Abstract
This investigation evaluated several factors associated with diverse participant enrollment of a clinical trial assessing safety, immunogenicity, and comparative viremia associated with administration of 17-D live, attenuated yellow fever vaccine given alone or in combination with human immune globulin. We obtained baseline participant information (e.g., sociodemographic, medical) and followed recruitment outcomes from 2005 to 2007. Of 355 potential Yellow Fever vaccine study participants, 231 cases were analyzed. Strong interest in study participation was observed among racial and ethnically diverse persons with 36.34% eligible following initial study screening, resulting in 18.75% enrollment. The percentage of white participants increased from 63.66% (prescreened sample) to 81.25% (enrollment group). The regression model was significant with white race as a predictor of enrollment (OR=2.744, 95% CI=1.415-5.320, p=0.003).In addition, persons were more likely to enroll via direct outreach and referral mechanisms compared to mass advertising (OR=2.433, 95% CI=1.102-5.369). The findings indicate that racially diverse populations can be recruited to vaccine clinical trials, yet actual enrollment may not reflect that diversity.
Keywords: Yellow fever vaccine, Immunization, Clinical trials, Willingness to participate, Ethnic minorities, Women
Introduction
Ensuring proportional representation of diverse populations in vaccine trials is an ethical and a scientific imperative in clinical research. The NIH Health Revitalization Act of 1993 underscored the importance of inclusion of women and minorities in research studies as a national policy objective to fulfill broad social justice aims, to promote generalizability of study findings, and to understand subgroup differences in health outcomes [1].
Involvement of diverse groups in vaccine clinical trials is critical for meaningful evaluation of vaccine products and to achieve overarching public health objectives. Women and minorities have historically remained underrepresented in clinical research studies [2,3]. A high level of mistrust about the medical system, vaccine safety issues, and misperceptions about vaccines may factor in the decision to join a clinical study and encourage others to receive immunizations in the future [4-8]. The legacy of the Tuskegee syphilis study also serves as a source of medical establishment mistrust [8,9]. However, greater participation of minorities in biomedical research has been achieved in recent years [10].
Monitoring recruitment outcomes is an essential practice for successful enrollment of diverse populations [11]. The development and implementation of dynamic tracking systems provides detail on subject accrual patterns [12], along with insight on recruitment approaches that yield desired outcomes [13]. A system was developed by our site in collaboration with a team of biostatisticians and computer programmers to provide dynamic evaluative monitoring and assessment of a yellow fever virus vaccine study [14]. Previous findings have indicated that “willingness-to-participate” (WTP) declines with the passage of time among groups [15-17]. Thus, factors that affect the attrition of target populations in clinical studies are also of interest to ensure that future enrollment goals are met.
The purpose of this study is to investigate factors associated with accrual of women and minorities from prerandomization to enrollment stages. Although much is known about the enormous challenges associated with recruitment and retention of populations in other types of health research [18-20] very little is known about the factors which impact the ability to enroll a diverse group of participants in vaccine safety studies [21-26]. Participant concerns in this arena extend beyond clinical trials to vaccine safety issues and other immunization fears (e.g., needles) [27].
Background on yellow fever immunization
Since its introduction in the 1930s the yellow fever vaccine has been considered one of the most effective and safest vaccines available to prevent infection with the potentially fatal flavivirus [28,29]. At least 500 million doses have been made available globally and the US Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO) recommend vaccination for persons ≥ 9 months of age who are traveling to or living in a yellow fever endemic area [28,30] However, recent reports of adverse events after yellow fever vaccine (17-D and 17-DD) have raised concern about its safety [31,32].
Our clinical research site conducted a double-blind, randomized outpatient study to determine if human Immune Globulin (IG) limits the viremic response to 17D yellow fever vaccine without compromising immunogenicity [33]. The hypothesis examined whether co-administration of yellow fever antibody and yellow fever vaccine (passive-active immunization) resulted in effective immunization. The clinical study was conducted in 80 healthy adults who were 18-40 years of age. A stratified randomization procedure was used to ensure equal distribution of study medications to participants by gender and race.
The recruitment study
We evaluated the sociodemographic and recruitment factors predictive of enrollment in this phase IV yellow fever vaccine clinical trial. More specifically, we examined differences in the enrollment patterns of a diverse sample of women and minorities. We sought to contribute to the evidence for the need for culturally sensitive approaches in the recruitment process by investigating potential disparities in enrollment at our site. For example, clinical screening processes can be enhanced through cultural competency trainings for research team members. Trainings would offer communication strategies sensitive to cultural beliefs, values, and needs of participants.
We also examined the role of our Integrated Marketing Communication (IMC) approach on the recruitment process to determine whether specific approaches had an effect on the enrollment outcome [34]. The IMC strategy focuses on key communication objectives (e.g., build study awareness) for target audiences with a campaign comprised of various approaches. Over time, the interaction of such tactics theoretically would generate sufficient study interest among priority groups to realize enrollment goals. Thus, IMC posits that an equilibrium of effect can be anticipated with the counteraction of advertising and promotional tactics over time [34].
Methods
Study sample
From May 2005 through June 2007 volunteers were actively recruited for a randomized, controlled, double-blind trial of comparative viremia, immunogenicity and safety of an attenuated yellow fever virus vaccine when given alone or in combination with human immune globulin [33]. Recruitment strategies focused on print materials such as flyers and posters; electronic resources such as websites, emails, and list-serves; and mass media outlets including newspaper ads. Of the 210 recruitment tactics employed, 42.38% involved direct outreach with interpersonal contacts and distribution of print materials to these contacts, 24.76% involved flyers and print materials, 20.0% involved mass media recruitment strategies, and the remaining tactics focused on electronic/internet awareness building (12.86%). Recruitment strategies targeted specific populations including college and graduate students, missionaries in need of the yellow fever vaccine for travel, and healthcare providers able to encourage patients’ participation and inoculation prior to travel to yellow fever endemic regions of the world. To avoid selection bias via our recruitment strategies, we widely advertised the study in places where diverse racial and ethnic groups of persons would be in need of yellow fever vaccination for travel purposes. Therefore we conducted outreach and advertised the study in a variety of community settings such as churches, travel clinics, and colleges and universities.
Most often, potential volunteers contacted the study site by a telephone hotline from which our staff was available to provide preliminary information about the study and to conduct a brief pre-screening for eligibility. Occasionally, volunteers walked in to study site, and the pre-screen was performed in person. Eligible volunteers were referred to study nurses for initiation of the informed consent process and clinical screening. For a consenting volunteer, once clinical screening was completed successfully, she/he was able enroll in the study. Potential volunteers who could not be contacted after three un-returned phone calls were considered to have lost interest in study participation.
For this vaccine study, inclusion and exclusion criteria were established by the study protocol. Eligible potential volunteers had the following characteristics (additional inclusion/exclusion criteria are listed at www.clinicaltrials.gov [NCT00254826]):
Between 18 and 40 years old.
Could understand and sign informed consent and HIPAA authorization forms.
No history of allergic reaction to the vaccine, vaccine components, human immune globulin, eggs, or vaccines prepared in eggs or chick embryo cultures (e.g., influenza, measles).
HIV, hepatitis B, and hepatitis C negative.
Healthy, with no major medical problems including a history of cancer, immunodeficiency or any other medical condition that might have endangered the health or safety of the volunteer.
No history of previous yellow fever, West Nile, dengue, St. Louis encephalitis, Japanese encephalitis, or tick-borne encephalitis vaccination or infection.
No history of travel to yellow fever endemic zones as defined by the Centers for Disease Control and Prevention.
Weighed more than 110 pounds at screening
For female volunteers, not pregnant and agreed to use effective birth control throughout the duration of study.
Data collection
During pre-screening, potential volunteers consented to the inclusion of contact information, demographic characteristics, and psycho-social factors in a volunteer database. Information collected included name, address, phone numbers, email address, age, gender, race/ethnicity, education, sexual orientation, motivation for participation and means of recruitment. Potential volunteers were permitted to skip any questions that they did not feel comfortable answering. Data were stored in a password protected, numerically encoded online volunteer database. Pre-screening took place during normal business hours when a potential volunteer contacted the study site.
Measurement
The primary outcome of interest in this analysis was enrollment in the clinical trial. Alternative outcomes included ineligible, lost to follow up, or eligible but not enrolled (i.e., passive or active refusal of participation). Educational attainment included the following levels: K-12 grade or some College (Vocational or technical training, some college without degree, or Associate degree), and Bachelor's degree or beyond (Bachelor's degree, Masters, Doctorate or Professional degree). Race/ethnicity included ‘white’ for those who self-identified as white/Caucasian respondents, ‘Non-white’ for those reporting other than white race including Black/African American, Hispanic/Latino/a, Asian/Pacific Islander, Multiracial or ‘Other’ race or ethnicity. Recruitment tactics were categorized as general promotion (print material distribution, educational presentations, special community events, word of mouth referral and multiple sources), internet-based (email, listservs, web banners), and mass media (television, radio, mass print advertising).
Statistical analysis
SAS version 9.1 (SAS Institute, Inc., Cary, NC, USA) and SPSS version 15.0 (SPSS, Inc., Chicago, IL., USA) were used for analyses. Descriptive statistics and cross-tabulations were generated for all of the variables of interest. An overall multivariate model (i.e., binary logit regression method) was performed along with similar analyses for the male subgroup. Participant motivation had a large proportion (≥35%) of missing data and therefore was not included in the regression analyses. Significant independent predictors of outcomes were assessed at p <0.05 levels. Differences in categorical sociodemographic information (e.g., gender and enrollment status) were assessed by Chi-square (χ2) tests and differences in continuous information (e.g., mean age) were assessed by paired t-tests (t).
Descriptive statistics were generated from the recruitment campaign records. These tactics were catalogued and continuously updated in a spreadsheet that detailed the target audience (community segment), date of activity, communication approach tactic, and associated recruitment strategies. Each tactic was coded (e.g., 1, 2, 3) to enumerate the campaign approach frequencies. Cross-tabulation procedures including chi-square (χ2) tests were employed to assess the differences in recruitment sources and enrollment outcomes for all participants and for minorities.
Results
Volunteer contacts, screening and enrollment
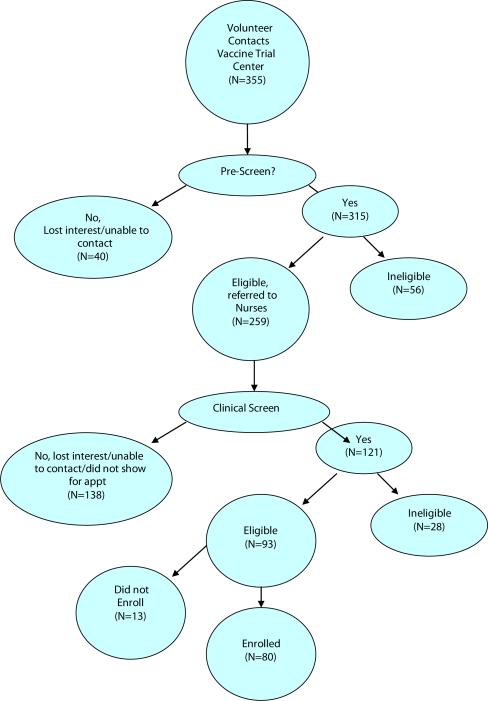
Of those individuals who initially contacted the study site (N=355), 88.7% (n=315) completed the pre-screening process (Figure 1). The remaining 40 potential volunteers either lost interest prior to pre-screening or were unable to be contacted. Of those individuals who completed the telephone pre-screen, 17.7% (n=56) were found to be ineligible for a variety of reasons including age, difficulty with study commitment, HIV positive status or other medical exclusion, and risk ineligibility through upcoming travel to endemic areas (Table 2). The remaining 259 potential volunteers were referred to the clinical nursing staff for clinical screening and informed consent processes.
Figure 1.
Flow chart of participant counts through screening and eligibility steps.
Table 2.
Frequency of reasons for Prescreen Ineligibility (n=96).
| Criteria | Frequency (Percent) |
|---|---|
| Age (<18 Years or >40 Years) | 12 (12.5%) |
| Difficulty with Study Commitment | 15 (15.63%) |
| Health Problem or medical exclusion (e.g. HIV positive) | 11 (11.46%) |
| Lost interest | 33 (34.37%) |
| Travel to endemic area or previous vaccination | 8 (8.33%) |
| Other and unknown reasons | 14 (14.58%) |
| Multiple | 3 (3.13%) |
Of these 259 pre-screened and potentially eligible volunteers, 53.2% (n=138) did not complete clinical screening. Many of these participants were unable to be contacted or lost interest prior to completing clinical screening, often because of an unpredicted time delay between volunteer's initial contact and actual opportunity for enrollment. Ten volunteers were enrolled at two sites each month and many potential volunteers lost interest while waiting in queue. 121 individuals completed informed consent and clinical screening, however 28 individuals were found to be ineligible (Table 3). Of the remaining 93 individuals, 80 volunteers (86.0%) enrolled in the yellow fever vaccine clinical trial.
Table 3.
Causes of Clinical Screen Ineligibility (n=28).
| Criteria | Frequency (Percent) |
|---|---|
| Travel to yellow fever endemic area within 2 weeks, or previous yellow fever vaccination | 2 (7.1%) |
| Health Problems (positive serology, anemia, allergies, seizure disorder, obesity, poor venous access, elevated blood pressure, HCV positive, hypertension, and aortic valve disease) | 24 (85.8%) |
| Other (borderline serology for other flaviviruses) | 2 (7.1%) |
Characteristics of the sample
The study population (Table 1) was created to characterize the differences between volunteers who enrolled in the trial (n=80) and volunteers who did not enroll (n=151). The non-enrolling groups is comprised of both the group of eligible volunteers who completed the clinical screening, but did not to enroll (n=13) as well as the group of potentially eligible volunteers who chose not to complete clinical screening (n=138).
Table 1.
Characteristics of Participants in the Vaccine Trial by Those who Enrolled and Those who Did Not Enroll.
| Characteristic | Consented and Clinically Screened Participants (N=231) | Not Enrolled Participants (N=151) | Enrolled Participants (N=80) |
|---|---|---|---|
| Age | |||
| Age in years | 26.6 | 26.5 | 27.4 |
| Missing | 10 | 5 | 0 |
| Gender | |||
| Female | 141 | 96 | 45 |
| Male | 90 | 55 | 35 |
| Missing | 0 | 0 | 0 |
| Race/Ethnicity | |||
| Asian | 14 | 8 | 6 |
| Black/African American | 48 | 40 | 8 |
| Caucasian, not Hispanic | 147 | 89 | 58 |
| Hispanic | 10 | 4 | 6 |
| Multiracial | 5 | 5 | 0 |
| Other or Native American/Pacific Islander | 4 | 2 | 2 |
| Missing | 3 | 3 | 0 |
| Sexual Orientation | |||
| Heterosexual | 187 | 122 | 65 |
| Homosexual | 20 | 15 | 5 |
| Bisexual | 13 | 9 | 4 |
| Missing | 11 | 5 | 6 |
| Education | |||
| Not a High School Graduate | 3 | 2 | 1 |
| High School Graduate | 8 | 6 | 2 |
| Some College, No Degree | 68 | 47 | 21 |
| Associates Degree | 6 | 6 | 0 |
| Vocational Training | 1 | 1 | 0 |
| Bachelors Degree | 99 | 66 | 33 |
| Post-graduate Training (Masters, Doctorate or Professional Training) | 37 | 18 | 19 |
| Missing | 9 | 5 | 4 |
| Recruitment | |||
| Newspaper Ad/magazine/ Radio/Television/Billboard/Indoor/Newsletter | 48 | 37 | 11 |
| Email/Listserv/Web Site | 73 | 52 | 21 |
| Flyer/ Health Fair/ Community Event/ Educational Presentation/Special Event/Other/ Word of Mouth/Provider Referral/Personal Letter/Mass Mailing | 106 | 60 | 46 |
| Missing | 4 | 2 | 2 |
| Motivation | |||
| Altruism | 35 | 22 | 13 |
| Compensation/Free Vaccine | 75 | 55 | 20 |
| Personal Connection to the Cause | 4 | 3 | 1 |
| Scientific or Medical Contribution | 51 | 31 | 20 |
| Other | 57 | 34 | 23 |
| Missing | 9 | 6 | 3 |
Participants in the enrolled group were more often women (56.3%, n=45) and most reported heterosexual orientation (87.8%, n=65 out of 74, excluded missing data). Nearly three-fourths (72.5%, n=58) of the enrolled participants reported white race, while less than one quarter (20.0%, n=16) reported race other than white or Hispanic ethnicity (7.5%, n=6). Education was not normally distributed with most volunteers having a “high” education level (BA, Masters, Doctorate or Professional degree, 68.4%, n=52 out of 76, excluded missing data), reflecting the large push for recruitment among college and graduate students. Many volunteers reported desire for compensation and vaccine (26.0%, n=20 out of 77, excluded missing data) as their motivation for participating in this yellow fever vaccine clinical trial and many reported directed outreach strategies such as flyers, community events, and health fairs (59.0%, n=46 out of 78, excluded missing data) were responsible for recruiting them to the study site.
Recruitment tactics
The campaign totaled 210 tactics implemented throughout the recruitment cycle at our site. General promotional activities including in-person outreach to faith leaders and their communities, educational presentations, and special events comprised a significant proportion of the recruitment endeavor (n=89 tactics; 42.38% of campaign approach). Print materials were also distributed (n=52 tactics; 24.76% of overall endeavor). Effort given to web-based recruitment (e.g., email outreach, listserv and web advertising) resulted in about 12.86% of the overall strategy (n=27). Finally, the investment in mass media was limited, resulting in 20.0% (n=42 tactics) of the overall effort.
Enrollment demographics and psycho-social factors
The mean age for the non-enrolled portion of the study population is 26.5 years and the average age for volunteers enrolled in the study is 27.4 years (Table 1). There is no statistical association between gender, educational attainment or sexual orientation and enrollment. Race, however, seems to be an important factor predicting enrollment with white volunteers more likely to enroll than non-white volunteers (χ2, 1df=7.65, p=0.0057). Motivation for participation had no association with enrollment, whereas recruitment approach had a weak association with enrollment (χ2, 6df=14.08, p=0.0288).
Predictive enrollment model
Multiple logistic regression analyses were used to determine the overall predictive ability of personal characteristics (e.g., race, gender, educational attainment level, and reported recruitment method) on the likelihood of enrollment among those eligible following clinic screening. These factors were selected to ensure parsimony of model measures.
Race was a significant predictor for enrollment (p=0.003). White participants were significantly more likely to enroll compared to participants who were from minority racial groups (OR=2.744; 95% CI=0.188-0.706). In addition, overall recruitment channel was a significant predictor for the enrollment (p=0.04). Those who were recruited by method 3 (flyer, health fair, community event, educational presentation, special event, word of mouth, provider referral, personal letter, or mass mailing) were more likely to enroll than those recruited through mass media messaging of method 1—newspaper ad, magazine, radio, television, billboard, or newsletter (p=0.01, OR=2.433, 95% CI=0.186-0.907).
Discussion
The 1994 NIH mandate prioritized the inclusion of women and minorities in research [35]. Since then, a greater emphasis has been placed on recruiting and retaining these populations. Recent evidence on minority participation in health research indicates a desire for information about the research activity in the community, greater demand to understand the relevance of the research efforts in addressing medical problems, and occasions to learn about clinical research entities and study volunteer participants [10,36]. Therefore, the creation of opportunities to serve these needs is necessary in order to effectively engage minority communities.
In this study, white participants were more likely to enroll than minority participants and were overrepresented in our sample. In Atlanta, blacks make up 32% of the population and 31% of the population of Georgia [37] while only constituting 10% of enrolled participants. Recruiting and retaining women and minorities in clinical research has been a challenge, which can be attributed to varying factors. Evidence has shown that distrust among minority populations of clinical research can be a strong deterrent for participation and the history of unethical behavior such as the Tuskegee trials continues to prevent minority groups from trusting the benefits of participation [38-40]. In addition, a lack of awareness of the study and its impact on the community may also serve as a factor to prevent enrollment [39]. It is important to continue efforts to include women and minorities in clinical trials and increasing the number of researchers and who are women or minorities will help to improve the proportion of those enrolled [39]. Researchers must improve how they communicate the intent and impact of the study and better strategize methods to increase the reach of that message into minority communities.
The CDC recommends the yellow fever vaccine to those who are traveling to countries where the disease is present, including parts of South America and Africa [41]. Due to the possibility of rare severe vaccine side effects, the CDC recommends the vaccine only for those who are at risk of contracting the virus [41,42]. Concerns about the vaccine mandated a high level of protectiveness in order to ethically conduct the study. As the results indicate, those who were recruited through direct channels, including health fairs, community events, referrals, and other person-to-person contact were more likely to enroll in the study. Recruiters were focused in their target population particularly on those who were traveling to areas where yellow fever is endemic [42,43]. The more personal methods of recruitment pursued a narrower audience than the mass media messaging strategies. In addition, the directed outreach techniques may have increased trust among potential participants compared to indirect outreach thus improving the proportion of those who enrolled in the study.
There is a need for directed strategies for vaccines that have the potential to cause harm, balancing the inclusion of participants who need the vaccine with more effective methods of outreach [43]. Directed strategies have the potential of immediately targeting only those who need the vaccine by interacting directly with populations who are at risk—for example, missionaries, health workers, or other travelers. These methods can prevent the inclusion of participants who will not encounter the virus and protect them from unnecessary exposure to the potentially severe outcomes of the vaccine. A preponderance of the rare, severe vaccine side effects occurs in older persons or those with predisposing disease conditions. In this study of healthy young adults, we did also include fully informed participants who had no immediate plans to travel to a yellow fever endemic area.
There are limitations to this study that include the small sample size and the directed scope of our recruitment. Furthermore, there may have been selection bias that differed between recruitment strategies since direct strategies were more easily able to target individuals who might require the yellow fever virus vaccine for upcoming travel compared to broader messaging strategies.
The main purpose of the study was to determine if the IG might reduce viremia, and thereby we might gain an understanding of why there has been an apparent increase in YFV vaccine-related side effects over the decade during which routine travel clinic use of IG for hepatitis A protection was discontinued due to the availability of the hepatitis A vaccine. However, we make the point in the primary study manuscript that for many years the YFV vaccine was co-administered with IVIG in travel clinics, and there was no problem with take [33]. Since this was an established standard of care, with no report of problem with take due to co-administration of IG with YFV vaccine, our IRB was fine with that approach.
This study highlights the importance of ethical considerations and the effectiveness of direct recruitment strategies in enrolling participants in a study of a potentially harmful vaccine. In addition, researchers must continue to improve understanding of clinical trials in minority communities to ensure that they receive the benefits of participation. This can be done by continuously building trust between minority communities and clinical researchers as well as through more effective ways of communicating the benefits and aims of research to minority participants.
Acknowledgement
We thank our study volunteers for their time and commitment to this trial. This research was supported by the Biostatistics and Clinical Cores of the Emory Center for AIDS Research (P30 AI050409), with additional support from the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR000454. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Citation: Frew PM, Shapiro ET, Lu L, Edupuganti S, Keyserling HL, et al. (2013) Enrollment in YFV Vaccine Trial: An Evaluation of Recruitment Outcomes Associated with a Randomized Controlled Double-Blind Trial of a Live Attenuated Yellow Fever Vaccine. Trop Med Surg 1: 117. doi:10.4172/tpms.1000117
References
- 1.Mastroianni AC, Faden R, Federman D. Women and health research: a report from the Institute of Medicine. Kennedy Inst Ethics J. 1994;4:55–62. doi: 10.1353/ken.0.0121. [DOI] [PubMed] [Google Scholar]
- 2.Moutsiakis DL, Chin PN. Why blacks do not take part in HIV vaccine trials. J Natl Med Assoc. 2007;99:254–257. [PMC free article] [PubMed] [Google Scholar]
- 3.BeLue R, Taylor-Richardson KD, Lin J, Rivera AT, Grandison D. African Americans and participation in clinical trials: differences in beliefs and attitudes by gender. Contemp Clin Trials. 2006;27:498–505. doi: 10.1016/j.cct.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Thapinta D, Jenkins RA, Morgan PA, Chiu J, Boenim W, et al. Recruiting volunteers for a multisite phase I/II HIV preventive vaccine trial in Thailand. J Acquir Immune Defic Syndr. 2002;30:503–513. doi: 10.1097/00126334-200208150-00006. [DOI] [PubMed] [Google Scholar]
- 5.Crosby RA, Holtgrave DR, Bryant L, Frew PM. Factors associated with the acceptance of an AIDS vaccine: an exploratory study. Prev Med. 2004;39:804–808. doi: 10.1016/j.ypmed.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Strauss RP, Sengupta S, Kegeles S, McLellan E, Metzger D, et al. Willingness to volunteer in future preventive HIV vaccine trials: issues and perspectives from three U.S. communities. J Acquir Immune Defic Syndr. 2001;26:63–71. doi: 10.1097/00126334-200101010-00010. [DOI] [PubMed] [Google Scholar]
- 7.King WD. Examining African Americans’ mistrust of the health care system: expanding the research question. Commentary on “Race and trust in the health care system”. Public Health Rep. 2003;118:366–367. doi: 10.1093/phr/118.4.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crawley LM. African-American participation in clinical trials: situating trust and trustworthiness. J Natl Med Assoc. 2001;93:14S–17S. [PMC free article] [PubMed] [Google Scholar]
- 9.Corbie-Smith G, Thomas SB, Williams MV, Moody-Ayers S. Attitudes and beliefs of African Americans toward participation in medical research. J Gen Intern Med. 1999;14:537–546. doi: 10.1046/j.1525-1497.1999.07048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wendler D, Kington R, Madans J, Van Wye G, Christ-Schmidt H, et al. Are racial and ethnic minorities less willing to participate in health research? PLoS Med. 2006;3:e19. doi: 10.1371/journal.pmed.0030019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salazar LF, Holtgrave D, Crosby RA, Frew P, Peterson JL. Issues related to gay and bisexual men's acceptance of a future AIDS vaccine. Int J STD AIDS. 2005;16:546–548. doi: 10.1258/0956462054679232. [DOI] [PubMed] [Google Scholar]
- 12.Charlson ME, Horwitz RI. Applying results of randomised trials to clinical practice: impact of losses before randomisation. Br Med J (Clin Res Ed) 1984;289:1281–1284. doi: 10.1136/bmj.289.6454.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agras WS, Marshall GD, Kraemer HC. Planning recruitment. Circulation. 1982;66:IV54–IV58. [PubMed] [Google Scholar]
- 14.Frew PM, del Rio C, Lu L, Clifton S, Mulligan MJ. Understanding differences in enrollment outcomes among high-risk populations recruited to a phase IIb HIV vaccine trial. J Acquir Immune Defic Syndr. 2009;50:314–319. doi: 10.1097/QAI.0b013e3181945eec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halpern SD, Metzger DS, Berlin JA, Ubel PA. Who will enroll? Predicting participation in a phase II AIDS vaccine trial. J Acquir Immune Defic Syndr. 2001;27:281–288. doi: 10.1097/00126334-200107010-00011. [DOI] [PubMed] [Google Scholar]
- 16.O'Connell JM, Hogg RS, Chan K, Strathdee SA, McLean N, et al. Willingness to participate and enroll in a phase 3 preventive HIV-1 vaccine trial. J Acquir Immune Def Syndr. 2002;21:521–528. doi: 10.1097/00126334-200212150-00010. [DOI] [PubMed] [Google Scholar]
- 17.Jenkins RA, Chinaworapong S, Morgan PA, Ruangyuttikarn C, Sontirat A, et al. Motivation, recruitment, and screening of volunteers for a phase I/ II HIV preventive vaccine trial in Thailand. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;18:171–177. doi: 10.1097/00042560-199806010-00009. [DOI] [PubMed] [Google Scholar]
- 18.Gadegbeku CA, Stillman PK, Huffman MD, Jackson JS, Kusek JW, et al. Factors associated with enrollment of African Americans into a clinical trial: results from the African American study of kidney disease and hypertension. Contemp Clin Trials. 2008;29:837–842. doi: 10.1016/j.cct.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galbreath AD, Smith B, Wood P, Forkner E, Peters JI. Cumulative recruitment experience in two large single-center randomized, controlled clinical trials. Contemp Clin Trials. 2008;29:335–342. doi: 10.1016/j.cct.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Lovato LC, Hill K, Hertert S, Hunninghake DB, Probstfield JL. Recruitment for controlled clinical trials: literature summary and annotated bibliography. Control Clin Trials. 1997;18:328–352. doi: 10.1016/s0197-2456(96)00236-x. [DOI] [PubMed] [Google Scholar]
- 21.Tello J, Soong SJ, Hunter B, Meriwether R, Hook EW, 3rd, et al. HIV vaccine acceptance among heterosexual clients of a sexually transmitted diseases clinic. Am J Med Sci. 1998;315:11–16. doi: 10.1097/00000441-199801000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Ness RB, Nelson DB, Kumanyika SK, Grisso JA. Evaluating minority recruitment into clinical studies: how good are the data? Ann Epidemiol. 1997;7:472–478. doi: 10.1016/s1047-2797(97)00080-x. [DOI] [PubMed] [Google Scholar]
- 23.Koblin BA, Heagerty P, Sheon A, Buchbinder S, Celum C, et al. Readiness of high-risk populations in the HIV Network for Prevention Trials to participate in HIV vaccine efficacy trials in the United States. AIDS. 1998;12:785–793. doi: 10.1097/00002030-199807000-00015. [DOI] [PubMed] [Google Scholar]
- 24.Newman PA, Duan N, Rudy ET, Anton PA. Challenges for HIV vaccine dissemination and clinical trial recruitment: if we build it, will they come? AIDS Patient Care STDS. 2004;18:691–701. doi: 10.1089/apc.2004.18.691. [DOI] [PubMed] [Google Scholar]
- 25.Koblin BA, Holte S, Lenderking B, Heagerty P. Readiness for HIV vaccine trials: changes in willingness and knowledge among high-risk populations in the HIV network for prevention trials. The HIVNET Vaccine Preparedness Study Protocol Team. J Acquir Immune Defic Syndr. 2000;24:451–457. doi: 10.1097/00126334-200008150-00010. [DOI] [PubMed] [Google Scholar]
- 26.de Bruyn G, Hudgens MG, Sullivan PS, Duerr AC. Participant retention in clinical trials of candidate HIV vaccines. J Acquir Immune Defic Syndr. 2005;39:499–501. doi: 10.1097/01.qai.0000148532.12329.df. [DOI] [PubMed] [Google Scholar]
- 27.Priddy FH, Cheng AC, Salazar LF, Frew PM. Racial and ethnic differences in knowledge and willingness to participate in HIV vaccine trials in an urban population in the Southeastern US. Int J STD AIDS. 2006;17:99–102. doi: 10.1258/095646206775455667. [DOI] [PubMed] [Google Scholar]
- 28.Barrett AD, Monath TP, Barban V, Niedrig M, Teuwen DE. 17D yellow fever vaccines: new insights.. Vaccine; A report of a workshop held during the World Congress on medicine and health in the tropics; Marseille, France. Monday 12 September 2005; 2007. pp. 2758–2765. [DOI] [PubMed] [Google Scholar]
- 29.Monath T. In: Yellow fever vaccine. Vaccines. 4 ed. Plotkin S, editor. WB Saunders; Philadelphia: 2004. pp. 1095–1176. [Google Scholar]
- 30.Centers for Disease Control and Prevention Yellow fever vaccine: Recommendations of the Advisory Committee on Immunization Practices (ACIP). Morbidity and Mortality Weekly (MMWR) 2002;51(17):1–11. [PubMed] [Google Scholar]
- 31.Pugachev KV, Guirakhoo F, Monath TP. New developments in flavivirus vaccines with special attention to yellow fever. Curr Opin Infect Dis. 2005;18:387–394. doi: 10.1097/01.qco.0000178823.28585.ad. [DOI] [PubMed] [Google Scholar]
- 32.Belsher JL, Gay P, Brinton M, DellaValla J, Ridenour R, et al. Fatal multiorgan failure due to yellow fever vaccine-associated viscerotropic disease. Vaccine. 2007;25:8480–8485. doi: 10.1016/j.vaccine.2007.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edupuganti S, Eidex RB, Keyserling H, Akondy RS, Lanciotti R, et al. A Randomized, Double-Blind, Controlled Trial of the 17D Yellow Fever Virus Vaccine Given in Combination with Immune Globulin or Placebo: Comparative Viremia and Immunogenicity. American Society of Tropical Medicine and Hygiene. 2013;88:172–177. doi: 10.4269/ajtmh.2012.12-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Percy L, Elliott R. Strategic Advertising Management. Second ed. Oxford University Press; Oxford: 2012. [Google Scholar]
- 35.Health NIo . NIH Guideline on the Inclusion of Women and Minorities as Subjects in Clinical Research. Services DoHaH, ed. Vol. 23. Bethesda, MD: 1994. [PubMed] [Google Scholar]
- 36.Smith YR, Johnson AM, Newman LA, Greene A, Johnson TR, et al. Perceptions of clinical research participation among African American women. J Womens Health (Larchmt) 2007;16:423–428. doi: 10.1089/jwh.2006.0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bureau USC. State and County QuickFacts. Data derived from Population Estimates, American Community Survey, Census of Population and Housing, County Business Patterns, Economic Census, Survey of Business Owners, Building Permits, Consolidated Federal Funds Report, Census of Governments. 2013.
- 38.Corbie-Smith G. The continuing legacy of the Tuskegee Syphilis Study: considerations for clinical investigation. Am J Med Sci. 1999;317:5–8. doi: 10.1097/00000441-199901000-00002. [DOI] [PubMed] [Google Scholar]
- 39.Shavers VL, Lynch CF, Burmeister LF. Racial differences in factors that influence the willingness to participate in medical research studies. Ann Epidemiol. 2002;12:248–256. doi: 10.1016/s1047-2797(01)00265-4. [DOI] [PubMed] [Google Scholar]
- 40.Shavers VL, Lynch CF, Burmeister LF. Knowledge of the Tuskegee study and its impact on the willingness to participate in medical research studies. J Natl Med Assoc. 2000;92:563–572. [PMC free article] [PubMed] [Google Scholar]
- 41.The Centers for Disease Control and Prevention Yellow Fever Vaccine. 2011 [Google Scholar]
- 42.Codeço CT, Luz PM, Coelho F, Galvani AP, Struchiner C. Vaccinating in disease-free regions: a vaccine model with application to yellow fever. J R Soc Interface. 2007;4:1119–1125. doi: 10.1098/rsif.2007.0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.London L, Kagee A, Moodley K, Swartz L. Ethics, human rights and HIV vaccine trials in low-income settings. J Med Ethics. 2012;38:286–293. doi: 10.1136/medethics-2011-100227. [DOI] [PubMed] [Google Scholar]