Abstract
IMPORTANCE
In the traditional model of acute appendicitis, time is the major driver of disease progression; luminal obstruction leads inexorably to perforation without timely intervention. This perceived association has long guided clinical behavior related to the timing of appendectomy.
OBJECTIVE
To evaluate whether there is an association between time and perforation after patients present to the hospital.
DESIGN, SETTING, AND PARTICIPANTS
Using data from the Washington State Surgical Care and Outcomes Assessment Program (SCOAP), we evaluated patterns of perforation among patients (≥18 years) who underwent appendectomy from January 1, 2010, to December 31, 2011. Patients were treated at 52 diverse hospitals including urban tertiary centers, a university hospital, small community and rural hospitals, and hospitals within multi-institutional organizations.
MAIN OUTCOMES AND MEASURES
The main outcome of interest was perforation as diagnosed on final pathology reports. The main predictor of interest was elapsed time as measured between presentation to the hospital and operating room (OR) start time. The relationship between in-hospital time and perforation was adjusted for potential confounding using multivariate logistic regression. Additional predictors of interest included sex, age, number of comorbid conditions, race and/or ethnicity, insurance status, and hospital characteristics such as community type and appendectomy volume.
RESULTS
A total of 9048 adults underwent appendectomy (15.8% perforated). Mean time from presentation to OR was the same (8.6 hours) for patients with perforated and nonperforated appendicitis. In multivariate analysis, increasing time to OR was not a predictor of perforation, either as a continuous variable (odds ratio = 1.0 [95% CI, 0.99-1.01]) or when considered as a categorical variable (patients ordered by elapsed time and divided into deciles). Factors associated with perforation were male sex, increasing age, 3 or more comorbid conditions, and lack of insurance.
CONCLUSIONS AND RELEVANCE
There was no association between perforation and in-hospital time prior to surgery among adults treated with appendectomy. These findings may reflect selection of those at higher risk of perforation for earlier intervention or the effect of antibiotics begun at diagnosis but they are also consistent with the hypothesis that perforation is most often a prehospital occurrence and/or not strictly a time-dependent phenomenon. These findings may also guide decisions regarding personnel and resource allocation when considering timing of nonelective appendectomy.
Acute appendicitis is the most common indication for urgent intra-abdominal surgery.1 The conventional pathophysiologic model of acute appendicitis is based on a relationship between time and disease progression; risk of perforation increases as time elapses from onset of disease to treatment. Delays can occur anywhere along the pathway from symptom onset to presentation, evaluation, and treatment and many factors come into play including aspects of the disease itself, patient characteristics, access to medical care, and characteristics of the health care system. Observational research has demonstrated an association between time to treatment and perforation;2-12 indirect evidence for this association has also come from studies linking impaired health care access to increased risk of perforation.13-15
It is challenging to establish the precise time of symptom onset and to characterize patients’ prehospital courses. While several previous studies have attempted this using record review or by incorporating time-based questions into clinical history taking, many have been hampered by small numbers of patients from single institutions, recall bias, and/or poor time discrimination. Previous studies evaluating time to treatment after patients arrive to the hospital have encountered similar difficulties. The question of an association between time and perforation raises the possibility that facilitating earlier treatment could reduce incidence of perforation.
The Washington State Surgical Care and Outcomes Assessment Program (SCOAP), a physician-led quality surveillance program, provides the following benefits to an evaluation of the relationship between time to treatment and perforation: a large number of diverse institutions, many patients, individualized review of the medical record by trained abstractors and specific data on hospital arrival time, time of diagnostic imaging, and operating room (OR) start time. Although our study was not able to investigate the impact of prehospital time on perforation, the precise capture of elapsed time after patients present to the hospital and the accurate pathology-based identification of clinical outcomes are advantages when compared with previous studies. Our objective was to evaluate the relationship between perforation and the amount of time patients wait for surgery after arriving at the hospital.
Methods
Study Population and Setting
Consecutive adult patients were included in this prospective cohort if they underwent nonelective appendectomy in 1 of 52 SCOAP hospitals in Washington State between January 1, 2010, and December 31, 2011. Recent estimates derived from the state’s abstract reporting system suggest that greater than 85% of nonelective appendectomies performed in Washington are captured by SCOAP. Unlike administrative data sets where billing codes are used to identify diagnoses, SCOAP relies on the direct review of clinical records by trained abstractors. Data in SCOAP are collected primarily for quality improvement but the abstracting protocol is also developed in a prospective evolving manner to answer new research questions. The University of Washington Human Subjects Division reviewed our protocol and deemed it not human-subjects research because the analytic team used anonymous data. The STROBE (Strengthening the Reporting of Observational studies in Epidemiology) Statement Checklist was used in planning and reporting this research.16
Descriptive Variables
Demographic and socioeconomic information, clinical characteristics, and pathology results were abstracted from clinical records using standardized definitions. Abstracted data were audited for quality control and to verify that records were being evaluated in a similar way across sites. A comorbidity score was calculated based on documentation of the following comorbid conditions: coronary artery disease, asthma, diabetes mellitus, human immunodeficiency virus/AIDS, and/or elevated serum creatinine level. White blood cell count was based on the last result prior to surgery.
Outcome and Predictor Variables
The outcome of interest was the presence or absence of perforation on the final pathologic report. The primary predictor of interest was elapsed time between hospital arrival and initiation of surgery. In this large data set, there were a small number of clear outliers, some with obviously misclassified times. For this reason, we restricted the analytic cohort to the 99th percentile of all patients. This resulted in exclusion of only 74 patients, all with time to treatment longer than 2.5 days.
Statistical Analysis
Patients with negative appendectomy were excluded from analysis. The remaining patients were then divided into those diagnosed as having perforated appendicitis and those having nonperforated appendicitis. Clinical and demographic characteristics of each group were compared. Continuous variables were compared using means (and the t test) and/or medians. Differences between categorical variables were tested using the Pearson χ2 test. Statistical significance was set at P = .05. Some demographic and socioeconomic characteristics were not recorded for every patient, in particular race and/or ethnicity and insurance status. Those observations with missing race and/or ethnicity data were not discarded but presented in the data tables as unknown. We also generated a variable that combined ethnicity with race (white/non-Hispanic, white/Hispanic, minority race/non-Hispanic, and minority race/Hispanic). Patients with unknown race were not included in these Hispanic designations except for those who were listed as Hispanic/Latino and did not have a race designated; these were included in the white/Hispanic category. If race was listed but there was no ethnicity included, the patient was assigned to the appropriate non-Hispanic category. Unknown insurance status was included with the uninsured and self-pay categories.
In the next univariate analysis, to identify potential predictors of delay, we produced a mean standard deviation and median time to treatment for each of the variables in the initial analysis. A perforation rate for each category was also generated. Statistical testing for differences in perforation was performed by univariate logistic regression.
Time from presentation to OR was compared for patients with and without perforation using the t test. Additionally, we subdivided the time from emergency department to OR into 2 segments, arrival to imaging and imaging to OR. Not all patients had the exact time of imaging recorded and some were imaged prior to hospital arrival. These were excluded, reducing the number of observations for this subset analysis. If a patient had more than 1 imaging modality, analysis was based on the time of the last study obtained.
To further analyze the relationship between time and perforation, we ordered the patients by time to treatment then divided the cohort into deciles. We calculated percentage of perforation and 95% CI for each decile. Percentage of perforation for each decile was compared with the first decile (fastest to OR) and univariate logistic regression was performed with decile 1 as referent.
Finally, to adjust for potential confounding, we developed a multivariate logistic regression model. Covariates were initially considered for inclusion based on clinical experience and review of the surgical literature. Variables were included in the final regression model if at least 1 of the predictor categories had P < .10 in univariate analysis. Using a priori criteria, sex, age, comorbidity index score, race, ethnicity, insurance status, and certain hospital characteristics were included in the multivariate model. In the fully adjusted model, ethnicity was included as a yes, no, or unknown variable instead of the race and ethnicity categories used in univariate analysis because the latter was colinear with race in the model. The STATA software version 12 (StataCorp) was used for analysis.
Results
Perforated vs Nonperforated Appendicitis
During the study period, 9048 patients underwent appendectomy (52% male; mean [SD] age, 39.8 [16.6] years). Four percent underwent negative appendectomy and were excluded from further analysis. Of the total cohort, 15.8% had a perforated appendix (Table 1). Patients with perforation were slightly more likely to be male (55.3% vs 52.1%, P < .001), older (48.8 years vs 38.2 years, P < .001), and more likely to have comorbid conditions than those without perforation. Race and ethnicity did not differ but there were significantly fewer patients with private insurance (54.2% vs 63.6%, P < .001) and more patients with Medicare (17.0% vs 6.4%, P < .001) in the perforated group.
Table 1. Clinical and Demographic Cohort Characteristicsa.
| Characteristic | All Appendectomy (n = 9048) |
Nonperforated (n = 7233) |
Perforated (n = 1421) |
P Value |
|---|---|---|---|---|
| Male, % | 52.0 | 52.1 | 55.3 | .03 |
| Age, mean (SD), y | 39.8 (16.6) | 38.2 (15.8) | 48.8 (17.6) | <.001 |
| Age distribution, %, y | ||||
| 18-29 | 34.6 | 37.5 | 17.5 | <.001 |
| 30-39 | 20.8 | 22.0 | 14.2 | <.001 |
| 40-49 | 16.3 | 16.6 | 17.7 | .16 |
| 50-59 | 14.0 | 12.8 | 21.3 | <.001 |
| 60-69 | 8.9 | 7.4 | 17.2 | <.001 |
| ≥70 (max = 97) | 5.4 | 4.2 | 12.0 | <.001 |
| WBC count, mean (SD) | 13.3 (4.8) | 13.2 (4.3) | 14.5 (4.8) | <.001 |
| Comorbidity index score, % | ||||
| 0 | 86.1 | 87.1 | 80.4 | <.001 |
| 1 | 11.3 | 10.7 | 14.1 | <.001 |
| 2 | 1.8 | 1.6 | 3.2 | <.001 |
| ≥3 | 0.9 | 0.6 | 2.3 | <.001 |
| Race, % | ||||
| White | 71.7 | 71.6 | 72.4 | .52 |
| African American | 2.7 | 2.7 | 8 | .06 |
| Asian | 5.3 | 5.4 | 4.3 | .08 |
| American Indian/Alaskan | 0.9 | 0.8 | 1.1 | .23 |
| Hawaiian/Pacific Islander | 0.6 | 0.7 | 0.4 | 8 |
| Unknown | 19.0 | 18.8 | 20.00 | .31 |
| Ethnicity, % | ||||
| White, non-Hispanic | 65.4 | 65.2 | 66.7 | .26 |
| White, Hispanic | 8.0 | 8. | 7.5 | .45 |
| Minority, non-Hispanic | 9.3 | 9.5 | 7.5 | .02 |
| Minority, Hispanic | 0.1 | 0.1 | 0.1 | .64 |
| Unknown | 17.3 | 17.2 | 18.2 | .36 |
| Insurance, % | ||||
| Private | 61.8 | 63.6 | 54.2 | <.001 |
| Medicare | 8. | 6.4 | 17.0 | <.001 |
| Medicaid | 8.4 | 8.2 | 8.2 | .96 |
| Uninsured, self-pay, unknown |
18.1 | 18.0 | 18.0 | .97 |
| Other government program |
3.6 | 3.9 | 2.7 | .03 |
| Preoperation imaging | 93.3 | 93.9 | 93.9 | .97 |
| Negative appendectomy | 4.1 | NA | NA | NA |
| Perforation | 15.8 | NA | NA | NA |
Abbreviations: NA, not applicable; WBC, white blood cell.
Significance testing for patient characteristics with more than binomial categories (eg, comorbidity score, race, race and ethnicity, and insurance status) was performed using the Pearson χ2 test. The proportion of each strata was compared between those with and without perforation. For example, the percentage of patients with Medicare among those with perforation was compared with the percentage of patients with Medicare among those without perforation. Differences in continuous variables (age and WBC count) were tested for significance using the t test.
Time to Treatment
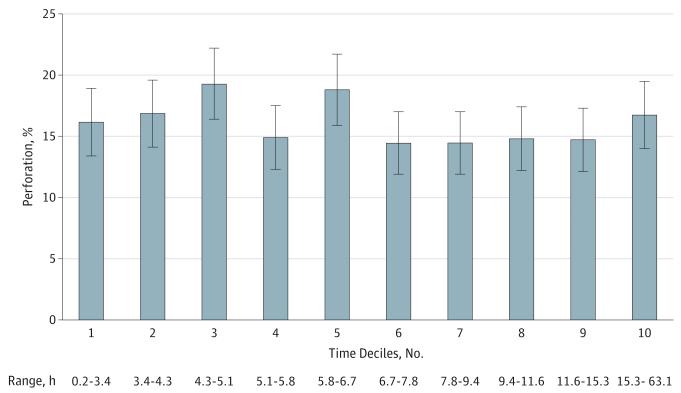
Seven thousand five hundred five patients had complete data for time of hospital admission and OR start. We compared the entire time elapsed from admission to OR and found that it was the same for patients with perforated and nonperforated appendicitis (8.6 hours vs 8.6 hours) (Table 2). We also compared time from presentation to imaging (3.2 hours vs 3.4 hours, P = .06) and time from imaging to the OR (7.8 hours vs 8.2 hours, P = .52). With the patients ordered into deciles (Figure), there were no differences in perforation compared with the first decile. Similarly, with time as a continuous variable, there was no difference in unadjusted odds of perforation for each incremental increase in time (odds ratio = 1.0 [95% CI, 0.99-1.01]). After adjusting for clinical and demographic factors, odds ratio of perforation remained 1.0 (95% CI, 0.99-1.01) (Table 3).
Table 2. Preoperative Wait Timesa.
| Wait Time | All Appendectomy | Nonperforated | Perforated | P Value |
|---|---|---|---|---|
| Presentation to OR, h | 8.6 (6.5) | 8.6 (6.4) | 8.6 (7.2) | .82 |
| Presentation to imaging, h |
3.2 (5.9) | 3.2 (5.5) | 3.4 (7.4) | .06 |
| Imaging to OR, h | 7.8 (7.0) | 7.8 (6.9) | 8.2 (7.8) | .52 |
Abbreviation: OR, operating room.
Table 2 presents elapsed time from hospital presentation to OR start. These datawere also broken down into time before and time after advanced imaging was obtained.
Figure. Percentage of Perforation by Deciles of Time From Presentation to Operating Room Start.
Patients were ordered by time to treatment and divided into deciles. Percentage of perforation and 95% CIs were calculated for each decile. The range of elapsed time from hospital presentation to operating room start time is shown for each decile (ie, the shortest time and the longest time for each decile).
Table 3. Odds of Perforation, Unadjusted and Fully Adjusted Regression Models.
| Characteristic | Unadjusted Odds Ratio (95% CI) | Fully Adjusted Odds Ratio (95% CI) |
|---|---|---|
| Presentation to OR time (continuous variable) | 1.0 (0.99-1.01) | 1.0 (0.99-1.01) |
| Sex | ||
| Female | 1 [Reference] | 1 [Reference] |
| Male | 1.17 (1.03-1.33) | 1.24 (1.08-1.43) |
| Age (continuous) | 1.04 (1.03-1.04) | 1.04 (1.08-1.43) |
| Comorbidity index score | ||
| 0 | 1 [Reference] | 1 [Reference] |
| 1 | 1.40 (1.16-1.69) | 0.99 (0.81-1.21) |
| 2 | 2.44 (1.69-3.52) | 1.32 (0.98-1.76) |
| ≥3 | 3.56 (2.12-5.96) | 2.18 (1.36-3.49) |
| Race | ||
| White | 1 [Reference] | 1 [Reference] |
| African American | 0.68 (0.43-1.07) | 0.65 (0.42-1.02) |
| Asian | 0.73 (0.53-1.00) | 0.68 (0.52-0.89) |
| American Indian/Alaskan | 0.94 (0.46-1.92) | 1.15 (0.52-2.56) |
| Hawaiian/Pacific Islander | 0.62 (0.25-1.58) | 0.83 (0.31-2.23) |
| Unknown | 1.03 (0.88-1.21) | 1.00 (0.83-1.21) |
| Ethnicity | ||
| Non-Hispanic | 1 [Reference] | 1 [Reference] |
| Hispanic | 0.85 (0.66-1.09) | 0.93 (0.71-1.21) |
| Unknown | 1.09 (0.95-1.24) | 1.08 (0.88-1.33) |
| Insurance | ||
| Private | 1 [Reference] | 1 [Reference] |
| Medicare | 3.05 (2.51-3.71) | 0.98 (0.76-1.26) |
| Medicaid | 1.1 (0.87-1.40) | 1.24 (0.96-1.61) |
| Uninsured, self-pay, unknown | 1.25 (1.06-1.48) | 1.43 (1.24-1.66) |
| Other government program | 0.63 (0.40-0.97) | 0.70 (0.44-1.09) |
| Hospital volume, quartiles | ||
| 1st (lowest) | 1 [Reference] | 1 [Reference] |
| 2nd | 0.81 (0.55-1.20) | 0.87 (0.62-1.21) |
| 3rd | 0.729 (0.50-1.06) | 0.90 (0.67-1.20) |
| 4th | 0.69 (0.48-0.99) | 0.84 (0.61-1.16) |
| Hospital location | ||
| Urban | 1 [Reference] | 1 [Reference] |
| Rural | 1.3 (1.05-1.61) | 1.15 (0.94-1.42) |
Abbreviations: NA, not applicable; OR, operating room.
Patient Characteristics, Time to Treatment, and Perforation
Mean and median wait times among various clinical, demographic, and socioeconomic strata were compared (Table 4). Although there was a higher percentage of perforation among male patients, they had shorter time to treatment than women (mean 8.2 hours vs 9.0 hours). Perforation increased with increasing age; the eldest group of patients had the highest rate of perforation and the longest time to treatment (10.0 hours). Both perforation and time to treatment increased with increasing number of comorbid conditions.
Table 4. Percentage of Perforation and Time to Treatment by Clinical and Demographic Groups.
| Characteristic | Perforation, % | Presentation to OR Time | Observations, No. | |
|---|---|---|---|---|
| Mean (SD) | Median | |||
| Sex | ||||
| Male | 17.3 | 8.2 (6.2) | 6.5 | 3847 |
| Female | 15.5 | 9.0 (6.8) | 7.1 | 3479 |
| Age distribution, y | ||||
| 18-29 | 8.4 | 9.0 (6.8) | 7.2 | 2600 |
| 30-39 | 11.3 | 8.3 (5.9) | 6.7 | 1520 |
| 40-49 | 17.7 | 7.9 (5.7) | 6.4 | 1180 |
| 50-59 | 24.7 | 8.3 (6.2) | 6.5 | 1004 |
| 60-69 | 31.5 | 8.4 (6.6) | 6.3 | 625 |
| ≥70 (max = 97) | 36.1 | 10.0 (8.8) | 7.0 | 399 |
| Comorbidity index score | ||||
| 0 | 15.4 | 8.4 (6.2) | 6.7 | 6308 |
| 1 | 20.5 | 9.4 (7.9) | 6.9 | 814 |
| 2 | 28.0 | 9.9 (7.5) | 7.6 | 143 |
| ≥3 | 43.4 | 11.1 (9.0) | 8.1 | 63 |
| Race | ||||
| White | 16.6 | 8.4 (6.4) | 6.6 | 5202 |
| African American | 11.7 | 11.2 (8.9) | 8.3 | 198 |
| Asian | 13.4 | 8.8 (5.8) | 7.4 | 370 |
| American Indian/Alaskan | 21.6 | 8.2 (6.7) | 6.5 | 61 |
| Hawaiian/Pacific Islander | 9.6 | 10.9 (8.7) | 7.8 | 47 |
| Unknown | 17.3 | 8.7 (6.3) | 6.9 | 1450 |
| Ethnicity | ||||
| White, non-Hispanic | 16.8 | 8.4 (6.5) | 6.6 | 4720 |
| White, Hispanic | 15.4 | 8.4 (5.9) | 6.8 | 615 |
| Minority, non-Hispanic | 13.4 | 9.6 (7.2) | 7.6 | 670 |
| Minority, Hispanic | 22.2 | 6.2 (2.4) | 6.7 | 6 |
| Unknown race | 17.2 | 8.6 (6.3) | 6.9 | 1317 |
| Insurance | ||||
| Private | 14.3 | 8.1 (6.0) | 6.5 | 4372 |
| Medicare | 34.4 | 9.6 (8.3) | 6.8 | 585 |
| Medicaid | 16.4 | 9.4 (7.2) | 7.2 | 648 |
| Uninsured, self-pay, unknown |
16.4 | 9.0 (6.7) | 7.0 | 1466 |
| Other government program | 12.0 | 9.1 (5.9) | 7.8 | 257 |
In terms of race and ethnicity, African American patients had the longest mean wait time but the second-lowest perforation rate. No race had significantly different odds of perforation from white patients in univariate analysis (Table 3) and after adjustments, only Asian American patients differed significantly (odds ratio = 0.68, P = .004). Asian American patients also had the third-longest wait time (8.8 hours). In unadjusted analysis, minority-race non-Hispanic patients had significantly reduced odds of perforation compared with white non-Hispanic patients (13.4% vs 16.8%, P = .005) but no other category differed from white non-Hispanic patients (Table 4). In the fully adjusted model (Table 3), there was no difference in odds of perforation between non-Hispanic and Hispanic patients.
Among insurance categories, Medicare beneficiaries had the longest mean wait time (9.6 hours) and the highest percentage of perforation (Table 4) but after adjustments there was no difference compared with privately insured patients (Table 3). Uninsured patients and patients with government-sponsored health care waited over an hour more on average than privately insured patients (Table 4). In both univariate and multivariate analyses, those patients in the uninsured, selfpay, and unknown category had substantially increased odds of perforation (odds ratio = 1.43, P < .001) (Table 3).
Sensitivity Analyses
We performed 2 sensitivity analyses. The first was an additional logistic regression of perforation vs time in which time was coded as a categorical variable (hours) and wait time of 1 to 2 hours was chosen as the reference category instead of the 0 to 1 hour. (A small group of patients [n = 37] had <1 hour wait time and a high perforation rate [27%], which might have obscured a difference in shorter vs longer wait times). Within the first 24 hours, there was no difference in perforation for any of the hourly increments. There was no pattern in perforation from 25 to 63 hours (the limit of the 99th percentile), although a few isolated hourly increments differed significantly from the referent (1-2 hours). Second, we performed logistic regression with time to treatment categorized into deciles in case the more granular hourly data were obscuring trends because some groups had few or no observations. In unadjusted logistic regression, no decile had significantly different odds of perforation compared with decile 1. This was repeated with patients from the first hour excluded (ie, reducing perforation in decile 1 and potentially enhancing a difference with later deciles). Still, there were no differences in odds of perforation. Finally, patient deciles were included in the full multivariate model (instead of time as a continuous variable) and, again, the results were unchanged.
Discussion
After patients presented to the hospital, we found no relationship between time to treatment and perforation. Clinical risk factors for perforation included male sex, advancing age, and 3 or more comorbid conditions. Among demographic and socioeconomic characteristics, Asian American patients had lower odds of perforation compared with white patients and the uninsured/self-pay group had a 43% increase in odds of perforation compared with the privately insured group. Hispanic ethnicity and African American race were not risk factors for perforation. Hospital appendectomy volume and rural or urban setting were not associated with perforation.
Beginning with several classic articles published in the late-19th and early-20th centuries,17,18 surgeons have maintained that appendicitis progresses in a time-dependent manner as luminal obstruction and visceral distension lead to venous congestion, blood supply compromise, gangrenous changes in the appendiceal wall, and, ultimately, perforation. Although both Fitz17 and McBurney18 believed that some cases of appendicitis resolved without intervention, the treatment imperative became early operation to forestall progression to perforation. Observational studies have demonstrated an association between perforation and the overall amount of time elapsed from symptom onset to definitive care.2-12 Although several studies have specifically evaluated time to treatment once patients reach the hospital (in both pediatric and adult populations), divergent conclusions have been reached about the association between in-hospital time and risk of perforation.8,10,12,19-21 Three substantial strengths for the current study compared with these previous investigations of in-hospital time are the large study population, multiinstitutional design, and level of discrimination with which time was defined; the data include exact time of hospital presentation and OR start. To illustrate this advantage, consider a study based on dates recorded in the medical record. A patient who presented at 11 pm on Monday and was taken to the OR at 3 am on Tuesday (4 hours) would be classified as having waited 1 day between presentation and OR whereas a patient who presented at 1 am on Monday and was taken to the OR at 11 pm that same evening (22 hours) would be classified as having waited less than a day. This can lead to substantial misclassification. Given that 97% of the patients in this series had surgery within 48 hours of presentation, a study based on precise hourly intervals offers significant advantage compared with previous studies based on 24-hour intervals, particularly when there is ongoing debate over the appropriate timing of appendectomy (eg, can the procedure be delayed a few hours to accomodate work force or health system priorities?).
Multiple investigators have found an association between markers of limited access to health care and increased risk of perforation, extrapolating that such health care barriers lead to delays in presentation and increased perforation. In adults, insurance status13-15 and race/ethnicity13,15 have been associated with perforation. Similarly, among children, race and ethnicity have been implicated as risk factors for perforation,22-25 as has insurance status.24-26 However, these studies into impaired access and perforation have not produced consistent results. For instance, Pieracci et al,27 Boomer et al,14 and Lee et al28 all found that, compared with white adults, other racial and ethnic groups had lower or similar odds of perforation (as did the current study). Moreover, many studies in the pediatric literature contradict one another in terms of which races or ethnicities have higher or lower proportions of perforation. It is not clear whether these divergent findings stem from differences in study populations, data sources, study designs, characteristics of treatment settings, or other causes. Most studies (including the current study) that have evaluated perforated appendicitis have detected 1 or more access issues that appear to be risk factors for perforation. This has been most consistent for insurance status; findings have varied considerably for race and/or ethnicity.
Using a California billing claims database and US Census data, Luckmann and Davis29calculated separate population-based incidences for perforating and nonperforating appendicitis and detected differences among racial and ethnic groups. Noting that “some cases of appendicitis [may] resolve spontaneously before an appendectomy is performed,” Luckmann and Davis29 described how differences in access and use of health care services may drive some of the differences in the incidence of nonperforating appendicitis. In another article, Luckmann30 illustrated how this might lead to inconsistencies in determining a denominator when calculating percentages of perforation. These methodological concerns, as well as diverging secular incidences of perforated and nonperforated appendicitis,31 have led some authors to dissent from the view that time to treatment is the predominant driver of perforation.29,30,32,33 Alternative theories to time dependence have been proposed to explain perforation including differences in microbiology34 or differences in host inflammatory response.29,35,36 By the time patients develop symptoms, a unique microbiology or host response profile may already have resulted in perforation. If true, this would suggest that perforation may not be a particularly time-sensitive (or modifiable) event, a contention that could also be supported by these in-hospital data.
This study has limitations. Initiation of antibiotics may stop the natural progression of appendicitis, muting the time-to-perforation effect. Unfortunately, SCOAP does not collect data on the initiation of antibiotics. An alternative explanation for lack of association is that surgeons’ clinical acumen may enable identification of those at highest risk for perforation, leading to a form of selection bias in which those most near to perforation (but not yet perforated) are taken expeditiously to the OR. However, given the lack of objective measures to identify such patients, it seems unlikely that surgeons are able to cherry pick with such a level of discrimination. Finally, patients present to the operating hospital at different stages in the progression of their disease, which means that analytic time 0 is not the same for every patient. However, if appendicitis were strictly time dependent and the interval for progression less than 2.5 days, some relationship between time and perforation should be evident in our data, especially because our definition of perforation is based on pathologic review that allows for the detection of small early perforations. Additionally, patients presenting with perforation and abscess are frequently taken for interventional radiology drain and would not be captured in our data set, removing a number of late-stage presenters from analysis, which might make the disease stage of the remaining patients somewhat more similar at presentation. Outcomes such as surgical site infection and length of stay might add additional insight to the question of timing when patients present with appendicitis.
Conclusions
In conclusion, perforation was not associated with elapsed time from hospital presentation to OR start among adult patients admitted for appendectomy across a large number of diverse hospitals. Our findings are consistent with the hypothesis that perforation is more often a prehospital event and that delays in presentation confer increased risk. However, these findings are also consistent with the view that disease progression in appendicitis has a more complex pathophysiology driven by factors other than time with disease. As some researchers have suggested, perforated and nonperforated appendicitis may be separate biological or host-response entities. Although observational data cannot prove that time to treatment does or does not impact outcomes in appendicitis, the current study suggests that perforation may not be strictly a time-dependent phenomenon. Additional factors may need to be identified on which to focus efforts aimed at reducing the incidence of perforated appendicitis.
Acknowledgments
Funding/Support: Dr Drake was supported by a T32 training fellowship from the National Institute of Diabetes and Digestive and Kidney Diseases and Ms Mottey was supported by the University of Washington Medical Student Research Training Program. The Life Discovery Fund of Washington State and the Agency for Healthcare Research and Quality support SCOAP-CERTAIN (Surgical Care and Outcomes Assessment Program and Clinical Effectiveness Research Translation Network) research projects.
Role of the Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Drs Drake and Flum had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Drake, Florence, Flum.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Drake, Flum. Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Drake.
Obtained funding: Flum.
Administrative, technical, or material support: Mottey, Farrohki, Florence, Flum.
Study supervision: Florence, Mock, Steele, Thirlby, Flum.
Conflict of Interest Disclosures: None reported.
Additional Information: The authors represent the writing group for the Washington State Surgical Care and Outcomes Assessment Program (SCOAP), a program of the Foundation for Health Care Quality and the Clinical Effectiveness Research Translation Network (CERTAIN). More information at http://www.scoap.org and http://www.becertain.org.
REFERENCES
- 1.Körner H, Söndenaa K, Söreide JA, et al. Incidence of acute nonperforated and perforated appendicitis: age-specific and sex-specific analysis. World J Surg. 1997;21(3):313–317. doi: 10.1007/s002689900235. [DOI] [PubMed] [Google Scholar]
- 2.Scher KS, Coil JA., Jr. Appendicitis: factors that influence the frequency of perforation. South Med J. 1980;73(12):1561–1563. [PubMed] [Google Scholar]
- 3.Temple CL, Huchcroft SA, Temple WJ. The natural history of appendicitis in adults: a prospective study. Ann Surg. 1995;221(3):278–281. doi: 10.1097/00000658-199503000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cappendijk VC, Hazebroek FW. The impact of diagnostic delay on the course of acute appendicitis. Arch Dis Child. 2000(1):64–66. doi: 10.1136/adc.83.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pittman-Waller VA, Myers JG, Stewart RM, et al. Appendicitis: why so complicated? analysis of 5755 consecutive appendectomies. Am Surg. 2000;66(6):548–554. [PubMed] [Google Scholar]
- 6.Redmond JM, Smith GW, Wilasrusmee C, Kittur DS. A new perspective in appendicitis: calculation of half time (T(1/2)) for perforation. Am Surg. 2002;68(7):593–597. [PubMed] [Google Scholar]
- 7.Bickell NA, Aufses AH, Jr, Rojas M, Bodian C. How time affects the risk of rupture in appendicitis. J Am Coll Surg. 2006;202(3):401–406. doi: 10.1016/j.jamcollsurg.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 8.Ditillo MF, Dziura JD, Rabinovici R. Is it safe to delay appendectomy in adults with acute appendicitis? Ann Surg. 2006;244(5):656–660. doi: 10.1097/01.sla.0000231726.53487.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sicard N, Tousignant P, Pineault R, Dubé S. Non-patient factors related to rates of ruptured appendicitis. Br J Surg. 2007;94(2):214–221. doi: 10.1002/bjs.5428. [DOI] [PubMed] [Google Scholar]
- 10.Kearney D, Cahill RA, O’Brien E, Kirwan WO, Redmond HP. Influence of delays on perforation risk in adults with acute appendicitis. Dis Colon Rectum. 2008;51(12):1823–1827. doi: 10.1007/s10350-008-9373-6. [DOI] [PubMed] [Google Scholar]
- 11.Busch M, Gutzwiller FS, Aellig S, Kuettel R, Metzger U, Zingg U. In-hospital delay increases the risk of perforation in adults with appendicitis. World J Surg. 2011;35(7):1626–1633. doi: 10.1007/s00268-011-1101-z. [DOI] [PubMed] [Google Scholar]
- 12.Papandria D, Goldstein SD, Rhee D, et al. Risk of perforation increases with delay in recognition and surgery for acute appendicitis. J Surg Res. 2013;184(2):723–729. doi: 10.1016/j.jss.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Braveman P, Schaaf VM, Egerter S, Bennett T, Schecter W. Insurance-related differences in the risk of ruptured appendix. N Engl J Med. 1994;331(7):444–449. doi: 10.1056/NEJM199408183310706. [DOI] [PubMed] [Google Scholar]
- 14.Boomer L, Freeman J, Landrito E, Feliz A. Perforation in adults with acute appendicitis linked to insurance status, not ethnicity. J Surg Res. 2010;163(2):221–224. doi: 10.1016/j.jss.2010.04.041. [DOI] [PubMed] [Google Scholar]
- 15.Paquette IM, Zuckerman R, Finlayson SR. Perforated appendicitis among rural and urban patients: implications of access to care. Ann Surg. 2011;253(3):534–538. doi: 10.1097/SLA.0b013e3182096d68. [DOI] [PubMed] [Google Scholar]
- 16.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, STROBE Initiative The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 17.Fitz RH. Perforating Inflammation of the Vermiform Appendix; With Special Reference to its Early Diagnosis and Treatment. Wm. J. Dornan; Philadelphia, PA: 1886. [Google Scholar]
- 18.McBurney C. II: the indications for early laparotomy in appendicitis. Ann Surg. 1891;13(4):233–254. doi: 10.1097/00000658-189101000-00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yardeni D, Hirschl RB, Drongowski RA, Teitelbaum DH, Geiger JD, Coran AG. Delayed versus immediate surgery in acute appendicitis: do we need to operate during the night? J Pediatr Surg. 2004;39(3):464–469. doi: 10.1016/j.jpedsurg.2003.11.020. discussion 464-469. [DOI] [PubMed] [Google Scholar]
- 20.Taylor M, Emil S, Nguyen N, Ndiforchu F. Emergent vs urgent appendectomy in children: a study of outcomes. J Pediatr Surg. 2005;40(12):1912–1915. doi: 10.1016/j.jpedsurg.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 21.Abou-Nukta F, Bakhos C, Arroyo K, et al. Effects of delaying appendectomy for acute appendicitis for 12 to 24 hours. Arch Surg. 2006;141(5):504–506. doi: 10.1001/archsurg.141.5.504. 506-507. [DOI] [PubMed] [Google Scholar]
- 22.Hu J. Increased incidence of perforated appendixes in Hmong children in California. N Engl J Med. 2001;344(13):1023–1024. doi: 10.1056/NEJM200103293441316. [DOI] [PubMed] [Google Scholar]
- 23.Guagliardo MF, Teach SJ, Huang ZJ, Chamberlain JM, Joseph JG. Racial and ethnic disparities in pediatric appendicitis rupture rate. Acad Emerg Med. 2003;10(11):1218–1227. doi: 10.1111/j.1553-2712.2003.tb00606.x. [DOI] [PubMed] [Google Scholar]
- 24.Ponsky TA, Huang ZJ, Kittle K, et al. Hospital- and patient-level characteristics and the risk of appendiceal rupture and negative appendectomy in children. JAMA. 2004;292(16):1977–1982. doi: 10.1001/jama.292.16.1977. [DOI] [PubMed] [Google Scholar]
- 25.Smink DS, Fishman SJ, Kleinman K, Finkelstein JA. Effects of race, insurance status, and hospital volume on perforated appendicitis in children. Pediatrics. 2005;115(4):920–925. doi: 10.1542/peds.2004-1363. [DOI] [PubMed] [Google Scholar]
- 26.Bratton SL, Haberkern CM, Waldhausen JH. Acute appendicitis risks of complications: age and Medicaid insurance. Pediatrics. 2000;106(1, pt 1):75–78. doi: 10.1542/peds.106.1.75. [DOI] [PubMed] [Google Scholar]
- 27.Pieracci FM, Eachempati SR, Barie PS, Callahan MA. Insurance status, but not race, predicts perforation in adult patients with acute appendicitis. J Am Coll Surg. 2007;205(3):445–452. doi: 10.1016/j.jamcollsurg.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 28.Lee SL, Shekherdimian S, Chiu VY. Effect of race and socioeconomic status in the treatment of appendicitis in patients with equal health care access. Arch Surg. 2011;146(2):156–161. doi: 10.1001/archsurg.2010.328. [DOI] [PubMed] [Google Scholar]
- 29.Luckmann R, Davis P. The epidemiology of acute appendicitis in California: racial, gender, and seasonal variation. Epidemiology. 1991;2(5):323–330. doi: 10.1097/00001648-199109000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Luckmann R. Incidence and case fatality rates for acute appendicitis in California: a population-based study of the effects of age. Am J Epidemiol. 1989;129(5):905–918. doi: 10.1093/oxfordjournals.aje.a115224. [DOI] [PubMed] [Google Scholar]
- 31.Livingston EH, Woodward WA, Sarosi GA, Haley RW. Disconnect between incidence of nonperforated and perforated appendicitis: implications for pathophysiology and management. Ann Surg. 2007;245(6):886–892. doi: 10.1097/01.sla.0000256391.05233.aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koepsell TD. In search of the causes of appendicitis. Epidemiology. 1991;2(5):319–321. [PubMed] [Google Scholar]
- 33.Andersson RE. The natural history and traditional management of appendicitis revisited: spontaneous resolution and predominance of prehospital perforations imply that a correct diagnosis is more important than an early diagnosis. World J Surg. 2007;31(1):86–92. doi: 10.1007/s00268-006-0056-y. [DOI] [PubMed] [Google Scholar]
- 34.Bennion RS, Baron EJ, Thompson JE, Jr, et al. The bacteriology of gangrenous and perforated appendicitis—revisited. Ann Surg. 1990;211(2):165–171. doi: 10.1097/00000658-199002000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rivera-Chavez FA, Wheeler H, Lindberg G, Munford RS, O’Keefe GE. Regional and systemic cytokine responses to acute inflammation of the vermiform appendix. Ann Surg. 2003;237(3):408–416. doi: 10.1097/01.SLA.0000055274.56407.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rivera-Chavez FA, Peters-Hybki DL, Barber RC, et al. Innate immunity genes influence the severity of acute appendicitis. Ann Surg. 2004;240(2):269–277. doi: 10.1097/01.sla.0000133184.10676.26. [DOI] [PMC free article] [PubMed] [Google Scholar]