Abstract
BACKGROUND. Autoimmune inner ear disease (AIED) is a rare disease that results in progressive sensorineural hearing loss. Patients with AIED initially respond to corticosteroids; however, many patients become unresponsive to this treatment over time, and there is no effective alternative therapy for these individuals.
METHODS. We performed a phase I/II open-label, single-arm clinical trial of the IL-1 receptor antagonist anakinra in corticosteroid-resistant AIED patients. Given that the etiology of corticosteroid resistance is likely heterogeneous, we used a Simon 2-stage design to distinguish between an unacceptable (≤10%) and an acceptable (≥30%) response rate to anakinra therapy. Subjects received 100 mg anakinra by subcutaneous injection for 84 days, followed by a 180-day observational period.
RESULTS. Based on patient responses, the Simon 2-stage rule permitted premature termination of the trial after 10 subjects completed the 84-day drug period, as the target efficacy for the entire trial had been achieved. Of these 10 patients, 7 demonstrated audiometric improvement, as assessed by pure tone average (PTA) and word recognition score (WRS). In these 7 responders, reduced IL-1β plasma levels correlated with clinical response. Upon discontinuation of treatment, 3 subjects relapsed, which correlated with increased IL-1β plasma levels.
CONCLUSION. We demonstrated that IL-1β inhibition in corticosteroid-resistant AIED patients was effective in a small cohort of patients and that IL-1β plasma levels associated with both clinical hearing response and disease relapse. These results suggest that a larger phase II randomized clinical trial of IL-1β inhibition is warranted.
TRIAL REGISTRATION. ClinicalTrials.gov NCT01267994.
FUNDING. NIH, Merrill & Phoebe Goodman Otology Research Center, and Long Island Hearing & Speech Society.
Introduction
Potentially reversible, immune-mediated, sensorineural hearing loss (SNHL) can be divided into 2 subgroups: autoimmune inner ear disease (AIED) and sudden SNHL (SSNHL). Patients with AIED usually experience multiple episodes of rapid hearing loss, either concurrently or sequentially, in both ears, whereas SSNHL is usually a unilateral, isolated event. The mechanisms that control corticosteroid-responsive immune-mediated SNHL remain enigmatic, in large part, because AIED is a rare disease, worthy of orphan disease classification, and SSNHL is likely heterogeneous in etiology. For patients that experience an acute, sensorineural decline in hearing, timely corticosteroid administration may result in preservation of some or all hearing in approximately 60% of patients with either AIED (1) or SSNHL (2). Unfortunately, alternate medical therapies for the 40% that fail to respond to corticosteroids have proven largely ineffectual. Therefore, the majority of interventions following corticosteroid failure are rehabilitative, involving either hearing aids or cochlear implants. Interestingly, the influence of plasma cytokines has not been investigated to any great degree in this disorder, largely because the events in the cochlea may not be reflected in the peripheral blood immune cells (PBMCs). Furthermore, since AIED is a rare disease, studies of these patients are often difficult to implement.
We initially examined patients with end-stage AIED, who were candidates for cochlear implants, and compared them with cochlear implant candidates with nonimmunologic etiologies of hearing loss. We harvested cochlear perilymph at the time of cochlear implant surgery and used it to stimulate autologous PBMCs. We identified that the 2 groups differed with respect to expression of the IL-1 type II decoy receptor (IL-1R2) in PBMCs in response to autologous perilymph (3). The decoy receptor sequesters IL-1, yet has no downstream signaling, thereby preventing inflammation (4). These initial observations led to further investigation of the cytokines in the peripheral blood of steroid-sensitive and steroid-resistant AIED patients. Consistent with the increased expression of the IL-1R2 decoy receptor in steroid-resistant AIED patients (3), we observed increased expression of IL-1β in these steroid-resistant individuals (5). Moreover, whereas dexamethasone was unable to prevent IL-1β release in the PBMCs of these patients, anakinra effectively prevented IL-1β release (5). Anakinra is an IL-1 receptor antagonist that competitively inhibits IL-1 binding to the cognate IL-1 type I receptor, thereby blocking the biologic activity of IL-1β and IL-1α (6). This observation became the rationale for the present clinical trial in steroid-resistant AIED patients.
SNHL related to IL-1β expression has also been observed in a rare disease, Muckle-Wells disease (MWS), in which a gain-of-function mutation in NLRP3 results in excessive IL-1β release (7, 8). In this autosomal-dominant disease, patients experience skin rashes, periodic fevers, and SNHL. Unlike MWS patients, AIED patients do not have an afflicted first-degree relative, skin rash, or fever. Moreover, although the majority of AIED patients are initially steroid responsive (70%), that response is lost over time in all but 14% of patients (9). Furthermore, whereas IL-1β inhibition is quite effective at controlling the systemic manifestations of MWS, only 3 of 14 patients experienced minor improvement in auditory acuity with IL-1 inhibition (10), despite isolated case reports of significant greater hearing recovery (11, 12).
Results
Characteristics of the study population.
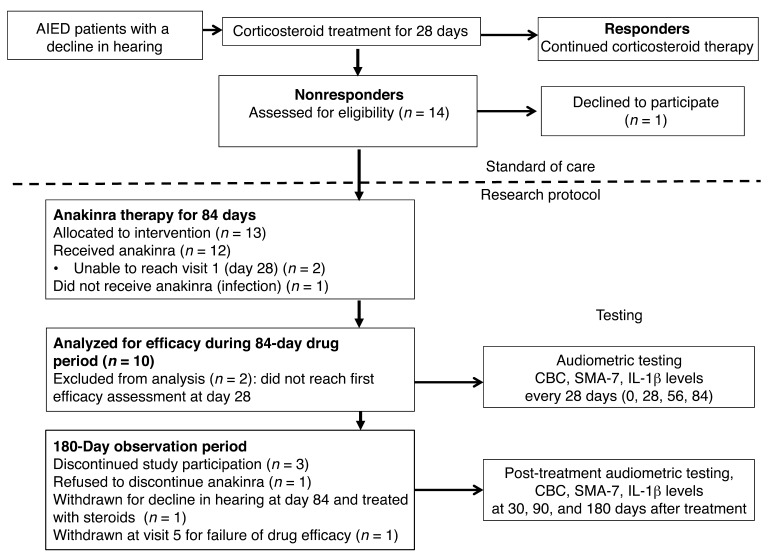
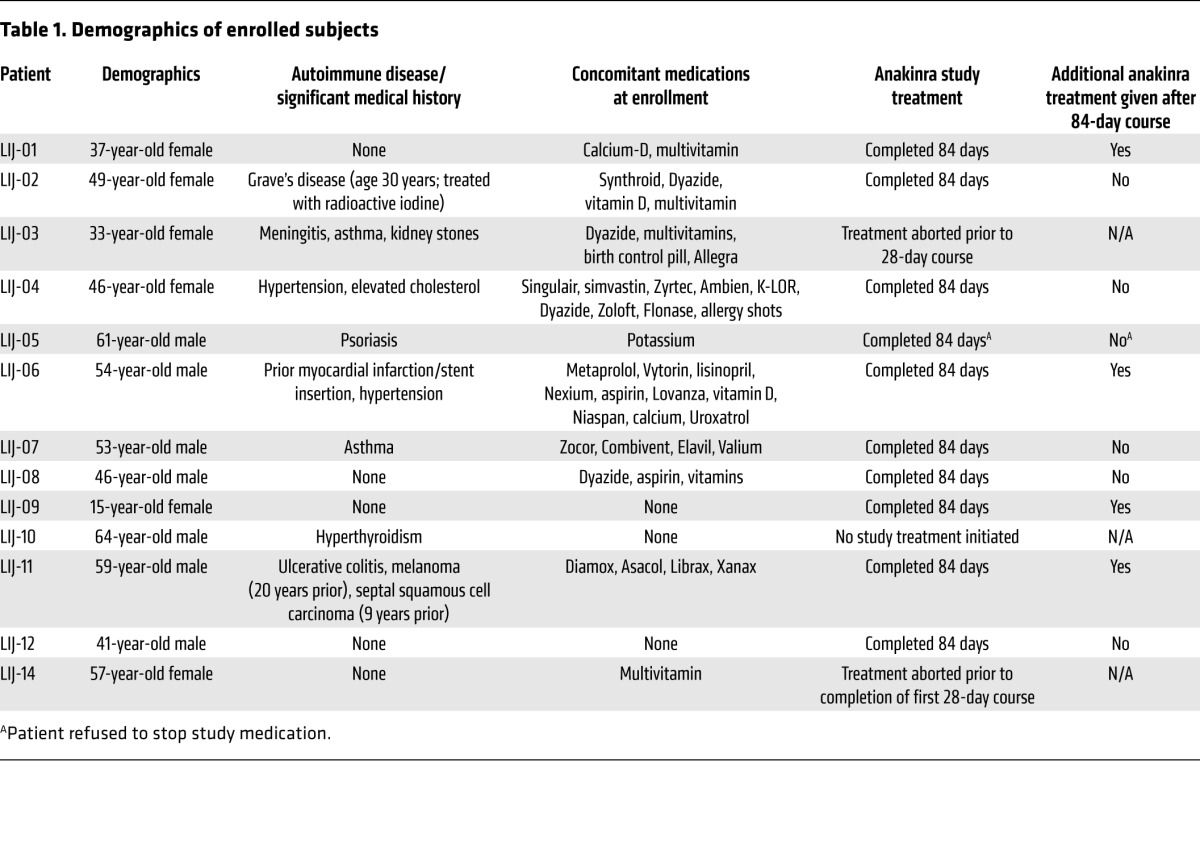
During a 3-year period (December 2010 to January 2014), 14 patients were screened, 13 of which enrolled into this single-center, phase I/II open-label study (Figure 1). Of these 13 patients, 12 received anakinra, as patient LIJ-10 passed the screening process, but was not started on drug because of development of a systemic infection in the interim. Demographics of age, gender, concurrent autoimmune conditions, other diseases, and medications are outlined in Table 1. Of the 12 patients, 10 were evaluable for efficacy (Figure 1), as 2 patients were unable to complete the requisite initial 28 days of therapy required to assess efficacy. Results reported follow the 2010 CONSORT guidelines as applicable (13). Notably, both the patient reporting uveitis (LIJ-03) and the single teenaged patient (LIJ-09) were identified to be sequence-negative for NLRP3 mutations, excluding a diagnosis of MWS.
Figure 1. CONSORT flow diagram.
For enrollment, all patients must have been treated with a 28-day course of prednisone and have experienced no clinical improvement, as assessed by PTA and WRS. All patients that met enrollment criteria were allocated to receive the intervention (n = 13). Of the 13 subjects, 1 never received anakinra. Of the 12 patients that received anakinra, 2 were unable to complete the initial 28 days of therapy, leaving 10 subjects evaluable for the efficacy assessment.
Table 1.
Demographics of enrolled subjects
Efficacy.
As per protocol, 10 patients were enrolled in the first stage, and 8 of these completed the 84-day therapy period at the time of interim analysis. Of these 8, 5 were classified as responders by PTA score, which triggered enrollment into the second stage. At the time enrollment began in the second stage, patients LIJ-09 and LIJ-10 had not yet completed the 84-day observation period (but ultimately yielded 1 responder and 1 that never instituted treatment). During this time, 3 stage II patients were enrolled, for a trial total of 13.
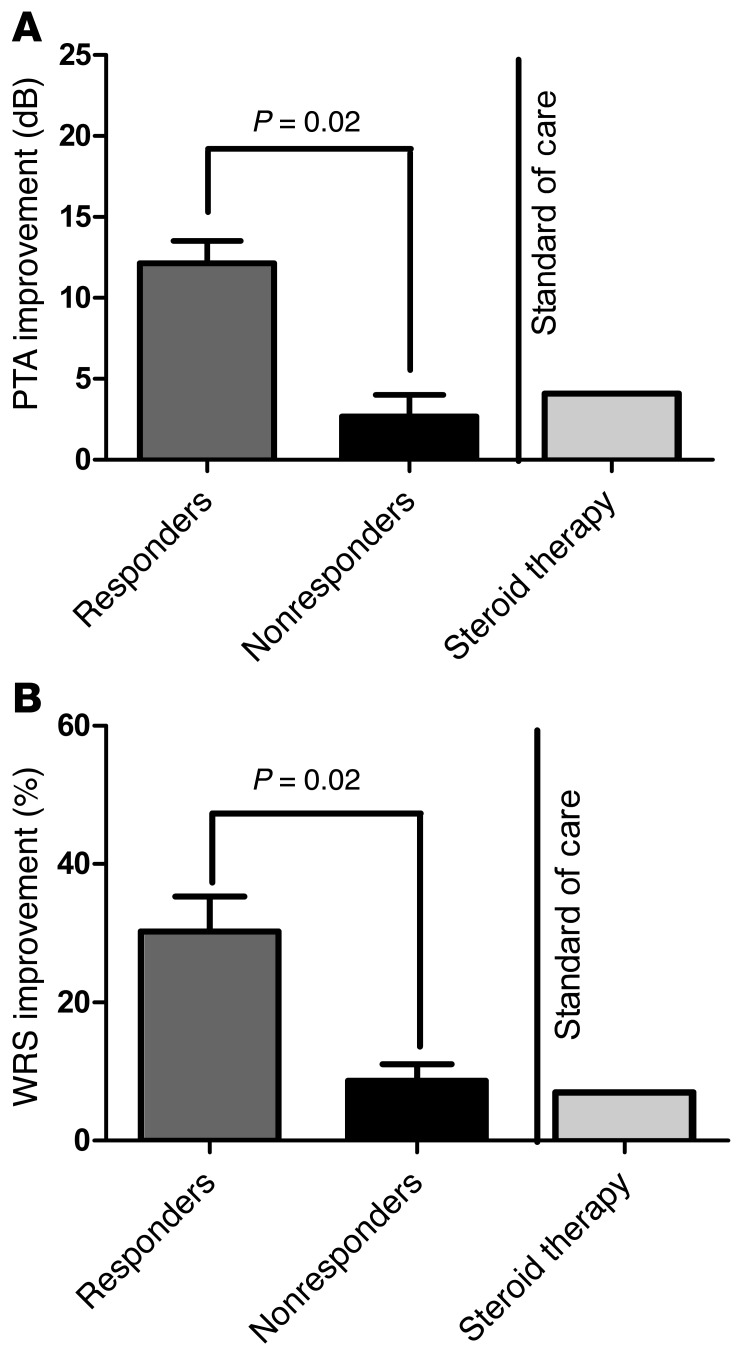
Of the total 13 subjects, 2 withdrew from treatment within the first 28 days, and 1 never initiated treatment, leaving 10 subjects evaluable for efficacy assessment. Per protocol, responsive patients were defined as having ≥5 decibel (dB) improvement in PTA in the active ear, or as having ≥12% improvement in WRS, in response to anakinra at any point during the 28th to 84th day. Among the 10 evaluable subjects (those who completed 84 days of therapy), 7 of 10 (patients LIJ-01, LIJ-02, LIJ-04, LIJ-05, LIJ-07, LIJ-09, and LIJ-11; 70%) met the criteria for response by PTA, and 7 of 10 (patients LIJ-01, LIJ-02, LIJ-04, LIJ-06, LIJ-07, LIJ-09, and LIJ-11; 70%) met the criteria of response by WRS. The results comparing anakinra responders and nonresponders were statistically significant for PTA and WRS (both P = 0.02, Mann-Whitney test; Figure 2). In a prior clinical trial that evaluated the efficacy of methotrexate in steroid-sensitive AIED, therapeutic efficacy as measured by pure tones was defined as either a 15 dB improvement at 1 frequency or a 10 dB improvement at 2 contiguous frequencies (14). This efficacy assessment was difficult to use in the present study because of its long duration (264 days) and multiple points of assessment, and would not account for possible simultaneous or sequential improvements and declines at different frequencies. Nonetheless, we did assess whether the enrolled subjects met the criteria for PTA improvement based on the existing criteria. Specifically, we queried whether the criteria for improvement were met during the treatment period, relative to the initial audiogram prior to starting anakinra. Notably, when using the criteria set forth in the methotrexate trial, the results yielded 1 additional responder: patient LIJ-06 now met the definition for responder by PTA as well. As a result, in a per-protocol assessment, an 80% response rate would be achieved using these alternate criteria for clinical improvement in PTA. WRS criteria for improvement were the same for this trial and prior trials. Interestingly, for patient LIJ-05, WRS improvement was limited, and may have been artificially low due to a ceiling effect: the patient’s starting WRS score was 88%, which improved to 90% at the end of 28 days and to 100% by the 84th day. By the best interval response (see Figure 3), this patient would be classified as a nonresponder. Because by both endpoints, PTA and WRS, the criterion of ≥6 responders of a maximum 29 to reject the null hypothesis was achieved, the trial was prematurely terminated at the recommendation of the Data and Safety Monitoring Board (DSMB), since evidence for efficacy had been supported. Of the patients treated with anakinra in this clinical trial, there did not appear to be a correlation between audiometric configuration and the ability to respond: patients derived benefit with up-sloping, down-sloping, flat, and U-shaped audiograms (data not shown).
Figure 2. Best hearing improvement observed while on anakinra therapy.
PTA improvement (A) and WRS improvement (B) during the 84-day drug period were both significantly higher in anakinra responders (n = 7) versus nonresponders (n = 3) (P = 0.02 for both, Mann-Whitney test). The result was also compared with the historic improvement with corticosteroid therapy (Standard of care) previously reported by Niparko et al. (1).
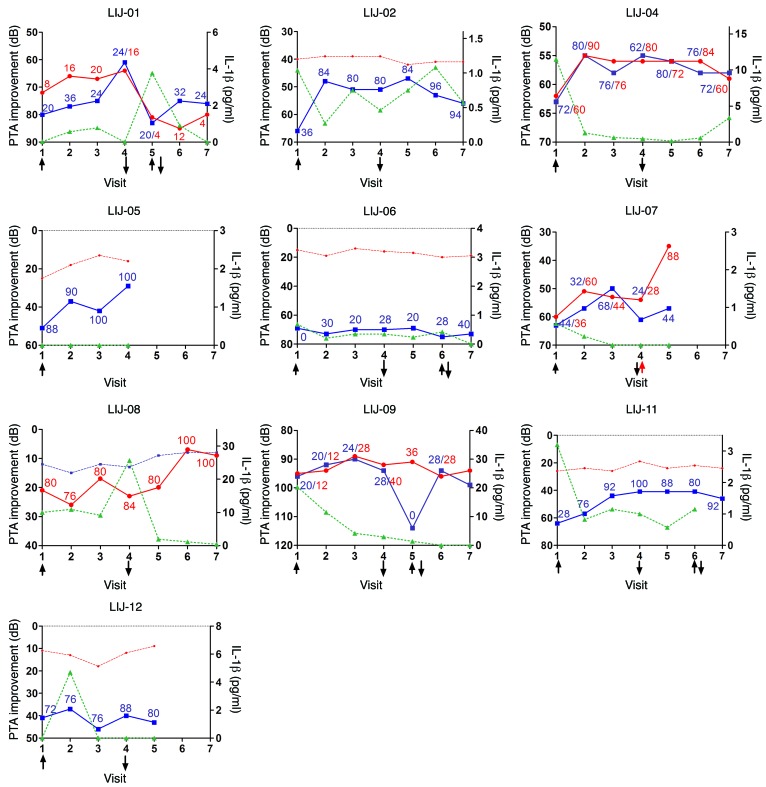
Figure 3. Hearing thresholds (PTA and WRS) relative to IL-1β plasma levels in a phase I/II trial of anakinra for corticosteroid-resistant AIED (NCT01267994).
At visit 1, anakinra (daily subcutaneous injectable medication) was instituted (↑) for the next 84 days, after which therapy was discontinued (↓). Interval audiograms were obtained at visits 2 (day 28), 3 (day 56), and 4 (day 84). The 180-day post-treatment observational period is shown in visits 5 (day 114), 6 (day 174), and 7 (day 264). The left axis shows PTA, plotted inversely, similar to an audiogram (lower numbers correspond to better hearing); solid lines denote active ears (blue, left; red, right), and dashed lines denote inactive ears. Numeric values show WRS for the active ear (blue, left; red, right), with a best possible score of 100. The right axis shows IL-1β plasma values (green). In some patients, after visit 4, anakinra treatment was reinstated for an additional 28 days (denoted by arrows) because of a decline in hearing. Institution of steroid therapy in patient LIJ-07 is denoted by a red arrow.
Although this was not a randomized clinical trial, the results could also be interpreted by an intention-to-treat (ITT) analysis. There were a total of 13 subjects enrolled, 1 of whom could not start the trial, due to logistical reasons unrelated to the trial itself. We would not include this subject in the ITT analysis. Of the remaining 12 patients, 2 dropped out due to toxicity (intolerable injection site reactions) and would be counted in the ITT analysis, yielding a response rate of 7 of 12 (58%). Even this more conservative ITT rate of 58% is substantially higher than our initial hypothesis of a 30% response rate being clinically meaningful.
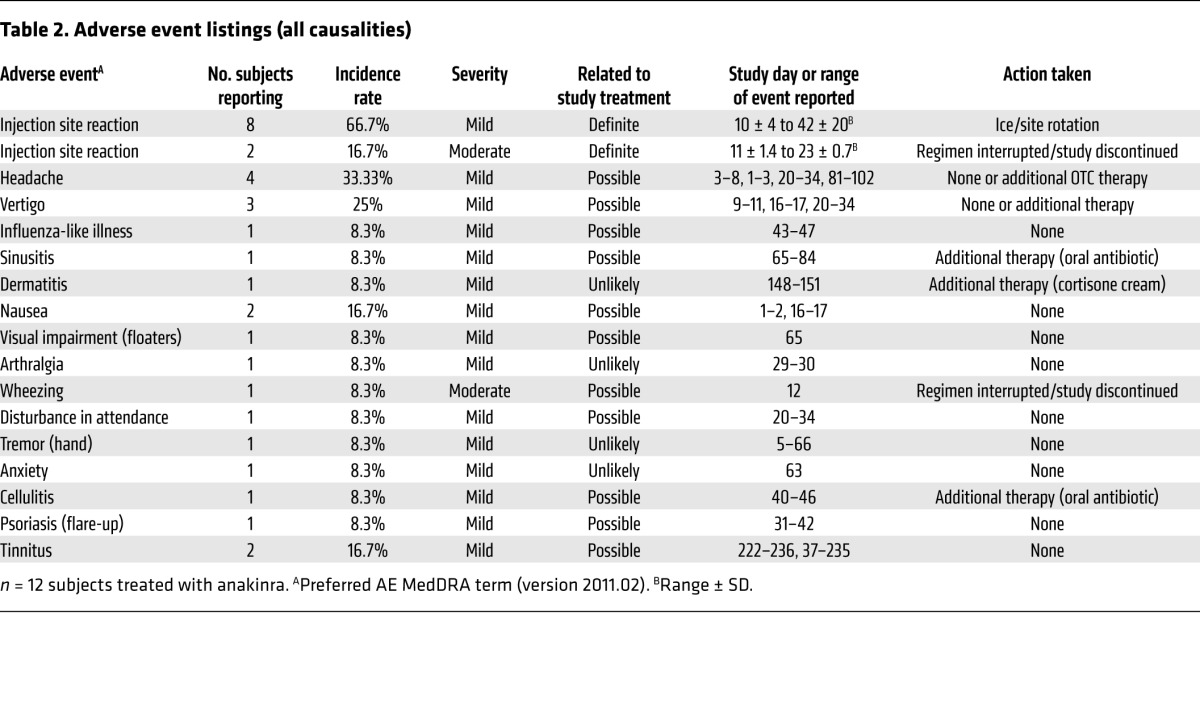
Adverse events and subject withdrawals.
Injection site reactions were the most common adverse event, observed in approximately 70% of patients. This observed rate was consistent with use of anakinra in other diseases (15). Of the 13 eligible patients, 2 were withdrawn from further study during the anakinra treatment phase: patients LIJ-03 and LIJ-14 did not complete the initial 28 days of treatment because of intolerable injection site reactions. During the observational period, 3 patients were withdrawn. Patient LIJ-05, one of the responders, was terminated from further trial participation at day 84, as the patient refused to discontinue drug. Patient LIJ-12 withdrew at visit 5 because of failure of drug efficacy. In patient LIJ-07, use of anakinra appeared to modulate the ability to respond to steroids: the patient initially responded to anakinra, but subsequently worsened by day 84. He was placed on prednisone at day 84, and rapidly became corticosteroid responsive at that point, whereas he previously failed to respond to prednisone. He withdrew from the study following the steroid therapy.
Laboratory studies demonstrated few abnormalities and no evidence of neutropenia. No elevations of creatinine were observed. Several patients were identified to have mild, asymptomatic hypokalemia during the course of the trial. These patients were treated with potassium replacement; however, the etiology of the hypokalemia was not felt to be a result of the anakinra therapy.
IL-1β plasma levels.
We sought to determine the association between clinical responsiveness and biomarkers. Based on the rationale for this trial that elevated IL-1β may be associated with corticosteroid resistance, we focused our analysis on IL-1β. Plasma levels of IL-1β were measured at all interval visits. Notably, IL-1β plasma levels were generally associated with clinical response and, perhaps more importantly, with clinical relapse (Figure 3). During the 180 day post-treatment observation phase, 3 patients who experienced initial improvement in hearing while taking anakinra exhibited relapse 30–90 days after its discontinuation (patients LIJ-01, LIJ-06, and LIJ-09). The relapse was associated with increasing IL-1β, although a common absolute threshold value was not identified for relapse. Notably, although patient LIJ-08 was considered an anakinra failure, upon discontinuing drug, her IL-1β levels declined and her hearing clinically improved (Figure 3). These findings suggest that although anakinra was not effective in modulating IL-1β levels in this patient, reduction in her circulating IL-1β appeared to be linked to audiometric improvement. Plasma levels of IL-1α were also measured at all time points in each subject. No IL-1α could be detected in the samples assayed (data not shown).
Discussion
Here, anakinra demonstrated clinical efficacy in this small sample of corticosteroid-resistant AIED patients. Prior to these studies, no effective therapies have been identified for corticosteroid-resistant AIED patients. Moreover, no alternative effective therapies have been identified in corticosteroid-sensitive patients (14, 16, 17). Finally, even gains in steroid-sensitive AIED patients using corticosteroid therapy may be limited. In the serial audiometry trial for AIED, the average hearing improvement in AIED patients after 1 month of corticosteroid therapy was 4.1 dB for PTA and 7% for WRS (1). During the anakinra trial, the average PTA improvement was 12 dB in anakinra responders compared with 3 dB in nonresponders (Figure 2A). Similarly, the WRS improvement was 28% in anakinra responders and 6% in nonresponders (Figure 2B). Especially in instances of more significant hearing loss, WRS improvements were more clinically significant, as WRS scores reflect speech comprehension and therefore can distinguish the ability (or lack thereof) to derive benefit from hearing aids. Although the anakinra study had substantially fewer enrollees, the response rate is certainly encouraging compared with the gold standard of corticosteroid responses.
In patients with MWS, only minor improvements in auditory acuity have been observed (10), which suggests that corticosteroid-resistant AIED is a distinct disease process. This distinction is further supported by the fact that unlike MWS, in which inheritance is autosomal-dominant, in AIED we are yet to identify an afflicted first-degree relative. Furthermore, whereas in MWS, the audiometric pattern observed is usually a high-frequency SNHL, in the AIED patient cohort, the audiometric pattern was highly variable (data not shown) and did not correlate with the ability to respond to anakinra.
One of the most difficult aspects in conducting studies on AIED patients is the fluctuating nature of the hearing loss, and the potential for interpretation of efficacy of an intervention rather than improvement as a natural fluctuation of disease. In these studies, reduction of plasma IL-1β correlated with clinical hearing improvement within the first 28 days of anakinra therapy in patients LIJ-02, LIJ-04, LIJ-07, LIJ-09, and LIJ-11. Although hearing fluctuation was observed during both the 84-day drug intervention period and the subsequent 180-day observational period, this fluctuation in response to unknown stimuli is one of the clinical features of AIED. Notably, no clinical hearing improvement or fluctuation was observed in response to the initial corticosteroid therapy, as was requisite for entrance into the trial. Even more intriguing, clinical relapse within 30–90 days in several patients once anakinra was discontinued correlated with elevation of their IL-1β levels. Reintroduction of anakinra in patients LIJ-01 and LIJ-09 resulted in clinical hearing improvement again, albeit not to the degree seen with initial treatment. This observation supports our hypothesis that IL-1β is a critical mediator of immune-mediated SNHL, yet also underscores the potential need for long-term inhibition and/or longer-acting agents. To date, none of the interventional clinical trials on AIED patients have successfully correlated a plasma biomarker with pharmaceutical efficacy. Correlation of IL-1β levels with disease severity and efficacy of anakinra is therefore novel, and an important avenue of further exploration and validation.
IL-1 inhibition in AIED may represent yet another example of the spectrum of diseases in which IL-1 plays a pivotal role (18). Validation of IL-1β inhibition through larger phase II and phase III trials in AIED will determine efficacy in terms of hearing restoration as well as the potential of plasma IL-1β as a predictive biomarker of hearing fluctuation. Future clinical trials will also identify whether similar efficacy can be achieved in a more ethnically and racially diverse patient population. These data provide strong evidence that a placebo-controlled phase II trial of IL-1β inhibition in the corticosteroid-resistant AIED patients is warranted.
Methods
Patients.
To be eligible for this clinical trial, the patient (age between 13 and 75) must have bilateral SNHL, with active deterioration in 1 ear, and must have failed to respond to high-dose corticosteroids (defined as taking 60 mg prednisone for 14 days followed by a taper, totaling 28 days of prednisone therapy). Specifically, patients must have SNHL of >30 dB at 1 or more frequencies in 1 or 2 ears with evidence of active deterioration (elevated threshold) in at least 1 ear of 15 dB at 1 frequency (excluding 250 or 8 kHz as a sole indicator) on their audiogram, or 10 dB at ≥2 frequencies developing in no less than 3 and no more than 90 days; if the hearing loss evolved in less than 3 days, the patient displayed features suggestive of an autoimmune disorder. Furthermore, hearing declines could not be accompanied by concurrent disabling vertigo, as this clinical presentation would be more consistent with Meniere’s disease.
The definition of steroid failure was exhibiting <5 dB PTA improvement (PTA defined as the average of 250, 500, 1,000, 2,000, and 4,000 Hz) and ≤12% WRS improvement. Since WRS was used as an endpoint, all enrolled patients were required to use English as their native language, as this test could not be administered in other languages by our study personnel. Concurrent intratympanic steroid therapy may have been used, but could not substitute for oral therapy. All patients were of mixed European descent; age and gender are listed in Table 1. Patients may have concurrent autoimmune diseases (Table 1). Because AIED is a rare disease, recruitment of an untreated control group was not feasible. Moreover, a placebo arm was equally problematic from ethical and practical standpoints, as anakinra requires daily subcutaneous injections. The likelihood of a placebo effect was highly improbable, as trial enrollment required 2 sequential audiograms, prior to and following steroids, which demonstrated no improvement with standard corticosteroid therapy.
Patients were excluded from study participation for radiologic abnormalities suggestive of alternate SNHL etiologies, evidence of chronic infection that would increase the risk of anakinra therapy, or laboratory abnormalities that would also increase the risk of anakinra therapy. MRI was used to exclude patients with retrocochlear pathology (vestibular schwannoma) or inner ear malformations. Patients with a history of chronic infections, or treatment of a malignancy within 3 years prior to enrollment, were similarly excluded. Additionally, patients receiving recent methotrexate or TNF antagonist therapy were also prevented from participating. Patients that demonstrated evidence of neutropenia or renal insufficiency or failure were also excluded. Finally, patients with latex allergy and allergy to E. coli–derived products were excluded because of the manner in which anakinra is formulated and packaged.
Experimental therapy.
All eligible patients were treated with 84 continuous days of 100 mg subcutaneous anakinra daily injections followed by a 180-day observational period to determine the stability of hearing following use of anakinra (Figure 1). Retreatment during the observational period was provided to patients that initially demonstrated clinical improvement on anakinra, but exhibited relapse in the absence of drug. This consisted of 28 continuous days of 100 mg subcutaneous anakinra injections, followed by continued observation.
Measures of clinical improvement.
Patients were reassessed a minimum of 7 times during this clinical trial. Following the initial audiogram at the point of steroid failure, patients were reassessed every 28 days while on anakinra (days 28, 56, and 84). They were reassessed on days 30, 90, and 180 of the observational period (days 114, 174, and 264, respectively, relative to therapy initiation). Additionally, if they reported a hearing decline at any point during the entire trial, they were asked to return for audiometric testing. If retreated with anakinra for a confirmed decline in hearing, an audiogram at the 28th day of anakinra therapy was obtained.
Hearing was measured by several audiologists, all masked to prior audiograms. PTA was measured on a GSI-61 audiometer, calibrated every 3 months. WRS was performed by recorded speech, 50 word lists, rotating the track number at each visit, maintaining the same level of difficulty.
A positive clinical response was classified as an improvement in PTA hearing of ≥5 dB at the 5-frequency average described above, or a ≥12% improvement in WRS, at any point between the 28th and 84th day of trial participation. Although the criteria of a 15 dB improvement at 1 frequency or a 10 dB improvement at 2 frequencies has been previously used to measure responsiveness in other studies (14), this model does not account for evaluation at multiple time points, and thus could not be used in the present study.
The DSMB recommended closing the trial when >6 of a total 29 evaluable patients achieved the efficacy target of PTA improvement between the 28th and 84th day of treatment with anakinra, as dictated by the Simon 2-stage design.
Safety laboratory measures.
To screen for eligibility and to assess safety during the trial, a CBC with differential, and SMA-7 was obtained from all participants at each visit. A β-HCG was obtained in all female participants at each visit. Notably, 1 patient initially failed the screen for trial inclusion because of a positive β-HCG. This patient was enrolled >12 months later once she met eligibility criteria again. No patients failed screening or were prevented from using anakinra during the trial for concerns of neutropenia.
Experimental laboratory measures.
At the time of initial enrollment and at all visits, safety blood and blood biomarker identification was obtained, with the hope that anakinra responders and nonresponders could be identified based on their peripheral blood expression of various cytokines, most importantly IL-1β and IL-1α. Plasma was collected and stored at –20°C until a complete set of samples was acquired for each enrolled subject. Frozen samples were thawed immediately prior to analysis, and none of the samples underwent repetitive freeze-thawing cycles prior to analysis. IL-1β and IL-1α levels in plasma were quantified using a sandwich ELISA (R&D Systems) as previously described (5). All samples were run in duplicate; mean variance was ≤0.02%.
Statistics and sample size calculation.
Because steroid-resistant subjects were to be enrolled, and because we assumed that multiple etiologies may contribute to the failure to respond to steroids, we proposed that a ≤10% response rate would be unacceptable, and a ≥30% response rate would be clinically meaningful and worthy of further study. A Simon 2-stage “optimal” design was used: 10 subjects would be treated in the first stage; if 2 or more responded, the trial would enroll 19 additional subjects; if ≥6 of all 29 responded, then anakinra would be considered effective and a candidate for further larger trials. This design corresponded to testing the null hypothesis that the true objective response rate was ≤10% versus the alternative hypothesis that the true response rate was ≥30%, with α = 0.05 and power = 0.80.
Given that 2 sequential audiograms (before and after steroid therapy) were required for trial eligibility, the likelihood of a placebo effect contributing to the observed hearing response was unlikely. Statistical significance between PTA and WRS of responders and nonresponders was calculated using 2-tailed Mann-Whitney test (Graph Pad Prism). A P value less than 0.05 was considered significant.
Study approval.
We conducted these studies as a phase I/II open-label, single-arm, single-institution study. An IND to perform these studies, IRB approval from the North Shore–LIJ Health System, and clinical trial registry (NCT01267994) was obtained prior to the inception of the trial. Because A. Vambutas and the Feinstein Institute for Medical Research have filed a patent for the use of IL-1 antagonists in the treatment of AIED, SSNHL, and Meniere’s disease, an external DSMB was assembled to periodically review safety (approximately biyearly), and an internal monitoring system to assess safety and efficacy as well as to verify source data was also established and conducted by the Feinstein Institute for Medical Research Office of Research Compliance. Written, informed consent was obtained from all enrolled patients from an investigator without a conflict of interest.
Assessment of adverse events.
In order to ascertain the number, frequency, and duration of adverse events, patients were required to maintain daily symptom logs in addition to periodic review during interval visits. Adverse events are shown in Table 2. The daily questionnaire consisted of questions related to anticipated injection site reactions, change in hearing, or associated symptoms of tinnitus or vertigo. Furthermore, an open response field permitted the enrolled patient to report any new symptoms. Logs were reviewed daily as received, and unanticipated symptoms prompted follow-up by telephone. In several cases, the patients were requested to return to the office for an unscheduled visit to better ascertain the severity of the adverse event. No serious adverse events were reported or recorded. In 2 cases, the drug was temporarily suspended because of an adverse event. In these cases, further drug was discontinued because of significant, intolerable injection site reactions, 1 in combination with a presumptive allergic reaction manifested by wheezing.
Table 2.
Adverse event listings (all causalities)
Supplementary Material
Acknowledgments
The authors thank the external DSMB for their commitment to the safety and well-being of the patients; the NIDCD program officer, Gordon Hughes, for his expert advice and guidance; and Betty Diamond for her critical review of this manuscript. This study was funded by NIH grant R21/R33DC011827 (to A. Vambutas), the Merrill & Phoebe Goodman Otology Research Center, and the Long Island Hearing & Speech Society at North Shore–LIJ Health System.
Footnotes
Conflict of interest: Andrea Vambutas and the Feinstein Institute for Medical Research have filed a patent for the use of IL-1 antagonists in the treatment of AIED, SSNHL, and Meniere’s disease.
Reference information:J Clin Invest. 2014;124(9):4115–4122. doi:10.1172/JCI76503.
See the related Attending physician beginning on page 3685.
References
- 1.Niparko JK, et al. Serial audiometry in a clinical trial of AIED treatment. Otol Neurotol. 2005;26(5):908–917. doi: 10.1097/01.mao.0000185081.28598.5c. [DOI] [PubMed] [Google Scholar]
- 2.Wilson WR, Byl FM, Laird N. The efficacy of steroids in the treatment of idiopathic sudden hearing loss. A double-blind clinical study. Arch Otolaryngol. 1980;106(12):772–776. doi: 10.1001/archotol.1980.00790360050013. [DOI] [PubMed] [Google Scholar]
- 3.Vambutas A, DeVoti J, Goldofsky E, Gordon M, Lesser M, Bonagura V. Alternate splicing of interleukin-1 receptor type II (IL1R2) in vitro correlates with clinical glucocorticoid responsiveness in patients with AIED. PLoS One. 2009;4(4):e5293. doi: 10.1371/journal.pone.0005293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Granowitz EV, Clark BD, Vannier E, Callahan MV, Dinarello CA. Effect of interleukin-1 (IL-1) blockade on cytokine synthesis: I. IL-1 receptor antagonist inhibits IL-1-induced cytokine synthesis and blocks the binding of IL-1 to its type II receptor on human monocytes. Blood. 1992;79(9):2356–2363. [PubMed] [Google Scholar]
- 5.Pathak S, Goldofsky E, Vivas EX, Bonagura VR, Vambutas A. IL-1β is overexpressed and aberrantly regulated in corticosteroid nonresponders with autoimmune inner ear disease. J Immunol. 2011;186(3):1870–1879. doi: 10.4049/jimmunol.1002275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.PDR.net. Drug summary: Kineret. http://www.pdr.net/drug-summary/kineret?druglabelid=2061
- 7.Hoffman HM, Mueller JL, Broide DH, Wanderer AA, Kolodner RD. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nat Genet. 2001;29(3):301–305. doi: 10.1038/ng756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dinarello CA. Unraveling the NALP-3/IL-1β inflammasome: a big lesson from a small mutation. Immunity. 2004;20(3):243–244. doi: 10.1016/S1074-7613(04)00055-X. [DOI] [PubMed] [Google Scholar]
- 9.Broughton SS, Meyerhoff WE, Cohen SB. Immune mediated inner ear disease: 10-year experience. Semin Arthritis Rheum. 2004;34(2):544–548. doi: 10.1016/j.semarthrit.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Ombrello MJ, Kastner DL. Autoinflammation in 2010: expanding clinical spectrum and broadening therapeuitc horizons. Nat Rev Rheumatol. 2011;7(2):82–84. doi: 10.1038/nrrheum.2010.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mirault T, et al. Recopvery from deafness in a patient with Muckle-Wells syndrome treated with anakinra. Arthritis Rheum. 2006;54(5):1697–1700. doi: 10.1002/art.21807. [DOI] [PubMed] [Google Scholar]
- 12.Yamazaki T, et al. Anakinra improves sensory deafness in a japanese patient with Muckle-Wells syndrome, possibly inhibiting the cryopyrin inflammasome. Arthritis Rheum. 2008;58(3):864–868. doi: 10.1002/art.23261. [DOI] [PubMed] [Google Scholar]
- 13.Schulz KF, Altman DG, Moher D, CONSORT Group CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med. 2010;152(11):726–732. doi: 10.7326/0003-4819-152-11-201006010-00232. [DOI] [PubMed] [Google Scholar]
- 14.Harris JP, et al. Treatment of corticosteroid-responsive autoimmune inner ear disease with methotrexate: a randomized controlled trial. JAMA. 2003;290(14):1875–1883. doi: 10.1001/jama.290.14.1875. [DOI] [PubMed] [Google Scholar]
- 15.Kaiser C, et al. Injection-site reactions upon Kineret (anakinra) administration: experiences and explanations. Rheumatol Int. 2012;32(2):295–299. doi: 10.1007/s00296-011-2096-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen S, Shoup A, Weisman MH, Harris J. Etanercept treatment for autoimmune inner ear disease: results of a pilot placebo-controlled study. Otol Neurotol. 2005;26(5):903–907. doi: 10.1097/01.mao.0000185082.28598.87. [DOI] [PubMed] [Google Scholar]
- 17.Matteson EL, et al. Etanercept therapy for immune-mediated cochleovestibular disorders: a multicenter, open-label, pilot study. Arthritis Rheum. 2005;53(3):337–342. doi: 10.1002/art.21179. [DOI] [PubMed] [Google Scholar]
- 18.Dinarello CA, Simon A, van der Meer JW. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov. 2012;11(8):633–652. doi: 10.1038/nrd3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.