Abstract
Purpose
To determine the pharmacokinetics (PK), maximum tolerated dose (MTD), safety, and antitumor activity of an oral formulation of rigosertib, a dual phosphoinositide 3-kinase (PI3K) and polo-like kinase 1 (Plk1) pathway inhibitor, in patients with advanced solid malignancies.
Experimental Design
Patients with advanced solid malignancies received rigosertib twice daily continuously in 21-day cycles. Doses were escalated until intolerable grade ≥ 2 toxicities, at which point the previous dose level was expanded to define the MTD. All patients were assessed for safety, PK, and response. Urinary PK were performed at the MTD. Archival tumors were assessed for potential molecular biomarkers with multiplex mutation testing. A subset of squamous cell carcinomas (SCC) underwent exome sequencing.
Results
Forty-eight patients received a median of 2 cycles of therapy at 5 dose levels. Rigosertib exposure increased with escalating doses. Dose-limiting toxicities were hematuria and dysuria. The most common grade ≥2 drug-related toxicities involved urothelial irritation. The MTD is 560 mg twice daily. Activity was seen in head and neck SCCs (1 complete response, 1 partial response) and stable disease for ≥ 12 weeks was observed in 8 additional patients. Tumors experiencing ≥partial response had PI3K pathway activation, inactivated p53, and unique variants in ROBO3 and FAT1, two genes interacting with the Wnt/β-catenin pathway.
Conclusions
The recommended phase II dose of oral rigosertib is 560 mg twice daily given continuously. Urinary toxicity is the dose-limiting and most common toxicity. Alterations in PI3K, p53, and Wnt/β-catenin pathway signaling should be investigated as potential biomarkers of response in future trials.
Introduction
Phosphoinositide 3-kinase (PI3K) is a key intracelluar kinase in the PI3K/AKT/mTOR signaling pathway that contributes to cell growth, survival, and resistance to therapy in many human malignancies (1, 2). PI3K is activated by upstream receptor tyrosine kinases and inhibited by the tumor suppressor PTEN (1). Amplification of PIK3CA, the gene encoding PI3K’s p110α catalytic subunit, is commonly seen in head and neck squamous cell carcinoma (HNSCC; ref. 3), non-small cell lung cancer (NSCLC; ref. 4), gastric, cervical, and ovarian cancer (2, 5). PIK3CA activating mutations occur in over 15% of breast, endometrial, colon and urinary tract cancers, and are one of the most common activating mutations in HNSCC (6–8). Mutations in the PIK3R1 gene encoding the p85a regulatory subunit occur in 10% of glioblastoma multiforme (9). PTEN loss of heterozygosity occurs in >25% of breast, gastric, glioblastoma, and prostate cancers, and mutations occur in >10% of glioblastoma, endometrial, colon, and prostate cancers (6, 10). In preclinical models and early clinical trials, PI3K inhibitors show significant promise (6).
The polo-like kinase 1 (Plk1)-centered regulatory loop is a critical cell-cycle mediator, controlling entry into the mitotic phase, spindle assembly, and centrosome maturation (11). Plk1 modulates the transition through the G2–M checkpoint by altering the activation of cyclin B1 and the phosphatase CDC25C (12). Plk1 also associates with c-Raf at the centrosome and spindle poles, and inhibition of this interaction impairs G2–M transition (13). High Plk1 expression is a poor prognostic feature in non-Hodgkin lymphoma, gastric, HNSCC, NSCLC, and ovarian cancer (11). Plk1 has been targeted using deletion mutants (14) and RNA interference (15). These strategies have been associated with antiproliferative effects in lung (16) and pancreatic cancer cell lines in vitro (17), and growth inhibition of cervical and lung cancer xenografts in vivo (15).
Rigosertib (Estybon; ON01910.Na) is a stryryl sulfone, ATP-independent, allosteric, multikinase inhibitor (18). Its complex mechanism of action involves indirect suppression of the PI3K and Plk1 pathways, likely resulting from rigosertib binding to c-Raf that, in turn, impairs c-Raf/coenzyme interactions (19–22). Rigosertib has shown efficacy in patient-derived breast, pancreatic, and myelodysplastic syndrome cancer models (19, 20, 22). In the first-in-human phase I solid tumor study of intravenous (i.v.) rigosertib, toxicity was modest and a patient with ovarian cancer had a prolonged objective response (23). A phase II/III study of i.v. rigosertib plus gemcitabine for pancreas cancer and a phase III study of i.v. rigosertib for myelodysplastic syndrome are ongoing.
The current phase I study represents the first-in-human experience with the oral formulation of rigosertib in patients with advanced solid malignancies. The primary objective was to determine the maximum tolerated dose (MTD) of rigosertib administered orally twice daily in a continuous 21-day cycle. Secondary objectives included (i) assessing toxicity; (ii) investigating the clinical pharmacology of rigosertib; (iii) identifying molecular biomarkers; and (iv) documenting antitumor activity.
Experimental Design
Patient eligibility
Patients with an incurable, histologically confirmed solid malignancy, age ≥18 years, Eastern Cooperative Oncology Group performance status ≤2, life expectancy ≥6 months, measurable disease per Response Evaluation Criteria In Solid Tumors (RECIST) 1.0 (24), adequate bone marrow, hepatic and renal function [white blood cell >4,000/mL; absolute neutrophil count > 1,500/mL; hemoglobin >9 g/dL; platelet ≥100,000/mL; bilirubin ≤1.5× the upper limit of normal (ULN); aspartate aminotransferase or alanine aminotransferase <2.5× ULN (<5×ULN if due to liver metastases); and creatinine ≤2×ULN] were eligible. Surgery or palliative radiotherapy >14 days or chemotherapy >21 days before treatment without residual grade >1 toxicity were allowed. Patients with irradiated, clinically stable brain metastases were eligible. Pregnant/nursing patients and those with clinically significant and/or uncontrolled medical conditions were excluded. The protocol was approved by the institutional review board and signed informed consent was mandatory.
Treatment plan
Rigosertib (ON 0910.Na, Onconova Therapeutics Inc.) was given orally in a fasting state twice daily in continuous 21-day cycles. The severely toxic dose in 10% of dogs and rats was 75 mg/kg twice daily, equivalent to 73 mg (12.2 mg/kg) in a 60 kg human with a safety factor of 10. Therefore, a flat starting dose of 70 mg twice daily was selected. In the absence of treatment delays due to adverse events (AE), rigosertib was continued until disease progression, intercurrent illness, unacceptable AE(s), or consent withdrawal. Retreatment required adequate laboratory parameters on every evaluation, and resolution of all nonhematologic toxicities (except alopecia and fatigue) to baseline or grade ≤ 1. In case of a delay >14 days, patients were removed from the study. A 12-patient subset in the expansion phase received either prophylactic or urinary symptom-emergent urine alkalinization with oral sodium bicarbonate (NaHCO3) 650 mg twice daily.
Assessments, follow-up, and monitoring
Toxicity was followed until resolution to baseline or grade ≤ 1. Before study entry and with each cycle, patients had a clinical history and physical examination, performance status assessment, and comprehensive laboratory testing. A disease assessment by computed tomography (CT) or MRI scan occurred at screening and after every 2 cycles. AEs were classified/graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) 4.0. Response was evaluated by RECIST 1.0 criteria. Patients were evaluable for toxicity once therapy commenced and for efficacy after completing one full cycle.
Definition of dose-limiting toxicity, MTD, and dose-escalation plan
Dose-limiting toxicity (DLT) was defined as any grade ≥3 AE at least possibly attributable to rigosertib occurring within 21 days of the first dose, or if rigosertib was held for 7 consecutive days due to AE(s). The MTD was defined as the maximal dose in which <33% of ≥6 patients experienced a DLT. An accelerated, adaptive dose-escalation plan was used. Patients were initially enrolled in 2-patient cohorts starting at 70 mg twice daily. In the absence of drug-related grade ≥2 AEs during cycle 1, the next 2 patients received a dose escalated by 100%. If drug-related grade ≥2 toxicity occurred in at least 1 of the initial patients during cycle 1, the cohort was expanded to 3 evaluable patients. If no DLT occurred in these 3 patients, then the next 3 patients were enrolled at a 50% dose increase. If one DLT was observed in the first 3 patients, then the dose level was expanded up to 6 patients. If ≤ 1 DLT was observed in these 6 patients during cycle 1, then the next 6 patients were treated at a 25% dose escalation. If ≥2 patients in any cohort experienced a DLT, then the MTD was exceeded and dose escalation ceased. Patients experiencing a DLT discontinued or reduced (at the discretion of the investigator) rigosertib to the lower dose level. If any nonhematologic toxicity of grade ≥3 was present on the day of rigosertib administration, the drug was held until resolution to grade ≤1.
Pharmacokinetic sampling and analytic assay
Blood samples were collected in all patients on days 1 and 21 during cycle 1 at the following time points: predose, 0.5, 1, 1.5, 2, 4, 6, and 8 hours after the morning dose. Urine pharmacokinetic (PK) samples were collected on days 1 and 21 of cycle 1 and on day 15 of the second cycle in all patients over the following intervals: predose, 0 to 4, 4 to 8, and 8 to 24 hours. Urine collections for PK analysis were repeated in all patients experiencing grade ≥2 urinary toxicity (see Supplementary Materials).
Molecular analysis
Optional archival tumor tissue blocks were assessed for the presence of common mutations in 15 genes using multiplex single base extension reactions (SNaPshot; Applied Biosystems; Life Technologies). Given rigosertib’s activity against squamous cell carcinomas (SCC), exome sequencing and FISH to determine PIK3CA and PTEN gene copy number were performed in a SCC subset (see Supplementary Materials).
Results
Patient characteristics
Forty-eight patients (24 males and 24 females) with a median age of 58 years (range, 20–79) were enrolled (Table 1 and Supplementary Table S1). Two patients (280- and 700-mg cohorts) developed rapid disease progression and were not assessable for response.
Table 1.
Patient and disease characteristics
| Rigosertib twice daily dosing (mg) |
||||||
|---|---|---|---|---|---|---|
| 70 | 140 | 280 | 560 | 700 | Total | |
| Patients | 3 | 2 | 3 | 33 | 7a | 48 |
| Sex | M1/F2 | F2 | M2/F1 | M15/F18 | M6/F1 | M24/F24 |
| Age (range) | 49–74 | 57–63 | 51–60 | 20–79 | 47–71 | 20–79 |
| Colorectal carcinoma | 10 | 10 | ||||
| HNSCC | 1 | 3 | 2 | 6 | ||
| Ovarian carcinoma | 2 | 2 | 4 | |||
| Esophageal adenocarcinoma | 4 | 4 | ||||
| Renal cell carcinoma | 3 | 3 | ||||
| Hepatocellular carcinoma | 2 | 2 | ||||
| Breast carcinoma | 1 | 1a | 2 | |||
| Uterine adenocarcinoma | 1 | 1 | ||||
| Uterine carcinosarcoma | 1 | 1 | ||||
| Leiomyosarcoma | 1 | |||||
| Cervical adenocarcinoma | 1 | 1 | ||||
| Transitional cell carcinoma | 1 | 1 | ||||
| Pancreatic neuroendocrine | 1 | 1 | ||||
| Vulvovaginal melanoma | 1 | 1 | ||||
| Carcinoid tumor | 1 | 1 | ||||
| Salivary gland carcinoma | 1 | 1 | ||||
| Gastrointestinal stromal tumor | 1 | 1 | ||||
| Craniopharyngioma | 1 | 1 | ||||
| Adrenocortical carcinoma | 1 | 1 | ||||
| SCC lung | 1 | |||||
| Osteosarcoma | 1 | |||||
| Prostate carcinoma | 1 | 1 | ||||
| SCC vulva | 1 | |||||
| Adenoid cystic nasopharyngeal | 1 | 1 | ||||
One patient in the 700-mg cohort was taken off study for progression 2 days after starting treatment and replaced by an additional patient treated at this dose level.
Dose escalation
Five dose levels (70, 140, 280, 560, and 700 mg) were explored. The 70-mg (unrelated grade 4 hyperbilirubinemia), 280-mg (unrelated grade 3 pleural effusion), and 560-mg (grade 2 drug-related dysuria, grade 2 unrelated liver enzyme elevation) cohorts were all expanded to 3 patients with no DLT observed. The 700-mg cohort was expanded to 6 patients after 1 patient developed grade 3 hyponatremia shortly after cycle 1 and the subsequent 2 patients experienced grade 2 AEs (drug-related dysuria, unrelated creatinine elevation). A DLT (grade 3 hematuria) was observed at the 700-mg expansion cohort. Given two grade 3 toxicities, one DLT, and one near-DLT, 700 mg twice daily was considered intolerable and the previous dose level (560 mg) was expanded. In the 30-patient 560-mg expansion, 1 patient developed a DLT (grade 3 dysuria and hematuria) and 15 patients experienced grade 2 or 3 urinary symptoms (Table 2 and Supplementary Table S2) that occurred in cycle 2 or later. Therefore, the recommended phase II dose (RP2D) is 560 mg twice daily despite the original definition of an MTD not being met.
Table 2.
Treatment-related AEs by dose level and symptom
| Rigosertib twice daily dosing (mg) | 70 | 140 | 280 | 560 | 700 | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Evaluable patients | 3 | 2 | 3 | 33 | 6 | 47 | ||||||
| Patients with a DLT | 0 |
0 |
0 |
1 |
1 |
2 |
||||||
| Grade | 2 | 3 | 2 | 3 | 2 | 3 | 2 | 3 | 2 | 3 | 2 | 3 |
| Dysuria | 0 | 0 | 0 | 0 | 0 | 0 | 13 | 2a | 4 | 0 | 17 | 2 |
| Cystitis | 0 | 0 | 1 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 4 | 0 |
| Micturition urgency | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 2 | 0 | 4 | 0 |
| Hematuria | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1a | 1 | 1b | 2 | 2 |
| Urinary hesitancy | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| Abdominal discomfort | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| Abdominal distension | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| Abdominal pain | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| Nausea | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| Fatigue | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 |
| Hypertonic bladder | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| Pelvic pain | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 |
| Hyponatremia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
DLT (grade 3 hematuria and dysuria week 3).
DLT (grade 3 hematuria week 2).
Safety
Forty-eight patients in the study were evaluable for toxicity (Table 2 and Supplementary Table S2). Doses were held and/ or reduced for toxicity in 14 and 13 patients, respectively. Urinary AEs were most common, with grade ≥2 urinary toxicity reported for 17 of 33 (52%) patients treated at the RP2D. Seven patients experienced grade 3 nonhematologic toxicity, including urinary symptoms, fatigue, pelvic pain, and hyponatremia (Table 2 and Supplementary Table S2). Of the 13 patients experiencing serious AEs, only 1 (grade 3 weakness) was considered possibly drug-related (Supplementary Table S3). Two patients discontinued treatment because of dysuria. Urinary symptoms occurred at a median of 4 weeks and typically resolved within 14 days of drug discontinuation. Because rigosertib is acidic in the urine, the last 12 subjects enrolled were alternately assigned to receive prophylactic or urinary symptoms-emergent urine alkalization. Prophylactic NaHCO3 had no significant effect on urinary toxicity incidence (data not shown).
Antitumor activity
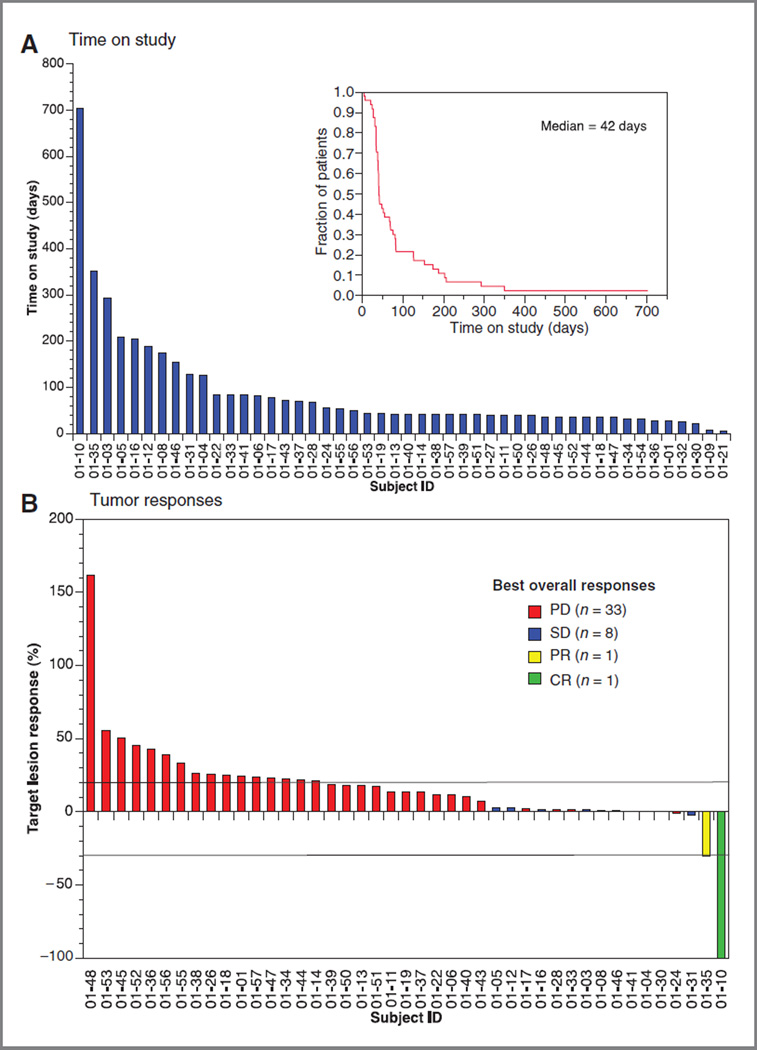
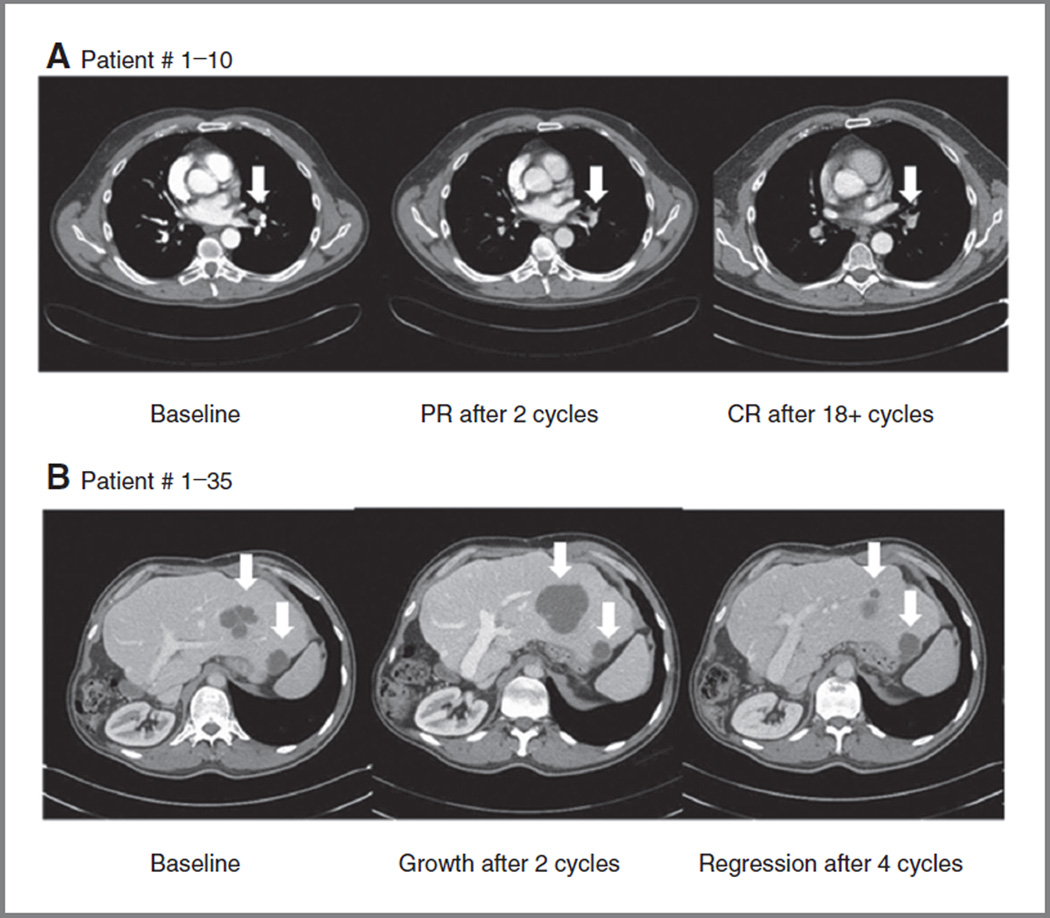
Forty-six patients were evaluable for efficacy with 43 patients receiving serial tumor measurements (Fig. 1 and Supplementary Table S1). The median time on study was 2 cycles (6 weeks; range, 1–101+; Fig. 1). One patient with metastatic, platinum-refractory, human papilloma virus (HPV)-negative HNSCC treated with rigosertib 280 mg twice daily achieved a complete response (CR) and remains on study at 101+ weeks (Fig. 2). A second patient with platinum-refractory, HPV-positive HNSCC treated with 560 mg twice daily experienced a partial response (PR) and remained on study for 50 weeks. His course was characterized by an initial increase in hepatic metastasis size and pain through cycle 2; however, his imaging suggested tumor necrosis and he remained on study (Fig. 2). At cycle 5, his tumor had decreased in size 30% from baseline and 40% from its maximum. Stable disease for ≥ 12 weeks was seen in 8 patients, yielding an overall response rate (RR) of 4.3% (2/46) and clinical benefit rate (CBR = CR + PR + stable disease) of 21.7% (10/46; Fig. 1 and Supplementary Table S1).
Figure 1.
A, the median time on study was 6 weeks (95% CI, 1–101+ weeks). Forty-three (89.5%) patients came off study because of progression of disease, 2 patients because of toxicity, and 1 patient died. One patient withdrew consent. One patient remained on study at the time of analysis. B, waterfall plot of tumor responses of the 43 patients for whom serial imaging studies were available. One patient achieved a CR, 1 patient a prolonged PR, and 8 patients achieved stable disease for12 or more weeks (range, 12–36 weeks).
Figure 2.
Objective responses to oral rigosertib in 2 patients with HNSCC. A, serial CT scan images of a patient with metastatic HNSCC, who achieved a CR. B, initial tumor growth then reduction in a patient with metastatic HNSCC who achieved a prolonged PR.
PK analysis
The PK profile is given in Table 3. Rigosertib plasma pharmacokinetics were linear and dose proportional (Supplementary Fig. S1). The plasma concentration-time profile exhibited a rapid distribution phase followed by a slower elimination phase. For patients treated at the RP2D, the Cmax was significantly lower at steady-state on day 21 than on day 1, and T1/2 and volume of distribution were greater (Table 3). There was not a strong relationship between plasma and urinary PKs. Peak plasma concentration and AUC0-∞ were similar in patients with or without urinary symptoms. Mean urine PK parameters ± SD were similar in symptomatic and nonsymptomatic groups: Cmax (2,489 ng/mL ± 2,671 vs. 2,021 ng/mL ± 1,312; P = 0.56), AUC0-∞ (5,886 ng*h/mL± 6,086 vs. 5,069 ng*h/mL± 3,517; P = 0.72), 24-hour urine excretion (16.6 mg ± 7.9 vs. 18.7 mg ± 2.8; P = 0.63).
Table 3.
Pharmacokinetics and bioavailability of oral rigosertib
| Oral rigosertib plasma PK parameters | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 70 mg |
140 mg |
280 mg |
560 mg |
700 mg |
||||||
| Parameter | Day 1 (n = 3; mean ± SD) |
Day 21 (n = 3; mean ± SD) |
Day 1 (n = 4; mean ± SD) |
Day 21 (n = 4; mean ± SD) |
Day 1 (n = 5; mean ± SD) |
Day 21 (n = 3; mean ± SD) |
Day 1 (n = 32; mean ± SD) |
Day 21 (n = 24; mean ± SD) |
Day 1 (n = 7; mean ± SD) |
Day 21 (n = 2; mean ± SD) |
| Cmax (µg/mL) | 0.40 ± 0.29 | NA | 0.20 ± 0.07 | 0.43 ± 0.26 | 1.84 ± 1.31 | 0.73 ± 0.44 | 4.04 ± 2.97 | 2.54 ± 2.35 | 5.93 ± 4.74 | 2.34 ± 1.28 |
| P = 0.0463a | ||||||||||
| AUC0-∞ (µg*h/mL) | 1.52 ± 1.36 | NA | 3.49 ± 5.25 | 4.59 ± 4.85 | 4.93 ± 4.06 | 2.61 ± 1.23 | 9.32 ± 6.92 | 6.99 ± 5.88 | 19.58 ±22.95 | 4.96 ± 1.71 |
| P = 0.1898a | ||||||||||
| Tmax (h) | 1.17 ±0.29 | NA | 1.13 ±0.25 | 0.88 ± 0.25 | 1.00 ± 0.35 | 0.83 ± 0.58 | 1.02 ± 0.32 | 1.08 ±0.41 | 1.14 ±0.48 | 1.25 ±0.35 |
| P = 0.54093 | ||||||||||
| Vss/F (L) | 439.4 ±512.8 | NA | 1101.8 ±424.4 | 787.9 ±415.4 | 498.5 ± 469.3 | 1061.4 ±603.4 | 314.9 ±285.2 | 573.8 ± 498.5 | 241.7 ± 211.8 | 472.8 ± 137.5 |
| P = 0.0173a | ||||||||||
| CI7F (I7h) | 71.54 ±42.87 | NA | 171.29 ± 155.74 | 55.64 ± 36.62 | 122.84 ± 123.67 | 127.45 ±66.13 | 93.28 ± 58.24 | 116.33 ±56.32 | 64.62 ± 34.70 | 149.9 ±51.79 |
| P = 0.1430a | ||||||||||
| T1/2 (h) | 3.34 ± 2.40 | NA | 21.56 ±36.39 | 25.69 ± 38.29 | 2.81 ± 0.77 | 5.29 ± 1.09 | 2.42 ±1.10 | 4.03 ± 2.92 | 3.09 ±2.18 | 1.99 ± 0.47 |
| P = 0.0059a | ||||||||||
NOTE: Individual patients underwent PK testing after oral dosing on days 1 and 21 of cycle 1. For patients treated at the RP2D of rigosertib (560 mg twice daily), Cmax was significantly lower at steady-state on day 21 than on day 1.
Abbreviations: AUC0-∞, area under the curve determined from time zero extrapolated to infinity; Cmax, maximal concentration; CL/F, clearance Tmax(h), time of peak concentration; T1/2 (h), terminal elimination phase half-life; Vss/F (L), apparent volume of distribution at steady state.
Unpaired t test at 95% confidence interval.
Mutational and genetic analyses
Archival tissue was obtained from 32 subjects. Multiplex mutational analysis did not reveal a correlation between common oncogenic mutations and response to therapy (Supplementary Table S4). In a subset of patients with SCCs (5 HNSCC and 1 lung SCC), PI3K pathway activation via PIK3CA amplification or PTEN loss was seen in both sensitive tumors (≥PRs) but did not predict response overall (Supplementary Table S4). Exome sequencing of these 6 SCCs provided 33–95 × coverage and 18 to 200 million reads. Limiting our analysis to published variants in HNSCC (8), there were 1,352 variants in common between sensitive and resistant tumors, 27 unique to sensitive tumors, and 103 unique to resistant tumors. There were 12 variants seen exclusively in both sensitive SCCs and 5 variants seen exclusively in all four resistant tumors (Table 4 and Supplementary Table S5). Sensitive tumors had unique variants in the cancer-related genes FAT1, ROBO3, ACIN1, and ABCC11. Resistant tumors had unique variants in cancer-related genes CDH23 and HCLS1.
Table 4.
Cancer-related exome variants seen exclusively in sensitive and resistant squamous cell carcinomas
| Gene | Description | Chromosome | Nucleotide change |
Amino acid change |
|---|---|---|---|---|
| Nonsynonymous | SNPs present in both sensitive tumors, but not in resistant tumors | |||
| ABCC11 | ATP-binding cassette, subfamily C (CFTR/MRP), member 11 | 16 | T>A | N1277Y |
| ACIN1 | Apoptotic chromatin condensation inducer 1 | 14 | T>C | N20S |
| FAT1 | FAT tumor suppressor homolog 1 (drosophila) | 4 | G>A | A131V |
| ROBO3 | Roundabout, axon guidance receptor, homolog 3 (drosophila) | 11 | G>A | R416H |
| Nonsynonymous | SNPs present in all resistant tumors, but not in sensitive tumors | |||
| CDH23 | Cadherin-related 23 | 10 | A>G | N1351D |
| HCLS1 | Hematopoietic cell-specific Lyn substrate 1 | 3 | C>T | E361K |
Discussion
This first-in-human study demonstrates that the oral formulation of rigosertib, a non-ATP competitive inhibitor of PI3K and the Plk1 pathways, is safe and well tolerated by patients with advanced solid malignancies. The RP2Dis560 mg twice daily continuously in 21-day cycles. Rigosertib has a unique toxicity profile and promising signals of efficacy, particularly in HNSCC.
Rigosertib has a side-effect pattern different from other PI3K and Plk1 inhibitors. Whereas many direct PI3K inhibitors cause mucocutaneous toxicity, nausea/vomiting, diarrhea, and hyperglycemia, though both PX-866 and XL147 do not cause hyperglycemia, rigosertib largely lacks these side effects (6, 25–27). Similarly, rigosertib does not have the hematologic toxicity of direct Plk1 inhibitors that are limited by hematologic toxicity (28, 29). Rather, the most common and clinically significant toxicity was dose dependent, delayed urothelial irritation. The high incidence of urinary AEs is in stark contrast to the low frequency of urinary toxicity with i.v. rigosertib despite higher Cmax and AUC0-∞ seen with the i.v. formulation (23). There were no differences in plasma or urine drug levels between patients with and without urinary symptoms, though this analysis is limited by large variance in the data. Urine alkalinization, although helpful in some patients with treatment-emergent urinary toxicity, did not prevent urinary symptoms in this study. At the RP2D, the Vss is higher at day 21 than at day 1 and exceeds the i.v. Vss (23). This may be due to increased urothelial binding with the oral drug and could account for the oral formulation’s delayed urinary toxicity. The urinary toxicity is unlikely to be related to the excipient, as both the i.v. and oral versions use the same agent and the oral formulation uses an order of magnitude less. It is likely that rigosertib or an unmeasured metabolite is an urothelial irritant; however, patient factors may help determine the severity of symptoms. One consideration is that oral rigosertib may undergo acidification in the stomach or first pass metabolism through the liver, and that these processes produce a toxic metabolite. This would account for the lack of urothelial irritation with the i.v. formulation of the drug. Further evaluation into the origin and amelioration of this toxicity are ongoing. Rigosertib’s lack of mucocutaneous or hematologic toxicity makes it an exciting candidate for combinations with myelosuppresive chemotherapy, radiation, and targeted agents like cetuximab where skin rash is the DLT.
Oral rigosertib’s PK profile is linear and dose dependent. There does not seem to be drug accumulation based upon AUC0-∞, though the high Vss suggests tissue binding. The lower Cmax at day 21 points toward a possible induction phenomenon, but the longer T1/2 and unchanged AUC0-∞ and clearance do not support this hypothesis. Given the oral formulation’s short T1/2, it is possible that increased drug exposure can be achieved with 3 times a day dosing. A phase I trial of 3 times a day dosing with PKs beyond cycle 1 is underway. Of note, despite lower Cmax at day 21, rigosertib maintained clinical activity.
Rigosertib demonstrated clinical activity in this phase I study. In contrast to other PI3K/mTOR or Plk1 inhibitors, rigosertib has the advantage of inhibiting both cell signaling and cycling. The RR of 4.3% (all HNSCCs) and a ≥12-week CBR of 21.7% compare favorably with other Plk1 and PI3K inhibitors. For instance, the phase I CBR for the Plk1 inhibitor BI-2536 was 32% (0% PR, 32% stable disease) and the RRs with the ATP-competitive PI3K inhibitors BKM120 and PX-866 were 2.8% and 0%, respectively (25, 26, 28). In our study, 1 patient with HPV-positive HNSCC experienced substantial tumor shrinkage following initial tumor growth in the liver. The tumor flare phenomenon has been observed in HPV-positive tumors treated with chemotherapy or radiation, as well as targeted immunotherapies (30, 31). It may be that, in select patients, initial tumor growth and pain reflects tumor necrosis and immune cell infiltration. Future studies should consider on-treatment tumor biopsies to investigate this phenomenon.
Predicting responders to PIK3 and Plk1 pathway inhibitors has been challenging. There are conflicting data suggesting that PI3K pathway activation may be required for PI3K inhibitor activity (32–34). Similarly inactivated p53 may be necessary for Plk1 inhibitors to be effective (35). Both patients with ≥PR in this study had PI3K pathway activation and inactivated p53, either via mutation or destruction by an HPV oncoprotein. In patient-derived HNSCC xenografts, p53 inactivation events and PI3K pathway activation were required for rigosertib antitumor activity (36). However, because patients with similar molecular profiles failed to respond, this suggests that PIK3 pathway activation and p53 inactivation may be necessary, but not sufficient, for rigosertib activity.
In the SCC subset undergoing exome sequencing, two variants in rigosertib-sensitive tumors point to possible interactions between Wnt/β-catenin, PI3K, and Plk1. FAT1 is a protocadherin mutated in 6.7% of HNSCCs that, when inactivated, leads to aberrant Wnt/β-catenin signaling, oral cancer carcinogenesis, migration, and invasion (37, 38). Similarly, ROBO3 operates downstream of Slit2, a tumor suppressor, and acts via Wnt/ β-catenin and PI3K/AKT; aberrant Slit2-ROBO3 signaling destabilizes these pathways in lung cancer and increases cell migration in oral SCCs (39, 40). Plk1 interacts with the Wnt/β-catenin scaffolding protein Axin in mitotic spindles (41). We propose that rigosertib’s unique mechanism of action allows it to impair aberrant Wnt/β-catenin via Plk1 and PI3K pathway inhibition, while also blocking G2–M transition and PI3K–driven cell signaling. Resistance to rigosertib may occur through HCLS1 (Lyn substrate 1), a target of the Src family kinase Lyn active in HNSCC cell lines and tumor specimens (42, 43). Interestingly, both sensitive tumors harbored variants in ACIN1, an apoptosis regulator, and ABCC11 (MRP8), an ATP-binding cassette membrane transporter, associated with chemotherapy resistance (44). PI3K and Plk1 inhibitors both impair a multidrug resistance protein similar to ABCC11, P-glycoproten, in vitro, and may allow for increased intracellular drug accumulation (45). These findings are only hypothesis-generating because a limited number of tumors underwent exome sequencing and germline specimens were not evaluated. An ongoing phase II trial will perform multiplex mutational analysis and exome sequencing in all eligible patients (n = 80) to clarify possible biomarkers (NCT01807546).
Although the exome analysis of a subset of SCCs is intriguing, the biomarkers analysis in the study had several limitations. For example, although the majority of subjects had archival tumor assessed for common mutations with multiplex mutation testing, few mutations were found. Similarly, immunohistochemical staining was not performed in archival specimens. The study would have been strengthened by pretreatment and on-treatment biopsies to assess changes in gene expression profiles and/or immunohistochemistry findings. In preclinical testing, for instance, FOXO3, FOXO4, reactive oxygen species, and c-jun-NH2-kinase signaling pathways alterations were associated with rigosertib’s anticancer activity (22). Pre- and postbiopsies would allow for this analysis and should be recommended in at least a subset of patients in future studies.
In summary, oral rigosertib has predictable PKs, a unique toxicity profile, and encouraging antitumor activity in patients with solid tumors, particularly SCCs. A phase II study for patients with SCCs of any origin, with a focus on HNSCC, stratified by HPV status is currently enrolling.
Supplementary Material
Translational Relevance.
The phosphoinositide 3-kinase (PI3K)/AKT/mTOR pathway is deregulated in a variety of solid tumors and may provide key growth and survival signals to tumor cells. Cell-cycle regulation through polo-like kinase 1 (Plk1) affects tumor growth. Therefore, coinhibitors of PI3K and PLK1 may impair both cell signaling and cell cycling. This article reports data from the phase I trial of the oral formulation of rigosertib, an indirect inhibitor of PI3K, PLK1, and other kinases. Results from this trial demonstrate that rigosertib may be administered with a tolerable, though unique, toxicity profile in patients with advanced solid tumors. Evidence of antitumor activity supports development as a single agent or in combination with other therapies. Exome sequencing is used to identify possible biomarkers in squamous cell carcinomas.
Acknowledgments
The authors thank the study subjects for their generous participation. Dr. Michael Reiss provided medical writing services on behalf of Onconova Therapeutics, Inc. Dr. Alexander Ree helped process the CT scan images.
Grant Support
The study was supported by Onconova Therapeutics, Inc and University of Colorado Cancer Center (P30CA046934).
Disclosure of Potential Conflicts of Interest
D.L. Aisner received a commercial research grant from Onconova Therapeutics, Inc., has received honoraria from the speakers bureau of Abbott Molecular, and is a consultant/advisory board member of Boehringher Ingelheim, GlaxoSmithKline, and Pfizer. F. Wilhelm is Chief Medical Officer and has ownership interest in Onconova Therapeutics, Inc. M. Maniar is Sr. Vice President in Onconova Therapeutics, Inc. S.G. Eckhardt is a consultant/ advisory board member of Onconova Therapeutics, Inc. W.A. Messersmith received a commercial research grant from Onconova Therapeutics, Inc. A. Jimeno is an ad hoc consultant, received a commercial research grant, and has ownership interests in Onconova Therapeutics, Inc.
Footnotes
Authors' Contributions
Conception and design: R.T. Anderson, F. Wilhelm, M. Maniar, S.G. Eckhardt, A. Jimeno
Development of methodology: D.P. Astling, R.T. Anderson, M. Varella-Garcia, A.-C. Tan, M. Maniar, A. Jimeno
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): D.W. Bowles, J.R. Diamond, E.T. Lam, C.D. Weekes, R.T. Anderson, S. Leong, L. Gore, M. Varella-Garcia, B.W. Vogler, S.B. Keysar, E. Freas, D.L. Aisner, C. Ren, A.-C. Tan, M. Maniar, S.G. Eckhardt, W.A. Messersmith, A. Jimeno
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): D.W. Bowles, J.R. Diamond, E.T. Lam, D. P. Astling, R.T. Anderson, M. Varella-Garcia, B.W. Vogler, A.-C. Tan, M. Maniar, S.G. Eckhardt, W.A. Messersmith, A. Jimeno
Writing, review, and/or revision of the manuscript: D.W. Bowles, J.R. Diamond, E.T. Lam, C.D. Weekes, D.P. Astling, R.T. Anderson, S. Leong, L. Gore, M. Varella-Garcia, S.B. Keysar, C. Ren, A.-C. Tan, F. Wilhelm, M. Maniar, S.G. Eckhardt, W.A. Messersmith, A. Jimeno
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): R.T. Anderson, E. Freas, A. Jimeno
Study supervision: R.T. Anderson, E. Freas, F. Wilhelm, A. Jimeno
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Prior presentations: American Society of Clinical Oncology Annual Meeting, 2012, Chicago, IL, abstract 3017; American Association for Cancer Research Annual Meeting, 2013, Washington, DC, abstract LB-198.
References
- 1.Yuan TL, Cantley LC. PI3K pathway alterations in cancer: variations on a theme. Oncogene. 2008;27:5497–5510. doi: 10.1038/onc.2008.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 3.Pedrero JM, Carracedo DG, Pinto CM, Zapatero AH, Rodrigo JP, Nieto CS, et al. Frequent genetic and biochemical alterations of the PI 3-K/ AKT/PTEN pathway in head and neck squamous cell carcinoma. Int J Cancer. 2005;114:242–248. doi: 10.1002/ijc.20711. [DOI] [PubMed] [Google Scholar]
- 4.Massion PP, Kuo WL, Stokoe D, Olshen AB, Treseler PA, Chin K, et al. Genomic copy number analysis of non-small cell lung cancer using array comparative genomic hybridization: implications of the phosphatidylinositol 3-kinase pathway. Cancer Res. 2002;62:3636–3640. [PubMed] [Google Scholar]
- 5.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 6.Bowles DW, Jimeno A. New phosphatidylinositol 3-kinase inhibitors for cancer. Expert Opin Investig Drugs. 2011;20:507–518. doi: 10.1517/13543784.2011.562192. [DOI] [PubMed] [Google Scholar]
- 7.Stransky N, Egloff AM, Tward AD, Kostic AD, Cibulskis K, Sivachenko A, et al. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011;333:1157–1160. doi: 10.1126/science.1208130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agrawal N, Frederick MJ, Pickering CR, Bettegowda C, Chang K, Li RJ, et al. Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science. 2011;333:1154–1157. doi: 10.1126/science.1206923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer. 2009;9:550–562. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- 11.Strebhardt K. Multifaceted polo-like kinases: drug targets and antitargets for cancer therapy. Nat Rev Drug Discov. 2010;9:643–660. doi: 10.1038/nrd3184. [DOI] [PubMed] [Google Scholar]
- 12.Jackman M, Lindon C, Nigg EA, Pines J. Active cyclin B1-Cdk1 first appears on centrosomes in prophase. Nat Cell Biol. 2003;5:143–148. doi: 10.1038/ncb918. [DOI] [PubMed] [Google Scholar]
- 13.Mielgo A, Seguin L, Huang M, Camargo MF, Anand S, Franovic A, et al. A MEK-independent role for CRAF in mitosis and tumor progression. Nat Med. 2011;17:1641–1645. doi: 10.1038/nm.2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mundt KE, Golsteyn RM, Lane HA, Nigg EA. On the regulation and function of human polo-like kinase 1 (PLK1): effects of overexpression on cell cycle progression. Biochem Biophys Res Commun. 1997;239:377–385. doi: 10.1006/bbrc.1997.7378. [DOI] [PubMed] [Google Scholar]
- 15.Spankuch B, Matthess Y, Knecht R, Zimmer B, Kaufmann M, Streb-hardt K. Cancer inhibition in nude mice after systemic application of U6 promoter-driven short hairpin RNAs against PLK1. J Natl Cancer Inst. 2004;96:862–872. doi: 10.1093/jnci/djh146. [DOI] [PubMed] [Google Scholar]
- 16.Spankuch-Schmitt B, Bereiter-Hahn J, Kaufmann M, Strebhardt K. Effect of RNA silencing of polo-like kinase-1 (PLK1) on apoptosis and spindle formation in human cancer cells. J Natl Cancer Inst. 2002;94:1863–1877. doi: 10.1093/jnci/94.24.1863. [DOI] [PubMed] [Google Scholar]
- 17.Gray PJ, Jr, Bearss DJ, Han H, Nagle R, Tsao MS, Dean N, et al. Identification of human polo-like kinase 1 as a potential therapeutic target in pancreatic cancer. Mol Cancer Ther. 2004;3:641–646. [PubMed] [Google Scholar]
- 18.Reddy MV, Venkatapuram P, Mallireddigari MR, Pallela VR, Cosenza SC, Robell KA, et al. Discovery of a clinical stage multi-kinase inhibitor sodium (E)-2-{2-methoxy-5-[(2′,4′ ,6′-trimethoxys-tyrylsulfonyl)methyl]phenylamino}acetate (ON 01910.Na): synthesis, structure-activity relationship, and biological activity. J Med Chem. 2011;54:6254–6276. doi: 10.1021/jm200570p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gumireddy K, Reddy MV, Cosenza SC, Boominathan R, Baker SJ, Papathi N, et al. ON01910, a non-ATP-competitive small molecule inhibitor of Plk1, is a potent anticancer agent. Cancer Cell. 2005;7:275–286. doi: 10.1016/j.ccr.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 20.Jimeno A, Rubio-Viqueira B, Rajeshkumar NV, Chan A, Solomon A, Hidalgo M. A fine-needle aspirate-based vulnerability assay identifies polo-like kinase 1 as a mediator of gemcitabine resistance in pancreatic cancer. Mol Cancer Ther. 2010;9:311–318. doi: 10.1158/1535-7163.MCT-09-0693. [DOI] [PubMed] [Google Scholar]
- 21.Prasad A, Park IW, Allen H, Zhang X, Reddy MV, Boominathan R, et al. Styryl sulfonyl compounds inhibit translation of cyclin D1 in mantle cell lymphoma cells. Oncogene. 2009;28:1518–1528. doi: 10.1038/onc.2008.502. [DOI] [PubMed] [Google Scholar]
- 22.Chapman CM, Sun X, Roschewski M, Aue G, Farooqui M, Stennett L, et al. ON 01910.Na is selectively cytotoxic for chronic lymphocytic leukemia cells through a dual mechanism of action involving PI3K/AKT inhibition and induction of oxidative stress. Clin Cancer Res. 2012;18:1979–1991. doi: 10.1158/1078-0432.CCR-11-2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jimeno A, Li J, Messersmith WA, Laheru D, Rudek MA, Maniar M, et al. Phase I study of ON 01910.Na, a novel modulator of the polo-like kinase 1 pathway, in adult patients with solid tumors. J Clin Oncol. 2008;26:5504–5510. doi: 10.1200/JCO.2008.17.9788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 25.Hong DS, Bowles DW, Falchook GS, Messersmith WA, George GC, O'Bryant CL, et al. A multicenter phase I trial of PX-866, an oral irreversible phosphatidylinositol 3-kinase inhibitor, in patients with advanced solid tumors. Clin Cancer Res. 2012;18:4173–4182. doi: 10.1158/1078-0432.CCR-12-0714. [DOI] [PubMed] [Google Scholar]
- 26.Bendell JC, Rodon J, Burris HA, de Jonge M, Verweij J, Birle D, et al. Phase I, dose-escalation study of BKM120, an oral pan-class I PI3K inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2012;30:282–290. doi: 10.1200/JCO.2011.36.1360. [DOI] [PubMed] [Google Scholar]
- 27.Edelman G, Bedell C, Shapiro GI, Pandya SS, Kwak EL, Scheffold C, et al. A phase I dose-escalation study of XL147 (SAR245408), a PI3K inhibitor administered orally to patients (pts) with advanced malignancies. J Clin Oncol. 2010;28 abstract 3004. [Google Scholar]
- 28.Hofheinz RD, Al-Batran SE, Hochhaus A, Jager E, Reichardt VL, Fritsch H, et al. An open-label, phase I study of the polo-like kinase-1 inhibitor, BI 2536, in patients with advanced solid tumors. Clin Cancer Res. 2010;16:4666–4674. doi: 10.1158/1078-0432.CCR-10-0318. [DOI] [PubMed] [Google Scholar]
- 29.Schoffski P, Awada A, Dumez H, Gil T, Bartholomeus S, Wolter P, et al. A phase I, dose-escalation study of the novel Polo-like kinase inhibitor volasertib (BI 6727) in patients with advanced solid tumours. Eur J Cancer. 2012;48:179–186. doi: 10.1016/j.ejca.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 30.Vu HL, Sikora AG, Fu S, Kao J. HPV-induced oropharyngeal cancer, immune response and response to therapy. Cancer Lett. 2010;288:149–155. doi: 10.1016/j.canlet.2009.06.026. [DOI] [PubMed] [Google Scholar]
- 31.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDer-mott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Janku F, Tsimberidou AM, Garrido-Laguna I, Wang X, Luthra R, Hong DS, et al. PIK3CA mutations in patients with advanced cancers treated with PI3K/AKT/mTOR axis inhibitors. Mol Cancer Ther. 2011;10:558–565. doi: 10.1158/1535-7163.MCT-10-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keysar SB, Astling DP, Anderson RT, Vogler BW, Bowles DW, Morton JJ, et al. A patient tumor transplant model of squamous cell cancer identifies PI3K inhibitors as candidate therapeutics in defined molecular bins. Mol Oncol. 2013;7:776–790. doi: 10.1016/j.molonc.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lui VW, Hedberg ML, Li H, Vangara BS, Pendleton K, Zeng Y, et al. Frequent mutation of the PI3K pathway in head and neck cancer defines predictive biomarkers. Cancer Discov. 2013;3:761–769. doi: 10.1158/2159-8290.CD-13-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanhaji M, Louwen F, Zimmer B, Kreis NN, Roth S, Yuan J. Polo-like kinase 1 inhibitors, mitotic stress and the tumor suppressor p53. Cell Cycle. 2013;12:1340–1351. doi: 10.4161/cc.24573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anderson RT, Keysar SB, Bowles DW, Glogowska MJ, Astling DP, Morton JJ, et al. The dual pathway inhibitor rigosertib is effective in direct-patient tumor xenografts of head and neck squamous cell carcinomas. Mol Cancer Ther. 2013;12:1994–2005. doi: 10.1158/1535-7163.MCT-13-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morris LG, Kaufman AM, Gong Y, Ramaswami D, Walsh LA, Turcan S, et al. Recurrent somatic mutation of FAT1 in multiple human cancers leads to aberrant Wnt activation. Nat Genet. 2013;45:253–261. doi: 10.1038/ng.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nishikawa Y, Miyazaki T, Nakashiro K, Yamagata H, Isokane M, Goda H, et al. Human FAT1 cadherin controls cell migration and invasion of oral squamous cell carcinoma through the localization of beta-catenin. Oncol Rep. 2011;26:587–592. doi: 10.3892/or.2011.1324. [DOI] [PubMed] [Google Scholar]
- 39.Bauer K, Dowejko A, Bosserhoff AK, Reichert TE, Bauer R. Slit-2 facilitates interaction of P-cadherin with Robo-3 and inhibits cell migration in an oral squamous cell carcinoma cell line. Carcinogenesis. 2011;32:935–943. doi: 10.1093/carcin/bgr059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tseng RC, Lee SH, Hsu HS, Chen BH, Tsai WC, Tzao C, et al. SLIT2 attenuation during lung cancer progression deregulates beta-catenin and E-cadherin and associates with poor prognosis. Cancer Res. 2010;70:543–551. doi: 10.1158/0008-5472.CAN-09-2084. [DOI] [PubMed] [Google Scholar]
- 41.Ruan K, Ye F, Li C, Liou YC, Lin SC, Lin SY. PLK1 interacts and phosphorylates Axin that is essential for proper centrosome formation. PLoS One. 2012;7:e49184. doi: 10.1371/journal.pone.0049184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu Z, Doondeea JB, Gholami AM, Janning MC, Lemeer S, Kramer K, et al. Quantitative chemical proteomics reveals new potential drug targets in head and neck cancer. Mol Cell Proteomics. 2011;10 doi: 10.1074/mcp.M111.011635. M111.011635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wheeler SE, Morariu EM, Bednash JS, Otte CG, Seethala RR, Chiosea SI, et al. Lyn kinase mediates cell motility and tumor growth in EGFRvIII-expressing head and neck cancer. Clin Cancer Res. 2012;18:2850–2860. doi: 10.1158/1078-0432.CCR-11-2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamada A, Ishikawa T, Ota I, Kimura M, Shimizu D, Tanabe M, et al. High expression of ATP-binding cassette transporter ABCC11 in breast tumors is associated with aggressive subtypes and low disease-free survival. Breast Cancer Res Treat. 2013;137:773–782. doi: 10.1007/s10549-012-2398-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ansbro MR, Shukla S, Ambudkar SV, Yuspa SH, Li L. Screening compounds with a novel high-throughput ABCB1-mediated efflux assay identifies drugs with known therapeutic targets at risk for multidrug resistance interference. PLoS One. 2013;8:e60334. doi: 10.1371/journal.pone.0060334. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.