Abstract
Despite the tremendous success of cisplatin and other platinum-based anticancer drugs, severe toxicity and resistance to tumors limit their applications. It is believed that the coordination (formation of covalent bond) of the metal (platinum) to the nitrogen bases of DNA cause the ruptures of the cancer as well as normal cells. A search for anticancer drugs with different modes of action resulted in the synthesis of variety of novel compounds. Many of them are in clinical trials now. Recently we synthesized a series of novel rhenium pentylcarbonato compounds (PC1–PC6). The rhenium atom in each compound is coordinated (bonded) to a planar polypyridyl aromatic ligand, thereby forcing each compound to intercalate between the DNA bases. We have investigated the DNA binding properties of one of the PC-series of compounds (PC6) using electronic spectroscopy. The UV absorption titration of PC6 with DNA shows hypochromic effect with concomitant bathochromic shift of the charge transfer band at 290 nm. These results suggest that the compound PC6 binds to DNA through intercalation. It is therefore likely that the other PC-series of compounds will behave in a similar manner. Thus it is expected that these compounds will exhibit negligible or no side effect. We have observed that the PC-series of compounds are strong cytotoxic agents against lymphosarcoma (average GI50 ≈ 2±2.6 µM), PC-3 prostate (average GI50 ≈ 3±2.8 µM) and myeloid leukemia (average GI50 ≈ 3±2.8 µM) cancer cell lines. The average GI50 values of the PC-series of compounds are 2–3 less than the corresponding GI50 values of cisplatin. Also each of the PC-series of compounds exhibits less toxicity than cisplatin in the glomerular mesangial cells.
Keywords: Rhenium compounds, lymphosarcoma, PC-3 prostate, myeloid leukemia, cancer cells, mesangial cells, DNA binding, electronic spectroscopy, hypochromic effect, bathochromic shift
INTRODUCTION
The serendipitous discovery of the anticancer property of cisplatin [1] contributed to the development of cancer chemotherapy. Due to its severe side effects and resistance to tumor, clinical effectiveness of cisplatin is limited. Second and third generation platinum drugs are not free from side effects and spontaneous or acquired resistance. As a result, it has been proposed that we should move away from the ‘platinum paradigm’ by developing drugs based on other metals which have different mechanisms of action [2,3]. Non-platinum complexes such as those of ruthenium [4], titanium [5], gold [6], tin [7], palladium [8], rhodium [9], bismuth [10], and gallium [11] exhibit very strong cytotoxicity and possess distinct chemical advantages over cisplatin and related drugs. In fact, some of them entered clinical trials. This opens the door for the exploration of non-platinum anticancer drugs.
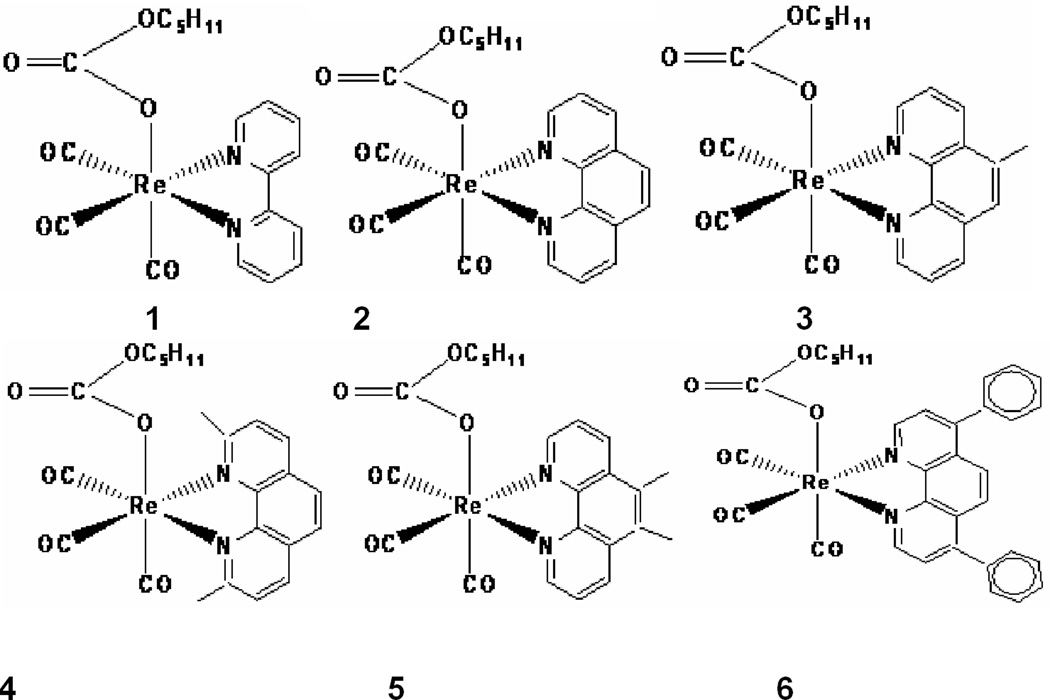
Rhenium organometallics are another very new class of promising antiproliferative compounds. For example, several tricarbonylrhenium compounds have been found to be cytotoxic against numerous cancer cell lines [12–15]. In the present study, we synthesized six novel rhenium pentylcarbanato compounds PC1–PC6 [16]. The structure each compound is depicted in Figure 1. Unlike the rhenium compounds noted above [12–15], the PC-series of compounds exhibit fluorescence [16]. Here we report the cytotoxic effects of the compounds PC1–PC6 on lymphosarcoma, PC-3 prostate, and myeloid leukemia cancer cells and mesangial cells and the DNA-binding study of the compound PC6 using electronic spectroscopy (UV-Visible spectrophotometric titrations).
Figure 1.
The structural formulae of the PC-series of compounds PC1–PC6
Materials and Methods
Tris buffer was prepared according to standard protocol: 5 mM Tris–HCl, 50 mM NaCl, pH 7.2. Calf thymus DNA (CT-DNA) was purchased from Sigma-Aldrich. Solution of CT-DNA in Tris buffer gave a ratio of UV absorbance (A260/A280) of ≈1.9 at 260 and 280 nm, indicating that the DNA was sufficiently free of protein [17]. The concentration of CT- DNA in nucleotide phosphate was determined spectrophotometrically using a molar absorptivity of 6600 M−1 cm−1 (260 nm) [18]. The UV-visible spectra were recorded at room temperature using a Varian Cary 50 Scan UV-Visible Spectrophotometer.
Cell Culture
Lymphosarcoma and myeloid leukemia cancer cell lines were obtained from Coriel Institute, MD(USA) and cultured in RPMI 1640 medium supplemented with 10% Fetal Bovine Serum, penicillin and streptomycin antibiotics, grown at 37° C in a CO2 incubator. PC-3 prostate cancer cell lines were obtained from ATCC, VA (USA) and cultured in the same growth conditions stated above. Mouse glomerular mesangial cells were donated by Dr. C. Reilly at Virginia Tech University, VA (USA). The culture conditions were same as above.
Synthesis of rhenium pentylcarbanato compounds (PC1–PC6)
The rhenium compounds PC1–PC6 were synthesized according to the published procedure [16](Mbagu et.al., 2012). In brief, a mixture of Re2(CO)10 and the corresponding polypyridyls in 1:2 mole ratio was refluxed in 1-pentanol while CO2 was bubbled through the solutions. The polypyridyls in compounds PC1–PC6 are 2,2′-bipyridyl, 1,10-phenanthroline, 5-methyl-1,10-phenanthroline, neocuproin, 5,6-dimethyl-1,10-phenanthroline, and bathophenanthroline, respectively. The reaction was discontinued after 24 hours. After cooling to room temperature, the solids PC1–PC6 were isolated by filtration.
UV Absorption Titrations
We selected only compound PC6 for UV absorption titrations. The titrations of PC6 in Tris buffer were carried out using a fixed concentration (20 µM) of PC6 to which DNA solutions were added. Initially, 3000 µL of the blank buffer and 3000 µL solution of PC6 (20 µM) were placed in the reference and sample cuvettes, respectively. The first spectrum was recorded in the range of 235–700 nm. During the titrations, a measured amount of DNA was added to each cuvette from the DNA stock solution so that the final concentration of the DNA becomes 10, 20, 30, 40, 50, and 60 µM. The solutions were mixed thoroughly by repeated inversion and were allowed to incubate for 10 minutes before the absorption spectra were recorded. The change in concentration of the compound PC6 due to each titration was negligible.
Cytotoxicity Assay
The cancer cell lines were grown in six well plates to confluency, the cells were then kept in serum-free medium overnight and then treated with different doses of drugs PC1–PC6 (dissolved in DMSO) along with untreated cells and DMSO vehicular control for 48 hours. DMSO was used as vehicular control, non-drug treated as negative control and cisplatin as positive control. A Trypan blue assay was done to measure cell viability using a standard hemocytometer and light microscope.
Statistical Analysis
Each experiment was repeated three times and statistical t test was used to analyze the results with P < 0.05.
Results and Discussion
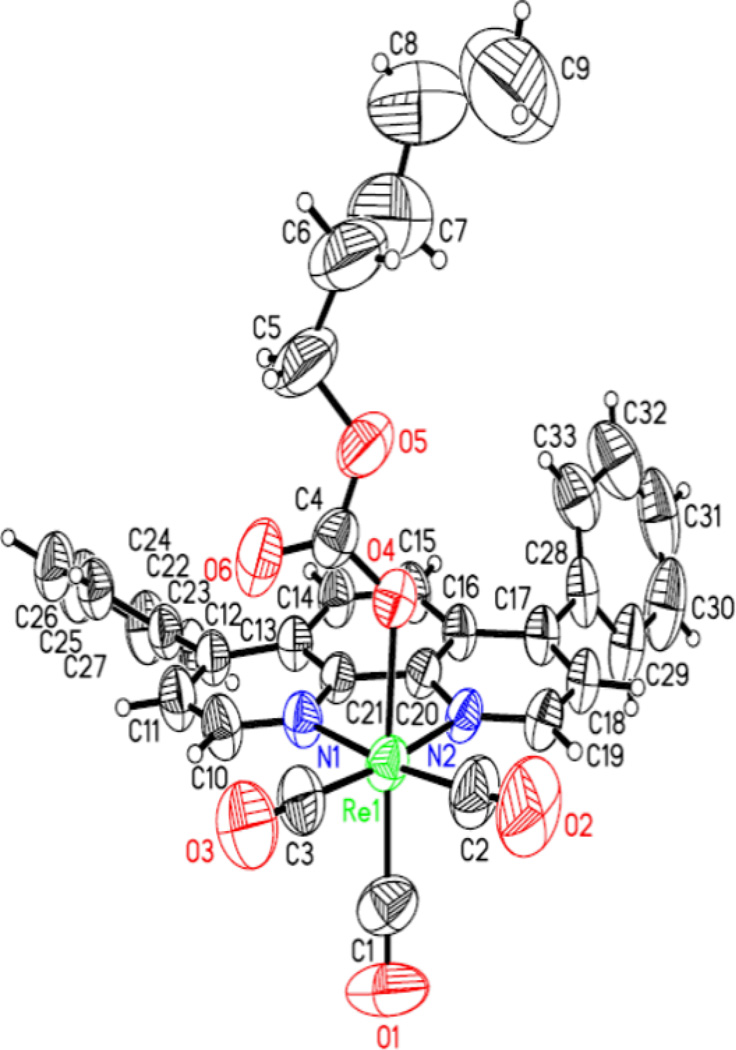
The PC-series of compounds were characterized spectroscopically. Additionally, PC1, PC4 and PC6 were characterized through X-ray crystallography. The X-ray structures of PC1, PC4, and PC6 which are shown in Figures 2–4, respectively, clearly indicate the planarity of the aromatic heterocyclic ligands (viz. 2,2’-bipyridyl, 2,9-dimethyl-1,10-phenanthroline, and 4,7-diphenyl-1,10-phenanthroline). The crystal structures of PC1 and PC6 were published earlier [16]. The crystallographic data for PC4 will be published elsewhere.
Figure 2.
X-ray structure of PC1
Figure 4.
X-ray structure of PC6
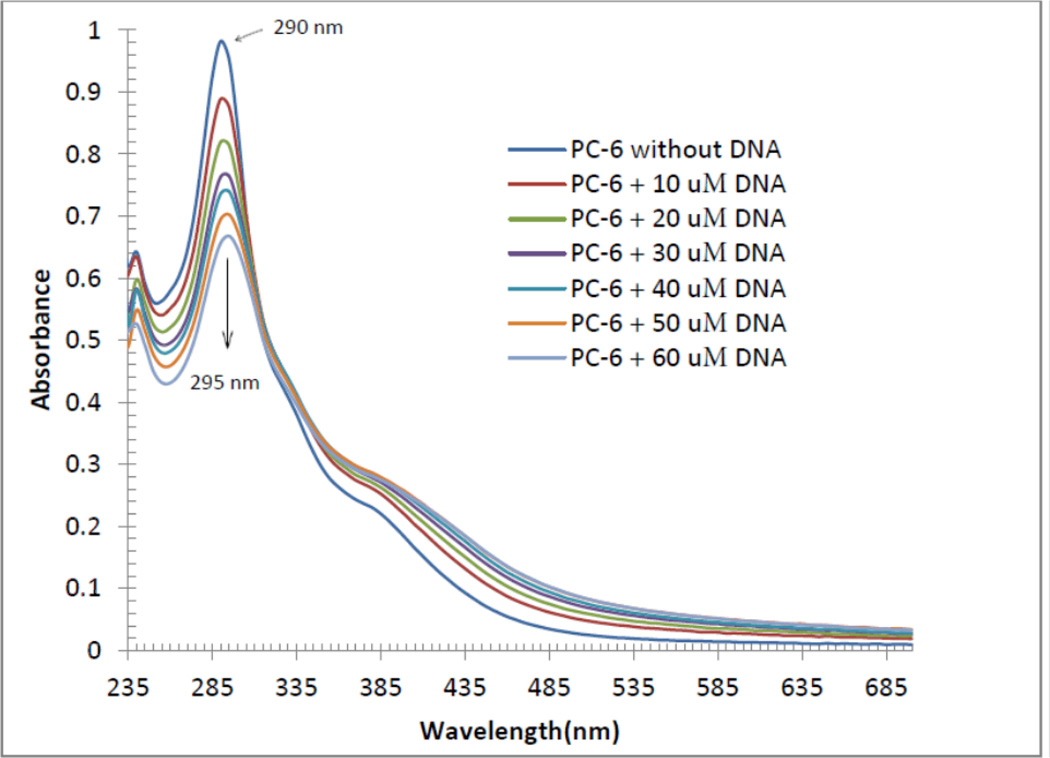
DNA binding studies are important because many anticancer agents work through binding to DNA causing DNA damage in cancer cells, interrupting replication and transcription processes, and inducing apoptosis. The electronic spectroscopy is the most common technique to find out the interaction of compounds with DNA [19]. When a compound binds to DNA through intercalation, the UV-Visible spectra usually shows hypochromism with a red shift. Each of the PC1–PC6 compounds is coordinated to a planar aromatic heterocyclic ligand (see, Figures 1–4). It is therefore possible that each compound will bind to DNA in an intercalative mode, involving a stacking interaction between the aromatic chromophores and DNA base pairs. Figure 5 depicts the UV-Visible spectra of the compound PC6 (20 µM in Tris buffer) in the absence and presence of calf thymus DNA. The intensity of the charge transfer band at 290 nm in PC6 decreases as the DNA concentration (range of 10–60 µM) increases. A small red shift of the band at 290 nm is observed concomitantly after each addition of DNA. A red shift to 295 nm is observed when 60 µM of DNA was added to fixed concentration (20 µM) of PC6. Based on the UV-visible spectra of the compound PC6, it may be concluded that PC6 binds to DNA in an intercalative fashion. Thus PC6 and may be other PC-series of compounds (PC1–PC5) do not bind to DNA via covalent interaction through nitrogen bases (adenine, cytosine, guanine and thymine). Cisplatin and other platinum metal drugs directly bind (form covalent bond through nitrogen bases of DNA) to the DNA. As a result normal cells are highly affected resulting in severe toxic side effects. It is therefore possible that the PC-series of compounds might not exhibit severe side effects. It is worth mentioning that analogous rhenium compounds exhibited very negligible toxicity towards fibroblasts [15]. We have not extensively studied the mechanism of action using other techniques and with other PC-series of compounds. Therefore it is not impossible that the PC compounds follow other modes of action. We chose to study the DNA binding study of PC6 because the planar aromatic heterocyclic ligand in PC6 has two additional planar aromatic (phenyl) rings which must facilitate PC6 to follow intercalaive mode of action. Although our prediction is right, the presence of planar aromatic ring is not the final arbiter of intercalation mechanism. For example, the mode of action of the complex [Ru(bpy)2(dppn)]Cl2 (where, dppn = benzo[i]dipyrido[3,2-a:2’,3’-c]phenazine) seems to involve interactions with cell membranes, rather than interactions with DNA (in this case intercalation of the dppn ligand was anticipated) [20].
Figure 5.
Electronic absorption spectra for the titration of 20 µM of compound PC6 in the absence and presence of varied amount of DNA
To determine the inhibitory potency of active compounds (GI50, 50% cell-growth inhibition), tests were extended for compounds PC1–PC6 to varying concentrations for different cancer cell lines. Most of the six drugs showed GI 50 around 3–4 µM (Table1). Interestingly, the Re drugs (PC1–PC6) showed less toxicity than cisplatin in the glomerular mesangial cells. Because nephrotoxicity is a major problem in cisplatin treatment, the titled Re compounds could be an ideal alternative. Significantly, all the Re compounds (PC1–PC6) showed efficacy against all the cancer cells treated at a lower dose than the cisplatin.
Table 1.
The effects of the PC-series of compounds (PC1–PC6) on lyphosarcoma, PC-3 prostate, and myeloid leukemia cancer cells and mesangial cells
| Compounds | Lymphosarcoma | PC-3 Prostate Cancer Cells |
Myeloid Leukemia Cancer Cells |
Mesangial Cells |
|---|---|---|---|---|
| PC1 | 3±.5 | 4±.5 | 4±.9 | 15±2.8 |
| PC2 | 2±.6 | 2±.7 | 3±.5 | 13±.5 |
| PC3 | 2±.7 | 4±.5 | 3±.6 | 12±2.5 |
| PC4 | 3±.6 | 2±.8 | 2±.5 | 14±3.5 |
| PC5 | 2±.8 | 3±.7 | 4±1.7 | 11±2.6 |
| PC6 | 2±.5 | 2±.6 | 2±.5 | 13±.8 |
| Cisplatin | 6±.5 | 8±2.5 | 10±3.7 | 4±.4 |
| DMSO | NA | NA | NA | NA |
GI50 (mean ± S.D.) refers to the concentration required to have 50% cell-growth inhibition; (−) indicates that the isolated is inactive (negative at 100 or GI50>100 µM); N/A: no data available.
Figure 3.
X-ray structure of PC4
Acknowledgments
Partially supported by US-NIH-RISE, US-National Science Foundation LSAMP institutional support Award to Elizabeth City State University, NC, TMCF-DOE award to Dr. H. Banerjee and the work at MSU was partially supported by grants from the National Institutes of Health (Grant No. G11HD038439) and Nuclear Regulatory Commission (Grant No. NRC-HQ-12-G-27-0086). Crystallographic data were collected through the SCrALS (Service Crystallography at Advanced Light Source) program at Beamline 11.3.1 at the Advanced Light Source (ALS), Lawrence Berkeley National Laboratory. The ALS is supported by the U.S. Department of Energy, Office of Energy Sciences Materials Sciences Division, under contract DE-AC02-05CH11231.
Abbreviations
- GI50
Concentration required to have 50% cell-growth inhibition
References
- 1.Rosenberg B, Vancamp L, Krigas T. Inhibition of cell division in Escherichia Coli by electrolysis products from a platinum electrode. Nature. 1965;205:698–699. doi: 10.1038/205698a0. [DOI] [PubMed] [Google Scholar]
- 2.Dyson PJ, Sava G. Metal-based antitumour drugs in the post genomic era. Dalton Trans. 2006;16:1929–1933. doi: 10.1039/b601840h. [DOI] [PubMed] [Google Scholar]
- 3.Fricker SP. Metal based drugs: from serendipity to design. Dalton Trans. 2007;43:4903–4917. doi: 10.1039/b705551j. [DOI] [PubMed] [Google Scholar]
- 4.Guidi F, Modesti A, Landini I, Nobili S, Mini E, Bini L, Puglia M, Casini A, Dyson PJ, Gabbiani C, Messori L. The molecular mechanisms of antimetastatic ruthenium compounds explored through DIGE proteomics. J Inorg Biochem. 2013;118:94–99. doi: 10.1016/j.jinorgbio.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Caruso F, Rossi M. Antitumor titanium compounds. Mini-Rev Med Chem. 2004;4:49–60. doi: 10.2174/1389557043487565. [DOI] [PubMed] [Google Scholar]
- 6.Ott I. On the medicinal chemistry of gold complexes as anticancer drugs. Coord Chem Rev. 2009;253:1670–1681. [Google Scholar]
- 7.Hadjikakou SK, Hadjiliadis N. Antiproliferative and anti-tumor activity of organotin compounds. Coord Chem Rev. 2009;253:235–249. [Google Scholar]
- 8.Reedijk J. Platinum Anticancer Coordination Compounds: Study of DNA Binding Inspires New Drug Design. Eur J Inorg Chem. 2009;10:1303–1312. [Google Scholar]
- 9.Leung C-H, Zhong H-J, Chan DS-H, Ma D-L. Bioactive iridium and rhodium complexes as therapeutic agents. Coord Chem Rev. 2013;257:1764–1776. [Google Scholar]
- 10.Tiekink ERT. Antimony and bismuth compounds in oncology. Crit Rev Oncol Hemat. 2002;42:217–224. doi: 10.1016/s1040-8428(01)00217-7. [DOI] [PubMed] [Google Scholar]
- 11.Jakupec MA, Keppler BK. Gallium in cancer treatment. Curr Top Med Chem. 2004;4:1575–1583. doi: 10.2174/1568026043387449. [DOI] [PubMed] [Google Scholar]
- 12.Yan Y-K, Cho SE, Shaffer KA, Rowell JE, Barnes BJ, Hall IH. Cytotoxicity of rhenium(I) alkoxo and hydroxo carbonyl complexes in murine and human tumor cells. Pharmazie. 2000;55:307–313. [PubMed] [Google Scholar]
- 13.Wang W, Yan YK, Hor TSA, Vittal JJ, Wheaton JR, Hall IH. Synthesis, X-ray structures, and cytotoxicity of rhenium(I) carbonyl 2-(Dimethylamino)ethoxide complexes. J Organomet Chem. 2002;21:1991–1999. [Google Scholar]
- 14.Zhang J, Vittal JJ, Henderson W, Wheaton JR, Hall IH, Andy Hor TS, Yan YK. Tricarbonylrhenium(I) complexes of phosphine-derivatized amines, amino acids and a model peptide: structures, solution behavior and cytotoxicity. J Organomet Chem. 2002;650:123–132. [Google Scholar]
- 15.Ho J, Lee WY, Koh KJT, Lee PPF, Yan YK. Rhenium(I) tricarbonyl complexes of salicylaldehyde semicarbazones: Synthesis, crystal structures and cytotoxicity. J Inorg Biochem. 2013;119:10–20. doi: 10.1016/j.jinorgbio.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 16.Mbagu MK, Kebulu D, Winstead A, Pramanik SK, Banerjee HN, Iwunze MO, Wachira JM, Greco GE, Haynes G, Sehmer A, Sarkar FH, Ho DM, Pike RD, Mandal SK. Fac-tricarbonyl (pentylcarbonato) (α-diimine) rhenium complexes: One-pot synthesis, characterization, fluorescence studies, and cytotoxic activity against human MDA-MB-231 breast, CCl-227 colon and BxPC-3 pancreatic carcinoma cell lines. Inorg Chem Comm. 2012;21:35–38. [Google Scholar]
- 17.Marmur J. A procedure for the isolation of deoxyribonucleic acid from microorganisms. J Mol Biol. 1961;3:208–218. [Google Scholar]
- 18.Reichmann ME, Rice SA, Thomas CA, Doty PJ. A further examination of the molecular weight and size of desoxypentose nucleic acid. J Am Chem Soc. 1954;76:3047–3053. [Google Scholar]
- 19.Barton JK, Danishefsky A, Goldberg J. Tris(phenanthroline)ruthenium(II): stereoselectivity in binding DNA. J Am Chem Soc. 1984;106:2172–2176. [Google Scholar]
- 20.Schatzschneider U, Niesel J, Ott I, Gust R, Alborzinia H, Wolfl S. Cellular uptake, cytotoxicity, and metabolic profiling of human cancer cells treated with ruthenium(II) polypyridyl complexes [Ru(bpy)2(N--N)]Cl2 with N--N=bpy, phen, dpq, dppz, and dppn. Chem Med Chem. 2008;3:1104–1109. doi: 10.1002/cmdc.200800039. [DOI] [PubMed] [Google Scholar]