Abstract
Objective
The significance of distinct B cell abnormalities in Primary Sjögren’s Syndrome (pSS) remains to be established. Therefore, we analyzed the phenotype and mRNA transcript profiles of B cell subsets in patients with pSS compared with patients with sicca symptoms, and healthy controls (HCs).
Methods
CD19pos B cells from pSS (n=26), sicca (n=27), and HCs (n=22) were analyzed by flow cytometry. Gene expression profiles of purified B cell subsets (n=3-5, per group, per test) were analyzed using Affymetrix gene arrays.
Results
pSS patients had lower CD27pos/IgDneg switched memory (SM) and CD27pos/IgDpos unswitched memory (UM) B cells compared with HCs. UM B cell frequencies were also lower in sicca patients and their levels correlated with serologic hyperactivity in both disease states. Further, pSS UM had lower expression of CD1c and CD21. Gene expression analysis of CD27pos memory B cells separated pSS from HCs and identified a subgroup of sicca with a pSS-like transcript profile. Moreover, UM B cell gene expression analysis identified 187 differentially expressed genes between pSS and HCs.
Conclusion
A decrease in UM B cells is characteristic of established pSS as well as sicca with serologic hyperactivity thereby suggesting their value as biomarkers of future disease progression and in understanding disease pathogenesis. Overall, the mRNA transcript analysis of UM B cells suggests their activation in pSS through innate immune pathways in the context of attenuated antigen-mediated adaptive signaling. Thus, our findings provide important insight into the mechanisms and potential consequences of decreased UM B cell in pSS.
Primary Sjögren’s Syndrome (pSS) is a systemic autoimmune disease characterized by abnormal lymphocytic infiltration in the lacrimal and the salivary glands. The most prevalent and distinctive features of this disease are keratoconjunctivitis sicca (dry eyes) and xerostomia (dry mouth) - sicca symptoms. pSS patients can also suffer from extra-glandular manifestations that may either precede full-blown disease or present late in the course of the disease [1]. Nevertheless, sicca symptoms can be present in the general population frequently accompanied by immunological abnormalities but in the absence of obvious autoimmune disease. Thus, in absence of definitive diagnostic tests, early diagnosis of pSS is difficult to make [2]. Accordingly, classification criteria have been proposed to assess disease activity and provide a more homogeneous case classification for research studies [3]. However, these classification criteria often fail to capture patients early in the course of the disease – before underlying immunological mechanisms lead to destructive pathology.
A role for B cells in the pathogenesis of pSS is strongly indicated by multiple lines of evidence, including elevated levels of total serum immunoglobulin, high levels of several autoantibodies, and greatly increased levels of B cell survival and differentiation factors like BAFF (B cell Activating Factor) and IL-21 [4–6]. Additionally, pSS patients have major disturbances of peripheral-blood B cell homeostasis [7–9] and have lymphocytic infiltrates in the salivary glands that frequently include the presence of ectopic germinal-center reactions [10]. The pathogenic significance of B cells is also supported by promising results obtained by B cell-targeting therapies [11, 12].
The precise contribution of B cells to pSS pathology remains to be fully understood, as is the potential diagnostic value of the observed B cell abnormalities. Studies of B cell profiling in pSS typically concentrate on univariate analysis of B cell populations in patients with definitive diagnosis. However, given disease heterogeneity and the multiple, often opposing functions of B cell populations [13, 14], it is important to understand the global B cell profile of autoimmune diseases. In this work, we examined the B cell memory phenotypic and gene expression profile of patients with a wide spectrum of disease. We found the loss of unswitched (IgDpos/CD27pos) memory B cells was associated with clinical disease indicators in pSS and that this loss was present in a subset of sicca patients lacking a conclusive pSS diagnosis. Furthermore, gene expression studies demonstrate unswitched memory B cells from pSS patients had an altered profile characterized by lower expression of cell signaling genes necessary for adaptive immunity. These findings may provide a model for eventual advanced diagnostics and rational design of B cell targeted therapies.
Patients and Methods
Study Subjects
This study was approved by the University of Rochester Research Subject Review Board and all subjects provided informed consent. Blood was drawn from 26 pSS patients meeting AECG classification criteria [3] (pSS), 27 sicca patients and 22 healthy control donors. Sicca patients comprised the following subgroups: patients with sicca symptoms in the context of a clinical diagnosis of pSS established by the managing rheumatologist but without fulfilling AECG criteria (non-AECG pSS; n=4); and patients with sicca symptoms but neither clinical nor AECG-based diagnosis of pSS (sicca; n=23). pSS patients included 25 females and 1 male, sicca patients included 27 females, and healthy controls included 22 females. Patient characteristics are detailed in Table 1. For microarray analysis, whole blood was acquired from 5 pSS patients defined by AECG classification criteria, 5 sicca patients, and 3-5 healthy control donors per experiment.
Table 1.
Patient Characteristics
| Characteristic | AECG pSS (n=26) |
Sicca (n=27) |
|---|---|---|
| Age (mean±SD) | 58 ± 13 | 55 ± 14 |
| anti-Ro positive (%) | 69 | 26 |
| anti-La positive ( %) | 42 | 11 |
| RF positive (%) | 35 | 11 |
| ANA positive (%) | 62 | 56 |
| WBC count (mean±SD) | 6 ± 3 | 6 ± 2 |
| % lymphocyte (mean±SD) | 26 ± 7 | 30 ± 9 |
| IgG, mg/dl (mean±SD) | 1353 ± 647 | 725 ± 668 |
| IgM, mg/dl (mean±SD) | 100 ± 55 | 140 ± 104 |
| ESR mm/hr (mean±SD) | 28 ± 19 | 19 ± 13 |
| C3 (mean±SD) | 120 ± 29 | 121 ± 25 |
| C4 (mean±SD) | 24 ± 9 | 21 ± 6 |
| years with sicca symptoms (mean±SD) | 27.5 ± 11 | 7 ± 6.01 |
| NSAIDS (%) | 15 | 11 |
| Corticosteroids (%) | 8 | 7 |
| Hydroxychloroquine (%) | 31 | 15 |
| Methotrexate (%) | 4 | 0 |
RF = rheumatoid factor; ANA = antinuclear antibody
WBC = white blood cell; ESR = erythrocyte sedimentation rate
Sample Preparation
Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Paque (Amersham Biosciences) gradient centrifugation of whole blood at 20°C. PBMCs were cryogenically frozen until flow cytometric analysis. Serum was isolated from serum separator tubes (SST) by centrifugation at 20°C and frozen until luciferase immunoprecipitation systems (LIPS) analysis.
Flow Cytometry
After thawing, single-cell suspensions of PBMCs were labeled at 4°C with the following mAb conjugates: anti-CD1c biotin (Miltenyi), anti-CD3 Pacific Orange (Invitrogen, Carlsbad, CA), anti-CD19 APC-Cy7 (BD Pharmingen, San Diego, CA), anti-CD21 PE-Cy5 (BD Pharmingen), anti-CD23 PE-Cy7 (eBioscience), anti-CD24 PE (BD Pharmingen), anti-CD27 Qdot605 (Invitrogen), anti-IgD FITC (BD Pharmingen), Streptavidin PE-Alexa610 (Invitrogen). Dead cells were excluded with Live/Dead fixable Aqua dead cell staining kit (Invitrogen). 5x105 – 106 events were collected for each sample on an LSRII flow cytometer (BD). FlowJo (Tree Star) was used for gating analysis.
Luciferase Immunoprecipitation Systems (LIPS) Assay
LIPS assay was performed as previously described [15]. 1-µL of serum or 5-µL of saliva was used for each test. Light units were calculated from the mean of at least two independent experiments.
Cell Sorting
Single-cell suspensions of freshly isolated PBMCs were labeled at 4°C with the following mAbs: anti-CD3 pacific orange (Invitrogen), anti-CD14 pacific orange (Invitrogen), anti-CD19 APC-Cy7 (BD Pharmingen), anti-CD27 PE (BD Pharmingen), and anti-IgD FITC. Live CD19pos B cells were subdivided into CD27pos memory and CD27pos/IgDneg naïve or separately, CD27pos/IgDneg switched memory and CD27pos/IgDpos unswitched memory, and sort-purified on a FACSAria cell sorter (BD) into FACS buffer. Samples were stored at −80°C in cell lysis buffer until RNA extraction for microarray analysis.
RNA Extraction
RNA was extracted with QIAGEN RNeasy Micro kits (QIAGEN) following the manufacturer’s protocol, which included on-column DNA digestion. Total RNA yield was determined with a Nanodrop spectrophotometer, and RNA integrity confirmed with an Agilent 2100 Bioanalyzer (Agilent Technologies).
cDNA Microarray
Global gene expression profiles were generated with Gene 1.0 ST arrays (Affymetrix). Biotinylated cDNA was generated from cDNA with the NuGEN Encore Biotin kit (NuGEN). The microarray probe intensity files were analyzed with the Affymetrix PLIER algorithm to produce normalized signals for the probe sets.
Statistics and Analysis
Statistical Analysis including, T-test, Mann-Whitney U test and ANOVA, were performed using Prism (GraphPad Software, Inc., La Jolla CA). Hierarchical clustering was accomplished using Matlab (The Mathworks, Inc., Natick MA). Ingenuity Pathways Analysis (Ingenuity Systems, Redwood City CA) was used for network and functional enrichment analysis.
Results
Patients with AECG+ pSS and a subset of subjects with sicca symptoms share B cell abnormalities
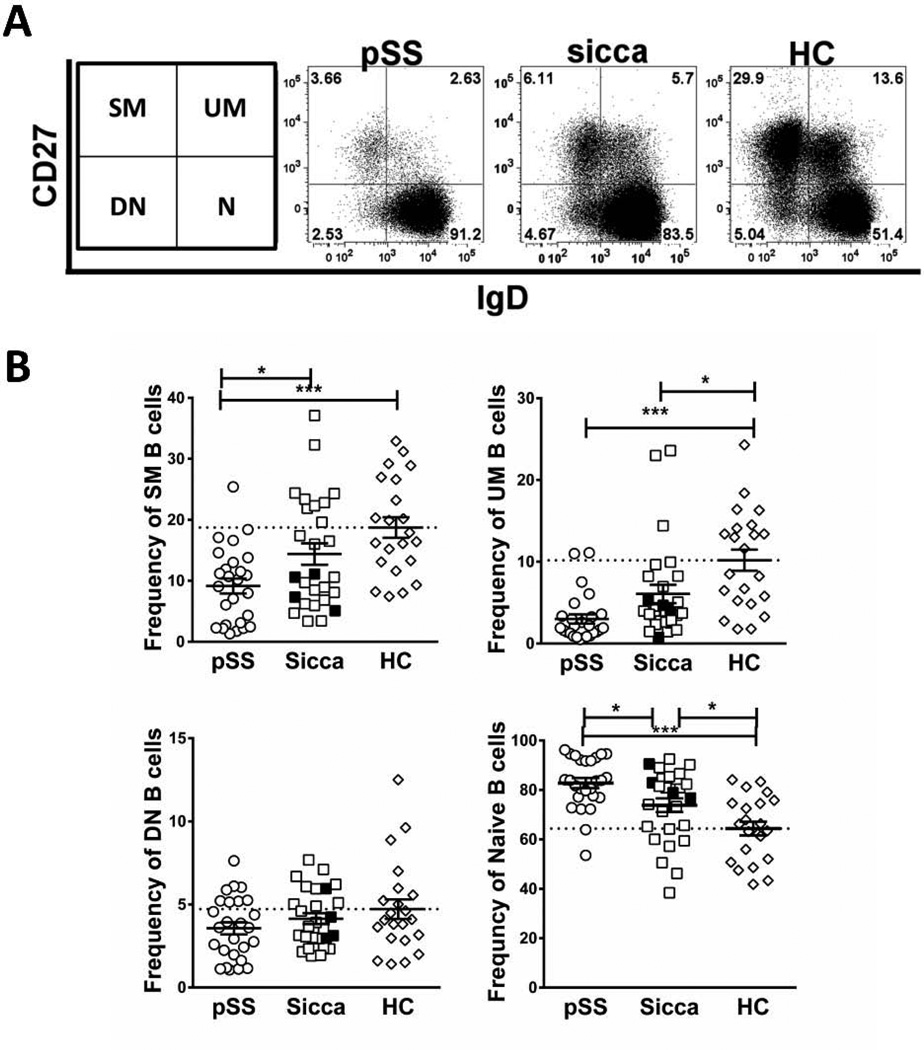
Because pronounced B cell abnormalities are commonly observed in well-established pSS [16–18], we hypothesized that undiagnosed patients with sicca symptoms (sicca patients) would share a similar B cell phenotype. Viable CD19pos/CD3neg B cells were analyzed by flow cytometry for surface expression of CD27 and IgD to define the major B cell subsets identified by these markers [19] (Figure 1A). As previously reported [9, 16, 20], pSS patients had significantly lower frequencies of switched memory (SM) and unswitched memory (UM) B cells together with increased frequencies of naïve B cells compared with HCs (Figure 1B) (all p < 0.0005,). As a group, sicca patients also had significantly lower frequencies of UM B cells compared with HCs (p < 0.05; Figure 1B). Interestingly, all 4 patients with non-AECG SS fell within the lower UM range. By contrast, sicca patients had SM frequencies that were significantly higher than pSS but not statistically different than HC. The differences between pSS and HCs were also observed when cell number per µl of peripheral blood was calculated for SM and UM subsets (p < 0.005 and p < 0.0005, respectively) (not shown). Therefore, lower peripheral B cell memory compartments (particularly UM) are shared phenotypic disturbances in pSS patients and sicca patients. There was a higher frequency of CD27neg/IgDpos naïve B cells in pSS and sicca compared to HC (p < 0.0005 and p < 0.05, respectively) (Figure 1B); however, the absolute numbers (not shown) of these cells were not significantly different between pSS and HCs. This suggests that reduced memory frequency in pSS results from a reduction in the number of circulating memory B cells whereas increased naïve cell frequencies, and not absolute numbers, reflects decreased memory cell numbers. Finally, there were no differences in isotype-switched CD27neg/IgDneg B cells, double negative (DN) B cells [21], in either pSS patients or sicca patients compared to HCs (Figure 1B).
Figure 1. pSS patients and patients with sicca symptoms have similar B-cell phenotypic abnormalities.
CD19+ B-cells from peripheral blood mononuclear cells (PBMCs) of AECG pSS patients, grouped non-AECG SS (filled squares) and sicca patients (open squares), and HCs were analyzed for CD27 and IgD expression by flow cytometry. (A) Representative flow cytometry plots showing CD27 and IgD expression of viable, single CD19+ gated lymphocytes from the indicated subjects. Values are the percentages of CD19+ B cells for each gated subset (indicated at left), switched memory (SM), unswitched memory (UM), double negative memory (DN), and naïve (N). (B) Quantitative comparison of frequencies of canonical B cell subset, [defined in (A)] among the indicated subject groups. Values are the percentages of CD19pos B cells for each gated subset. Statistical significance calculated by Tukey’s post-hoc test after a one-way analysis of variance (ANOVA), *p < 0.05, ***p < 0.0005. Filled symbols are non-AECG SS and open squares within the same group are sicca symptoms.
Integrated B cell profiling allows clustering of pSS and sicca patients
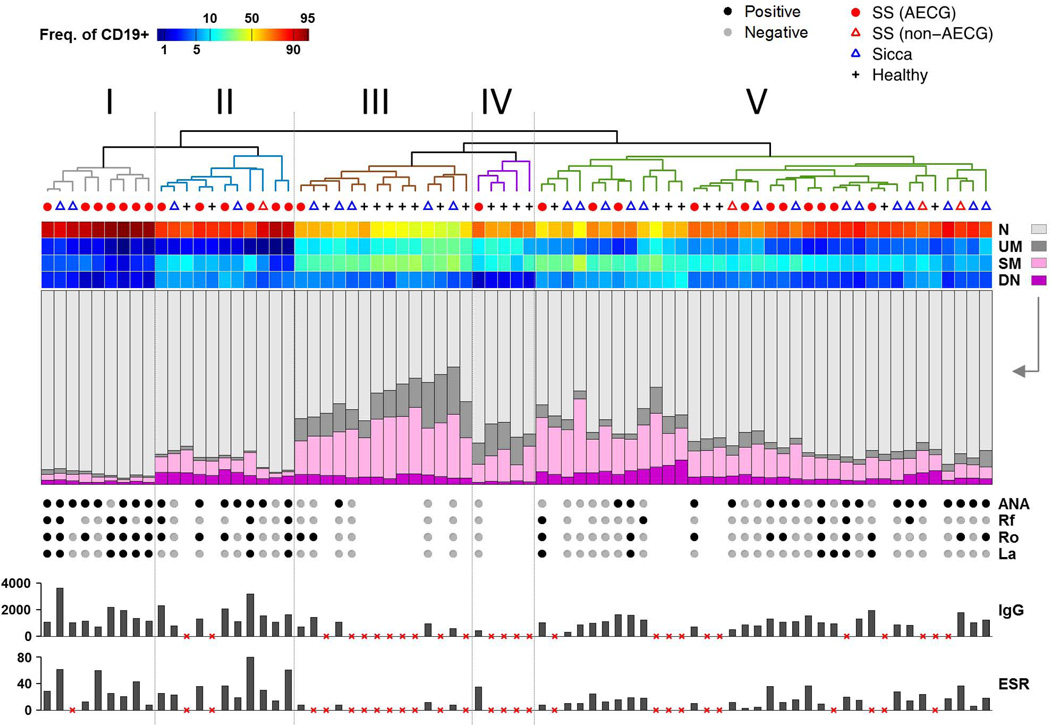
Figure 1 shows significant inter-individual variability for all B cell subsets. Therefore, we clustered subjects based on B cell frequencies, using simplicial distance [22] and average linkage, and displayed as a heatmap with clinical and immunological variables aligned to the heatmap (Figure 2, middle bar graphs and bottom dots and bars, respectively). We identified up to 5 distinct B cell-defined clusters, with AECG-defined pSS patients (Figure 2) concentrated in clusters I, II and V whereas HC were preferentially grouped in clusters III and IV. By contrast, sicca patients segregated either with pSS patients (Clusters I, II & V) or with HCs in Cluster III. Cluster I, which only comprised diseased subjects with a predominance of pSS, was characterized by profound contraction of memory B cells (both SM and UM) and levels of DN cells on the low-normal range together with a relative expansion of naïve B cells. Cluster V was also enriched for diseased subjects with an almost equal mix of pSS and sicca patients, including 3 out of 4 AECG-negative SS patients. This cluster shared with Cluster I a significant decrease in UM and SM B cells albeit at a lower degree for the SM subset. Cluster II contained a mix of pSS and sicca patients with low UM but a range of SM frequencies. Cluster III contained most of the HCs with a characteristic normative range of all major B cell subsets as well as a number of sicca patients with a B cell profile comparable to HC. Finally, cluster IV included HC with low SM frequencies essentially representing the low normal quartile, however UM was not reduced. Of interest, the one pSS patient included in this cluster lacked all the serological abnormalities typical of this disease. Collectively, Figure 2 shows that clustering allows the categorization of sicca patients based on the relatedness of their B cell phenotypic profile to either pSS patients or to HCs.
Figure 2. Cluster Analysis and heatmap display of multicolor flow cytometry data.
Data from 75 patient samples (columns) and the four IgD/CD27 B cell subsets (rows). Sample group membership is indicated by the row of symbols above the heat map and below the dendrogram. Sample clustering was performed on cell subset frequency data using simplicial distance and average linkage. Five clusters were delineated. In the heat map, color corresponds to logit-transformed frequency (log(f / (100 – f)) to aid in visualization of rare and abundant subsets. Immediately beneath the heat map, the compositions of the four subsets are shown using stacked bar graphs. Additionally, the presence of autoantibodies, as well as IgG and ESR levels are shown where available (missing autoantibody data is indicated by the lack of a gray or black dot; lack of IgG or ESR data indicated by red “x”).
Unswitched memory (UM) B cell frequency associates with clinical and immunological parameters in sicca and Sjögren’s patients
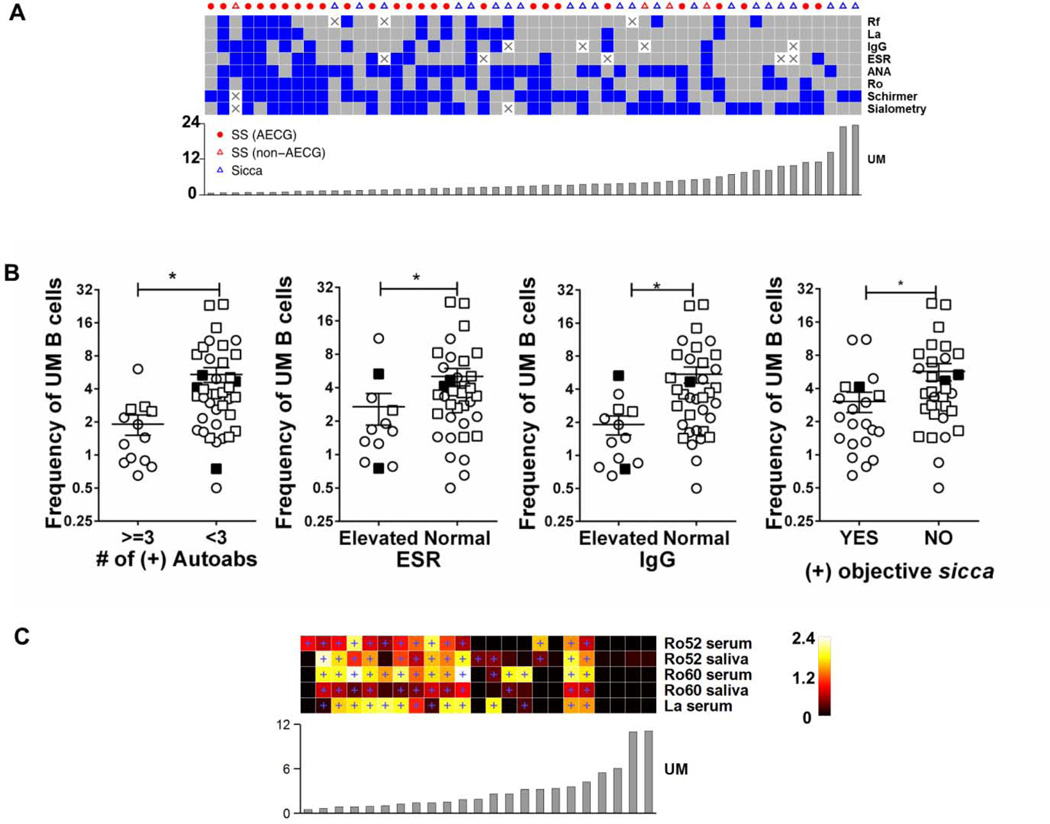
Patients in Cluster I (Figure 2, left) displayed a high frequency of serological abnormalities, (autoantibodies, IgG and ESR) compared with patients in the other clusters. Moreover, the main abnormality shared between pSS and sicca patients was low frequency of UM B cells. Accordingly, we analyzed the relationship between UM B cell frequency and several clinical and serological parameters of disease (Figure 3A). When patients were clustered based on these parameters there were more clinical and serological abnormalities in subjects with lower frequencies of UM B cells irrespective of their diagnostic categorization (Figure 3A). UM frequency is closely associated with these parameters as the UM frequency follows clinical and immunological clustering in an almost rank order (Figure 3A, bottom). Interestingly, some patients with objective signs of sicca, measured by sialometry and Schrimer’s test, had low UM frequency in the absence of anti-Ro and anti-La antibodies suggesting that low UM frequency may be an early immunological sign in pSS, preceding development of disease-associated autoantibodies.
Figure 3. UM B cell frequency correlates with clinical hyperactivity in pSS and sicca patients.
(A) Data from 53 patients (columns) for eight binary variables (rows). Blue is positive and gray is negative. Columns and rows were clustered based on Hamming distance and average linkage (dendrograms not shown). UM frequency (of CD19+) is shown below the heat map. White squares with “X” indicate missing data. (B) Plots show UM frequencies of AECG pSS (open circles) non-AECG SS (filled squares) and sicca patients (open squares) that were separated into two groups based on the indicated clinical parameters. Statistical significance was calculated using Mann-Whitney test. (C) Serum and saliva from AECG pSS patients were analyzed for autoantibodies using Luciferase Immunoprecipitation Systems Assay (LIPS). Autoantibody titers of 23 of the AECG criteria pSS were displayed as a heatmap, where columns are patients and rows are autoantibodies measured in the indicated fluids. Blue plus signs in the heat map indicate above-threshold values. Color scale is in millions light units. UM frequency (of CD19+) in ascending order, left - right (bar graph), is shown below the heat map.
To further measure the significance of these observations, we analyzed the univariate relationship between UM frequencies and corresponding clinical parameters. UM frequencies were lower in patients (pSS and sicca) that had elevated serum IgG and ESR (p = 0.002 and p = 0.03, respectively) (Figure 3B). Additionally, UM frequencies were lower in the patients that were positive for objective sicca measurements, unstimulated whole saliva flow rate and Schirmer’s test (p = 0.02) (Figure 3B). Patients were also separated based on their autoantibody load (anti-Ro, anti-La, ANA, and RF). Patients with 3 or greater positive autoreactivities had significantly lower UM frequencies (p =0.001) (Figure 3B).
The Luciferase Immunoprecipitation System (LIPS) assay provides quantitative measurement of autoantibodies with a very wide dynamic range [15]. We used this assay to assess the association of autoantibody titers and the UM frequency on serum and saliva of pSS patients. Even using the sensitive LIP assays, AECG+ pSS patients with the highest UM frequencies had negative titers for anti-Ro and anti-La (Figure 3C, right). Overall, the pSS patients with lower UM frequencies had a higher autoantibody score with more autoreactivities and higher individual autoantibody titers (Figure 3C, left). Thus, subjects with lower UM frequencies had the highest titers of autoantibodies of anti-Ro-60 and Ro-52 in serum (p < 0.008, p < 0.02, respectively, Fishers Test) and saliva (p < 0.008, p < 0.02, respectively, Fishers Test). The same relationship was observed for anti-La in the serum (p < 0.005).
Phenotype of unswitched memory (UM) B cells in sicca and Sjögren’s patients
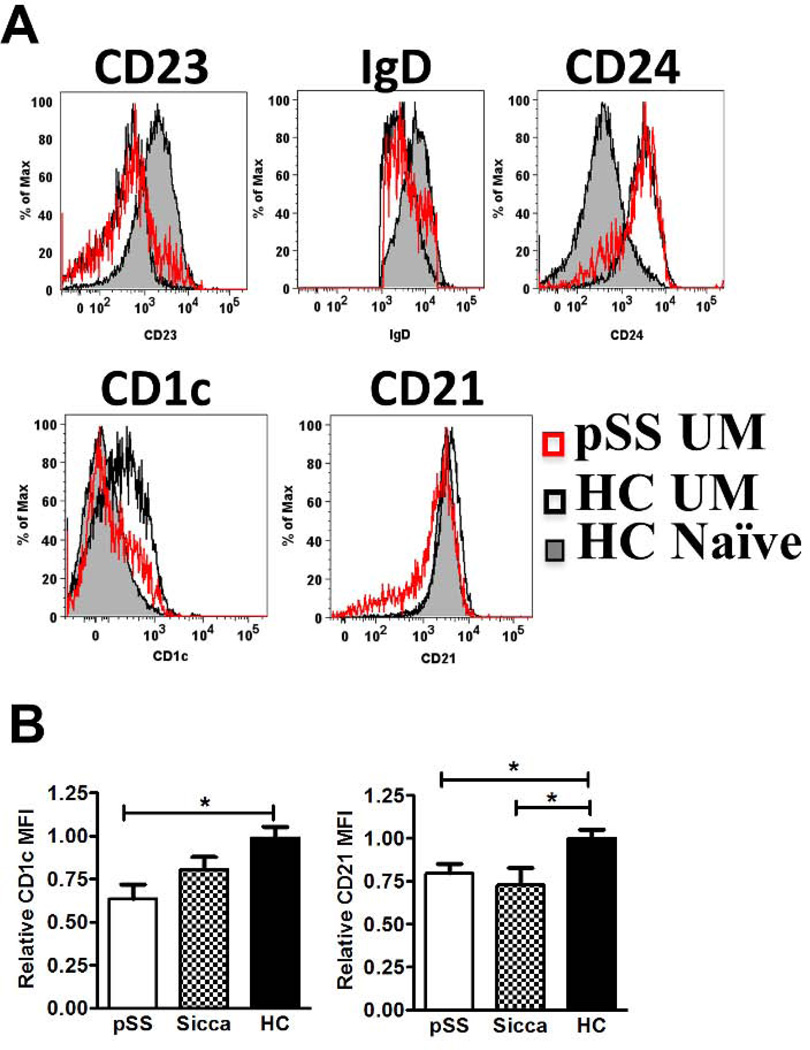
The phenotype of unswitched memory B cells was analyzed by flow cytometry to better understand UM B cell abnormalities in pSS and sicca patients. Because IgDpos/CD27pos UM in the peripheral blood have been proposed to represent a recirculating equivalent of spleen marginal zone B cells (MZB) [23], the expression of MZB-associated markers was examined (Figure 4 A). HC naïve B cells were used as a reliable phenotypic control because of established differences with the UM compartment [23]. HC naïve B cells were CD23pos and IgDhi compared with UM B cells, which were CD23neg and IgDlo in both HC and pSS UM B cells (Figure 4A). HC UM B cells expressed relatively higher levels of CD21 and the MZB cell marker CD1c compared to naïve B cells (Figure 4A). Interestingly, UM B cells from pSS patients expressed lower levels of CD1c and CD21 when compared to UM B cells of HCs (Figure 4 A, B) two markers with high expression in human spleen marginal zone B cells [24] [25] and which are also expressed in the recirculating MZ-like cells of healthy control [24]. A pattern similar to the UM of pSS was shared by UM B cells of sicca patients. Thus, while HC UM B cells in our cohort displayed a relatively homogenous MZB–like phenotype [24], pSS patients’ UM B cells differed from this phenotype by the lower expression of both CD1c and CD21 (Figure 4B).
Figure 4. Unswitched memory (UM)-phenotype B cells in AECG pSS patients, sicca patients and HCs.
UM B cells (as in Figure 1A) were analyzed by flow cytometry for expression of the indicated markers. (A) Representative flow cytometry histograms for expression of CD23, IgD, CD24, CD1c, and CD21 of HC naïve B cells (shaded grey), HC UM B cells (black line), and pSS UM B cells (red line). (B) Quantitative comparison of CD1c MFI and CD21 MFI among UM B cells in the indicated subject groups, pSS (n=14), sicca (n=8) and HC (n=14). Statistical significance calculated by Tukey’s post-hoc test after a one-way analysis of variance (ANOVA) *p > 0.05.
Unswitched Memory B cells express a distinctive transcriptome in patients with pSS
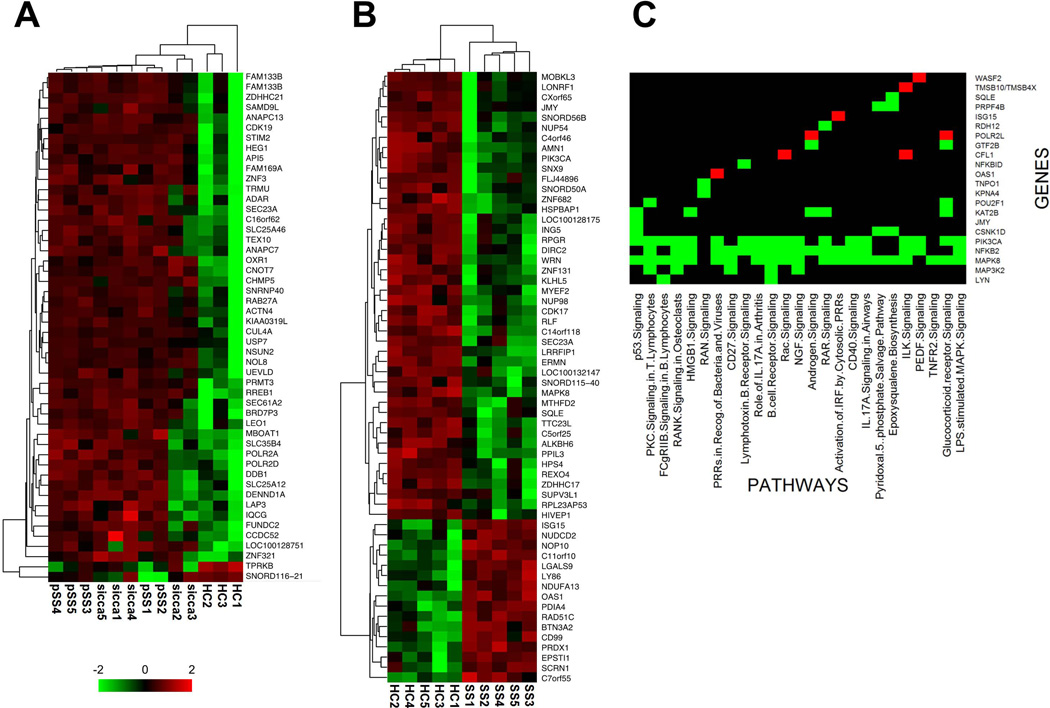
We have shown that memory B cell phenotypic aberrations are characteristic of pSS and at least a subset of sicca subjects; therefore, we examined the transcriptome of the different memory B cell populations. First, we analyzed gene expression of total CD27pos memory and naive B cell subsets of a cohort of pSS (N=5) and sicca patients (N=5) and HCs (N=3). Differentially expressed genes (DEG) (p<0.005, >1.5 fold) were identified between pSS and HCs. This analysis identified 135 DEGs between pSS and HC total CD27pos memory B cells (Supplementary file 1). In pSS patients, 69 genes had higher expression whereas 66 genes had lower expression. The DEGs were rank ordered and the top 50, using expression levels from pSS, sicca and HC, were displayed in a heatmap. Unsupervised hierarchal clustering of these genes showed a separation of pSS patients from HCs, as expected, but also revealed a subset of sicca patients (3 of 5; sicca 1, 4 and 5) with global CD27pos memory B cell gene expression profile that segregates with pSS patients whereas the other 2 (sicca 2 and 3) had an intermediate profile (Figure 5A). The sicca patients analyzed by gene expression were all without a clinical diagnosis of Sjögren’s syndrome. The sicca subject with a UM frequency in the normal range (SS2) clustered with HC. All other sicca subjects had UM frequencies in the lower range of normal.
Figure 5. Gene expression and Network Analysis data for total CD27pos memory B cells and UM B cells.
Data were log-transformed and then for each gene, value is expressed as the z-score (number of standard deviations away from the mean). Columns are patients and rows are genes. Clustering was based on Euclidean distance and average linkage. This clustering follows feature selection, so it is used to illustrate patterns of expression in significant genes. (A) Top 50 most differentially expressed genes among total CD27pos memory B cells from AECG pSS patients, sicca patients and HCs. (B) Top 60 most differentially expressed genes among UM B cells from AECG pSS patients and HCs. (C) Ingenuity Pathways Analysis (IPA) was used for pathway and network analysis of significantly differentially expressed genes (DEG) in Sjögren’s Unswitched Memory (UM) B cells. Heatmap summary of the top 25 significant pathways associated with UM DEGs. The names of the pathways are indicated at the bottom, left to right with decreasing significance, and gene names indicated to the right of the figure. Red indicates fold increase in expression level and green indicates fold decrease in expression level comparing pSS to HCs. Black indicates that the listed gene does not participate in the indicated pathway.
Whereas the analysis of unfractionated CD27pos memory B cells provided informative clustering of clinical groups, such population is heterogeneous as it is comprised of both UM and SM B cells whose relative abundance may significantly differ between subjects. This difference in proportionality of UM and SM B cells in pSS, sicca and HC (Figure 1) likely results in the differences observed in Figure 5A. Consequently, these gene differences reflect disproportionate cellular make-up of the heterogeneous CD27pos memory population among pSS and HCs. Therefore, we performed gene expression analysis on sort-purified pSS and HC SM and UM B cell subsets. Interestingly, there was no difference in gene expression between SM B cell gene expression in pSS patients and HCs. In contrast, UM B cell gene expression was markedly different between the two groups with 187 unique differentially expressed DEGs (Supplementary file 2). 135 genes were down-regulated in pSS compared to HC, whereas 52 genes were up-regulated. The 60 genes (Figure 5B) with greater differential expression in either direction were displayed in a heatmap after hierarchical clustering and provided a separation of pSS patients and HC with very consistent profiles within each patient group. Overall, most differentially transcribed genes were down regulated in pSS compared to HC (44 genes; Figure 5B, top) whereas 16 genes were upregulated in pSS UM cells (Figure 5B, bottom). It is important to note that DEGs were uniformed across pSS patients irrespective of different standard of care treatments. Moreover subjects did not cluster based on treatment, nor did the treatment naive patient cluster as an outlier.
Pathway analysis on the 187 DEGs in UM B cells revealed that the most relevant intracellular signaling pathways in which the DEGs are known to be involved include B cell (BCR) signaling, FcγRIIB signaling, CD27 signaling, lymphotoxin β receptor signaling, and CD40 signaling (Figure 5C). Many of these pathways share one or more genes that were down-regulated in pSS UM B cells, including PIK3CA, LYN, MAP3K2 (MEKK2), MAPK8 (JNK1) and NFκB2 (Figure 5C). Interestingly, all five of these genes participate in the B cell receptor-signaling pathway. Moreover, ours is the first description of interferon signaling genes, in UM B cells in pSS, including ISG15, OAS1, NUP54, IFITM2, KPNA4, and TRIM25. Moreover, genes involved in the regulation of actin cytoskeleton, WRN, CFL1, TMSB4X- which plays a role in modulating BCR responses [26, 27], were also associated with this analysis. Overall these results show that UM B cells in pSS patients are dissimilar from HCs not only proportionally but also at the level of gene expression on a per-subset basis that may result in functional abnormalities.
Discussion
The significance of B cells in Sjögren’s syndrome is indicated by abnormalities in peripheral blood B cell homeostasis [7, 9, 16, 20]. Yet, the value of these abnormalities remains to be formally explored. Our results demonstrate that clinical and immunological parameters in pSS associate with phenotypic and gene-expression patterns of peripheral blood memory cells. In particular, a decrease in the relative and total numbers of UM B cells below the mean values of HC is an almost universal feature of patients with pSS. Decreased UM B cells were also observed in a subgroup of patients with sicca. Moreover, the relative frequency of UM B cells demonstrates a strong inverse association with clinical abnormalities including the number and titer of disease-associated autoantibodies. Combined, these results demonstrate that alterations in the UM B cells are characteristic of Sjögren’s Syndrome and occur in a subgroup of patients with sicca symptoms in the absence of a diagnosis of Sjögren’s. These observations based on our training set remain to be confirmed using a longitudinal validation cohort.
When defined on the basis of IgD and CD27 co-expression, UM B cells have been variously proposed to represent natural memory cells, innate memory cells and a recirculating marginal zone memory cell equivalent [23]. From a functional standpoint, UM B cells contain at least a significant portion of populations described by other groups as IL-10 producing regulatory B10 cells and B1 cells [28–31]. Of note, IL-10 mediated regulatory functions have also been ascribed to mouse MZ B cells [29]. It is important to note that other work has shown that at least a significant fraction of pSS patients have an increased number of B10 cells [29], and is therefore unlikely that a deficiency in these particular regulatory cells would account for our observations. On the other hand, the decreased expression of CD1c and CD21 observed in pSS UM B cells suggest that the observed phenotype could be explained at least in part by a loss of the MZ-like component of the UM population. Interestingly, MZ-type B cells have been shown to be a part of the lymphocytic infiltrates of the salivary glands of SS patients [32].
A similar decrease of UM B cells has been observed in other but not all autoimmune diseases studied by different groups. Thus, decreased UM B cells appears to be a feature of both SLE [33] and Crohn’s disease [34] and in both cases it might reflect a functional decline in splenic function. Interestingly, in Crohn’s disease this defect and the decline in UM B cells may be reversed by pharmacological TNF inhibition [35]. Whether similar mechanisms, namely defective spleen function and/or excess TNF may also be at play in SS remains to be further examined. Whatever the specific mechanism for their decline may be, pathological consequences downstream of a defective MZ compartment could be explained in multiple ways as this compartment can play a protective role not only through regulatory B cells but also by compartmentalization of autoreactive B cells away from the follicular compartment thereby minimizing their participation in GC reactions and the generation of pathogenic autoreactive long-lived memory and plasma cells. Moreover, CD1c can be downregulated, together with CD1d, in human MZ B cells by CD40L stimulation [36] and CD1c downregulation can inhibit CD1d-mediated activation of NKT cells [37] a phenomenon that has been associated with human SLE [38].
Our important observation, that UM B cell gene-expression profiling distinguished pSS patients from healthy controls, suggests that the function of UM B cells is altered. The BCR signaling pathway in particular contained five genes, PIK3CA, LYN, MAP3K2 (MEKK2), MAPK8 (JNK1) and NFκB2, that had decreased expression in pSS compared to HCs. Moreover, genes involved in the regulation of the actin cytoskeleton, WRN, CFL1, TMSB4X, were also dysregulated in pSS. Cytoskeletal reorganization plays a role in modulating BCR responses by providing positive and negative feed-back loops during B cell activation [26, 27]. Additionally, dysregulation of the actin-cytoskeleton can lead to alterations in B cell development [39]. These observations suggest that there may be defective BCR signaling in pSS UM B cells which could have an effect on the function and homeostasis of these cells. Moreover, multiple other pathways significantly associated with this analysis shared decreased expression of one or more genes in pSS UM B cells, including CD40 signaling pathway. Interestingly, patients with liver cirrhosis have reduced frequencies of CD27pos/IgMpos B cells (UM), and the remaining UM B cells in these patient are hypo-responsive to CD40 compared with UM from healthy controls [40]. Similar mechanisms may be occurring in pSS, as suggested by the lower expression of genes associated with CD40 signaling – PIK3CA, NFKB2, MAPK8.
Whereas previous studies of pSS PBMCs identified genes associated with interferon signaling [41–43], ours is the first description of over-expression of type I IFN signaling genes in pSS UM B cells - ISG15, OAS1, IFITM2, and TRIM25. Additionally, there was upregulation of TRIM25 a ubiquitin E3 ligase and ISG15 E3 ligase involved in innate immune defense by different mechanisms including the triggering the cytosolic signal transduction that leads to the production of interferons in response to viral infection [44, 45]. The over-expression of type I IFN-regulated genes indicated by ISG15 was part of a larger pattern of induction of genes regulated by this cytokine family including OAS1 and IFITM. Finally, the potential impact of innate immune activation on pSS UM B cells is also consistent with the significant upregulation found for MD1 (Ly86; Figure 5B). MD-1 is a CD180 (RP105) co-factor capable of inhibiting the activity of the TLR4-MD2 complex induced in response to LPS stimulation [46]. RP105/MD-1 is highly expressed in mouse MZ B cells and most human B cells. In the mouse, TLR4 plus anti-RP105 co-stimulation induces dramatic proliferative responses and IgM secretion by MZ B cells and is indispensable for such type of responses [47]. Also of significant interest, RP105 is known to promote disease progression in MRL/lpr mice through tonic B cell activation [48]. Irrespective of the precise mechanism, our observations suggest that the unswitched memory (UM) B cell subset is involved in disease pathophysiology whether as a primary defect or as a consequence of disease activity.
Some underlying immune mechanisms are on-going, cumulative, and persistent in autoimmune patients before diagnosis [49, 50]. Interestingly, the phenotypic abnormalities in sicca patients precede disease-associated autoantibodies. By evaluating the B cell subset profiles in sicca patients, we showed B cell phenotypic and CD27pos B cell gene expression abnormalities, characteristic of pSS patients, in a subgroup of these patients without diagnostic classification criteria. Essentially, our results very strongly suggest that sicca patients with a pSS B cell signature, predominated by alterations in the UM B cell compartment, represent an intermediate state preceding clinical manifestation of pSS. Ultimately, the validation of our findings and the assessment of their predictive value for disease diagnosis and progression would greatly contribute to our ability to design rational B cell targeted intervention for early treatment of pSS.
Supplementary Material
Acknowledgements
We thank Sunil Kemshetti, Tracey Sanford, and Charlene Chung for patient recruitment, Dr. Timothy Bushnell and the URMC Flow Cytometry Core for flow cytometry assistance and Dr. Steve Welle and the URMC Functional Genomics Core for microarray assistance.
Supported by NIH-NIDCR R01 DE017585-0; NIH-NIAID ACE U19 AI56390 and NIH-NIAID P01 AI078907 to IS and the Intramural Research Program of the NIDCR
Abbreviations
- AECG
American European Consensus Group
- BAFF
B cell activating factor
- DN
Double Negative
- ESR
erythrocyte sedimentation rate
- HC
healthy control
- IgD
immunoglobulin D
- IgG
immunoglobulin G
- LIPS
luciferase immunoprecipitation system
- MZ
marginal zone
- pSS
primary Sjögren’s Syndrome
- SM
switched memory
- UM
unswitched memory
Footnotes
The authors have received no financial support or other benefits from commercial sources in relation to this work and have no potential conflict of interests.
References
- 1.Kassan SS, Moutsopoulos HM. Clinical manifestations and early diagnosis of Sjogren syndrome. Arch Intern Med. 2004;164(12):1275–1284. doi: 10.1001/archinte.164.12.1275. [DOI] [PubMed] [Google Scholar]
- 2.Galvez J, Saiz E, Lopez P, Pina MF, Carrillo A, Nieto A, Perez A, Marras C, Linares LF, Tornero C, et al. Diagnostic evaluation and classification criteria in Sjogren's Syndrome. Joint Bone Spine. 2009;76(1):44–49. doi: 10.1016/j.jbspin.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 3.Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, Daniels TE, Fox PC, Fox RI, Kassan SS, et al. Classification criteria for Sjögren's syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Annals of the Rheumatic Diseases. 2002;61(6):554–558. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosen A, Casciola-Rosen L. Altered autoantigen structure in Sjogren's syndrome: implications for the pathogenesis of autoimmune tissue damage. Crit Rev Oral Biol Med. 2004;15(3):156–164. doi: 10.1177/154411130401500304. [DOI] [PubMed] [Google Scholar]
- 5.Youinou P, Saraux A, Pers JO. B-Lymphocytes govern the Pathogenesis of Sjogren's Syndrome. Curr Pharm Biotechnol. 2012 doi: 10.2174/138920112802273100. [DOI] [PubMed] [Google Scholar]
- 6.Scofield RH. IL-21 and Sjogren's syndrome. Arthritis Research & Therapy. 2011;13(6):137. doi: 10.1186/ar3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hansen A, Odendahl M, Reiter K, Jacobi AM, Feist E, Scholze J, Burmester GR, Lipsky PE, Dorner T. Diminished peripheral blood memory B cells and accumulation of memory B cells in the salivary glands of patients with Sjogren's syndrome. Arthritis Rheum. 2002;46(8):2160–2171. doi: 10.1002/art.10445. [DOI] [PubMed] [Google Scholar]
- 8.Binard A, Le Pottier L, Devauchelle-Pensec V, Saraux A, Youinou P, Pers JO. Is the blood B-cell subset profile diagnostic for Sjogren syndrome? Ann Rheum Dis. 2009;68(9):1447–1452. doi: 10.1136/ard.2008.096172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bohnhorst JO, Thoen JE, Natvig JB, Thompson KM. Significantly depressed percentage of CD27+ (memory) B cells among peripheral blood B cells in patients with primary Sjogren's syndrome. Scand J Immunol. 2001;54(4):421–427. doi: 10.1046/j.1365-3083.2001.00989.x. [DOI] [PubMed] [Google Scholar]
- 10.Stott DI, Hiepe F, Hummel M, Steinhauser G, Berek C. Antigen-driven clonal proliferation of B cells within the target tissue of an autoimmune disease. The salivary glands of patients with Sjögren's syndrome. The Journal of Clinical Investigation. 1998;102(5):938–946. doi: 10.1172/JCI3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coca A, Sanz I. B cell depletion in lupus and Sjogren's syndrome: an update. Current Opinion in Rheumatology. 2009;21(5):483–488. doi: 10.1097/BOR.0b013e32832efe55. 410.1097/BOR.1090b1013e32832efe32855. [DOI] [PubMed] [Google Scholar]
- 12.Coca A, Sanz I. Updates on B-cell immunotherapies for systemic lupus erythematosus and Sjogren's syndrome. Current Opinion in Rheumatology. 2012;24(5):451–456. doi: 10.1097/BOR.0b013e32835707e4. 410.1097/BOR.1090b1013e32835707e32835704. [DOI] [PubMed] [Google Scholar]
- 13.Manjarrez-Orduno N, Quach TD, Sanz I. B Cells and Immunological Tolerance. J Invest Dermatol. 2009;129(2):278–288. doi: 10.1038/jid.2008.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanz I, Lee FE. B cells as therapeutic targets in SLE. Nat Rev Rheumatol. 2010;6(6):326–337. doi: 10.1038/nrrheum.2010.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ching KH, Burbelo PD, Gonzalez-Begne M, Roberts ME, Coca A, Sanz I, Iadarola MJ. Salivary anti-Ro60 and anti-Ro52 antibody profiles to diagnose Sjogren's Syndrome. J Dent Res. 2011;90(4):445–449. doi: 10.1177/0022034510390811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bohnhorst JO, Bjorgan MB, Thoen JE, Natvig JB, Thompson KM. Bm1-Bm5 classification of peripheral blood B cells reveals circulating germinal center founder cells in healthy individuals and disturbance in the B cell subpopulations in patients with primary Sjogren's syndrome. J Immunol. 2001;167(7):3610–3618. doi: 10.4049/jimmunol.167.7.3610. [DOI] [PubMed] [Google Scholar]
- 17.Hansen A, Gosemann M, Pruss A, Reiter K, Ruzickova S, Lipsky PE, Dorner T. Abnormalities in peripheral B cell memory of patients with primary Sjogren's syndrome. Arthritis Rheum. 2004;50(6):1897–1908. doi: 10.1002/art.20276. [DOI] [PubMed] [Google Scholar]
- 18.Hansen A, Lipsky PE, Dorner T. B cells in Sjogren's syndrome: indications for disturbed selection and differentiation in ectopic lymphoid tissue. Arthritis Res Ther. 2007;9(4):218. doi: 10.1186/ar2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanz I, Wei C, Lee FE-H, Anolik J. Phenotypic and functional heterogeneity of human memory B cells. Seminars in Immunology. 2008;20(1):67–82. doi: 10.1016/j.smim.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bohnhorst JO, Bjorgan MB, Thoen JE, Jonsson R, Natvig JB, Thompson KM. Abnormal B cell differentiation in primary Sjogren's syndrome results in a depressed percentage of circulating memory B cells and elevated levels of soluble CD27 that correlate with Serum IgG concentration. Clin Immunol. 2002;103(1):79–88. doi: 10.1006/clim.2002.5199. [DOI] [PubMed] [Google Scholar]
- 21.Wei C, Anolik J, Cappione A, Zheng B, Pugh-Bernard A, Brooks J, Lee EH, Milner EC, Sanz I. A new population of cells lacking expression of CD27 represents a notable component of the B cell memory compartment in systemic lupus erythematosus. J Immunol. 2007;178(10):6624–6633. doi: 10.4049/jimmunol.178.10.6624. [DOI] [PubMed] [Google Scholar]
- 22.Aitchison J. The statistical analysis of compositional data. London; New York: Chapman and Hall; 1986. [Google Scholar]
- 23.Weller S, Braun MC, Tan BK, Rosenwald A, Cordier C, Conley ME, Plebani A, Kumararatne DS, Bonnet D, Tournilhac O, et al. Human blood IgM "memory" B cells are circulating splenic marginal zone B cells harboring a prediversified immunoglobulin repertoire. Blood. 2004;104(12):3647–3654. doi: 10.1182/blood-2004-01-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weill JC, Weller S, Reynaud CA. Human marginal zone B cells. Annu Rev Immunol. 2009;27:267–285. doi: 10.1146/annurev.immunol.021908.132607. [DOI] [PubMed] [Google Scholar]
- 25.Ettinger R, Sims GP, Robbins R, Withers D, Fischer RT, Grammer AC, Kuchen S, Lipsky PE. IL-21 and BAFF/BLyS Synergize in Stimulating Plasma Cell Differentiation from a Unique Population of Human Splenic Memory B Cells. The Journal of Immunology. 2007;178(5):2872–2882. doi: 10.4049/jimmunol.178.5.2872. [DOI] [PubMed] [Google Scholar]
- 26.Song W, Liu C, Seeley-Fallen MK, Miller H, Ketchum C, Upadhyaya A. Actin-mediated feedback loops in B-cell receptor signaling. Immunol Rev. 256(1):177–189. doi: 10.1111/imr.12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Batista FD, Treanor B, Harwood NE. Visualizing a role for the actin cytoskeleton in the regulation of B-cell activation. Immunol Rev. 237(1):191–204. doi: 10.1111/j.1600-065X.2010.00943.x. [DOI] [PubMed] [Google Scholar]
- 28.Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28(5):639–650. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 29.Iwata Y, Matsushita T, Horikawa M, Dilillo DJ, Yanaba K, Venturi GM, Szabolcs PM, Bernstein SH, Magro CM, Williams AD, et al. Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood. 2011;117(2):530–541. doi: 10.1182/blood-2010-07-294249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Griffin DO, Holodick NE, Rothstein TL. Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20+CD27+CD43+CD70−. J Exp Med. 2011;208(1):67–80. doi: 10.1084/jem.20101499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Griffin DO, Rothstein TL. Human "orchestrator" CD11b(+) B1 cells spontaneously secrete interleukin-10 and regulate T-cell activity. Mol Med. 2012;18:1003–1008. doi: 10.2119/molmed.2012.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Le Pottier L, Devauchelle V, Fautrel A, Daridon C, Saraux A, Youinou P, Pers JO. Ectopic germinal centers are rare in Sjogren's syndrome salivary glands and do not exclude autoreactive B cells. J Immunol. 2009;182(6):3540–3547. doi: 10.4049/jimmunol.0803588. [DOI] [PubMed] [Google Scholar]
- 33.Rodriguez-Bayona B, Ramos-Amaya A, Perez-Venegas J, Rodriguez C, Brieva J. Decreased frequency and activated phenotype of blood CD27 IgD IgM B lymphocytes is a permanent abnormality in systemic lupus erythematosus patients. Arthritis Research & Therapy. 2010;12(3):R108. doi: 10.1186/ar3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Di Sabatino A, Rosado MM, Ciccocioppo R, Cazzola P, Morera R, Corazza GR, Carsetti R. Depletion of Immunoglobulin M Memory B Cells is Associated with Splenic Hypofunction in Inflammatory Bowel Disease. Am J Gastroenterol. 2005;100(8):1788–1795. doi: 10.1111/j.1572-0241.2005.41939.x. [DOI] [PubMed] [Google Scholar]
- 35.Di Sabatino A, Rosado MM, Cazzola P, Biancheri P, Tinozzi FP, Laera MR, Cantoro L, Vanoli A, Carsetti R, Corazza GR. Splenic function and IgMmemory B cells in Crohn's disease patients treated with infliximab. Inflammatory Bowel Diseases. 2008;14(5):591–596. doi: 10.1002/ibd.20374. [DOI] [PubMed] [Google Scholar]
- 36.Allan LL, Stax AM, Zheng D-J, Chung BK, Kozak FK, Tan R, van den Elzen P. CD1d and CD1c Expression in Human B Cells Is Regulated by Activation and Retinoic Acid Receptor Signaling. The Journal of Immunology. 2011;186(9):5261–5272. doi: 10.4049/jimmunol.1003615. [DOI] [PubMed] [Google Scholar]
- 37.Li D, Hong A, Lu Q, Gao GF, Jin B, Screaton GR, Xu X-N. A novel role of CD1c in regulating CD1d-mediated NKT cell recognition by competitive binding to Ig-like transcript 4. International Immunology. 2012;24(11):729–737. doi: 10.1093/intimm/dxs082. [DOI] [PubMed] [Google Scholar]
- 38.Bosma A, Abdel-Gadir A, Isenberg David A, Jury Elizabeth C, Mauri C. Lipid- Antigen Presentation by CD1d+ B Cells Is Essential for the Maintenance of Invariant Natural Killer T Cells. Immunity. 2012;36(3):477–490. doi: 10.1016/j.immuni.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Westerberg LS, Dahlberg C, Baptista M, Moran CJ, Detre C, Keszei M, Eston MA, Alt FW, Terhorst C, Notarangelo LD , et al. Wiskott-Aldrich syndrome protein (WASP) and N-WASP are critical for peripheral B-cell development and function. Blood. 2012;119(17):3966–3974. doi: 10.1182/blood-2010-09-308197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Doi H, Iyer TK, Carpenter E, Li H, Chang KM, Vonderheide RH, Kaplan DE. Dysfunctional B-cell activation in cirrhosis due to hepatitis C infection associated with disappearance of CD27+ B-cell population. Hepatology. 2011 doi: 10.1002/hep.24689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Devauchelle-Pensec V, Cagnard N, Pers JO, Youinou P, Saraux A, Chiocchia G. Gene expression profile in the salivary glands of primary Sjogren's. Rheum. 2010;62(8):2262–2271. doi: 10.1002/art.27509. [DOI] [PubMed] [Google Scholar]
- 42.Emamian ES, Leon JM, Lessard CJ, Grandits M, Baechler EC, Gaffney PM, Segal B, Rhodus NL, Moser KL. Peripheral blood gene expression profiling in Sjogren's syndrome. Genes Immun. 2009;10(4):285–296. doi: 10.1038/gene.2009.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hjelmervik TO, Petersen K, Jonassen I, Jonsson R, Bolstad AI. Gene expression profiling of minor salivary glands clearly distinguishes primary Sjogren's syndrome patients from healthy control subjects. Arthritis Rheum. 2005;52(5):1534–1544. doi: 10.1002/art.21006. [DOI] [PubMed] [Google Scholar]
- 44.Munir M. TRIM proteins: another class of viral victims. Sci Signal. 3(118):jc2. doi: 10.1126/scisignal.3118jc2. [DOI] [PubMed] [Google Scholar]
- 45.Zhao C, Hsiang TY, Kuo RL, Krug RM. ISG15 conjugation system targets the viral NS1 protein in influenza A virus-infected cells. Proc Natl Acad Sci U S A. 107(5):2253–2258. doi: 10.1073/pnas.0909144107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Divanovic S, Trompette A, Atabani SF, Madan R, Golenbock DT, Visintin A, Finberg RW, Tarakhovsky A, Vogel SN, Belkaid Y, et al. Negative regulation of Toll-like receptor 4 signaling by the Toll-like receptor homolog RP105. Nat Immunol. 2005;6(6):571–578. doi: 10.1038/ni1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nagai Y, Yanagibashi T, Watanabe Y, Ikutani M, Kariyone A, Ohta S, Hirai Y, Kimoto M, Miyake K, Takatsu K. The RP105/MD-1 complex is indispensable for TLR4/MD–2-dependent proliferation and IgMsecreting plasma cell differentiation of marginal zone B cells. Int Immunol. 2012;24(6):389–400. doi: 10.1093/intimm/dxs040. [DOI] [PubMed] [Google Scholar]
- 48.Kobayashi T, Takahashi K, Nagai Y, Shibata T, Otani M, Izui S, Akira S, Gotoh Y, Kiyono H, Miyake K. Tonic B cell activation by Radioprotective105/MD-1 promotes disease progression in MRL/lpr mice. Int Immunol. 2008;20(7):881–891. doi: 10.1093/intimm/dxn049. [DOI] [PubMed] [Google Scholar]
- 49.Jonsson R, Theander E, Sjostrom B, Brokstad K, Henriksson G. Autoantibodies present before symptom onset in primary Sjogren syndrome. JAMA. 310(17):1854–1855. doi: 10.1001/jama.2013.278448. [DOI] [PubMed] [Google Scholar]
- 50.Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, Harley JB. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349(16):1526–1533. doi: 10.1056/NEJMoa021933. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.