Abstract
Purpose.
To examine factors contributing to eye–hand coordination deficits in children with amblyopia and impaired stereovision.
Methods.
Participants were 55 anisometropic or strabismic children aged 5.0 to 9.25 years with different degrees of amblyopia and abnormal binocularity, along with 28 age-matched visually-normal controls. Pilot data were obtained from four additional patients studied longitudinally at different treatment stages. Movements of the preferred hand were recorded using a 3D motion-capture system while subjects reached-to-precision grasp objects (two sizes, three locations) under binocular, dominant eye, and amblyopic/nonsighting eye conditions. Kinematic and “error” performance measures were quantified and compared by viewing condition and subject group using ANOVA, stepwise regression, and correlation analyses.
Results.
Movements of the younger amblyopes (age 5–6 years; n = 30) were much slower, particularly in the final approach to the objects, and contained more spatial errors in reaching (∼×1.25–1.75) and grasping (∼×1.75–2.25) under all three views (P < 0.05) than their age-matched controls (n = 13). Amblyopia severity was the main contributor to their slower movements with absent stereovision a secondary factor and the unique determinant of their increased error-rates. Older amblyopes (age 7–9 years; n = 25) spent longer contacting the objects before lifting them (P = 0.015) compared with their matched controls (n = 15), with absence of stereovision still solely related to increases in reach and grasp errors, although these occurred less frequently than in younger patients. Pilot prospective data supported these findings by showing positive treatment-related associations between improved stereovision and reach-to-grasp performance.
Conclusions.
Strategies that children with amblyopia and abnormal binocularity use for reach-to-precision grasping change with age, from emphasis on visual feedback during the “in-flight” approach at ages 5 to 6 years to more reliance on tactile/kinesthetic feedback from object contact at ages 7 to 9 years. However, recovery of binocularity confers increasing benefits for eye–hand coordination speed and accuracy with age, and is a better predictor of these fundamental performance measures than the degree of visual acuity loss.
Keywords: reaching, grasping, visuomotor control
Abnormal binocular stereovision, rather than reduced visual acuity, in children with amblyopia aged 5 to 9 years is associated with “arrested” development of eye–hand coordination skills, suggesting that future clinical amblyopia management should promote restoration of binocular function.
Introduction
Binocular vision provides a number of well-established perceptual advantages over monocular viewing, most notably in enhancing distance and depth discrimination for solid objects and surfaces (stereopsis) in near-space. This stereoscopic information also affords significant advantages for evaluating the visual scene during the planning of goal-directed movements of the limbs in immediate 3D space, such as the hand for grasping objects1 and the feet for stepping down or over obstacles.2,3 Indeed, even human infants just weeks after the normal emergence of binocular distance and depth sensitivity (aged between 3–6 months) are better able to judge whether objects are within their reach and to execute movements toward them using two eyes rather than with one eye covered.4–6 It is also clear that stereovision confers major advantages for providing the fast and reliable feedback required for “online” movement guidance and adjustment of the grasping hand relative to the goal object,1,7–11 although use of such feedback for improving performance does not appear to be prioritized until later in normal development, at approximately ages 7 to 8 years.12–14
Unfortunately, children with unequal refractive errors between the two eyes (anisometropia) or a squint (strabismus) between birth and these later ages commonly have defective stereovision15 and often develop amblyopia. This disorder is associated with abnormal responses to the affected (amblyopic) eye in the visual cortex,16 with reductions in its visual acuity (VA) accompanied by “higher level” deficits in spatial, object, and motion perception,15–19 along with impaired performance on a variety of visually guided, real-world three-dimensional (3D) tasks.20 For example, we quantified reach-to-precision grasp actions in a sample of 21 children aged 4 to 8 years with amblyopia of either subtype and found that their movements were slow, “uncertain,” and poorly coordinated under natural viewing conditions (i.e., with both eyes open) and when using just their amblyopic eye—and even their better eye alone—compared with the equivalent binocular and monocular performance of age-matched, normally sighted peers.21 There were also indications that the clumsiest performers across all views were those lacking measurable stereoacuity, suggesting that some degree of binocularity is needed for the acquisition of skilled eye–hand coordination abilities in childhood.
Importantly, some of these eye–hand coordination problems during habitual binocular—although not dominant eye—viewing also apply to adults with persistent amblyopia22 and even to adults whose VA deficits had previously been “cured” by childhood amblyopia treatment, but who remain stereo-impaired.23 This latter situation further suggests that loss of binocularity cannot be compensated for by more efficient use of numerous alternative, monocular cues to object distance and/or depth, even with long-term practice. We did, however, obtain evidence23 of a strategic change underlying a partial grasping adaptation, whereby adults with stereovision losses placed greater reliance on tactile/kinesthetic (i.e., nonvisual) feedback derived from initial digit–object contact to modify their grip and ensure its stability.
These findings have significant implications for current amblyopia therapies, which primarily aim to reverse the VA loss in the affected eye, first by a period of optical correction via spectacle wear (termed “refractive adaptation”), usually followed by daily periods of patching (occlusion) of the dominant eye. Most children who comply with these treatments achieve significant gains in VA,24–27 and in those with anisometropia or small-angle strabismus, stereoacuity may also improve.28,29 However, if binocular recovery really is the key to reversing the real-world visuomotor deficits in amblyopia, a greater focus on reinstating the stereovision—rather than visual acuity—loss would be warranted, as we and others have recently suggested.16,20–23,30–33
Here we extend our preliminary observations by examining eye–hand coordination abilities in a much larger cross-sectional group of 55 children with varying degrees of VA and stereoacuity loss at different stages of amblyopia therapy, and in another four children studied prospectively during their routine clinical management. The aims were to determine whether this more extensive analysis would support our initial conclusions regarding the importance of binocular visual recovery for enhancing reaching and grasping skills in these patients, distinct from other potential contributory factors such as their age, severity of amblyopia, or its treatment stage. For example, we previously confirmed21 that normally-sighted children aged 5 to 6 years generally adopt a feed-forward reach-to-grasp strategy, in which vision is used mainly for movement planning, with visual feedback used to reduce spatial errors (i.e., inaccuracies) in movement execution employed only at later ages. But any such analogous use of online control or of adaptive strategies by older children with amblyopia that may have improved their performance independently of their visual deficits—an important issue from both biological and clinical management perspectives—could not be determined, as only a few (5/21) of the patients tested were aged 7 years or older.
Materials and Methods
Cross-sectional data were obtained from 83 children aged 5.0 to 9.25 years, of whom 55 were either undergoing (n = 24) or had recently completed (n = 31) treatment for amblyopia (with varying degrees of success) and 28 were normally sighted controls. All subjects had standard clinical vision tests conducted by one of the authors just before their eye–hand coordination abilities were assessed. For the patients, this was always on the day of a routine clinical appointment and included evaluations of logMAR distance VA in each eye, of stereoacuity, and in those deemed necessary, of suppression (Bagolini lenses, followed by Worth 4-dot lights, when this was inconclusive). The initial stereo test was for “gross” (3000 arc seconds) stereopsis using the Titmus fly test. Patients passing the fly test were examined further with the Titmus circles and then, in most cases, the Frisby test. As the results of these tests were well correlated (R2 = 0.89, P < 0.001; n = 19), the lowest (best) score was recorded as the stereo-threshold. Solid stereograms were used because they are more akin to real-world 3D stimuli (which also contain monocular depth cues) and are easier for children to understand (and, hence, to administer) than random-dot displays. When not directly tested, other patient data, for example, on refractive error, suppression, cover test, ocular motility, convergence, and prism fusion ranges, were obtained from their earlier clinical appointment record.
Patient details are summarized in Table 1, grouped by younger (5.00–6.92 years) and older (7.0–9.25 years) ages, in anticipation of differences in hand movement control strategies, at least in the visually normal children at these matching ages.12–14,21 In brief, 19 of the patients had “pure” anisometropia (>1 diopter [D] interocular difference in the most ametropic meridian) and 36 were strabismic (including microtropia and four of mixed type). The severity of amblyopia present, as quantified by the range and mean interocular difference (IOD) in logMAR VA between the affected and dominant eyes, was similar in the two age-groups (t-test, P = 0.5). Around 20% of the children in each group no longer had amblyopia, as conventionally defined, since their IODs were less than two lines (i.e., <0.20 logMAR). These subjects were initially labeled as “cured,” with others designated as having “mild” or “moderate” amblyopia, based on IOD values of 0.20 to 0.39 and 0.40 to 1.10, respectively. A similar proportion (20%) of the 7- to 9-year-old patients (all anisometropic) had stereoacuity thresholds of 50 to 85 arc seconds, indicating that their binocular vision had recovered (two were also no longer amblyopic) and were initially classed as having “cured” stereovision—as was one child with moderate amblyopia among the 5- to 6-year-olds—with the rest classified as having “coarse” (100–600 arc seconds), “gross” (3000 arc seconds; fly only), or “nil” stereovision. Pilot data were obtained from four additional amblyopic children (two anisometropic; one mixed; one strabismic) studied longitudinally on 2 to 3 separate occasions before and/or during their normal treatment regime. Control children at ages 5.33 to 6.92 (n = 13) and at 7.0 to 9.08 (n = 15) years met inclusion criteria by having normal or corrected-to-normal VA in each eye with IODs < 0.20 (respective means were 0.03 [±0.06 SD] and 0.01 [±0.07 SD] logMAR), stereo-thresholds of ≤85 arc seconds, and no history of ocular disorder. Informed consent/assent was obtained for participation, and conduct was in accordance with the Declaration of Helsinki and both National Research Ethics Service UK and Senate Ethics Committee of City University London approval.
Table 1.
Summary of Patient Details
|
Patient Status |
Ages 5–6 y, n= 30 |
Ages 7–9 y, n= 25 |
|||
| Visual acuity | logMAR IOD range | 0.02–1.10 | 0.02–1.10 | ||
| Mean IOD (±SD) | 0.39 (±0.28) | 0.43 (±0.28) | |||
| Cause | Aniso (8) | Strab (22) | Aniso (11) | Strab (14) | |
| Amblyopia | Cured | 1 | 6 | 3 | 1 |
| Mild | 1 | 11 | 3 | 9 | |
| Moderate | 6 | 5 | 5 | 4 | |
| Stereo acuity | Cured (50–85 arc″) | 1 | 0 | 5 | 0 |
| Coarse (100–600 arc″) | 2 | 8 | 5 | 3 | |
| Gross (3000 arc″) | 0 | 1 | 1 | 0 | |
| Nil | 5 | 13 | 0 | 11 | |
| Treatment | Refractive adaptation | 1 | 2 | 4 | 1 |
| Partial occlusion | 3 | 6 | 3 | 4 | |
| Completed/follow-up | 4 | 14 | 4 | 9 | |
Apparatus and Hand Movement Recordings
Participants reached for and precision grasped cylindrical household objects of “small” (24 mm) or “large” (48 mm) diameter, but similar (100 mm) height, placed at a “near” midline or at two “far” locations (10° across their midline or 10° uncrossed) with respect to the start position of their preferred hand (see Fig. 1). Handedness was assessed with the abbreviated (10-question) version of the Edinburgh inventory.34 Children ticked the answers themselves following explanation of each question by the experimenter; and, in fact, the preference expressed was always the hand in which they held the pen to complete the test. Two sets of target distances were used, scaled according to the child's arm length. Lightweight (<5 g) infrared reflective markers were placed on the wrist and tips of the thumb and index finger of this hand, and on top of the center of each object. Instantaneous marker positions in the three-dimensions of their resultant movements were recorded (at 60 Hz) throughout each movement by three infrared motion capture cameras (ProReflex; Qualisys, Gothenburg Sweden), with spatial tracking errors of <0.4 mm. Subjects sat with eyes closed between trials and, following a verbal “go” signal, were required to open their eye(s) and reach out “as naturally and accurately as possible” to pick up the object “at approximately half its height, using a precision grip,” place it to one side and return to the start position. All participants performed several (typically 2–4 per view) practice movements to a neutral object placed at random table locations prior to the start of the testing, to ensure that they understood these instructions and were comfortable with the general task conditions. They then performed 12 or 18 experimental trials (i.e., two object sizes × three locations, each repeated two or three times) in separate blocks with both eyes open or with a patch occluding their dominant or nondominant eye, these being established for the control subjects by simple sighting versus nonsighting eye tests. Participants wore any habitual (i.e., most recent prescription) glasses when performing the tasks, with the patch placed under their glasses during monocular testing. Object presentations were in the same pseudorandomized order for each of the viewing conditions, which were counterbalanced between subjects in each group.
Figure 1.

The experimental setup showing (top) the 3-wall mounted infrared motion capture cameras (Qualisys) triangulating the (bottom) black workspace table from above. On this, the start button is in the foreground, with the “large” object shown at a “near, midline” location; the “small” object at an “ipsi, far” location (for a right-handed subject); and the neutral object used only for practice at a “contra, far” location. The locations shown were used for children with arm lengths ≥35 cm; blue stickers indicate those used for subjects with shorter arms.
Hand Movement Analyses
Analyses focused on seven kinematic and seven “error” measures that reflect different aspects of the efficacy of movement planning or of online control, several of which were seen to be defective among the amblyopic children of our earlier study.21 Five kinematic measures related to the movement dynamics: the overall movement durations (from leaving the start position to picking up the object); the time to peak velocity after movement onset (a measure of reach planning); the proportions (percentages) of the movement duration spent in the “low velocity” final approach (feedback) phase of the reach (between its peak deceleration and initial object contact); and in the final postcontact (feedback) phase of the grasp (between initially touching the object and picking it up); and in the actual time spent closing the grip (between its peak aperture at initial hand opening and the first moment of object contact). Two further kinematic parameters concerned spatial indices of grasping performance: the peak grip aperture between thumb and finger (a measure of planning accuracy in preparation for grasping the target); and the grip size at initial object contact (an index of end-point accuracy). Error measures related to the reach were late-velocity corrections, object collisions, and spatial path (trajectory) corrections, indicative of undershooting, overshooting, and misdirection of the reach, respectively. The grasp errors were adjustments to the grip occurring (1) just before object contact or (2) during grip application; (3) excessively wide grips at initial contact; and (4) prolonged grip applications, indicative of inaccurate (errors 1–3) or uncertain (error 4) performance. An example of a misdirected reach is shown in Figure 4B; readers are referred to our previous, open-access publications10,11,21 for depictions of the other error types.
Figure 4.

Profiles of (A) a normal and (B) a corrected spatial reach path during binocular movements toward the same “near, midline” target (filled circle) in (A) a left-handed control subject aged 7.25 years and (B) a right-handed stereo nil patient aged 7.33 years. The origin (0) of both movements on the x-axis corresponds to the starting hand positions; solid traces show the reach paths collapsed into lateral and forward directions and terminating just short of the target (as they were recorded from the marker on the wrist). In (A) the movement of the left hand follows a typical, slightly curved trajectory in a leftward (x-axis, -ve) lateral direction, but in (B) the trajectory is not a rightward mirror-image. Instead, the patient initially moved slightly rightward (open arrow), but misdirected his reach toward the midline well short of the target's location, necessitating a subsequent trajectory correction (filled arrow)—defined as a spatial path error—in order to acquire it.
Statistical Analyses
Median values of the above seven kinematic parameters, along with the frequency of total reach and grasp errors and of the seven specific error types committed during the (12 or 18) movements performed under each viewing condition were calculated for each subject and analyzed using statistical software (IBM SPSS, package version 21; IBM Corp., Armonk, NY, USA). Initial analyses were undertaken by repeated measures ANOVA (Huynh-Feldt corrected for lack of sphericity, when necessary) with view (binocular, dominant eye, nondominant eye) as the within-subjects factor and with group (controls, patients) as the between-subject factor in each age-range. Post hoc tests were conducted with the Bonferroni adjustment for multiple pair-wise comparisons to examine the origin of any main within-subject effects of view—with the (unadjusted) least significant difference test applied in cases where this yielded no explanation (see Table 2 footnotes)—followed by 1-way ANOVA to examine between-group differences under each separate view. Subsequent comparisons by age-range were then carried out to assess possible factors (i.e., depth of amblyopia, stereovision loss, treatment history) contributing to the generally poorer performance of the patients. To provide adequate numbers in each patient subgroup for these purposes, we condensed the original classifications (Table 1) into three sets of just two ordinal categories. Specifically: for amblyopia severity, those originally labeled as “cured” or as “mild” were combined into a single “mild” category for comparison with the “moderate” class; for stereovision, those with “cured,” “coarse,” or “gross” stereo-thresholds (Table 1) were combined into a single stereovision present (or “Stereo+”) category for comparison with the “Stereo Nil” patients; and for treatment stage, those who had only completed refractive adaptation and/or some occlusion therapy were classed as treatment “ongoing” for comparison with those in “follow-up” who were no longer undergoing treatment of any kind. The significance level was set at P < 0.05.
Table 2.
Mean (±SEM) Hand Movement Kinematics by Age, Group, and Viewing Condition
|
Dependent Measures |
Group |
View |
||
|
Binocular |
Dom Eye |
NonDom Eye |
||
| Ages 5–6 y | ||||
| Movement duration, ms* | Control | 829 (74) | 911 (65)L | 931 (77)L |
| Patient | 1075 (49) | 1106 (43) | 1136 (51) | |
| Time to peak velocity, ms† | Control | 266 (21) | 293 (19) | 283 (22) |
| Patient | 322 (14) | 336 (14) | 337 (14) | |
| Low velocity phase, %* | Control | 18.8 (3.7) | 18.6 (3.1) | 22.4 (3.7) |
| Patient | 32.8 (2.1) | 31.0 (1.8) | 32.8 (2.0) | |
| Postcontact time, % | Control | 15.8 (1.2) | 14.9 (1.2) | 14.8 (1.5) |
| Patient | 15.6 (0.8) | 16.0 (0.7) | 15.5 (0.9) | |
| Peak grip aperture, mm† | Control | 77.7 (2.1) | 81.8 (2.1)B | 83.0 (2.0)BB |
| Patient | 75.4 (1.4) | 75.6 (1.4) | 76.0 (1.3) | |
| Grip closure time, ms† | Control | 166 (12) | 197 (19)L | 204 (21)L |
| Patient | 269 (25) | 251 (20) | 263 (27) | |
| Grip size at contact, mm | Control | 51.7 (1.7) | 58.4 (2.2)BB | 60.2 (2.7)BB |
| Patient | 51.4 (1.1) | 54.9 (1.4) | 56.3 (1.8)B | |
| Ages 7–9 y | ||||
| Movement duration, ms | Control | 850 (41) | 966 (45)BB | 978 (43)BB |
| Patient | 915 (32) | 985 (35)L | 981 (33)L | |
| Time to peak velocity, ms | Control | 292 (17) | 292 (15) | 293 (14) |
| Patient | 313 (13) | 326 (11) | 326 (11) | |
| Low-velocity phase, % | Control | 28.3 (2.5) | 31.0 (2.9) | 32.1 (2.0)L |
| Patient | 28.0 (2.8) | 29.3 (2.0) | 29.4 (2.2) | |
| Postcontact time, %† | Control | 14.8 (1.1) | 15.3 (1.2) | 14.3 (1.2) |
| Patient | 17.2 (0.8) | 17.5 (0.9) | 17.4 (0.9) | |
| Peak grip aperture, mm | Control | 74.7 (2.7) | 77.7 (2.7)L | 79.1 (3.1)L |
| Patient | 75.1 (1.8) | 75.3 (2.1) | 74.8 (2.4) | |
| Grip closure time, ms | Control | 196 (14) | 233 (21) | 241 (20) |
| Patient | 198 (11) | 219 (13) | 214 (13) | |
| Grip size at contact, mm | Control | 49.5 (1.3) | 52.8 (1.4)L | 52.3 (1.5)L |
| Patient | 49.2 (1.4) | 51.2 (1.2) | 52.4 (1.4)L | |
Asterisks and daggers denote significant between-group differences in the given measure. Letters denote significant within-group differences in dominant (Dom) or NonDom eye performance compared with binocular viewing according to post hoc tests. B, P < 0.05, Bonferroni; BB, P ≤ 0.01, Bonferroni; L, P < 0.05, least significant difference.
* P < 0.01.
† P ≤ 0.05.
Two further approaches were taken to assess the impact of these factors in each patient age group. First, preliminary stepwise (hierarchical) multiple regression analyses were undertaken to evaluate relations between the three sets of ordinal categories above and their potentially unique (or combined) contribution(s) to the patient deficits. We say “preliminary” because the patient sample sizes were relatively low for this type of analysis. To counter this, we report adjusted R2 values to indicate the proportion of the variance in the data attributable to the specific ordinal factor in question, their beta coefficients (indicating the unique contribution of that variable when overlapping effects of any other factors are removed statistically), and the t-statistics representing the significance of that contribution. Potential for a unique contribution was confirmed by Spearman's rank order correlation analyses, which revealed no significant associations between any set of the variables at either age. This included only a general tendency—as might be expected—for the patients still undergoing therapy at 5 to 6 years (P = 0.23, P = 0.23) and 7 to 9 years (P = 0.11, P = 0.59) to have a greater VA loss than those who had completed it. In fact, average IODs were in the lower range of moderate amblyopia in the former subgroups of patients at the two ages (both, coincidentally, 0.50 ± 0.3 SD) and in the upper mild category in those sampled at follow-up, this difference not quite achieving statistical significance at ages 5 to 6 years (0.31 ± 0.2 SD, P = 0.056) and not significant at 7 to 9 years (0.37 ± 0.2 SD, P = 0.24), and with no relationships at all between the patient's stereovision and their VA loss or treatment stage. In accordance with this, colinearity diagnostics derived from the regression models routinely confirmed that the three ordinal variables were largely independent by consistently yielding very low variance inflation factor scores of <1.1. Second, Spearman's correlation analyses were conducted between each hand movement index and the stereoacuity thresholds recorded in each of the patients in which it was measurable and their IOD in VA, to determine whether the levels of stereovision or VA loss present in individual subjects—rather than just across broader categories—were associated with progressive reach-to-grasp effects at each age-range.
Results
Overview
Tables 2 and 3 summarize the kinematic and error data obtained from the children in the two age-ranges and results of ANOVA conducted by viewing condition for each parameter within- and between-subject groups. Control children at both ages exhibited significant binocular advantages for most parameters, including key measures of their movement speed (e.g., total durations) and accuracy (e.g., grip sizes at object contact; reach and grasp error-rates). That is, post hoc comparisons of the main effect of view showed that these movement components were performed better when using binocular vision compared with their dominant or their nonsighting eyes alone, with the advantages being relatively small for kinematic measures (range, ∼5%–20%; Table 2), but much more marked for error rates (range, ∼33%–200%; Table 3). Such binocular advantages over the dominant (“better”) eye were absent among the 5- and 6-year-olds with amblyopia and involved only one parameter—marginally (∼70 ms) shorter movement durations—in the 7- to 9-year-old patients, since the few other main effects of view in these subjects were due to poorer performance only with their amblyopic eye (Tables 2, 3) relative to binocular viewing.
Table 3.
Mean (±SEM) Movement Error Rates/Trial by Age, Group, and Viewing Condition
|
Dependent Measures |
Group |
View |
||
|
Binocular |
Dom Eye |
NonDom Eye |
||
| Ages 5–6 y | ||||
| Total reach errors‡ | Control | 0.47 (0.10) | 0.74 (0.09)BB | 0.72 (0.08)BB |
| Patient | 0.83 (0.07) | 0.91 (0.06) | 0.92 (0.06) | |
| Late velocity corrections‡ | Control | 0.36 (0.04) | 0.46 (0.04)L | 0.48 (0.03)L |
| Patient | 0.51 (0.05) | 0.59 (0.04) | 0.60 (0.05) | |
| Object collisions* | Control | 0.06 (0.03) | 0.17 (0.04)L | 0.14 (0.04)L |
| Patient | 0.05 (0.01) | 0.05 (0.01) | 0.07 (0.02) | |
| Spatial path corrections* | Control | 0.05 (0.04) | 0.11 (0.05) | 0.10 (0.03) |
| Patient | 0.27 (0.03) | 0.27 (0.03) | 0.26 (0.03) | |
| Total grasp errors‡ | Control | 0.47 (0.06) | 0.65 (0.07) | 0.73 (0.10) |
| Patient | 1.09 (0.09) | 1.13 (0.07) | 1.30 (0.08)L | |
| Precontact grip adjustments‡ | Control | 0.02 (0.01) | 0.04 (0.02) | 0.05 (0.03) |
| Patient | 0.18 (0.03) | 0.16 (0.03) | 0.17 (0.03) | |
| Postcontact grip adjustments* | Control | 0.15 (0.04) | 0.30 (0.07)L | 0.35 (0.07)L |
| Patient | 0.50 (0.05) | 0.47 (0.05) | 0.55 (0.05) | |
| Prolonged object contacts‡ | Control | 0.07 (0.02) | 0.06 (0.03) | 0.06 (0.03) |
| Patient | 0.24 (0.04) | 0.17 (0.03) | 0.18 (0.03) | |
| Wide grips at contact‡ | Control | 0.23 (0.03) | 0.25 (0.03) | 0.27 (0.03) |
| Patient | 0.27 (0.03) | 0.33 (0.03) | 0.40 (0.03)BB | |
| Ages 7–9 y | ||||
| Total reach errors | Control | 0.45 (0.05) | 0.61 (0.07) | 0.74 (0.08)B |
| Patient | 0.62 (0.06) | 0.70 (0.06) | 0.76 (0.05)L | |
| Late-velocity corrections | Control | 0.30 (0.05) | 0.38 (0.05) | 0.47 (0.06)L |
| Patient | 0.38 (0.04) | 0.41 (0.04) | 0.46 (0.04)L | |
| Object collisions | Control | 0.07 (0.05) | 0.08 (0.03) | 0.06 (0.02) |
| Patient | 0.04 (0.01) | 0.05 (0.01) | 0.07 (0.02) | |
| Spatial path corrections‡ | Control | 0.08 (0.02) | 0.15 (0.03)B | 0.22 (0.04)BB |
| Patient | 0.20 (0.03) | 0.25 (0.04) | 0.22 (0.02) | |
| Total grasp errors | Control | 0.49 (0.06) | 0.93 (0.08)BB | 0.84 (0.10)BB |
| Patient | 0.84 (0.08) | 0.91 (0.06) | 0.95 (0.08) | |
| Precontact grip adjustments | Control | 0.05 (0.02) | 0.06 (0.02) | 0.10 (0.02) |
| Patient | 0.07 (0.02) | 0.09 (0.02) | 0.13 (0.03)L | |
| Postcontact grip adjustments | Control | 0.22 (0.04) | 0.53 (0.07)BB | 0.43 (0.08)BB |
| Patient | 0.34 (0.04) | 0.35 (0.05) | 0.32 (0.04) | |
| Prolonged object contacts* | Control | 0.06 (0.02) | 0.06 (0.02) | 0.09 (0.02) |
| Patient | 0.19 (0.03) | 0.18 (0.03) | 0.17 (0.02) | |
| Wide grips at contact | Control | 0.16 (0.02) | 0.28 (0.03)BB | 0.22 (0.03) |
| Patient | 0.24 (0.04) | 0.29 (0.03) | 0.33 (0.03)L | |
Conventions as in Table 2, except between-group differences.
‡ P ≤ 0.001.
There were numerous other differences (involving 14 of the 16 parameters examined) between the 5- and 6-year-old control and amblyopic subjects, with only one indicative of worse performance by the normal participants. The 7- to 9-year-old children with amblyopia, by contrast, showed few significant differences (on only three measures) compared with their age-matched normally sighted peer-group (Tables 2, 3). However, as will be seen, this implied improvement in eye–hand coordination abilities with age in amblyopic children was partly due to different age-related, reach-to-grasp strategies adopted by the control-versus-patient groups, as well as to a developmental progression in the beneficial effects of recovered binocular stereovision in the amblyopic subjects.
Age- and Vision-Dependent Differences in Overall Movement Times
Total movement durations in the 5- and 6-year-old patients were much (∼200–250 ms or ∼20%–30%) longer than those of the age-matched controls across each of the three views, with evidence that significantly prolonged periods spent in both the early, planned (e.g., time to peak velocity), and later guidance (e.g., grip closure times) movement phases contributed to this main effect of group. Separate ANOVA by each ordinal patient category revealed significant relationships between total movement durations and amblyopia severity, stereovision (see Fig. 2A) and treatment stage, but no interactions with viewing conditions. More specifically, post hoc analyses showed that patients with moderate amblyopia (P < 0.001) or who were stereo nil (P = 0.003) or whose treatment was ongoing (P = 0.02) produced slower movements across all three views than control children, with prolonged movements also associated with moderate compared to mild amblyopia (P = 0.027). Regression analysis supported these findings, in that models examining the influence of the three ordinal variables in the patients indicated that their severity of amblyopia alone accounted for most of the variance in their movement durations under binocular (adjusted R2 = 0.16, β = 0.43, t = 2.5, P = 0.017); dominant eye (adjusted R2 = 0.14, β = 0.38, t = 2.2, P = 0.041); and nondominant/affected eye (adjusted R2 = 0.13, β = 0.40, t = 2.3, P = 0.027) conditions, although the models were improved for each view (e.g., all three now t > 2.5, P < 0.015) when further significant contributions of their stereo loss (but not treatment) were added to the stepwise analysis. Broadly similar findings applied, as well, to the patient's prolonged grip closure times, but not to their delayed time to peak velocity which was significant only for non-dominant eye conditions in the stereo nil subjects.
Figure 2.
Average movement durations by age (A) 5 to 6 years, (B) 7 to 9 years, viewing condition and stereovision. Movement times increased successively between control, stereo+ and stereo nil subjects at ages 5 and 6 years, but were similar in the control and stereo+ participants at ages 7 to 9 years, including faster performance with binocular compared with monocular vision, whereas there were no differences across views in the older stereo nil subjects. Errors bars: SEMs.
By contrast, there was no equivalent overall group difference in movement durations between the children aged 7 to 9 years nor any main effects of amblyopia, stereovision or treatment stage. But there was a stereovision x view interaction (F[4,74] = 3.9, P = 0.006). This arose because, like the control subjects, the patients who were stereo+ exhibited a binocular advantage over monocular viewing (of ∼15%; Fig. 2B) for this parameter, while the stereo nil participants did not. The absence of an overall group difference was, therefore, partly driven by the similarities in binocular movement times between visually normal and stereo-recovered subjects, along with similarities in performance by all participants when using one eye alone. Regression analysis supported these findings too, by indicating that the degree of stereovision present in the patients was uniquely sufficient to account for their binocular movement durations (adjusted R2 = 0.18, β = 0.48, t = 2.7; P = 0.015). Unlike in younger subjects, there were no main effects or interactions for times to peak velocity or grip closure.
Age-Dependent Changes in Reach and Grasp Strategies
Age-related between-group differences in approach to the tasks contributed to these effects. Most notably, there was a major change in behavior among control children who, at 5 and 6 years, spent only ∼20% of their total movement time across all views in the low velocity/visual feedback phase of the reach, compared with ∼30% at ages 7 to 9 years (Table 2). Indeed, Spearman's correlation analysis revealed a moderate positive correlation between this reach parameter and their continuous ages (5.33–9.08) in years (P = 0.42, P = 0.025), such that this aspect of online reach control in the older children, including their small binocular advantage—at least over the nondominant eye (of ∼13%)—began to resemble that of normal adults.10
The 5- and 6-year-old children with amblyopia, however, appeared to place as much reliance on visual feedback as the normal 7- to 9-year-olds, since they too spent a similarly greater proportion (∼32%) of their movements in the low velocity reach phase across all views compared with their age-matched controls. Indeed, it was the much longer period of actual time (some 150–175 ms) spent by the younger patients finally approaching the targets that accounted for most (70%–75%) of the between-group difference in movement durations at this age (Fig. 2A). It was also mainly the normal change in reach strategy that eliminated any between-group difference at ages 7 to 9 years, although the patients appeared to contribute to this via a small reduction in relative approach times (of 3.3%) after ages 5 to 6 years.
There was also an important age-dependent change in grasping strategy related to the percent time spent in contact with the object before it was lifted but, in this case, mainly involving the older children with amblyopia (Table 2). Specifically, whereas all control subjects and the younger patients spent equivalent relative periods of time (∼15%) in this movement phase across all views, the 7- to 9-year-old children with amblyopia increased their postcontact times to a mean of 17.4%, this increase being significant relative both to the younger patient counterparts (F[1,53] = 5.0, P = 0.03)—although not quite correlating with age (5.0–9.25) in years (Spearman's P = 0.21, P = 0.10)—and to their age-matched controls. Indeed, it was the relative increase in actual postcontact time (some 30–50 ms) by the older patients that accounted for most (∼80%) of the between-group difference in movement durations at ages 7 to 9 years (Fig. 2B).
Stereovision-Dependent Benefits for Reaching Performance
The 5- to 6-year-old children with amblyopia made significantly more total reaching errors than their age-matched controls (Table 3) particularly when using both eyes, but also under dominant and nondominant eye conditions. These differences were due to increased rates of late corrections to the reach velocity (i.e., “undershoots”) and, most markedly, to its spatial path by the patients, whereas the 5- to 6-year-old control children—uniquely among all subjects groups—produced more high-velocity collisions with the objects (i.e., overshoots) when viewing monocularly, consistent with their distinctively ballistic reaching behavior. Analysis of variance showed main effects of stereo loss on total reach errors (Fig. 3A), late velocity, and spatial path corrections (all F[2,40] > 3.0, P < 0.05), due to their increased occurrence in the stereo nil compared with control subjects and compared to the stereo+ participants in the cases of total reach and spatial path errors. There were no equivalent effects of amblyopia or treatment. Regression analysis suggested that stereovision loss was the sole factor responsible for the increased total reach errors in these patients under binocular (adjusted R2 = 0.16, β = 0.50, t = 3.0, P = 0.007); dominant eye (adjusted R2 = 0.14, β = 0.43, t = 2.5, P = 0.020); and nondominant/affected eye (adjusted R2 = 0.13, β = 0.48, t = 2.9, P = 0.007) conditions and for their misdirected reaches across all views (adjusted R2 ≥ 0.25, β ≥ 0.53, t ≥ 3.30, P ≤ 0.003), but provided no model explaining their increased late-velocity corrections.
Figure 3.
Average total reach error-rates per trial by age (A) 5 to 6 years, (B) 7 to 9 years, viewing condition and stereovision. Stereo nil subjects made the most reaching errors at both ages with no differences across the three views, whereas performance was similar in the control and stereo+ participants, particularly at ages 7 to 9 years with fewest errors occurring in the binocular condition. Errors bars: SEMs.
There was no overall between-group difference in total reach errors in the older children (Table 3). But there were main effects of stereovision (F[2,37] = 4.8, P = 0.014) and a stereo x view interaction (F[4,74] = 3.3, P = 0.015). These were due to consistently more error-prone performance, particularly in misdirected reaching (see Fig. 4B), by the stereo nil compared with control and stereo+ subjects, combined with an obvious binocular advantage (of ∼33%–50%), similar to the controls (∼45%–60%), among those who were stereo+ (Fig. 3B). Stepwise regression models indicated that stereovision loss was the only significant contributor to the increased total reach errors in these patients with binocular (adjusted R2 = 0.15, β = 0.43, t = 2.5, P = 0.019); dominant eye (adjusted R2 = 0.14, β = 0.41, t = 2.2, P = 0.048), and nondominant/affected eye (adjusted R2 = 0.25, I = 0.58, t = 3.2, P = 0.004) viewing, and to the increased spatial path errors in the binocular condition (adjusted R2 = 0.17, β = 0.50, t = 2.6, P = 0.016). Thus, improved reaching accuracy, indicative of better target localization, were consistent benefits of stereovision across both subject ages and groups.
Stereovision-Dependent Benefits for Grasping Performance
Normal binocularity provided additional benefits for grip planning. Control children of both ages formed significantly wider initial peak grip apertures with monocular viewing (Table 2), an effect typically associated with an increase to its “safety margin” produced by normal adults under similar conditions of increased visual “uncertainty.”7–11 But this cautious approach was absent in the patients who produced virtually identical peak grips at both ages across all views. The aperture of the peak grip usually increases quite linearly with increasing target size and in fact, all participants did this, by a factor of ×0.84–0.89 when preparing to grasp the “large” compared with “small” object. This relative scaling behavior was, however, closer to the normal adult mean (of ×0.8235) in the control children who, as exemplified in Figure 5, also showed significant view x object size interactions, in which they produced wider monocular peak grips only for the smaller object, as normal adults do on our task.10 But the patients did not, suggesting that they were equally “uncertain” when planning to grasp the smaller object when using two eyes as with one eye alone.
Figure 5.

Average peak grip apertures produced by 5- to 6-year-old participants prior to grasping the (A) “small” and (B) “large” objects, by viewing condition and stereovision. Only the control subjects selectively opened their grip much wider when preparing to grasp the smaller object when using monocular vision, this being classically designed to increase the “safety margin” for error under conditions of perceptual uncertainty. Error bars: SEM.
Despite these effects, there were no between-group differences at either age in the grip size achieved on initial contact with the objects (Table 2). Moreover, all subjects made contact with the smallest grip sizes closer to the average width of the two targets (36 mm) when viewing binocularly, an improvement in accuracy that was independent of the object's size, although only significant in relation to poorer amblyopic eye performance by the patients.
For the control children, the suggested binocular advantage for grip precision was supported by their reduced total grasp error-rates when using both eyes and, particularly, in their need to make fewer subsequent adjustments to their grip after object contact (Table 3). The amblyopic children, however, generally committed more grasping errors than the controls and showed no evidence of a binocular advantage for reducing postcontact grip adjustments. Indeed, total grasp error rates in the patients aged 5 to 6 years were roughly doubled across all views compared with the matched control group and involved increases in every subtype of “inaccurate” and “uncertain” grasping measure (see Methods). There was also a main effect of view for cumulative grasping errors in this patient cohort, mainly due to the increased occurrence of abnormally wide (i.e., inaccurate) grips at initial object contact under amblyopic eye compared with binocular viewing, consistent with the above findings related to their “grip size at contact” (Table 2), for which this is a related “error” parameter. There were no other significant effects, including of stereovision loss (Fig. 6A), or interactions with view.
Figure 6.

Average total grasp error rates per trial by age (A) 5 to 6 years, (B) 7 to 9 years, viewing condition and stereovision. Grasp error rates increased successively between control, stereo+ and stereo nil subjects at ages 5 to 6 years, but were similar in the control and stereo+ participants at ages 7 to 9 years, including generally better performance with binocular compared with monocular vision, whereas there were no differences across views in the older stereo nil subjects. Errors bars: SEMs.
In contrast, total grasping error rates were similar in the 7- to 9-year-old patients versus controls, although abnormally prolonged contacts with the objects were more common in the amblyopic children across all views, this partly reflecting their increased “postcontact times” (Table 2). The patients also more frequently adjusted their grip just before object contact and made contact with abnormally wide grips under amblyopic eye compared with binocular viewing. Additional ANOVA, however, revealed a main effect of stereovision (F[2,37] = 5.3, P = 0.010) and a stereo x view interaction (F[4,74] = 2.8, P = 0.034) for cumulative grasp error rates. These were, again, due to consistently worse performance—particularly in producing abnormally wide grips at contact—by the stereo nil compared with control and stereo+ subjects, combined with a small binocular advantage (of ∼22%) relative to control subjects (∼70%–90%), among those who were stereo+ (Fig. 6B). Stepwise regression models indicated that stereovision loss was the only significant contributor to the increased total grasp errors in these patients when using binocular vision (adjusted R2 = 0.18, β = 0.43, t = 2.3, P = 0.032)—but not either eye alone—and abnormally wide binocular grips at contact (adjusted R2 = 0.21, β = 0.47, t = 2.5, P = 0.021). Thus, improved grasping accuracy, indicative of better endpoint control, were consistent benefits provided by stereovision across subject ages and groups.
Correlation Analyses
Among the 12 younger stereo+ patients (Table 1) there were small-to-moderate positive Spearman's correlations (P = 0.32–0.60) between progressive increases in their IODs in VA and movement durations, total reach error rates and grip sizes at contact across all views, but only the correlation between grip size and amblyopic eye viewing achieved significance (P = 0.039). Increases in total reach error rates across all views (P = 0.57–0.69, P < 0.05) and in grip size at contact with binocular viewing (P = 0.73, P = 0.007), however, were moderately to strongly associated with increasing stereo-thresholds in this patient subgroup, in line with some of the foregoing analyses. However, there were no significant correlations between either IODs or stereo-thresholds for any movement parameter across the 14 stereo+ subjects aged 7 to 9 years.
The “Cured” Stereovision and Longitudinal Cases
“Treatment stage” was never found to be a significant factor in our regression analyses, implying that the patients' therapeutic history per se had little or no impact on their reach-to-grasp abilities. However, average data obtained from the five older patients (all anisometropes) with “cured” stereoacuities (three of whom had residual mild or moderate amblyopia) were all within the upper bound 95% confidence limits of (i.e., indistinguishable from) age-matched controls. As indicated in Table 4, key movement time and total error measures in the four patients studied longitudinally at different stages of their treatment also suggested that this can benefit eye–hand coordination, provided it resulted in binocular recovery. Specifically, the initial performance of the two anisometropic cases (A1, A2) whose stereovision was essentially normal at treatment cessation improved markedly, culminating in binocular advantages for both movement speed and accuracy within the normal range. But this did not occur in either the mixed (M1) or strabismic (S1) cases whose amblyopia severities were, respectively, mild and “cured” at the end of their therapy, but who remained “stereo blind” throughout. The following two cases highlight these differences.
Table 4.
Reach-to-Grasp Performance of the Longitudinal Cases Compared With Age-Matched Controls
|
Subjects |
Age |
IOD, SA, arc secs |
Move Times |
Reach Errors |
Grasp Errors |
||||||
|
Bino |
Dom |
NonDom |
Bino |
Dom |
NonDom |
Bino |
Dom |
NonDom |
|||
| Px A1 | |||||||||||
| Untreated | 7.75 | 1.88, NIL | * | * | * | * | * | * | * | * | |
| Refracted | 8.0 | 0.78, 100 | * | * | * | ||||||
| Occluded | 8.75 | 0.52, 60 | |||||||||
| Px A2 | |||||||||||
| Untreated | 7.33 | 0.88, 300 | * | * | * | * | * | * | * | ||
| Refracted | 7.8 | 0.44, 170 | * | * | |||||||
| Occluded | 8.2 | 0.30, 100 | * | ||||||||
| Px M1 | |||||||||||
| Refracted | 6.2 | 0.62, NIL | * | * | * | * | * | * | * | ||
| Occluded | 7.5 | 0.34, NIL | * | * | * | * | * | * | * | ||
| Px S1 | |||||||||||
| Refracted | 7.0 | 0.40, NIL | * | * | * | * | * | * | * | * | * |
| Occluded | 7.5 | 0.14, NIL | * | * | * | * | * | * | |||
Bino, binocular; Dom, dominant eye; move times, total movement durations; NIL, unmeasurable; occluded, tested immediately after patching therapy or at 3 to 6 months follow-up; Px, patient; refracted, tested after 3 to 4 months optical correction; untreated, tested prior to spectacle wear.
Data obtained for the given parameter were above the upper 95% confidence limit of the matched controls.
Case A1: Binocular Recovery, but Persistent Moderate Amblyopia
This patient initially presented at age 7.8 years with marked anisometropia (right [R] eye +8.25/−1.50 × 180; left [L] eye +2.00/–0.25 × 180) and severe amblyopia (R eye VA 2.00 logMAR, IOD 1.88). Cover test revealed an exophoria, and there was complete R eye Bagolini lens suppression with nil stereovision. He was first tested (uncorrected) on the day before he received his spectacles (untreated), when his reaching and grasping movements were notably slow and inaccurate, especially when using the amblyopic eye (Fig. 7). Repeat testing occurred immediately after 3.5 months of refractive adaptation and, finally, 8 months later (aged 8.75 years) following part-time (4 h/d) occlusion therapy with which he was reported to have been compliant, but had just been withdrawn due to signs of decompensation (i.e., cover test showed a flick R exotropia). Following spectacle correction his amblyopia was moderate (VA 0.78, IOD 0.78) and was associated with a dramatic improvement in both his stereovision (to 100 arc seconds) and reach-to-grasp performance across all three views, although the data obtained in the binocular condition were all beyond the upper 95% confidence limits of those in matched control subjects. However, after further gains in VA (to 0.54, IOD 0.52) and in stereoacuity (to 60 arc seconds) following occlusion therapy, there was evidence of binocular advantage, such that his eye–hand coordination abilities were indistinguishable from those of the control subjects (and from his normally sighted older brother, who we tested at age 9.3 years).
Figure 7.
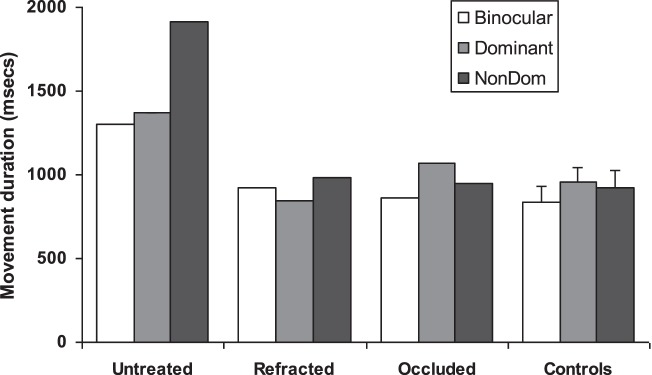
Recovery of stereovision and eye–hand coordination deficits in a child with anisometropic amblyopia (case A1). Patient data are median movement durations (12 trials per view) compared with 12 control subjects of equivalent ages (range, 7.0–8.75 years) over which the patient was tested between initial presentation (untreated) and the end of his spectacle adaptation (refracted) and occlusion therapy (occluded). For further patient details, see text. NonDom, the amblyopic eye in the patient; the nonsighting eye in the controls. Error bars are upper bound 95% confidence limits.
Case M1: Absent Binocularity, With Mild Amblyopia
This patient initially presented at age 5.8 years with marked anisometropia (R eye +1.00/–0.25 × 180; L eye −8.00/–0.50 × 30) and strabismus. She had moderate-to-severe amblyopia (L eye VA 1.00 logMAR, IOD 0.85) and absent stereovision with complete L eye suppression. She was first tested at age 6.2 years after 4 months of spectacle correction and again at follow-up age 7.5 years, 3 months after completing a year of prescribed occlusion (5 h/d) with which she was reported to have been reasonably compliant, but was no longer showing improvement. Her L eye VA had improved after refractive adaptation (0.62 logMAR, IOD 0.62) and after occlusion (0.36; IOD 0.34), so that she still had mild amblyopia at the end of her treatment. But her lack of stereovision remained unchanged, as did her eye–hand coordination abilities (Fig. 8), which were persistently poorer than that of matched controls and showed little evidence of binocular advantage, except compared with her amblyopic eye performance. Note that, as with case S1, there was also little evidence of a learning effect on our tests between her visits (Table 4), supporting the view that the improved performance of patients A1 and A2 were associated with their stereo-recovery rather than due to simple task-specific practice.
Figure 8.
Persistent eye–hand coordination deficits with absent stereovision in a child with mixed (anisometropic and strabismic) amblyopia (case M1) at the end of her spectacle adaptation (refracted) and at follow-up, after occlusion therapy (occluded) compared with control subjects. Other conventions are as in Figure 7.
Discussion
Binocular vision normally provides essential 3D spatial information for improving the initial planning and subsequent guidance of reach-to-precision grasp movements. Commensurate with this, removing binocular information (e.g., from vergence and disparity7–11) by covering one eye in our normally sighted children resulted in slower, yet less accurate, movements. We interpret this as due to “uncertainties” under these reduced-cue conditions about the precise location and intrinsic 3D properties (size, shape, curvature) of the cylindrical goal objects during encoding of these features for respective planning of the reach and the grasp, and when attempting to monitor relative depth changes between the moving hand/digits and intended endpoint grip positions on the objects in the guidance phase. Restricting vision to one eye also resulted in generally greater performance deficits relative to habitual binocular viewing in the older compared with younger control children, consistent with previous findings of increasing binocular advantage, especially in online control, for improving eye–hand coordination with age.4–14,21 In anticipation of this and of impaired reach-to-grasp abilities in amblyopic children with naturally degraded binocular 3D vision,21 the present study addressed two main questions: Do the impaired abilities in amblyopic children relative to matched controls also change with age between 5 to 6 and 7 to 9 years; and are they mainly related to their reduced visual (spatial) acuity in the affected eye or defective stereovision?
Age-Related Differences
The answer to the first question is clearly “yes.” Although not necessarily in the expected direction, because differences in reach-to-grasp performance were much more marked, regardless of viewing condition, in the 5- to 6-year-old children with amblyopia compared with control subjects than between the older patient and control groups. A key reason for the marked differences at ages 5 and 6 years was that, in agreement with previous findings, the normal children appeared to use vision mainly for feed-forward (planning) of their hand movements,12–14,21 whereas the patients adopted a more balanced dual-strategic approach, spending longer than the controls in the early planned (e.g., time to peak velocity) phase of their reach, but much longer in its later “in-flight” visual feedback (guidance) period. This suggests that 5- to 6-year-olds with normal 3D spatial vision are sufficiently confident that the information it provides for target localization during reach preparation will be adequate to achieve the desired outcome and/or satisfied to accept the “cost” of occasional errors (e.g., collisions with the object; Table 2) that might have been rectified by more use of feedback, whereas amblyopic children of the same age are not. We conclude from this that their degraded 3D vision generates uncertainty about object location and size/shape at the planning stage, of which they may well be consciously aware. This, in turn, causes them to slow down during movement execution and to engage in frequent online corrections of their initial misreaching (e.g., in required distance or direction; Fig. 4B) and subsequent grasping errors, while attempting to do this using degraded visual feedback information as well.
Our results further suggest that problems associated with visually encoding these spatial target properties during movement preparation were not completely resolved in the 7- to 9-year-old patient cohort. This was because they, too, made numerous corrections to their reach path and also appeared to adopt (consciously or otherwise) a compensatory grasping strategy across all views of prolonging their grip contact with the objects prior to lifting them (Tables 2, 3). We have previously23 observed similar selective increases in postcontact times among stereo-reduced adults and found that they were related to significant increases, compared with controls, in trial-by-trial variability of their initial digit placements when repeatedly grasping the same objects, this imprecision being especially marked along the depth axis of the targets. We argued that the prolonged contacts were likely designed to acquire extra tactile and kinesthetic feedback from the digits, so ensuring that they were appropriately positioned to apply the grip forces necessary to lift the objects and that such a cross-modal sensory adaptation represented a sensible mechanism to compensate for variable errors in visually guiding the thumb (especially23,36) and finger to the optimal object contact points for this subsequent purpose.
The current data would, therefore, suggest that this strategy is also implemented by amblyopic children as young as 7 to 9 years, presumably to counter problems of endpoint uncertainty they had already experienced in trying to use vision to plan and guide their grasps earlier in life. Indeed, increased reliance on nonvisual information for motor control may be a general response to amblyopia, because—anecdotally—we had to persuade several of our current patients not to employ another tactile/kinesthetic compensation for their reaching difficulties during the initial practice trials prior to the main testing, which involved sliding their hand along the table surface to acquire the objects. We did not analyze digit contact variability in the present work. However, the peak grip apertures formed by the amblyopic children of both ages showed evidence of “uncertainty” about the spatial dimensions of the goal objects, in that they adopted a similarly wide safety margin when preparing to grasp the smaller target under all viewing conditions and less appropriate aperture scaling for the larger object (Fig. 5).
Advantages of Recovered Stereovision
The answer to the second question was slightly more mixed. Repeat measures ANOVA and regression analyses indicated that amblyopia was the main contributor to the prolonged movement durations and grip closure times of the 5- to 6-year-old patients across all views. But these were the only performance deficits for which the degree of VA loss was the most important factor at either age, and also showed secondary influences of their abnormal binocularity. Absence of stereovision, by contrast, was revealed as the exclusive factor in a wider range of the patient's deficits, including their increased reaching errors across all views at both ages, along with aspects of slower (prolonged movement durations) and inaccurate (e.g., spatial reach errors, imprecise endpoint grips) binocular performance in the older children. Indeed, the effects of recovered binocularity in the 7- to 9-year-old stereo+ patients appeared to provide such notable benefits for movement speed (Fig. 2B) and for reach and grasp accuracy (Figs. 3B, 6B)—despite their similar VA losses to those of the stereo nil subgroup (respective mean IODs, 0.45 ± 0.30 SD versus 0.40 ± 0.27 SD, P = 0.62)—that those with stereoacuities in the normal range could not be distinguished from control subjects.
A different approach to the data, summarized in Table 5, supports these conclusions by showing each of the specific kinematic and error-type measures that were defective, according to one-way ANOVA, under each of the three viewing conditions in the patients with different VA versus stereovision losses compared with the matched controls. At both ages, deficits in performance occurred with almost equal likelihood in those with mild compared with moderate amblyopia, whereas they were much more common in the stereo nil than stereo+ patients aged 5 to 6 years, with the stereo nil 7- to 9-year-olds also having the worst binocular performance. That is, this alternative approach indicated that the severity of stereovision loss was a better predictor of the patient's eye–hand coordination skills at both ages than their degree of amblyopia. Evidence from the prospectively studied cases (Table 4) points to this same conclusion, since it was the two patients (M1, S1) with unmeasurable stereovision at the end of their therapeutic regime whose reach-to-grasp abilities did not appear to benefit much from treatment, despite normalization of their initial visual acuity deficits.
Table 5.
Movement Deficits by Age, View, Visual Acuity, and Stereovision Loss
|
Binocular Vision |
Dominant Eye |
Nondominant Eye |
||||||||||
|
Mild |
Mod |
S+ |
S– |
Mild |
Mod |
S+ |
S– |
Mild |
Mod |
S+ |
S– |
|
| Dependent measures: ages 5–6, y | ||||||||||||
| Movement durations | *† | † | † | † | † | † | ||||||
| Grip closure times | † | † | *† | † | ||||||||
| Late velocity corrections | † | † | † | |||||||||
| Spatial path corrections | † | † | † | † | † | *† | † | † | *† | |||
| Precontact grip adjustments | † | † | † | † | † | |||||||
| Postcontact grip adjustments | † | † | † | † | † | † | † | |||||
| Prolonged object contacts | † | † | † | † | † | † | † | |||||
| Wide grips at contact | † | |||||||||||
| n | 5 | 6 | 1 | 7 | 2 | 4 | 0 | 5 | 2 | 4 | 0 | 6 |
| Dependent measures: ages 7–9, y | ||||||||||||
| Spatial path corrections | † | *† | ||||||||||
| Prolonged object contacts | † | † | † | † | † | † | ||||||
| Wide grips at contact | † | † | † | |||||||||
| n | 0 | 2 | 1 | 3 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 1 |
Mod, moderate amblyopia; S+, stereo+; S−, stereo nil.
Significant difference between the two patient categories (Bonferroni corrected).
Significantly different from age-matched control subjects (Bonferroni corrected).
Profile of Fine Visuomotor Skill Development?
A surprising and important finding in our earlier study21 was that children with amblyopia performed worse than controls on a variety of measures when using their better (dominant/sighting) eye alone. Our current data (e.g., Table 5) confirms this finding, but only for the 5- to 6-year-old children, which was the age-range of the majority of patients tested previously. This clarification may be clinically relevant, because it implies that difficulties initially associated with use of the dominant eye (slower movements, yet more errors) in younger amblyopic children become largely resolved by age 7 to 9 years, not in teenage years as we originally speculated. Moreover, these data further suggest that most of the initial reach-to-grasp impairments at ages 5 to 6 years when using two eyes and even just the amblyopic eye alone also quite rapidly resolve, since there were few significant differences evident between the 7- to 9-year-old patients and control children under these other viewing conditions as well.
This raises a further important question: given this new evidence, what accounts for that fact that we and others have observed eye–hand coordination deficits on a variety of 3D tasks with binocular and/or affected eye viewing in adults with persistent amblyopia or stereo-deficiency?22,23,33,37–39 We believe that two main factors are involved. One is the inherent within- and between-subject variability in the performance of both amblyopic and visually normal children at the ages tested here so that between-group differences of as much as 25%–33% that might appear to be biologically important (e.g., binocular movement durations in stereo+ versus control subjects at age 5 to 6 years, Fig. 2A; object collisions at ages 7–9 years, Table 3) do not always achieve statistical significance due to overlapping confidence limits, whereas the more consistent behavior of both types of adult participant reduces this problem. The other is that eye–hand coordination skills continue to improve quite markedly beyond aged 9 years in the normally sighted, but less so in amblyopic individuals. For example, comparisons of performance on equivalent reach-to-precision grasp tasks that we have conducted during the past few years imply that reach error-rates decline by factors of approximately ×6 to 8 years between age 7 to 9 years and adulthood in control subjects, but by only approximately ×4 to 5 over the same developmental period in mild to moderately amblyopic or “stereo-blind” patients.21–23
The above and other existing evidence suggests that abnormal binocularity is, again, the key factor resulting in this relatively “arrested” visuomotor development. For example, Wilson and Welch40 examined longitudinal motor skill development, using a battery of eye–hand coordination and other tasks, in a birth cohort of ∼1000 children at ages 7, 9, and 11 years, and found no differences at any age between children with amblyopia or with good posttreatment recovery of VA compared with control subjects. However, subdividing the same study members by their stereoacuity levels, while controlling for the presence of amblyopia, revealed direct positive correlations between their degree of reduced binocularity and delayed visuomotor skill acquisition. Correlation analyses in some other large-scale, cross-sectional studies31–33 have revealed similar relationships between stereo-thresholds and fine motor skill development, as we did here for a few parameters in our younger, but not older, stereo+ children. This latter failure may have been due to the stereo-thresholds of most (11/14) of these older patients being clustered over a narrow (50–200 arc seconds) range and/or to variability in the data obtained from this small patient sample. Good stereovision also appears necessary for normal visuomotor learning in adulthood. Mazyn et al.41 trained control and stereo-deficient (400 arc seconds or worse) adults with similarly poor baseline catching skills on a task in which they attempted to catch one-handed ∼1500 tennis balls in eight sessions over a period of 2 weeks. Control subjects increased their average catching success on the trained task by a factor of ×5.4, whereas performance gains after this intensive training were only around half that among those who were stereo-reduced. This would imply that people with poor stereovision may require twice as much practice to achieve—if at all—the same motor skill levels as subjects with normal binocularity.
Mechanisms underlying normal procedural motor learning, in which continual movement rehearsal and practice—often involving cognitive effort and feedback evaluation—eventually becomes ingrained as a more automatic skill are known to involve extensive parietofrontal cortical, basal ganglia, and cerebellar networks. The visual input to this network underlying important neural transformations (e.g., internal representations) for action control mainly involves the “dorsal stream” cortical system emanating from occipital visual areas and terminating in posterior parietal regions,1 many of which are specifically recruited during the stereoscopic extraction of object location and 3D properties.42–45 These considerations may offer a general explanation for the arrested visuomotor development and performance deficits in individuals lacking the neural machinery needed to process this information and, more specifically, why our children aged 5 to 6 years with measurable but reduced stereovision, including patient A1 after optical correction and patient A2 when she was first tested (Table 4), had still yet to acquire skilled control of their hand movements.
Task Difficulty
Our eye–hand testing procedure involved presenting objects of two sizes at different locations in order to reduce the occurrence of overrepetitive, stereotypical, movements. But in general accordance with Fitts law,46,47 they should also vary task difficulty, whereby the larger amplitude reaches to the far locations for the smaller (less stable) object would be expected to generate more noise in the motor system and greater accuracy demands than to the near midline position for the larger target. Since it is often reported that impaired binocular performance under conditions of reduced stereopsis may be exacerbated by increased difficulty on some 3D visuomotor tasks,11,31–33,48 we compared the movement durations (timing) and total reach and grasp error rates (accuracy) of the patients when they executed binocular movements under “harder” (far-uncrossed, small object) versus “easier” (near, large object) trial combinations. ANOVA revealed expected significant effects of task difficulty and of stereovision—but not of amblyopia severity—with all three parameters increasing by factors of approximately ×1.2 to 1.5 between harder and easier tasks and between stereo nil and stereo+ patients. But there were no task x stereo interactions, suggesting that the stereo nil subjects were no more relatively impaired on the more difficult task than those with better binocularity.
Cause: Strabismus Versus Anisometropia
Amblyopia due to these different underlying causes is known to result in different patterns of visual anomaly, so an obvious question is whether the two subtypes also exhibit different reach-to-grasp impairments. For example, strabismic subjects typically show greater deficits in positional acuity and spatial localization in their affected eye than anisometropic individuals with similar VA losses.15–17 Some strabismic adults also exhibit systematic directional errors in two-dimensional manual pointing that have been directly linked to their mislocalization problems.49 We were able to age-match only small subsets (n = 7 to 8 each) of our strabismic and anisometropic patients by mild amblyopia and stereo+ binocularity or by moderate amblyopia with similar mixtures of SA losses. Although contrary to evidence of similarly altered reach planning in adults with amblyopia independent of cause,50,51 ANOVA suggested that the subset of mildly amblyopic and stereo+ anisometropic children produced significantly longer times to peak reach velocity and to complete their movements under all views than the strabismics (by ∼17.5%–30%) with the same (largely recovered) visual characteristics. And there were no other cause-related differences, including in misdirected reaches (i.e., spatial path errors) that might be expected to be specifically increased among those with strabismus.
Conclusions and Implications for Amblyopia Therapies
Our findings add to a growing consensus that the impaired performance of children and adults with a history of amblyopia on a variety of real-world visuomotor tasks, especially—but not limited to20—those with obvious 3D components is primarily related to their degree of abnormal binocularity, the reason being that use of alternative monocular visual or of nonvisual information, even with practice or familiarity,41,52,53 cannot completely compensate for their particular difficulties in planning and/or guiding movements in the depth plane.23,39 This even includes apparent failure to exploit motion parallax as a potentially highly reliable alternative depth cue, since our current children, as with subjects in our previous studies,21–23 had freedom of head motion before and during their movements, a likely reason being that the neural processing times required to extract such information, as with other monocular 3D cues (e.g., texture), are too long9,33 to be of generalized use for the immediate demands of action control.
These considerations emphasize the benefits of recovering or improving binocular function, rather than just vision in the affected eye, in children with amblyopia. While such enhancements in stereovision occur in many individual cases via conventional treatments with glasses, occlusion and/or squint surgery, several novel approaches, involving antisuppression16,54 and active perceptual learning or video game playing,55–58 have shown additional promise in this regard. However, amblyopia management needs to be carefully calibrated, and it could be that greater targeting of binocular recovery is at the detriment of restoring higher-level functions (e.g., in spatial or object vision) to the affected eye. Another caveat is that while stereo nil subjects are consistently revealed to have significant visuomotor deficits compared with controls, this is not so generally true of individuals with reduced (e.g., coarse) stereopsis, who may be impaired only on specific tasks, the identity of which have yet to be fully determined. It is also notable that the patients for whom recovering stereoacuity poses both the greatest challenge and risk of inducing intractable diplopia tend to be those with large-angle squint who may have been stereo nil for a considerable time at presentation.28,29 Further studies are required to better evaluate these issues and to weigh the relative benefits of targeting binocular visual recovery against the possible risks.
Acknowledgments
We thank Alison Finlay, children attending Hugh Myddelton Primary School, and the staff and patients of Moorfields Eye Hospital and of the City University London Optometry Clinic.
Supported by City University London School of Health Sciences, the Special Trustees of Moorfields Eye Hospital, Moorfields National Institute of Health Research Biomedical Research Centre for Ophthalmology, and Wellcome Trust Grant 066282.
Disclosure: S. Grant, None; C. Suttle, None; D.R. Melmoth, None; M.L. Conway, None; J.J. Sloper, None
References
- 1. Goodale MA. Transforming vision into action. Vision Res. 2011; 51: 1567–1587 [DOI] [PubMed] [Google Scholar]
- 2. Chapman GJ, Scally A, Buckley JG. Importance of binocular vision in foot placement accuracy when stepping onto a floor-based target during gait initiation. Exp Brain Res. 2012; 216: 71–80 [DOI] [PubMed] [Google Scholar]
- 3. Buckley JG, Panesar GK, MacLellan MJ, Pacey IE, Barrett BT. Changes to the control of adaptive gait in individuals with long-standing reduced stereoacuity. Invest Ophthalmol Vis Sci. 2010; 51: 2487–2495 [DOI] [PubMed] [Google Scholar]
- 4. von Hofsten C. Binocular convergence as a determinant of reaching behaviour in infancy. Perception. 1977; 6: 139–144 [DOI] [PubMed] [Google Scholar]
- 5. von Hofsten C, , Fazel-Zandy S. Development of visually guided hand orientation in reaching. J Exp Child Psychol. 1984; 38: 208–219 [DOI] [PubMed] [Google Scholar]
- 6. Granrud CE, Yonas A, Pettersen L. A comparison of monocular and binocular depth perception in 5- and 7-month old infants. J Exp Child Psychol. 1984; 38: 19–32 [DOI] [PubMed] [Google Scholar]
- 7. Watt SJ, Bradshaw MF. Binocular cues are important in controlling the grasp but not the reach in natural prehension movements. Neuropsychologica. 2000; 38: 1473–1481 [DOI] [PubMed] [Google Scholar]
- 8. Loftus A, Servos P, Goodale MA, Mendarozqueta N, Mon-Williams M. When two eyes are better than one in prehension: monocular viewing and end-point variance. Exp Brain Res. 2004; 158: 317–327 [DOI] [PubMed] [Google Scholar]
- 9. Greenwald HS, Knill DC, Saunders JA. Integrating visual cues for motor control: a matter of time. Vision Res. 2005; 45: 1975–1989 [DOI] [PubMed] [Google Scholar]
- 10. Melmoth DR, Grant S. Advantages of binocular vision for the control of reaching and grasping. Exp Brain Res. 2006; 171: 371–388 [DOI] [PubMed] [Google Scholar]
- 11. Melmoth DR, Storoni M, Todd G, Finlay AL, Grant S. Dissociation between vergence and binocular disparity cues in the control of prehension. Exp Brain Res. 2007; 183: 283–298 [DOI] [PubMed] [Google Scholar]
- 12. Hay L. Spatio-temporal analysis of movements in children: motor programs versus feedback in the development of reaching. J Motor Behav. 1979; 11: 189–200 [DOI] [PubMed] [Google Scholar]
- 13. Smyth MM, Peacock KA, Katamba J. The role of sight of the hand in the development of prehension in childhood. Q J Exp Psychol. 2004; 57: 269–296 [DOI] [PubMed] [Google Scholar]
- 14. Watt SJ, Bradshaw MF, Clarke TJ, Elliott KM. Binocular vision and prehension in middle childhood. Neuropsychologia. 2003; 41: 415–420 [DOI] [PubMed] [Google Scholar]
- 15. McKee SP, Levi DM, Movshon JA. The pattern of visual deficits in amblyopia. J Vision. 2003; 3: 380–405 [DOI] [PubMed] [Google Scholar]
- 16. Hess RF, Mansouri B, Thompson B. Restoration of binocular vision in amblyopia. Strabismus. 2013; 19: 110–118 [DOI] [PubMed] [Google Scholar]
- 17. Sireteanu R, Fronius M, Singer W. Binocular interaction in the peripheral visual field of humans with strabismic and anisometropic amblyopia. Vision Res. 1981; 21: 1065–1074 [DOI] [PubMed] [Google Scholar]
- 18. Simmers AJ, Ledgeway T, Mansouri B, Hutchinson CV, Hess RF. The extent of the dorsal extra-striate deficit in amblyopia. Vision Res. 2006; 46: 2571–2580 [DOI] [PubMed] [Google Scholar]
- 19. Barrett BT, Pacey IE, Bradley A, Thibos LN, Morrill P. Nonveridical visual perception in human amblyopia. Invest Ophthalmol Vis Sci. 2003; 44: 1555–1567 [DOI] [PubMed] [Google Scholar]
- 20. Grant S, Moseley MJ. Amblyopia and real-world visuomotor tasks. Strabismus. 2011; 19: 119–128 [DOI] [PubMed] [Google Scholar]
- 21. Suttle CM, Melmoth DR, Finlay AL, Sloper JJ, Grant S. Eye-hand coordination skills in children with and without amblyopia. Invest Ophthalmol Vis Sci. 2011; 52: 1851–1864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Grant S, Melmoth DR, Morgan MJ, Finlay AL. Prehension deficits in amblyopia. Invest Ophthalmol Vis Sci. 2007; 48: 1139–1148 [DOI] [PubMed] [Google Scholar]
- 23. Melmoth DR, Finlay AL, Morgan MJ, Grant S. Grasping deficits and adaptations in adults with stereo vision losses. Invest Ophthalmol Vis Sci. 2009; 50: 3711–3720 [DOI] [PubMed] [Google Scholar]
- 24. Stewart CE, Moseley MJ, Fielder AR, Stevens DA;, Cooperative MOTAS. Refractive adaptation in amblyopia: quantification of effect and implications for practice. Br J Ophthalmol. 2004; 88: 1552–1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Simmers AJ, Gray LS, McGraw PV, Winn B. Functional visual loss in amblyopia and the effect of occlusion therapy. Invest Ophthalmol Vis Sci. 1999; 40: 2859–2871 [PubMed] [Google Scholar]
- 26. Stewart CE, Moseley MJ, Stevens DA, Fielder AR. Treatment dose-dependence in amblyopia therapy: the monitored occlusion treatment of amblyopia study (MOTAS). Invest Ophthalmol Vis Sci. 2004; 45: 3048–3054 [DOI] [PubMed] [Google Scholar]
- 27. Wallace DK; Pediatric Eye Disease Investigator Group. A randomized trial to evaluate 2 hours of daily patching for strabismic and anisometropic amblyopia in children. Ophthalmology. 2006; 113: 904–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee SY, Isenberg SJ. The relationship between stereopsis and visual acuity after occlusion therapy for amblyopia. Ophthalmology. 2003; 110: 2088–2092 [DOI] [PubMed] [Google Scholar]
- 29. Stewart CE, Wallace MP, Stephens DA, Fielder AR, Moseley MJ;, Cooperative MOTAS. The effect of amblyopia treatment on stereoacuity. JAAPOS. 2013; 17: 166–173 [DOI] [PubMed] [Google Scholar]
- 30. Hess RF, Mansouri B, Thompson B. A binocular approach to treating amblyopia: antisuppression therapy. Optom Vis Sci. 2010; 87: 697–704 [DOI] [PubMed] [Google Scholar]
- 31. Hrisos S, Clarke MP, Kelly T, Henderson J, Wright CM. Unilateral visual impairment and neurodevelopmental performance in preschool children. Br J Ophthalmol. 2006; 90: 836–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. O'Connor AR, Birch EE, Anderson S, Draper H. The functional significance of stereopsis. Invest Ophthalmol Vis Sci. 2010; 51: 2019–2023 [DOI] [PubMed] [Google Scholar]
- 33. Schiller PH, Kendall GL, Kwak MC, Slocum WM. Depth perception, binocular integration and hand-eye coordination in intact and stereo impaired human subjects. J Clin Exp Ophthalmol. 2012; 3: 2 [Google Scholar]
- 34. Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologica. 1971; 9: 97–112 [DOI] [PubMed] [Google Scholar]
- 35. Smeets JBJ, Brenner E. A new view on grasping. Motor Control. 1999; 3: 237–271 [DOI] [PubMed] [Google Scholar]
- 36. Melmoth DR, Grant S. Getting a grip: different actions and visual guidance of the thumb and finger in precision grasping. Exp Brain Res. 2012; 222: 265–276 [DOI] [PubMed] [Google Scholar]
- 37. Mazyn LIN, Lenoir M, Montagne G, Savelsbergh GJP. The contribution of stereo vision to one-handed catching. Exp Brain Res. 2004; 157: 383–390 [DOI] [PubMed] [Google Scholar]
- 38. Barry GPB, Simon JW, Auringer D, Dunnican W, Zobal-Ratner J. Performance of strabismic subjects using a validated surgical training module: a pilot study. JAAPOS. 2009; 13: 350–355 [DOI] [PubMed] [Google Scholar]
- 39. Niechwiej-Szwedo E, Goltz HC, Chandrakumar M, Wong AF. The effect of sensory uncertainty due to amblyopia (lazy eye) on the planning and execution of visually-guided 3D reaching movements. PLoS One. 2012; 7: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wilson GA, Welch D. Does amblyopia have a functional impact? Findings from the Dunedin Multidisciplinary Health and Development study. Clin Exp Ophthalmol. 2013; 41: 127–134 [DOI] [PubMed] [Google Scholar]
- 41. Mazyn LIN, Lenoir M, Montagne G, Delaey C, Savelsbergh GJP. Stereo vision enhances the learning of a catching skill. Exp Brain Res. 2007; 179: 723–726 [DOI] [PubMed] [Google Scholar]
- 42. Verhagen L, Dijkerman HC, Grol MJ, Toni I. Perceptuo-motor interactions during prehension movements. J Neurosci. 2008; 28: 4726–4735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Króliczak G, McAdam TD, Quinlan DJ, Culham JC. The human dorsal stream adapts to real actions and 3D shape processing: a functional magnetic resonance imaging study. J Neurophysiol. 2008; 100: 2627–2639 [DOI] [PubMed] [Google Scholar]
- 44. Georgieva S, Peeters R, Kiolster H, Todd JT, Orban GA. The processing of three-dimensional shape from disparity in the human brain. J Neurosci. 2009; 29: 727–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Preston TJ, Kourtzi Z, Welchman AE. Adaptive estimation of three-dimensional structure in the human brain. J Neurosci. 2009; 29: 1688–1698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fitts PM. The information capacity of the human motor system in controlling the amplitude of movement. J Exp Psychol. 1954; 47: 381–391 [PubMed] [Google Scholar]
- 47. Harris CM, Wolpert DM. Signal-dependent noise determines motor planning. Nature. 1998; 394: 780–784 [DOI] [PubMed] [Google Scholar]
- 48. Piano MEF, O'Connor AR. The effect of degrading binocular single vision on fine visuomotor task performance. Invest Ophthalmol Vis Sci. 2013; 54: 8208–8213 [DOI] [PubMed] [Google Scholar]
- 49. Fronius M, Sireteanu R. Pointing errors in strabismics: complex patterns of distorted visuomotor coordination. Vision Res. 1994; 34: 689–707 [DOI] [PubMed] [Google Scholar]
- 50. Niechwiej-Szwedo E, Goltz HC, Chandrakumar M, Hirji Z, Crawford JD, Wong AM. Effects of anisometropic amblyopia on visuomotor behavior, part 2: visually guided reaching. Invest Ophthalmol Vis Sci; 2011; 52: 795–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Niechwiej-Szwedo E, Goltz HC, Chandrakumar M, Wong AM. Effects of strabismic amblyopia on visuomotor behavior, II: visually-guided reaching. Invest Ophthalmol Vis Sci. 2014; 55: 3857–3865 [DOI] [PubMed] [Google Scholar]
- 52. Marotta JJ, Goodale MA. Role of familiar size in the control of grasping. J Cogn Neurosci. 2001; 13: 8–17 [DOI] [PubMed] [Google Scholar]
- 53. Christensen A, Borchers S, Himmelbach M. Effects of pictorial cues on reaching depend on the distinctiveness of target objects. PLoS One. 2013; 8: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Knox PJ, Simmers AJ, Gray LS, Cleary M. An exploratory study: prolonged periods of binocular stimulation can provide an effective treatment for childhood amblyopia. Invest Ophthalmol Vis Sci. 2012; 53: 817–824 [DOI] [PubMed] [Google Scholar]
- 55. Chen PL, Chen JT, Fu JJ, Chien KH, Lu DW. A pilot study of anisometropic amblyopia improved in adults and children by perceptual learning: an alternative treatment to patching. Ophthal Physiol Opt. 2008; 28: 422–428 [DOI] [PubMed] [Google Scholar]
- 56. Astle AT, McGraw PV, Webb BS. Recovery of stereo acuity in adults with amblyopia. BMJ Case Rep. 2011. doi:10.1136/bcr.07.2010.3143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ding J, Levi DM. Recovery of stereopsis through perceptual learning in human adults with abnormal binocular vision. Proc Nat Acad Sci U S A. 2011; 108: E733–E741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Li RW, Ngo C, Nguyen J, Levi DM. Video-game play induces plasticity in the visual system of adults with amblyopia. PLoS Biology. 2011; 9: [DOI] [PMC free article] [PubMed] [Google Scholar]





