and 1% (w/v) deoxycholate to release associated proteins, and FLAG-tagged proteins recovered with anti-FLAG Sepharose, a method previously used to study the ubiquitination of T-cell receptor α (TCRα [27]. In agreement with our earlier findings, immunoblotting with ubiquitin linkage-specific antibodies revealed that p97 knockdown dramatically increased K11 and K48 ubiquitinated CD3δ (Figure 6A, compare lane 3 to 4).
FIGURE 6. p97 depletion or inhibition promotes accumulation of CD3δ and stabilizes its ubiquitinated form.
(A) 293 cells were transfected with CD3δ-FLAG and non-targeting control or p97-specific siRNAs. FLAG-tagged proteins were immunoprecipitated from RIPA extracts using anti-FLAG Sepharose, and ubiquitinated CD3δ was detected by immunoblotting the elutates with ubiquitin linkage-specific antibodies. (B) 293 cells were transfected as in (A), fractionated into cytosol and microsomes, and analyzed by immunoblotting. (C) 293 cells were transfected with FLAG-tagged CD3δ, and forty hours post-transfection treated with EerI (10 μM) or bortezomib (Bort, 3 μM) for 8 hours. Proteins were solubilized in RIPA buffer and incubated with anti-FLAG Sepharose. Ubiquitinated CD3δ-FLAG was detected with ubiquitin linkage-specific antibodies.
To examine if changes in CD3δ ubiquitination alter its association with the ER membrane, we looked at the levels of microsomal CD3δ in p97 knockdown cells. Subcellular fractionation revealed that CD3δ is localized exclusively to the microsomal fraction, and is not detectable in the cytosol (Figure 6B, compare lane 2 to 5 and 3 to 6), in agreement with a previous study [38]. P97 knockdown increased the levels of microsomal CD3δ (compare lane 5 to 6), indicating compromised CD3δ dislocation.
To explore this further, we evaluated the effects of EerI and bortezomib on CD3δ ubiquitination by immunoprecipitating CD3δ-FLAG from RIPA extracts prepared from EerI or bortezomib treated cells. Low levels of K11 and K48 ubiquitinated CD3δ were detected in mock-treated cells, which increased with EerI and bortezomib treatment (Figure 6C, compare lane 4 to 5 and 6). Taken together, these observations suggest that K11 and K48 ubiquitination of CD3δ is involved in its p97-dependent dislocation from the ER, preceding the targeting of the protein to the proteasome.
Catalytically inactive YOD1 binds and stabilizes ubiquitinated CD3δ
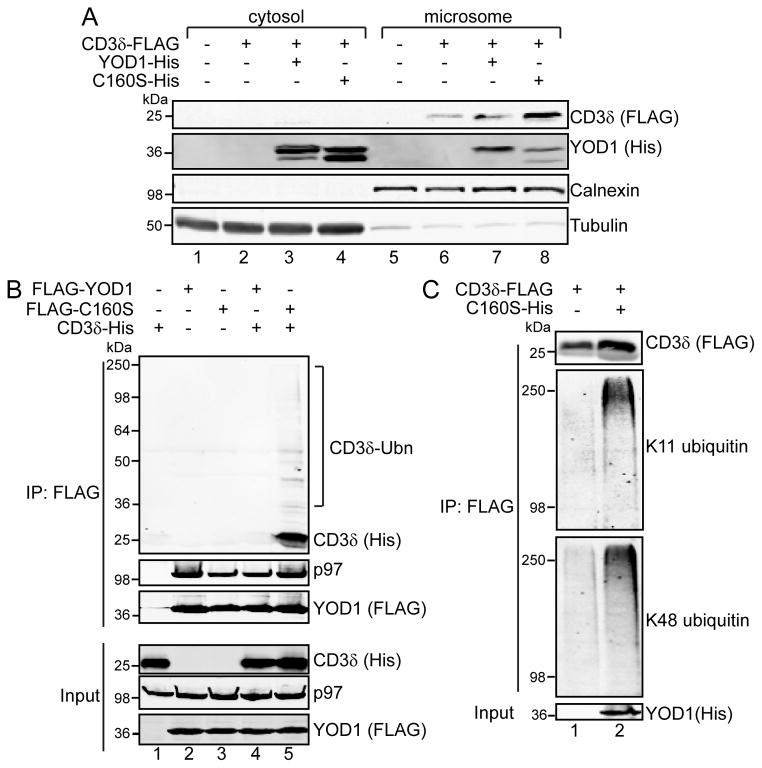
Having established that catalytically inactive YOD1 (C160S) inhibits the p97-dependent clearance of K11 and K48 ubiquitinated proteins (Figure 4C), we next studied the effects of C160S over-expression on CD3δ. We found that over-expression of C160S increased the levels of CD3δ at the ER membrane (Figure 7A, compare lane 8 to 6 and 7). We reasoned that C160S may be increasing CD3δ at the ER by binding ubiquitinated CD3δ, but not deubiquitinating and releasing it for p97-dependent processing. In this case, C160S could be acting as a “substrate trap”, similar to observations on other catalytically inactive deubiquitinases [39].
FIGURE 7. YOD1-C160S binds and stabilizes ubiquitinated CD3δ.
(A) 293 cells were transfected with CD3δ-FLAG and His-tagged YOD1 or C160S, fractionated into cytosol and microsomes, and analyzed by immunoblotting with the indicated antibodies. (B) 293 cells were transfected with CD3δ-His and FLAG-tagged YOD1 or C160S, and cell extracts prepared with Triton X-100 lysis buffer, which maintains protein-protein interactions. FLAG-tagged proteins were captured with anti-FLAG Sepharose, and elutates analyzed by immunoblotting. (C) CD3δ-FLAG was expressed in 293 cells with or without C160S-His. Cells were solubilized in RIPA buffer, and incubated with anti-FLAG Sepharose. Ubiquitinated CD3δ was detected with ubiquitin linkage specific antibodies. The input represents 5% of that used for immunoprecipitation.
To test this possibility, we expressed FLAG-tagged YOD1 or C160S with His-tagged CD3δ and immunoprecipitated FLAG-tagged proteins from whole-cell extracts. Both YOD1 and C160S co-immunoprecipitated endogenous p97, but only C160S recovered CD3δ (Figure 7B, lane 5, upper panel). We noticed that C160S also co-immunoprecipitated high molecular weight His-tag reactive species, indicative of ubiquitinated CD3δ. Furthermore, C160S increased the levels of K11 and K48 polyubiquitinated CD3δ as judged by immunoblotting CD3δ-FLAG immunoprecipitated from RIPA cell extracts co-expressing C160S (Figure 7C). We conclude that the dominant negative effect of C160S results from binding but not releasing K11 and K48 ubiquitinated dislocation substrates, potentially trapping p97 in stalled dislocation complexes. As a result, ERAD is compromised and ER stress ensues.
DISCUSSION
P97 has a central role in the degradation of ubiquitinated proteins from the endoplasmic reticulum. However, the type of ubiquitin linkages associated with p97 during this process is poorly understood. We found that p97 binds and modulates the level of K11 and K48 ubiquitinated proteins at the ER membrane. We also found that the model ERAD substrate CD3δ is modified with K11 and K48 ubiquitin when associated with the p97 complex. Accordingly, disruption of p97 through chemical or genetic inhibition, siRNA-mediated depletion, or expression of a catalytically inactive p97-associated deubiquitinase all result in the accumulation of K11 and K48 polyubiquitin.
Our data provide evidence linking K11 ubiquitination to p97 in human cells with a role in ERAD substrate clearance. While the role of K48 ubiquitin in targeting cytosolic and ER proteins for degradation has been known for some time, the importance of K11 ubiquitination has only recently been appreciated [21]. A hint that K11 ubiquitin might be involved with p97 came from a proteomic analysis of the UBA-UBX protein interaction network, which were enriched for K11 linked ubiquitin [26]. UBA-UBX proteins simultaneously bind p97 and ubiquitinated proteins, potentially linking p97 to a wide array of substrates in diverse cellular pathways [40]. Two of these UBA-UBX proteins, UBXD8 and SAKS1, have roles in ERAD [41, 42], lending support for the involvement of K11 ubiquitin modifications in this process.
A role for K11 ubiquitination in maintaining ER homeostasis has also been suggested by proteomic studies in yeast, which found K11 chains as abundant as K48, and accumulated when ER stress was induced with tunicamycin [22]. However, a subsequent report failed to identify appreciable levels of K11 linkages in yeast [43], and unstimulated, non-mitotic human embryonic kidney cells have only low levels of K11 linkages [25]. We did not observe any changes in the levels of ubiquitinated proteins at the ER or interacting with p97 when ER stress was induced with tunicamycin or Brefeldin A in 293 cells (Figure 3). However, blocking ERAD substrate dislocation with EerI or protein degradation by the proteasome with bortezomib resulted in the accumulation of ubiquitinated proteins on both ER-associated and cytoplasmic p97 (Figure 2).
The up-regulation of ERAD is an important facet of the unfolded protein response (UPR) to alleviate ER stress [10]. It is possible that the accumulation of ubiquitinated proteins occurs only when ERAD and the UPS are specifically inhibited or overwhelmed such as through p97 and proteasome inhibition. It is interesting to note that K11 polyubiquitin has been discovered in protein aggregates that characterize the pathology of neurodegenerative diseases, such as Alzheimer’s and Huntington’s [44], and that p97 is often observed in these structures [45]. In addition, impaired ER homeostasis resulting from p97 dysfunction contributes to polyQ toxicity in models of Huntington’s disease [46].
An important issue for future study is the identity of the enzymes responsible for the synthesis of K11 ubiquitin conjugates associated with p97. In mammals, at least three ubiquitin conjugating enzymes (UBE2J1, UBE2J2, and UBE2G2) and five ubiquitin ligases (HRD1, GP78, TRC8, RMA1, and TEB4) have been implicated in ERAD [47]. Interestingly, deletion of UBC6, the yeast orthologue of UBE2J1 and UBE2J2, reduces the total cellular K11 ubiquitin linkages by 40% [22]. Both UBE2J1 and UBE2J2 have been implicated in the degradation of T-cell receptor subunits [48, 49], as have the ubiquitin ligases HRD1 and GP78 [27, 50, 51]. Given our finding that CD3δ is modified with K11- and K48-linked polyubiquitin (Figure 6), it will now be possible to identify the E2(s) and E3(s) responsible for directing these modifications.
Polyubiquitin is an important dislocation signal for the recruitment of the p97 complex to ERAD substrates [18], and our results indicate that both K11 and K48 linkages are associated with p97 during this process. Recent studies have shown that deubiquitination is integral to dislocation of ERAD substrates from the ER membrane [34, 35]. This requirement has been proposed to facilitate the p97-driven extraction of an ERAD substrate, potentially by allowing it to be threaded through the hexameric p97 structure. Such a model would require a second round of ubiquitination to target the substrate to the proteasome [47]. While this model remains untested, over-expression of a catalytically inactive p97-associated DUB, YOD1-C160S, stalls ERAD substrates at the dislocation step [35]. Interestingly, YOD1-C160S has recently been shown to negatively regulate the retro-translocation of non-ubiquitinated substrates, such as cholera toxin A1 peptide and pro-α-factor [52]. Since the retro-translocation of these substrates does not require functional p97, it may point to p97-independent roles for YOD1 in retro-translocation, such as by regulating the ERAD machinery [52]. Here, we used C160S as a way to disrupt p97 function and found that it preferentially binds p97 at the ER membrane and causes increases in K11 and K48 polyubiquitin associated with p97 (Figure 4C). We propose that YOD1 and other p97-associated deubiquitinases disassemble K11 and/or K48 ubiquitin linkages from substrates during their dislocation.
Summary statement.
This study provides evidence that p97 interacts with proteins modified with K11- and K48-linked ubiquitin chains at the endoplasmic reticulum (ER) membrane, suggesting roles for these signals in protein quality control.
Acknowledgments
We thank Vishva Dixit for providing the anti-K11 ubiquitin linkage specific antibody, Ze’ev Ronai for providing the CD3δ open reading frame and Ze’ev Ronai, Dieter Wolf, and Tasha Toro for helpful discussions and suggestions. This work was supported by a Scholars Award from the V Foundation for Cancer Research, American Cancer Society Research Scholars Grant RSG-11-224-01-DMC, and National Institutes of Health grant RC2CA148414 to MDP.
Abbreviations
- EerI
Eeyarestatin I
- ERAD
endoplasmic reticulum associated degradation
- UPR
unfolded protein response
- UPS
ubiquitin-proteasome system
Footnotes
AUTHOR CONTRIBUTIONS
Matthew Locke, Julia Toth, and Matthew Petroski designed the research, performed the experiments and analyzed the data. Matthew Locke and Matthew Petroski wrote the paper. Matthew Petroski supervised the research.
References
- 1.Glickman MH, Ciechanover A. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev. 2002;82:373–428. doi: 10.1152/physrev.00027.2001. [DOI] [PubMed] [Google Scholar]
- 2.Rumpf S, Jentsch S. Functional division of substrate processing cofactors of the ubiquitin-selective Cdc48 chaperone. Mol Cell. 2006;21:261–269. doi: 10.1016/j.molcel.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 3.Yamanaka K, Sasagawa Y, Ogura T. Recent advances in p97/VCP/Cdc48 cellular functions. Biochim Biophys Acta. 2012;1823:130–137. doi: 10.1016/j.bbamcr.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Wolf DH, Stolz A. The Cdc48 machine in endoplasmic reticulum associated protein degradation. Biochim Biophys Acta. 2012;1823:117–124. doi: 10.1016/j.bbamcr.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Braun S, Matuschewski K, Rape M, Thoms S, Jentsch S. Role of the ubiquitin-selective CDC48(UFD1/NPL4 )chaperone (segregase) in ERAD of OLE1 and other substrates. EMBO J. 2002;21:615–621. doi: 10.1093/emboj/21.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jarosch E, Taxis C, Volkwein C, Bordallo J, Finley D, Wolf DH, Sommer T. Protein dislocation from the ER requires polyubiquitination and the AAA-ATPase Cdc48. Nat Cell Biol. 2002;4:134–139. doi: 10.1038/ncb746. [DOI] [PubMed] [Google Scholar]
- 7.Rabinovich E, Kerem A, Frohlich KU, Diamant N, Bar-Nun S. AAA-ATPase p97/Cdc48p, a cytosolic chaperone required for endoplasmic reticulum-associated protein degradation. Mol Cell Biol. 2002;22:626–634. doi: 10.1128/MCB.22.2.626-634.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ye Y, Meyer HH, Rapoport TA. The AAA ATPase Cdc48/p97 and its partners transport proteins from the ER into the cytosol. Nature. 2001;414:652–656. doi: 10.1038/414652a. [DOI] [PubMed] [Google Scholar]
- 9.Healy SJ, Gorman AM, Mousavi-Shafaei P, Gupta S, Samali A. Targeting the endoplasmic reticulum-stress response as an anticancer strategy. Eur J Pharmacol. 2009;625:234–246. doi: 10.1016/j.ejphar.2009.06.064. [DOI] [PubMed] [Google Scholar]
- 10.Tsai YC, Weissman AM. The Unfolded Protein Response, Degradation from Endoplasmic Reticulum and Cancer. Genes Cancer. 2010;1:764–778. doi: 10.1177/1947601910383011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fribley A, Zeng Q, Wang CY. Proteasome inhibitor PS-341 induces apoptosis through induction of endoplasmic reticulum stress-reactive oxygen species in head and neck squamous cell carcinoma cells. Mol Cell Biol. 2004;24:9695–9704. doi: 10.1128/MCB.24.22.9695-9704.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fiebiger E, Hirsch C, Vyas JM, Gordon E, Ploegh HL, Tortorella D. Dissection of the dislocation pathway for type I membrane proteins with a new small molecule inhibitor, eeyarestatin. Mol Biol Cell. 2004;15:1635–1646. doi: 10.1091/mbc.E03-07-0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Q, Mora-Jensen H, Weniger MA, Perez-Galan P, Wolford C, Hai T, Ron D, Chen W, Trenkle W, Wiestner A, Ye Y. ERAD inhibitors integrate ER stress with an epigenetic mechanism to activate BH3-only protein NOXA in cancer cells. Proc Natl Acad Sci U S A. 2009;106:2200–2205. doi: 10.1073/pnas.0807611106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Q, Shinkre BA, Lee JG, Weniger MA, Liu Y, Chen W, Wiestner A, Trenkle WC, Ye Y. The ERAD inhibitor Eeyarestatin I is a bifunctional compound with a membrane-binding domain and a p97/VCP inhibitory group. PLoS One. 2010;5:e15479. doi: 10.1371/journal.pone.0015479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pickart CM, Fushman D. Polyubiquitin chains: polymeric protein signals. Curr Opin Chem Biol. 2004;8:610–616. doi: 10.1016/j.cbpa.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 16.Hicke L, Schubert HL, Hill CP. Ubiquitin-binding domains. Nat Rev Mol Cell Biol. 2005;6:610–621. doi: 10.1038/nrm1701. [DOI] [PubMed] [Google Scholar]
- 17.Chau V, Tobias JW, Bachmair A, Marriott D, Ecker DJ, Gonda DK, Varshavsky A. A multiubiquitin chain is confined to specific lysine in a targeted short-lived protein. Science. 1989;243:1576–1583. doi: 10.1126/science.2538923. [DOI] [PubMed] [Google Scholar]
- 18.Flierman D, Ye Y, Dai M, Chau V, Rapoport TA. Polyubiquitin serves as a recognition signal, rather than a ratcheting molecule, during retrotranslocation of proteins across the endoplasmic reticulum membrane. J Biol Chem. 2003;278:34774–34782. doi: 10.1074/jbc.M303360200. [DOI] [PubMed] [Google Scholar]
- 19.Skaug B, Jiang X, Chen ZJ. The role of ubiquitin in NF-kappaB regulatory pathways. Annu Rev Biochem. 2009;78:769–796. doi: 10.1146/annurev.biochem.78.070907.102750. [DOI] [PubMed] [Google Scholar]
- 20.Peng J, Schwartz D, Elias JE, Thoreen CC, Cheng D, Marsischky G, Roelofs J, Finley D, Gygi SP. A proteomics approach to understanding protein ubiquitination. Nat Biotechnol. 2003;21:921–926. doi: 10.1038/nbt849. [DOI] [PubMed] [Google Scholar]
- 21.Bremm A, Komander D. Emerging roles for Lys11-linked polyubiquitin in cellular regulation. Trends Biochem Sci. 2011;36:355–363. doi: 10.1016/j.tibs.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 22.Xu P, Duong DM, Seyfried NT, Cheng D, Xie Y, Robert J, Rush J, Hochstrasser M, Finley D, Peng J. Quantitative proteomics reveals the function of unconventional ubiquitin chains in proteasomal degradation. Cell. 2009;137:133–145. doi: 10.1016/j.cell.2009.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wickliffe KE, Williamson A, Meyer HJ, Kelly A, Rape M. K11-linked ubiquitin chains as novel regulators of cell division. Trends Cell Biol. 2011;21:656–663. doi: 10.1016/j.tcb.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bremm A, Freund SM, Komander D. Lys11-linked ubiquitin chains adopt compact conformations and are preferentially hydrolyzed by the deubiquitinase Cezanne. Nat Struct Mol Biol. 2010;17:939–947. doi: 10.1038/nsmb.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsumoto ML, Wickliffe KE, Dong KC, Yu C, Bosanac I, Bustos D, Phu L, Kirkpatrick DS, Hymowitz SG, Rape M, Kelley RF, Dixit VM. K11-linked polyubiquitination in cell cycle control revealed by a K11 linkage-specific antibody. Mol Cell. 2010;39:477–484. doi: 10.1016/j.molcel.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 26.Alexandru G, Graumann J, Smith GT, Kolawa NJ, Fang R, Deshaies RJ. UBXD7 binds multiple ubiquitin ligases and implicates p97 in HIF1alpha turnover. Cell. 2008;134:804–816. doi: 10.1016/j.cell.2008.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishikura S, Weissman AM, Bonifacino JS. Serine residues in the cytosolic tail of the T-cell antigen receptor alpha-chain mediate ubiquitination and endoplasmic reticulum-associated degradation of the unassembled protein. J Biol Chem. 2010;285:23916–23924. doi: 10.1074/jbc.M110.127936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee AS. The ER chaperone and signaling regulator GRP78/BiP as a monitor of endoplasmic reticulum stress. Methods. 2005;35:373–381. doi: 10.1016/j.ymeth.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 29.DeLaBarre B, Christianson JC, Kopito RR, Brunger AT. Central pore residues mediate the p97/VCP activity required for ERAD. Mol Cell. 2006;22:451–462. doi: 10.1016/j.molcel.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 30.Kobayashi T, Tanaka K, Inoue K, Kakizuka A. Functional ATPase activity of p97/valosin-containing protein (VCP) is required for the quality control of endoplasmic reticulum in neuronally differentiated mammalian PC12 cells. J Biol Chem. 2002;277:47358–47365. doi: 10.1074/jbc.M207783200. [DOI] [PubMed] [Google Scholar]
- 31.Cross BC, McKibbin C, Callan AC, Roboti P, Piacenti M, Rabu C, Wilson CM, Whitehead R, Flitsch SL, Pool MR, High S, Swanton E. Eeyarestatin I inhibits Sec61-mediated protein translocation at the endoplasmic reticulum. J Cell Sci. 2009;122:4393–4400. doi: 10.1242/jcs.054494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Q, Li L, Ye Y. Inhibition of p97-dependent protein degradation by Eeyarestatin I. J Biol Chem. 2008;283:7445–7454. doi: 10.1074/jbc.M708347200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Samali A, Fitzgerald U, Deegan S, Gupta S. Methods for monitoring endoplasmic reticulum stress and the unfolded protein response. Int J Cell Biol. 2010;2010:830307. doi: 10.1155/2010/830307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ernst R, Claessen JH, Mueller B, Sanyal S, Spooner E, van der Veen AG, Kirak O, Schlieker CD, Weihofen WA, Ploegh HL. Enzymatic blockade of the ubiquitin-proteasome pathway. PLoS Biol. 2011;8:e1000605. doi: 10.1371/journal.pbio.1000605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ernst R, Mueller B, Ploegh HL, Schlieker C. The otubain YOD1 is a deubiquitinating enzyme that associates with p97 to facilitate protein dislocation from the ER. Mol Cell. 2009;36:28–38. doi: 10.1016/j.molcel.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Q, Li L, Ye Y. Regulation of retrotranslocation by p97-associated deubiquitinating enzyme ataxin-3. J Cell Biol. 2006;174:963–971. doi: 10.1083/jcb.200605100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhong X, Pittman RN. Ataxin-3 binds VCP/p97 and regulates retrotranslocation of ERAD substrates. Hum Mol Genet. 2006;15:2409–2420. doi: 10.1093/hmg/ddl164. [DOI] [PubMed] [Google Scholar]
- 38.Yang M, Omura S, Bonifacino JS, Weissman AM. Novel aspects of degradation of T cell receptor subunits from the endoplasmic reticulum (ER) in T cells: importance of oligosaccharide processing, ubiquitination, and proteasome-dependent removal from ER membranes. J Exp Med. 1998;187:835–846. doi: 10.1084/jem.187.6.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Edelmann MJ, Iphofer A, Akutsu M, Altun M, di Gleria K, Kramer HB, Fiebiger E, Dhe-Paganon S, Kessler BM. Structural basis and specificity of human otubain 1-mediated deubiquitination. Biochem J. 2009;418:379–390. doi: 10.1042/BJ20081318. [DOI] [PubMed] [Google Scholar]
- 40.Schuberth C, Buchberger A. UBX domain proteins: major regulators of the AAA ATPase Cdc48/p97. Cell Mol Life Sci. 2008;65:2360–2371. doi: 10.1007/s00018-008-8072-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.LaLonde DP, Bretscher A. The UBX protein SAKS1 negatively regulates endoplasmic reticulum-associated degradation and p97-dependent degradation. J Biol Chem. 2011;286:4892–4901. doi: 10.1074/jbc.M110.158030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mueller B, Klemm EJ, Spooner E, Claessen JH, Ploegh HL. SEL1L nucleates a protein complex required for dislocation of misfolded glycoproteins. Proc Natl Acad Sci U S A. 2008;105:12325–12330. doi: 10.1073/pnas.0805371105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ziv I, Matiuhin Y, Kirkpatrick DS, Erpapazoglou Z, Leon S, Pantazopoulou M, Kim W, Gygi SP, Haguenauer-Tsapis R, Reis N, Glickman MH, Kleifeld O. A perturbed ubiquitin landscape distinguishes between ubiquitin in trafficking and in proteolysis. Mol Cell Proteomics. 2011;10 doi: 10.1074/mcp.M111.009753. M111.009753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cripps D, Thomas SN, Jeng Y, Yang F, Davies P, Yang AJ. Alzheimer disease-specific conformation of hyperphosphorylated paired helical filament-Tau is polyubiquitinated through Lys-48, Lys-11, and Lys-6 ubiquitin conjugation. J Biol Chem. 2006;281:10825–10838. doi: 10.1074/jbc.M512786200. [DOI] [PubMed] [Google Scholar]
- 45.Hirabayashi M, Inoue K, Tanaka K, Nakadate K, Ohsawa Y, Kamei Y, Popiel AH, Sinohara A, Iwamatsu A, Kimura Y, Uchiyama Y, Hori S, Kakizuka A. VCP/p97 in abnormal protein aggregates, cytoplasmic vacuoles, and cell death, phenotypes relevant to neurodegeneration. Cell Death Differ. 2001;8:977–984. doi: 10.1038/sj.cdd.4400907. [DOI] [PubMed] [Google Scholar]
- 46.Duennwald ML, Lindquist S. Impaired ERAD and ER stress are early and specific events in polyglutamine toxicity. Genes Dev. 2008;22:3308–3319. doi: 10.1101/gad.1673408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Claessen JH, Kundrat L, Ploegh HL. Protein quality control in the ER: balancing the ubiquitin checkbook. Trends Cell Biol. 2011;22:22–32. doi: 10.1016/j.tcb.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lenk U, Yu H, Walter J, Gelman MS, Hartmann E, Kopito RR, Sommer T. A role for mammalian Ubc6 homologues in ER-associated protein degradation. J Cell Sci. 2002;115:3007–3014. doi: 10.1242/jcs.115.14.3007. [DOI] [PubMed] [Google Scholar]
- 49.Tiwari S, Weissman AM. Endoplasmic reticulum (ER)-associated degradation of T cell receptor subunits. Involvement of ER-associated ubiquitin-conjugating enzymes (E2s) J Biol Chem. 2001;276:16193–16200. doi: 10.1074/jbc.M007640200. [DOI] [PubMed] [Google Scholar]
- 50.Cattaneo M, Otsu M, Fagioli C, Martino S, Lotti LV, Sitia R, Biunno I. SEL1L and HRD1 are involved in the degradation of unassembled secretory Ig-mu chains. J Cell Physiol. 2008;215:794–802. doi: 10.1002/jcp.21364. [DOI] [PubMed] [Google Scholar]
- 51.Kikkert M, Doolman R, Dai M, Avner R, Hassink G, van Voorden S, Thanedar S, Roitelman J, Chau V, Wiertz E. Human HRD1 is an E3 ubiquitin ligase involved in degradation of proteins from the endoplasmic reticulum. J Biol Chem. 2004;279:3525–3534. doi: 10.1074/jbc.M307453200. [DOI] [PubMed] [Google Scholar]
- 52.Bernardi KM, Williams JM, Inoue T, Schultz A, Tsai B. A deubiquitinase negatively regulates retro-translocation of non-ubiquitinated substrates. Mol Biol Cell. 2013;24:3545–3556. doi: 10.1091/mbc.E13-06-0332. [DOI] [PMC free article] [PubMed] [Google Scholar]