Abstract
Angiogenesis is a critical process for tumor growth and metastatic dissemination. There is tremendous interest in the development of noninvasive methods for imaging tumor angiogenesis, and ultrasound (US) is an emerging platform technology to address this challenge. The introduction of intravascular microbubble contrast agents not only allows real-time visualization of tumor perfusion during an US examination, but they can be functionalized with specific ligands to permit molecular US imaging of angiogenic biomarkers that are overexpressed on the tumor endothelium. In this article, we will review current concepts and developing trends for US imaging of tumor angiogenesis, including relevant preclinical and clinicsal findings.
Keywords: angiogenesis, contrast agent, microbubble, molecular imaging, ultrasound
Cancer is one of the leading causes of death and, in 2013 alone, as many as 1.6 million new cases are expected to occur, bearing tremendous costs on the healthcare system. A key mechanism of cancer development is angiogenesis [1]. Angiogenesis is a process by which new blood vessels develop to help support the metabolic demands of a growing tumor. This process is a fundamental step for tumor growth, metastasis and a prediction of behavior for certain cancers [2-4]. Many tumors, in fact, cannot grow beyond 1–2 mm3 in size without the angiogenic progression to sufficiently provide oxygen and nutrients to the tumor [5]. The interplay between proangiogenic and antiangiogenic signaling molecules control and maintain tumor angiogenesis. Several angiogenic biomarkers and signaling molecules are present and overexpressed within a tumor. The most characterized and the main molecular regulator that is present on endothelial cells is the VEGF receptor (VEGFR) [6,7]. Overexpression of VEGFR has been linked to tumor progression and poor prognosis [8]. Other important biomarkers that play a role in angiogenesis include (but are not limited to) αvβ3 integrin [9], P-selection [10] and endoglin [11].
Newly formed tumor vessels have inherent abnormal phenotypes, with many gaps between the endothelial cells, abnormal basement membrane thicknesses and overall tortuous organization [12]. The intrinsic leakiness of the new vessels results in increased interstitial fluid pressure causing issues with delivery of drugs and therapeutics due to the slow diffusion process [13]. Additionally, flow stasis occurs which can lead to hypoxia and upregulation of angiogenic markers such as VEGF resulting in a cycle of tumor growth [14]. Thus, the difficulty in effectively delivering therapeutic drugs has proven to be a challenge in the treatment of many cancer types.
The ability to image and quantify tumor perfusion is highly desirable in the clinical evaluation of cancer changes. Angiogenesis provides a valid opportunity to differentiate between normal and cancerous tissues [15,16]. Morphologically defining a tumor requires outlining of the microvessel density (MVD), which has been shown to be an independent predictor of malignant disease and a useful prognostic tool [15,17,18]. Thereunto, ultrasound (US) has shown great potential in providing clinically relevant information such as tumor perfusion, structure and functional feature [19,20]. US has progressed vastly since its inception and has become one of the most widely used modalities for diagnostic imaging. This reputation stems from its ease of accessibility, portability, low cost, superior safety, excellent spatial resolution and real-time imaging capabilities. US has set itself apart from other modalities in imaging breast [21], liver [22] and other tissues due to these aforementioned favorable properties [23].
With the advent of microbubble (MB) contrast agents, characterization of tissue perfusion and vascular volume is possible with the application of a dynamic contrast-enhanced US (DCE-US) imaging session. MBs considerably improve the sensitivity and specificity of US imaging [24]. These US contrast agents are gas-filled, lipid-shelled, micron-sized bubbles that circulate strictly within the vasculature, and have a circulatory lifetime on the order of minutes. The outer lipid shell stabilizes the inner gaseous core and allows for flexibility in composition and properties of the MB, including the ability to use targeted molecules [25,26].
Owing to nonlinear oscillations and acoustic impedance mismatch between the contrast agent and surrounding blood and soft tissue, MBs produce a unique US signal. Using this knowledge, we can selectively isolate the MB signal using various techniques to enhance the contrast agent signal-to-noise ratio. In addition, MBs can boost grayscale echogenicity up to 27 dB [24]. One unique quality of MBs is the deliberate and rapid destruction of these agents using a short high-intensity pulse sequence. Once MBs are destroyed in a particular region-of-interest (ROI), the subsequent reperfusion can be visualized using DCE-US imaging. This US imaging technique is termed MB destruction–replenishment imaging and the acquired time-intensity curve data (either for a discrete spatial location or a tissue encompassing ROI) reflects the history and kinetics of MB flow. Since MBs strictly remain in the vasculature space, tracking MB circulation enables reconstruction of 2D or 3D [18] spatial representation of blood flow patterns and extract parametric measures of tissue perfusion [18,27-30]. Time-intensity curve data have proven to be especially useful and can be processed to form image maps of various tissues [28,31,32]. More importantly, the correlation between pathologic vascularity, such as MVD and DCE-US-derived vascularity measurements can be directly quantified [33,34].
Determining treatment response in oncology is of critical importance. It is also important to assess how well this tumor response to therapy is progressing, especially at an early time period, in order to reduce potential side effects. Currently, histological analysis is most commonly used to assess tumor angiogenesis and therapeutic outcomes. With the recent characterization of angiogenesis using DCE-US, this invasive process of obtaining histological data can be supplemented. In this article, we will first explore recent developments in the field of DCE-US imaging for the visualization and characterization of tumor angiogenesis in both the preclinical and clinical settings. We then explore the new field of molecular imaging as it pertains to DCE-US and the noninvasive assessment of key angiogenic biomarkers.
Preclinical US imaging of tumor angiogenesis
Animal models have an important role in new oncological advances. It is therefore important to assess the recent preclinical studies that pave the way for DCE-US imaging of angiogenesis. The plethora of information obtained from preclinical studies not only showcases the diagnostic value of DCE-US, but also illuminates the molecular events occurring during angiogenesis and tumor development.
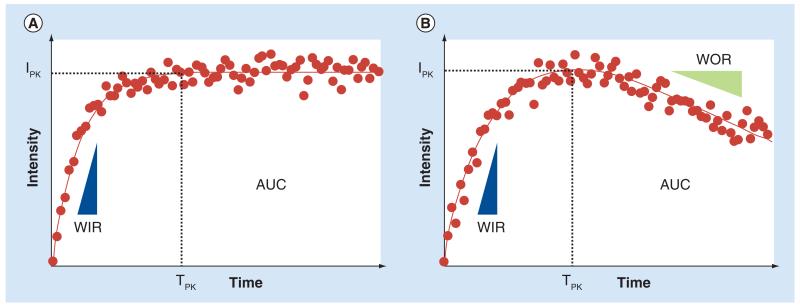
Utilizing functional imaging concepts, the temporal evolution of DCE-US image intensities from a user or computer-defined ROI can be extracted. For imaging studies, using an intravenous infusion of contrast media, this otherwise noisy time–intensity curve can be fit with a mathematical model following an exponential function, where the initial slope correlates to MB velocity and the plateau represents the fractional blood volume [31]. Conversely, for studies that necessitate a bolus injection of contrast media, a γ-variate function can be used for fitting to this otherwise noisy time–intensity curve data [35]. Regardless of the injection type, more data-specific parameters can be derived from either the mathematical model or raw time–intensity curve data, which serve as surrogate measures of tissue perfusion and vascular function, namely, peak intensity (IPK), time-to-peak intensity (TPK), area under the curve (AUC), wash-in rate (WIR) and wash-out rate (WOR) [36,37]. Figure 1 illustrates these time–intensity curve-derived tissue perfusion parameters following either an infusion or bolus injection of MB contrast agent and DCE-US imaging. After perfusion parameter derivation, a spatial map of the tumor vessels can be reconstructed or summarizing statistics can be generated for a given ROI. While these traditional analyses of time–intensity curve data have been widely described in the literature, it is important to note that various other mathematical modeling approaches have been reported in the literature that aim to more accurately describe tumor perfusion and microvascular properties [38-44].
Figure 1. Representative time–intensity curves.
Describes the history of microbubble circulation following administration of either (A) a continuous infusion or (B) bolus injection of contrast agent. Parametric perfusion measures indicate, namely, IPK, TPK, AUC, WIR and WOR when applicable.
AUC: Area under the curve; IPK: Peak intensity; TPK: Time-to-peak instensity; WIR: Wash-in rate; WOR: Wash-out rate.
A rich body of literature has convincingly demonstrated the utility of DCE-US imaging in the preclinical setting. Of interest, Lucidarme et al. established an angiogenesis murine model for imaging research utilizing Matrigel plugs in lieu of the more conventional xenograft tissue [45]. These Matrigel implants allowed for a translucent, anechoic, homogenous system that was previously used for modeling cellular events regarding angiogenesis [46]. These plug implants were injected with basic FGF (bFGF) to induce angiogenesis [45]. After a bolus injection of MB contrast agent, the group showed that implants that contained bFGF-induced neovascularity had higher maximum intratumoral enhancement versus controls that did not get any bFGF [47]. This novel animal model introduces a stable system that can be imaged several times over the angiogenic time course.
Monitoring angiogenesis and microvascularity patterns with concurrent treatment with antiangiogenic drugs has been extensively explored by several research groups. Since MB contrast agents are restricted to the vascular space and function as excellent blood vessel tracers, DCE-US imaging is a promising modality for characterizing any early neovascular change in response to these vascular disrupting treatments. To highlight, it has been shown in a preclinical murine model that cancer treatment with the antiangiogenic drug, bevacizumab, in conjunction with DCE-US imaging, produced considerable decreases in the AUC perfusion parameter (a surrogate measure of blood volume), which coincided with decreases in MVD immunohistologic measures [48]. Given MB contrast agents are excellent vascular tracers, Yoshida et al. used DCE-US imaging to monitor liver tumor response to anti-angiogenic treatment (i.e., sorafenib) in a rabbit model [49]. During this study, it was shown that the TPK perfusion parameter markedly increased in tumors treated with the antiangiogenic drug, which was attributed to a corresponding decrease in MVD. A comparable study investigated the feasibility of using DCE-US imaging for depicting early antiangiogenic treatment response of advanced osteolytic lesions [50]. While no changes in tumor volume were observed during the 6-day observation time, significantly decreased values for the IPK, AUC and WOR measurements were found in animals treated with sunitinib due to microvascular disruption. In another model, Liu et al. focused on vascular pathology and tumor microvascular perfusion was correlated with histopathology of the tumors [51]. A characteristic enhancement pattern that began from the tumor peripheral region was noted and was consistent with immunohistologic maps of tumor microvascularity. Collectively, these results demonstrate that DCE-US-based microvascular measurements are in good agreement with immunohistologic-derived results. Further results suggest that repeated tumor perfusion measurements using DCE-US imaging can effectively detect early response to anticancer therapy.
The above studies utilized 3D DCE-US imaging strategies for detecting microvascular distributions. While results were promising, it is known that planar DCE-US imaging is susceptible to changes in the imaging window due to user and/or subject motion. Specifically, it was shown that slight changes in transducer orientation (on a millimeter scale) can produce significant changes in time intensity curve measurements ranging from 6.4 to 40.3% and is dependent on the particular perfusion parameter of interest [18,27]. Since these deviations could complicate measurement reproducibility in a multiday longitudinal study, development of whole tumor (4D) DCE-US imaging of tumor perfusion is also underway. To that end, both planar and volumetric imaging probes have been used to collect this multidimensional spatial data. For those studies using a planar imaging probe, the general strategy is to mechanically step a linear array transducer across a ROI, while collecting temporal sequences of DCE-US images after contrast agent injection [27,52,53]. Conversely, DCE-US imaging using a 4D transducer can be performed for real-time visualization of perfusion throughout the entire ROI [18,54-58]. Specifically, Hoyt et al. showed that 4D DCE-US imaging can sensitively detect early breast cancer response to antiangiogenic therapy in a mouse animal model [55], which was in agreement with other studies detailed above. Overall, preclinical studies have thus far demonstrated that DCE-US imaging is a promising modality for both visualizing tumor microvascularity and predicting early response to drug treatment before any changes in physical size manifest.
Clinical US imaging of tumor angiogenesis
Clinical imaging of angiogenesis can add an adjunct functional measure to the traditional Response Evaluation Criteria in Solid Tumors (RECIST) assessment. The RECIST model takes into account morphological factors, such as tumor size to grade tumor response to therapy [59]. However, several clinical studies have shown that early changes in tumor perfusion manifest before any physical change in tumor size is observed [60,61]. Therefore, supplements to the RECIST criteria are needed, whereby morphological features of tumor phenotype and functional imaging are combined. Functional imaging with MRI and US are excellent candidates due to their nonionizing radiation, although both face challenges in the standardization of tumor measurements. Notwithstanding, DCE-US provides a cost advantage over MRI, which is an important consideration given the evolving healthcare landscape.
The clinical use of US contrast agents has slowly grown over the last decade with concurrent advances in US imaging technology and widening regulatory approval. While Definity (Lantheus Medical Imaging) and Optison (GE Healthcare) are the only MB products approved by the US FDA for use in the USA, there are other brands such as Sonovue (Bracco) that are also routinely used in other countries. The use of either of the former MB brands is indicated for patients with suboptimal echocardiograms to opacify the left ventricle and to improve delineation of the left ventricular endocardial borders. While these limitations have hampered DCE-US imaging trials, especially in the USA, there are several reports that have appeared in the literature that are important to consider as they highlight the evolving clinical landscape and promising future of DCE-US imaging.
Several early studies detailing the clinical use of DCE-US imaging ranked contrast agent uptake in the target tumor via a predetermined qualitative scoring criteria [62,63]. While important for demonstrating feasibility, the subjective nature of the analysis makes reproducibility a concern for inexperienced readers of imaging data. More recent studies have employed time–intensity curve analyses to quantitatively measure the kinetic properties of tumor perfusion [64]. Using such methods, DCE-US was able to differentiate between malignant and benign breast tumors based on a combination of perfusion parameters and morphological differences [65]. In a different study, DCE-US was used for response prediction and early response evaluation in patients receiving anti-angiogenic (bevacizumab) therapy for metastasized colorectal cancer [66]. Of interest, results from this study revealed that baseline TPK measures were significantly lower in the group of treatment responders compared to nonresponders, which indicates that low baseline TPK measures may predict tumor response according to RECIST. Furthermore, a strong increase in the TPK measure was observed during the treatment window for the group of responders and correlated with the antiangiogenic effect of bevacizumab. The prognostic role of DCE-US imaging for determining early therapeutic response has been studied for several other cancer types. In patients with gastrointestinal stromal tumors receiving imatinib (a tyrosine kinase inhibitor), DCE-US assessed treatment response as early as 7 days in some patients [67]. Patients with hepatocellular carcinoma undergoing treatment with bevacizumab therapy, showed considerable reductions in tumor vascularity as early as 3 days after treatment initiation [68]. Metastatic renal cell carcinomas treated with sunitinib showed a decrease in DCE-US-derived perfusion measures within 15 days [69]. Good and poor responders showed a significant difference in overall survival and progression-free survival within 3 weeks of treatment initiation in patients with renal cancer [67].
A pivotal study reported by Lassau and colleagues in collaboration with the French National Cancer Institute helped structure a standardization of DCE-US imaging for measuring tumor response to drug treatment [70]. As many as 539 patients were enrolled and over 2000 examinations performed on patients with breast cancer, colon cancer, melanoma, gastrointestinal stromal tumor, and renal cell carcinomas across 19 oncology centers. The authors of this study illustrated that a good predictor of response is available within 4 weeks after starting antiangiogenic therapy in a host of tumor types. It is important to highlight again that the changes in DCE-US-derived perfusion parameters were well before any morphological variations occurred as required for the RECIST criteria.
Overall, the collective studies detailed above showcase the potential of DCE-US imaging of angiogenesis in a clinical setting for the purpose of differentiating benign from malignant tumor types and monitoring tumor response to drug treatment.
Preclinical molecular US imaging of tumor angiogenesis
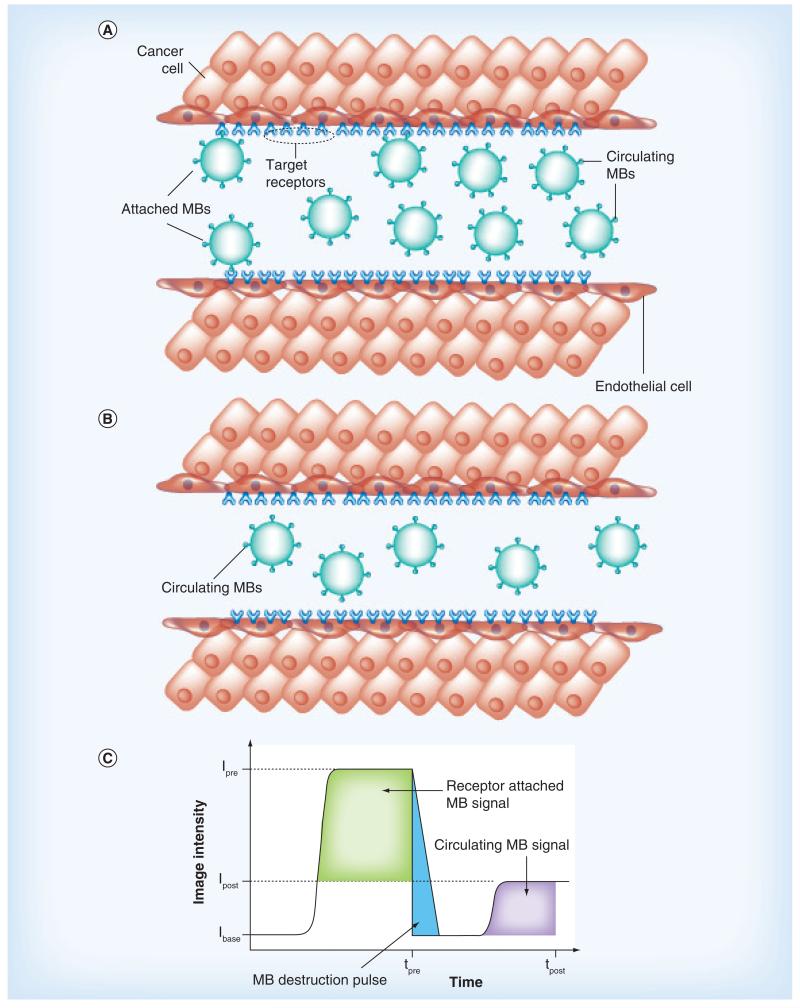
A more recent innovation in DCE-US imaging is the development of MB contrast agents targeted to angiogenic biomarkers overexpressed on tumor endothelial cells. While the acoustic and physical properties of these targeted MBs are similar to the nontargeted contrast agents described previously, the distinguishing feature is that targeted MBs are conjugated with ligands (e.g., monoclonal antibodies or peptides) to promote specific biomarker adhesion. Moreover, MBs that bind and accumulate within the intravasculature space can be differentiated from freely circulating (unbound) MBs using an array of strategies. The most common approach is to utilize MB destruction-replenishment imaging [71,72]. To detail, after systemic injection of the targeted MBs, a sufficient dwell time is allowed to pass (on order of minutes) so that the MBs can circulate and bind to the target biomarkers. After an image is acquired using a low-intensity MB-sensitive US imaging mode (e.g., pulse-inversion harmonic imaging), a short high-intensity pulse sequence is used to destroy all MBs in the field-of-view and then a second image is collected using low-intensity US imaging following MB reperfusion. During the initial phase of the imaging examination, US images depict both bound and systemically flowing MB contrast agents, whereas after destruction, US images depict flowing MBs only. The intensity difference between these two image sequences represents a surrogate measure of the molecular US signal (Figure 2). While MB destruction-replenishment is a robust technique for molecular US imaging, the US pressure necessary for MB destruction may cause unwarranted biological effects that are still not fully characterized [73-76]. To this end, several researchers have detailed alternative molecular US imaging strategies for isolating the targeted MB signal. One common strategy is to use spectral filtering of DCE-US images to selectively detect bound MBs while suppressing the signal from unbound contrast agents [77-80]. Alternatively, time–intensity curve data can be fit to a parametric model to mathematically describe the molecular US signal of interest [81].
Figure 2. Contrast-enhanced ultrasound imaging with microbubble contrast agents.
(A) Targeted ultrasound (US) contrast agents (MBs) attached to receptors overexpressed on tumor endothelial cells after intravenous administration and systemic circulation. A portion of injected MBs do not attach to the target receptors and freely circulate through tumor microvascularity. After destruction of all MBs in the tumor volume (both attached and freely circulating) using a high-intensity US pulse sequence, (B) reperfusion with only freely circulating MBs occurs. (C) The difference in contrast-enhanced US image intensity before and after MB destruction is a surrogate measure of attached MBs to the target angiogenic biomarkers representing the molecular US signal. I base: Baseline image intensity; Ipost: Image intensity after MB destruction; Ipre: Image intensity before MB destruction; MB: Microbubble; tpost: Image time after MB destruction; tpre: Image time before MB destruction.
The general appeal of molecular US imaging is that it allows detection of specific overexpressed biomarker targets in order to characterize molecular activities otherwise inaccessible using conventional US techniques. A direct correlation between receptor expression and MB binding has been established both in vitro [82] and in vivo [71]. For molecular US imaging of tumor angiogenesis, several highly expressed biomarkers on activated endothelium have been targeted, such as VEGFR2 [83-93], αVβ3 integrin [29,91-99] and endoglin [93,100,101]. Using molecular US imaging to assess the level of expression of these angiogenic biomarkers, it was shown in a longitudinal study that early-stage breast and ovarian tumors exhibit higher levels of endoglin expression than both αVβ3 integrin and VEGFR2 [93]. This study confirmed that molecular US imaging allows noninvasive assessment of angiogenic biomarkers, which can vary during tumor growth.
Leong-Poi et al. was one of the first groups to characterize molecular US imaging of tumor angiogenesis with targeted MB contrast agents [98]. This seminal in vivo study showed that αVβ3 integrin-targeted MBs significantly increased US image enhancement over control nontargeted MBs. Thereafter, several other groups confirmed the efficacy of a VEGFR2-targeted MB for molecular US imaging of angiogenic biomarkers. Collectively, these studies further demonstrated that targeted MBs produce an improved US signal-to-background ratio compared with conventional contrast agents [90,102,103]. Another molecular US imaging study targeted αVβ3 integrin receptors and showed a similar enhancement pattern [97]. Interestingly, due to higher target receptor density levels in the tumor periphery, the US signal from targeted MBs was more enhanced in this region, emphasizing the spatial heterogeneity of the tumor angiogenic process.
To improve the adhesion efficiency of MB contrast agents used for molecular US imaging, multitargeted strategies have been proposed. A dual-targeted MB directed at both VEGFR2 and αVβ3 integrin produced improved visualization of tumor angiogenesis in a murine model of ovarian cancer [92]. Results from this study revealed a 2.1- and 1.5-fold increase in the mean molecular US signal when using dual-targeted MBs versus the VEGFR2 and αVβ3 integrin-targeted MBs, respectively. Similar molecular US image enhancement patterns were observed when using a dual-targeted MB to both EGF receptor and extracellular matrix metalloproteinase inducer (CD147) compared with the single-targeted MB strategies [104]. As shown by Warram et al., multitargeted MBs produce a synergistic effect when used for molecular US imaging of tumor angiogenesis [91]. This threefold increase in biomarker-binding efficiency was attributed to the enhanced performance of the targeting ligands working in combination to achieve greater binding than the ligands working independently. Another advantage of targeting multiple receptors simultaneously is the improved potential for MB binding when endothelial receptor expression is unknown.
An alternative strategy for enhancing the selective binding of MB contrast agents is to use acoustic radiation forces to physically displace the circulating MBs toward the endothelial targets and promote sustained interaction [105]. As shown by Frinkling et al. in a murine model of prostate cancer, the use of acoustic radiation forces significantly enhanced binding of VEGFR2-targeted MBs by a factor of 7 at sites of active angiogenesis compared with targeted MB binding without the use of acoustic radiation force manipulation [106]. Beyond improved targeting, application of acoustic radiation forces during molecular US imaging studies may allow reduced contrast agent doses and help minimize potential side effects [107].
Molecular US imaging of angiogenesis is not only a promising strategy for cancer detection and disease staging, but also for therapeutic monitoring. To this end, several research groups have explored the feasibility of molecular US imaging of early tumor response to antiangiogenic treatment [72,85,99]. An early report by Palmowski et al. used both VEGFR2 and αVβ3 integrin-targeted MBs to investigate changes in biomarker expression during antiangiogenic treatment (a matrix metalloproteinase inhibitor, AG3340) of tumor-bearing animals [85]. Molecular US imaging at baseline and 7 days after treatment revealed a significantly lower level of targeted MB binding. By contrast, untreated tumors exhibited significantly increased binding of targeted MBs throughout the same time period. It was then shown that molecular US imaging of can sensitively detect tumor response to antiangiogenic therapy (bevacizumab) in animal models within 3 days of treatment initiation and before any changes in physical tumor size manifest [72,99]. Overall, these encouraging preclinical results suggest that noninvasive molecular US imaging may be used to determine the efficacy of antiangiogenic therapy.
Biodistribution studies of targeted MB contrast agents are important for assessing the temporal and spatial pharmacokinetics. To this end, whole-body distribution studies of radiolabeled MBs targeted to tumor angiogenesis-related VEGFR2 were performed in angiosarcoma-bearing animals using in vivo dynamic micro-PET imaging and traditional ex vivo radioactivity measurements [89]. Results revealed that a majority of the targeted MBs were rapidly cleared from circulation with minutes of injection. These targeted MBs were found to be localized within hepatic Kupffer cells and splenic macrophages. Importantly, the target tumor had significantly more uptake and retention of the VEGFR2-targeted MBS than adjacent skeletal muscle tissue.
Clinical molecular US imaging of tumor angiogenesis
US is one of the most widespread diagnostic imaging modalities used clinically. While the clinical significance of contrast-enhanced US is still being defined, the next developmental iteration of this promising technology involves the translation of molecular US imaging. The ultimate goal is to noninvasively image genetic and cellular alterations before any visible morphological or anatomic changes manifest. A vast majority of preclinical studies have used a streptavidin–biotin complex to conjugate moieties to the MB surface due to both the flexibility and high dissociation constant of streptavidin and the commercial availability of biotinylated-targeting ligands, such as antibodies, peptides or other small molecules. This approach also represents a practical platform for rapid testing of new targeting strategies in animal models of cancer. Notwithstanding, because the streptavidin–biotin complex can be highly immunogenic in human [108], other conjugation chemistries need to be considered that forgo this link [109].
Several clinically translatable targeted MBs for the purpose of molecular US imaging have been developed in recent years [81,83,84,86-88,110-112]. The first reported agent for clinical application was BR55 (Bracco Research, Geneva, Switzerland), which is a VEGFR2-targeted contrast agent for molecular US imaging of angiogenesis. This targeted contrast agent was prepared by incorporation of a biospecific lipopeptide into the MB membrane that binds to the endothelium of prostate [87] and breast [88] cancer. Molecular US imaging using BR55 was shown to be suitable for characterizing and distinguishing breast cancers with different angiogenesis and aggressiveness in animal models [83,84]. It was also shown that molecular US imaging using BR55 was useful for monitoring antiangiogenic therapy (bevacizumab) in colon cancer-bearing animals [86]. Given these promising preclinical results, BR55 is currently being evaluated in an exploratory clinical trial for its ability to identify prostate cancers on the basis of their increased VEGFR2 expression (using a visual score in comparison with histopathology results).
Another clinically translatable MB contrast agent for molecular US imaging of angiogenesis was developed whereby E-selectin was the target biomarker [110]. For this application, an E-selectin-specific peptide was synthesized and covalently attached to the MB surface. Similarly, it was shown that single-chain VEGF can also be covalently conjugated to the surface of select MB contrast agents for the purpose of molecular US imaging of angiogenic biomarkers [112]. This targeted MB (Visistar VEGFR2, Targeson Inc., CA, USA) is available commercially to the research community, in addition to a different MB product that targets the αVβ3 integrin (Visistar Integrin, Targeson Inc.). Since covalent chemistry was used to conjugate the respective ligands to the MB surface, molecular US imaging approaches using these targeted agents could potentially be applied in the clinical setting.
Conclusion
There is a critical need for noninvasive strategies for imaging tumor angiogenesis. Recent developments in US technology have made fulfilling this objective tenable. In this article, we summarized contrastenhanced US imaging techniques for describing tumor angiogenesis and perfusion properties, including preclinical and clinical studies that have been reported in the literature. Furthermore, the current status of molecular US imaging of angiogenic biomarkers was reviewed under the same context including emerging trends in targeted MB design and application.
Future perspective
The last decade has shown tremendous progress in the field of DCE-US imaging at both the preclinical and clinical frontiers of scientific discovery. US contrast agents are currently only approved by the FDA for cardiac applications, but additional procedure reimbursements are anticipated in the relatively near future. While the more widespread clinical use of DCE-US imaging will augment diagnostic procedures, a major contribution in the next 5–10 years is expected to be in the area of monitoring tumor response to anticancer therapy. These surrogate biomarkers of early tumor response are critically important in order to help determine early pharmaceutical treatment efficacy before harmful side effects emerge. Next-generation MBs are being used preclinically for assessing biological processes at the molecular level and clinically translatable targeted contrast agents are now being introduced by the scientific community. In 10 years, molecular US imaging certainly has the potential to improve our ability to make much earlier cancer diagnoses and to further guide treatment options. While more research and validation studies are needed before these US technologies can be integrated into routine clinical practice, the future of US imaging is very promising.
Executive summary.
Preclinical ultrasound imaging of tumor angiogenesis
Information obtained from preclinical studies not only showcase the diagnostic value of dynamic contrast-enhanced (DCE) ultrasound (US), but also illuminates molecular events occurring during angiogenesis and tumor development.
After injection of an intravascular microbubble (MB) contrast agent, the temporal evolution of DCE-US image intensities can be analyzed to provide information on tumor angiogenesis.
Preclinical studies have demonstrated that DCE-US imaging is a promising modality for both visualizing tumor microvascularity and predicting early response to drug treatment before any changes in physical size manifest.
Clinical US imaging of tumor angiogenesis
Clinical imaging of angiogenesis can add an adjunct functional measure to the traditional Response Evaluation Criteria in Solid Tumors (RECIST) assessment.
The clinical use of MB contrast agents has slowly grown over the last decade with concurrent advances in US imaging technology and widening regulatory approval.
Recent clinical studies showcase the potential of DCE-US imaging of angiogenesis in a clinical setting for the purpose of differentiating benign from malignant tumor types and monitoring tumor response to drug treatment.
Preclinical molecular US imaging of tumor angiogenesis
A more recent innovation in DCE-US imaging is the development of MB contrast agents targeted to angiogenic biomarkers overexpressed on tumor endothelial cells.
Targeted contrast agents that bind and accumulate within the intravasculature space can be differentiated from freely circulating (unbound) MBs using an array of strategies.
Molecular US imaging of angiogenesis is not only a promising strategy for cancer detection and disease staging, but also for therapeutic monitoring in preclinical animal models.
Clinical molecular US imaging of tumor angiogenesis
While the clinical significance of DCE-US is still being defined, the next developmental iteration of this promising technology involves the translation of molecular US imaging.
The ultimate goal is to noninvasively image genetic and cellular alterations before any visible morphological or anatomic changes manifest.
Several clinically translatable targeted MBs for the purpose of molecular US imaging have been developed in recent years and exploratory clinical trials are currently underway (or planned) for select agents.
Acknowledgments
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat. Rev. Cancer. 2003;3(6):401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 2.Schor AM, Schor SL. Tumour angiogenesis. J. Pathol. 1983;141(3):385–413. doi: 10.1002/path.1711410315. [DOI] [PubMed] [Google Scholar]
- 3.Gasparini G, Harris AL. Clinical importance of the determination of tumor angiogenesis in breast carcinoma: much more than a new prognostic tool. J. Clin. Oncol. 1995;13(3):765–782. doi: 10.1200/JCO.1995.13.3.765. [DOI] [PubMed] [Google Scholar]
- 4.Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis – correlation in invasive breast carcinoma. N. Engl. J. Med. 1991;324(1):1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- 5.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat. Med. 1995;1(1):27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 6.Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr. Rev. 1997;18(1):4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 7.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307(5706):58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 8.Brekken RA, Thorpe PE. VEGF–VEGF receptor complexes as markers of tumor vascular endothelium. J. Control. Release. 2001;74(1-3):173–181. doi: 10.1016/s0168-3659(01)00333-9. [DOI] [PubMed] [Google Scholar]
- 9.Sheldrake HM, Patterson LH. Function and antagonism of beta3 integrins in the development of cancer therapy. Curr. Cancer Drug Targets. 2009;9(4):519–540. doi: 10.2174/156800909788486713. [DOI] [PubMed] [Google Scholar]
- 10.Geng JG, Chen M, Chou KC. P-selectin cell adhesion molecule in inflammation, thrombosis, cancer growth and metastasis. Curr. Med. Chem. 2004;11(16):2153–2160. doi: 10.2174/0929867043364720. [DOI] [PubMed] [Google Scholar]
- 11.Nassiri F, Cusimano MD, Scheithauer BW, et al. Endoglin (CD105): a review of its role in angiogenesis and tumor diagnosis, progression and therapy. Anticancer Res. 2011;31(6):2283–2290. [PubMed] [Google Scholar]
- 12.Baluk P, Hashizume H, McDonald DM. Cellular abnormalities of blood vessels as targets in cancer. Curr. Opin. Genet. Dev. 2005;15(1):102–111. doi: 10.1016/j.gde.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 13.Stohrer M, Boucher Y, Stangassinger M, Jain RK. Oncotic pressure in solid tumors is elevated. Cancer Res. 2000;60(15):4251–4255. [PubMed] [Google Scholar]
- 14.Jain RK. Normalizing tumor microenvironment to treat cancer: bench to bedside to biomarkers. J. Clin. Oncol. 2013;31(17):2205–2218. doi: 10.1200/JCO.2012.46.3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weidner N, Folkman J, Pozza F, et al. Tumor angiogenesis: a new significant and independent prognostic indicator in early-stage breast carcinoma. J. Natl Cancer Inst. 1992;84(24):1875–1887. doi: 10.1093/jnci/84.24.1875. [DOI] [PubMed] [Google Scholar]
- 16.Folkman J. What is the evidence that tumors are angiogenesis dependent? J. Natl Cancer Inst. 1990;82(1):4–6. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- 17.Brawer MK, Bigler SA, Deering RE. Quantitative morphometric analysis of the microcirculation in prostate carcinoma. J. Cell Biochem. Suppl. 1992;16H:62–64. doi: 10.1002/jcb.240501213. [DOI] [PubMed] [Google Scholar]
- 18.Hoyt K, Sorace A, Saini R. Quantitative mapping of tumor vascularity using volumetric contrast-enhanced ultrasound. Invest. Radiol. 2012;47(3):167–174. doi: 10.1097/RLI.0b013e318234e6bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferrara KW, Merritt CR, Burns PN, Foster FS, Mattrey RF, Wickline SA. Evaluation of tumor angiogenesis with US: imaging, Doppler, and contrast agents. Acad. Radiol. 2000;7(10):824–839. doi: 10.1016/s1076-6332(00)80631-5. [DOI] [PubMed] [Google Scholar]
- 20.Fleischer AC. Sonographic depiction of tumor vascularity and flow: from in vivo models to clinical applications. J. Ultrasound Med. 2000;19(1):55–61. doi: 10.7863/jum.2000.19.1.55. [DOI] [PubMed] [Google Scholar]
- 21.Benson SR, Blue J, Judd K, Harman JE. Ultrasound is now better than mammography for the detection of invasive breast cancer. Am. J. Surg. 2004;188(4):381–385. doi: 10.1016/j.amjsurg.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 22.Cohen MP, Herman P, Chojniak R, Poli MR, Barbosa PN, Bitencourt AG. Focused abdominal ultrasound in preoperative liver surgery staging: a prospective study. World J. Surg. Oncol. 2013;11:138. doi: 10.1186/1477-7819-11-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D’Onofrio M, Malagò R, Zamboni G, et al. Contrast-enhanced ultrasonography better identifies pancreatic tumor vascularization than helical CT. Pancreatology. 2005;5(4-5):398–402. doi: 10.1159/000086540. [DOI] [PubMed] [Google Scholar]
- 24.Eisenbrey JR, Forsberg F. Contrast-enhanced ultrasound for molecular imaging of angiogenesis. Eur. J. Nucl. Med. Mol. Imaging. 2010;37(Suppl. 1):S138–S146. doi: 10.1007/s00259-010-1449-6. [DOI] [PubMed] [Google Scholar]
- 25.Borden MA, Kruse DE, Caskey CF, Zhao S, Dayton PA, Ferrara KW. Influence of lipid shell physicochemical properties on ultrasound-induced microbubble destruction. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2005;52(11):1992–2002. doi: 10.1109/tuffc.2005.1561668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bordon MA, Dayton P, Shukui Z, Ferrara KW. Physicochemical properties of the microbubble lipid shell; Presented at: Ultrasonics Symposium, 2004 IEEE.; Montreal, Canada. 23-27 August 2004. [Google Scholar]
- 27.Feingold S, Gessner R, Guracar IM, Dayton PA. Quantitative volumetric perfusion mapping of the microvasculature using contrast ultrasound. Invest. Radiol. 2010;45(10):669–674. doi: 10.1097/RLI.0b013e3181ef0a78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoyt K, Warram JM, Umphrey H, et al. Determination of breast cancer response to bevacizumab therapy using contrast-enhanced ultrasound and artificial neural networks. J. Ultrasound Med. 2010;29(4):577–585. doi: 10.7863/jum.2010.29.4.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson CR, Hu X, Zhang H, et al. Ultrasound molecular imaging of tumor angiogenesis with an integrin targeted microbubble contrast agent. Invest. Radiol. 2011;46(4):215–224. doi: 10.1097/RLI.0b013e3182034fed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Forsberg F, Ro RJ, Fox TB, et al. Contrast enhanced maximum intensity projection ultrasound imaging for assessing angiogenesis in murine glioma and breast tumor models: a comparative study. Ultrasonics. 2011;51(3):382–389. doi: 10.1016/j.ultras.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wei K, Jayaweera AR, Firoozan S, Linka A, Skyba DM, Kaul S. Quantification of myocardial blood flow with ultrasound-induced destruction of microbubbles administered as a constant venous infusion. Circulation. 1998;97(5):473–483. doi: 10.1161/01.cir.97.5.473. [DOI] [PubMed] [Google Scholar]
- 32.Eisenbrey JR, Dave JK, Merton DA, Palazzo JP, Hall AL, Forsberg F. Parametric imaging using subharmonic signals from ultrasound contrast agents in patients with breast lesions. J. Ultrasound Med. 2011;30(1):85–92. doi: 10.7863/jum.2011.30.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Forsberg F, Kuruvilla B, Pascua MB, et al. Comparing contrast-enhanced color flow imaging and pathological measures of breast lesion vascularity. Ultrasound Med. Biol. 2008;34(9):1365–1372. doi: 10.1016/j.ultrasmedbio.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chaudhari MH, Forsberg F, Voodarla A, et al. Breast tumor vascularity identified by contrast enhanced ultrasound and pathology: initial results. Ultrasonics. 2000;38(1-8):105–109. doi: 10.1016/s0041-624x(99)00146-8. [DOI] [PubMed] [Google Scholar]
- 35.Keller MW, Feinstein SB, Watson DD. Successful left ventricular opacification following peripheral venous injection of sonicated contrast agent: an experimental evaluation. Am. Heart J. 1987;114(3):570–575. doi: 10.1016/0002-8703(87)90754-x. [DOI] [PubMed] [Google Scholar]
- 36.Correas JM, Bridal L, Lesavre A, Méjean A, Claudon M, Hélénon O. Ultrasound contrast agents: properties, principles of action, tolerance, and artifacts. Eur. Radiol. 2001;11(8):1316–1328. doi: 10.1007/s003300100940. [DOI] [PubMed] [Google Scholar]
- 37.Broumas AR, Pollard RE, Bloch SH, Wisner ER, Griffey S, Ferrara KW. Contrast-enhanced computed tomography and ultrasound for the evaluation of tumor blood flow. Invest. Radiol. 2005;40(3):134–147. doi: 10.1097/01.rli.0000152833.35744.7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hudson JM, Karshafian R, Burns PN. Quantification of flow using ultrasound and microbubbles: a disruption replenishment model based on physical principles. Ultrasound Med. Biol. 2009;35(12):2007–2020. doi: 10.1016/j.ultrasmedbio.2009.06.1102. [DOI] [PubMed] [Google Scholar]
- 39.Quaia E, Nocentini A, Torelli L. Assessment of a new mathematical model for the computation of numerical parameters related to renal cortical blood flow and fractional blood volume by contrast-enhanced ultrasound. Ultrasound Med. Biol. 2009;35(4):616–627. doi: 10.1016/j.ultrasmedbio.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 40.Sehgal CM, Cary TW, Arger PH, Wood AK. Delta-projection imaging on contrast-enhanced ultrasound to quantify tumor microvasculature and perfusion. Acad. Radiol. 2009;16(1):71–78. doi: 10.1016/j.acra.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strouthos C, Lampaskis M, Sboros V, McNeilly A, Averkiou M. Indicator dilution models for the quantification of microvascular blood flow with bolus administration of ultrasound contrast agents. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2010;57(6):1296–1310. doi: 10.1109/TUFFC.2010.1550. [DOI] [PubMed] [Google Scholar]
- 42.Kuenen MP, Saidov TA, Wijkstra H, Mischi M. Contrastultrasound dispersion imaging for prostate cancer localization by improved spatiotemporal similarity analysis. Ultrasound Med. Biol. 2013;39(9):1631–1641. doi: 10.1016/j.ultrasmedbio.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 43.Kuenen M, Herold I, Korsten H, de la Rosette J, Wijkstra H, Mischi M. Maximum-likelihood estimation for indicator dilution analysis. IEEE Trans. Biomed. Eng. 2013 doi: 10.1109/TBME.2013.2290375. doi:10.1109/TBME.2013.2290375. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 44.Arditi M, Frinking PJ, Zhou X, Rognin NG. A new formalism for the quantification of tissue perfusion by the destruction-replenishment method in contrast ultrasound imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2006;53(6):1118–1129. doi: 10.1109/tuffc.2006.1642510. [DOI] [PubMed] [Google Scholar]
- 45.Lucidarme O, Nguyen T, Kono Y, et al. Angiogenesis model for ultrasound contrast research: exploratory study. Acad. Radiol. 2004;11(1):4–12. doi: 10.1016/s1076-6332(03)00575-0. [DOI] [PubMed] [Google Scholar]
- 46.Jain RK, Schlenger K, Höckel M, Yuan F. Quantitative angiogenesis assays: progress and problems. Nat. Med. 1997;3(11):1203–1208. doi: 10.1038/nm1197-1203. [DOI] [PubMed] [Google Scholar]
- 47.Lucidarme O, Kono Y, Corbeil J, et al. Angiogenesis: noninvasive quantitative assessment with contrast-enhanced functional US in murine model. Radiology. 2006;239(3):730–739. doi: 10.1148/radiol.2392040986. [DOI] [PubMed] [Google Scholar]
- 48.Zhang HP, Shi QS, Li F, et al. Regions of interest and parameters for the quantitative analysis of contrast-enhanced ultrasound to evaluate the anti-angiogenic effects of bevacizumab. Mol. Med. Rep. 2013;8(1):154–160. doi: 10.3892/mmr.2013.1499. [DOI] [PubMed] [Google Scholar]
- 49.Yoshida K, Hirokawa T, Moriyasu F, et al. Arterial-phase contrast-enhanced ultrasonography for evaluating anti-angiogenesis treatment: a pilot study. World J. Gastroenterol. 2011;17(8):1045–1050. doi: 10.3748/wjg.v17.i8.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Merz M, Komljenovic D, Semmler W, Bäuerle T. Quantitative contrast-enhanced ultrasound for imaging antiangiogenic treatment response in experimental osteolytic breast cancer bone metastases. Invest. Radiol. 2012;47(7):422–429. doi: 10.1097/RLI.0b013e31824f635a. [DOI] [PubMed] [Google Scholar]
- 51.Liu Y, Ren W, Liu C, et al. Contrast-enhanced ultrasonography of the rabbit VX2 tumor model: analysis of vascular pathology. Oncol. Lett. 2012;4(4):685–690. doi: 10.3892/ol.2012.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hwang M, Hariri G, Lyshchik A, Hallahan DE, Fleischer AC. Correlation of quantified contrast-enhanced sonography with in vivo tumor response. J. Ultrasound Med. 2010;29(4):597–607. doi: 10.7863/jum.2010.29.4.597. [DOI] [PubMed] [Google Scholar]
- 53.Gessner RC, Aylward SR, Dayton PA. Mapping microvasculature with acoustic angiography yields quantifiable differences between healthy and tumor-bearing tissue volumes in a rodent model. Radiology. 2012;264(3):733–740. doi: 10.1148/radiol.12112000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hoyt K, Sorace A. Volumetric contrast-enhanced ultrasound for characterizing tumor vascularity: preliminary studies in an animal model. Proc. IEEE Ultrasonics Sympos. 2011;1:1292–1295. [Google Scholar]
- 55.Hoyt K, Sorace A, Saini R. Volumetric contrast-enhanced ultrasound imaging to assess early response to apoptosis-inducing anti-death receptor 5 antibody therapy in a breast cancer animal model. J. Ultrasound Med. 2012;31(11):1759–1766. doi: 10.7863/jum.2012.31.11.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mahoney M, Sorace AG, Harbin B, Hoyt K. Real-time volumetric contrast-enhanced ultrasound and microbubble destruction-replenishment imaging: Preliminary in vitro and in vivo results. Proc. IEEE Ultrasonics Sympos. 2012;1:2157–2160. [Google Scholar]
- 57.Eisenbrey JR, Sridharan A, Machado P, et al. Three-dimensional subharmonic ultrasound imaging in vitro and in vivo. Acad. Radiol. 2012;19(6):732–739. doi: 10.1016/j.acra.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sridharan A, Eisenbrey JR, Liu JB, et al. Perfusion estimation using contrast-enhanced 3-dimensional subharmonic ultrasound imaging: an in vivo study. Invest. Radiol. 2013;48(9):654–660. doi: 10.1097/RLI.0b013e3182925160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J. Natl Cancer Inst. 2000;92(3):205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 60.Lassau N, Brule A, Chami L, Benatsou B, Péronneau P, Roche A. Evaluation of early response to antiangiogenic treatment with dynamic contrast enhanced ultrasound. J. Radiol. 2008;89(5 Pt 1):549–555. doi: 10.1016/s0221-0363(08)71480-x. [DOI] [PubMed] [Google Scholar]
- 61.Lassau N, Chami L, Benatsou B, Peronneau P, Roche A. Dynamic contrast-enhanced ultrasonography (DCE-US) with quantification of tumor perfusion: a new diagnostic tool to evaluate the early effects of antiangiogenic treatment. Eur. Radiol. 2007;17(Suppl. 6):F89–F98. doi: 10.1007/s10406-007-0233-6. [DOI] [PubMed] [Google Scholar]
- 62.Giorgi U, Aliberti C, Benea G, Conti M, Marangolo M. Effect of angiosonography to monitor response during imatinib treatment in patients with metastatic gastrointestinal stromal tumors. Clin. Cancer Res. 2005;11(17):6171–6176. doi: 10.1158/1078-0432.CCR-04-2046. [DOI] [PubMed] [Google Scholar]
- 63.Hochedez P, Lassau N, Bonvalot S, Bidault S, Leclère J, Avril MF. Treatment of local recurrent melanomas by isolated limb perfusion: value of Doppler ultrasonography. J. Radiol. 2003;84(5):597–603. [PubMed] [Google Scholar]
- 64.Dietrich CF, Averkiou MA, Correas JM, Lassau N, Leen E, Piscaglia F. An EFSUMB introduction into dynamic contrast-enhanced ultrasound (DCE-US) for quantification of tumour perfusion. Ultraschall. Med. 2012;33(4):344–351. doi: 10.1055/s-0032-1313026. [DOI] [PubMed] [Google Scholar]
- 65.Zhao H, Xu R, Ouyang Q, Chen L, Dong B, Huihua Y. Contrast-enhanced ultrasound is helpful in the differentiation of malignant and benign breast lesions. Eur. J. Radiol. 2010;73(2):288–293. doi: 10.1016/j.ejrad.2009.05.043. [DOI] [PubMed] [Google Scholar]
- 66.Schirin-Sokhan R, Winograd R, Roderburg C, et al. Response evaluation of chemotherapy in metastatic colorectal cancer by contrast enhanced ultrasound. World J. Gastroenterol. 2012;18(6):541–545. doi: 10.3748/wjg.v18.i6.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lassau N, Lamuraglia M, Chami L, et al. Gastrointestinal stromal tumors treated with imatinib: monitoring response with contrast-enhanced sonography. AJR Am. J. Roentgenol. 2006;187(5):1267–1273. doi: 10.2214/AJR.05.1192. [DOI] [PubMed] [Google Scholar]
- 68.Lassau N, Koscielny S, Chami L, et al. Advanced hepatocellular carcinoma: early evaluation of response to bevacizumab therapy at dynamic contrast-enhanced US with quantification - preliminary results. Radiology. 2011;258(1):291–300. doi: 10.1148/radiol.10091870. [DOI] [PubMed] [Google Scholar]
- 69.Lassau N, Koscielny S, Albiges L, et al. Metastatic renal cell carcinoma treated with sunitinib: early evaluation of treatment response using dynamic contrast-enhanced ultrasonography. Clin. Cancer Res. 2010;16(4):1216–1225. doi: 10.1158/1078-0432.CCR-09-2175. [DOI] [PubMed] [Google Scholar]
- 70.Lassau N, Chapotot L, Benatsou B, et al. Standardization of dynamic contrast-enhanced ultrasound for the evaluation of antiangiogenic therapies: the French multicenter Support for Innovative and Expensive Techniques Study. Invest. Radiol. 2012;47(12):711–716. doi: 10.1097/RLI.0b013e31826dc255. [DOI] [PubMed] [Google Scholar]
- 71.Saini R, Sorace AG, Warram JM, Mahoney MJ, Zinn KR, Hoyt K. An animal model allowing controlled receptor expression for molecular ultrasound imaging. Ultrasound Med. Biol. 2013;39(1):172–180. doi: 10.1016/j.ultrasmedbio.2012.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sorace AG, Saini R, Mahoney M, Hoyt K. Molecular ultrasound imaging using a targeted contrast agent for assessing early tumor response to antiangiogenic therapy. J. Ultrasound Med. 2012;31(10):1543–1550. doi: 10.7863/jum.2012.31.10.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Miller DL, Averkiou MA, Brayman AA, et al. Bioeffects considerations for diagnostic ultrasound contrast agents. J. Ultrasound Med. 2008;27(4):611–632. doi: 10.7863/jum.2008.27.4.611. quiz 633-616. [DOI] [PubMed] [Google Scholar]
- 74.Heath CH, Sorace A, Knowles J, Rosenthal E, Hoyt K. Microbubble therapy enhances anti-tumor properties of cisplatin and cetuximab in vitro and in vivo. Otolaryngol. Head Neck Surg. 2012;146(6):938–945. doi: 10.1177/0194599812436648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sorace AG, Warram JM, Umphrey H, Hoyt K. Microbubble-mediated ultrasonic techniques for improved chemotherapeutic delivery in cancer. J. Drug Target. 2012;20(1):43–54. doi: 10.3109/1061186X.2011.622397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sorace A, Saini R, Rosenthal E, Warram J, Zinn K, Hoyt K. Optical fluorescent imaging to monitor temporal effects of microbubble-mediated ultrasound therapy. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2013;60(2):281–289. doi: 10.1109/TUFFC.2013.2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhao S, Kruse DE, Ferrara KW, Dayton PA. Selective imaging of adherent targeted ultrasound contrast agents. Phys. Med. Biol. 2007;52(8):2055–2072. doi: 10.1088/0031-9155/52/8/002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Needles A, Couture O, Foster FS. A method for differentiating targeted microbubbles in real time using subharmonic micro-ultrasound and interframe filtering. Ultrasound Med. Biol. 2009;35(9):1564–1573. doi: 10.1016/j.ultrasmedbio.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 79.Patil AV, Rychak JJ, Allen JS, Klibanov AL, Hossack JA. Dual frequency method for simultaneous translation and real-time imaging of ultrasound contrast agents within large blood vessels. Ultrasound Med. Biol. 2009;35(12):2021–2030. doi: 10.1016/j.ultrasmedbio.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hu X, Zheng H, Kruse DE, Sutcliffe P, Stephens DN, Ferrara KW. A sensitive TLRH targeted imaging technique for ultrasonic molecular imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2010;57(2):305–316. doi: 10.1109/TUFFC.2010.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sugimoto K, Moriyasu F, Negishi Y, et al. Quantification in molecular ultrasound imaging: a comparative study in mice between healthy liver and a human hepatocellular carcinoma xenograft. J. Ultrasound Med. 2012;31(12):1909–1916. doi: 10.7863/jum.2012.31.12.1909. [DOI] [PubMed] [Google Scholar]
- 82.Saini R, Warram JM, Sorace AG, Umphrey H, Zinn KR, Hoyt K. Model system using controlled receptor expression for evaluating targeted ultrasound contrast agents. Ultrasound Med. Biol. 2011;37(8):1306–1313. doi: 10.1016/j.ultrasmedbio.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bzyl J, Lederle W, Rix A, et al. Molecular and functional ultrasound imaging in differently aggressive breast cancer xenografts using two novel ultrasound contrast agents (BR55 and BR38) Eur. Radiol. 2011;21(9):1988–1995. doi: 10.1007/s00330-011-2138-y. [DOI] [PubMed] [Google Scholar]
- 84.Bzyl J, Palmowski M, Rix A, et al. The high angiogenic activity in very early breast cancer enables reliable imaging with VEGFR2-targeted microbubbles (BR55) Eur. Radiol. 2013;23(2):468–475. doi: 10.1007/s00330-012-2594-z. [DOI] [PubMed] [Google Scholar]
- 85.Palmowski M, Huppert J, Ladewig G, et al. Molecular profiling of angiogenesis with targeted ultrasound imaging: early assessment of antiangiogenic therapy effects. Mol. Cancer Ther. 2008;7(1):101–109. doi: 10.1158/1535-7163.MCT-07-0409. [DOI] [PubMed] [Google Scholar]
- 86.Pysz MA, Foygel K, Rosenberg J, Gambhir SS, Schneider M, Willmann JK. Antiangiogenic cancer therapy: monitoring with molecular US and a clinically translatable contrast agent (BR55) Radiology. 2010;256(2):519–527. doi: 10.1148/radiol.10091858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tardy I, Pochon S, Theraulaz M, et al. Ultrasound molecular imaging of VEGFR2 in a rat prostate tumor model using BR55. Invest. Radiol. 2010;45(10):573–578. doi: 10.1097/RLI.0b013e3181ee8b83. [DOI] [PubMed] [Google Scholar]
- 88.Pochon S, Tardy I, Bussat P, et al. BR55: a lipopeptide-based VEGFR2-targeted ultrasound contrast agent for molecular imaging of angiogenesis. Invest. Radiol. 2010;45(2):89–95. doi: 10.1097/RLI.0b013e3181c5927c. [DOI] [PubMed] [Google Scholar]
- 89.Willmann JK, Cheng Z, Davis C, et al. Targeted microbubbles for imaging tumor angiogenesis: assessment of whole-body biodistribution with dynamic micro-PET in mice. Radiology. 2008;249(1):212–219. doi: 10.1148/radiol.2491072050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Willmann JK, Paulmurugan R, Chen K, et al. US imaging of tumor angiogenesis with microbubbles targeted to vascular endothelial growth factor receptor type 2 in mice. Radiology. 2008;246(2):508–518. doi: 10.1148/radiol.2462070536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Warram JM, Sorace AG, Saini R, Umphrey HR, Zinn KR, Hoyt K. A triple-targeted ultrasound contrast agent provides improved localization to tumor vasculature. J. Ultrasound Med. 2011;30(7):921–931. doi: 10.7863/jum.2011.30.7.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Willmann JK, Lutz AM, Paulmurugan R, et al. Dual-targeted contrast agent for US assessment of tumor angiogenesis in vivo. Radiology. 2008;248(3):936–944. doi: 10.1148/radiol.2483072231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Deshpande N, Ren Y, Foygel K, Rosenberg J, Willmann JK. Tumor angiogenic marker expression levels during tumor growth: longitudinal assessment with molecularly targeted microbubbles and US imaging. Radiology. 2011;258(3):804–811. doi: 10.1148/radiol.10101079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kiessling F, Gaetjens J, Palmowski M. Application of molecular ultrasound for imaging integrin expression. Theranostics. 2011;1:127–134. doi: 10.7150/thno/v01p0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Streeter JE, Gessner RC, Tsuruta J, Feingold S, Dayton PA. Assessment of molecular imaging of angiogenesis with three-dimensional ultrasonography. Mol. Imaging. 2011;10(6):460–468. [PMC free article] [PubMed] [Google Scholar]
- 96.Palmowski M, Peschke P, Huppert J, et al. Molecular ultrasound imaging of early vascular response in prostate tumors irradiated with carbon ions. Neoplasia. 2009;11(9):856–863. doi: 10.1593/neo.09540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ellegala DB, Leong-Poi H, Carpenter JE, et al. Imaging tumor angiogenesis with contrast ultrasound and microbubbles targeted to alpha(v)beta3. Circulation. 2003;108(3):336–341. doi: 10.1161/01.CIR.0000080326.15367.0C. [DOI] [PubMed] [Google Scholar]
- 98.Leong-Poi H, Christiansen J, Klibanov AL, Kaul S, Lindner JR. Noninvasive assessment of angiogenesis by ultrasound and microbubbles targeted to alpha(v)-integrins. Circulation. 2003;107(3):455–460. doi: 10.1161/01.cir.0000044916.05919.8b. [DOI] [PubMed] [Google Scholar]
- 99.Sirsi SR, Flexman ML, Vlachos F, et al. Contrast ultrasound imaging for identification of early responder tumor models to anti-angiogenic therapy. Ultrasound Med. Biol. 2012;38(6):1019–1029. doi: 10.1016/j.ultrasmedbio.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Korpanty G, Carbon JG, Grayburn PA, Fleming JB, Brekken RA. Monitoring response to anticancer therapy by targeting microbubbles to tumor vasculature. Clin. Cancer Res. 2007;13(1):323–330. doi: 10.1158/1078-0432.CCR-06-1313. [DOI] [PubMed] [Google Scholar]
- 101.Zhang Y, Yang Y, Hong H, Cai W. Multimodality molecular imaging of CD105 (endoglin) expression. Int. J. Clin. Exp. Med. 2011;4(1):32–42. [PMC free article] [PubMed] [Google Scholar]
- 102.Lyshchik A, Fleischer AC, Huamani J, Hallahan DE, Brissova M, Gore JC. Molecular imaging of vascular endothelial growth factor receptor 2 expression using targeted contrast-enhanced high-frequency ultrasonography. J. Ultrasound Med. 2007;26(11):1575–1586. doi: 10.7863/jum.2007.26.11.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Korpanty G, Carbon JG, Grayburn PA, Fleming JB, Brekken RA. Monitoring response to anticancer therapy by targeting microbubbles to tumor vasculature. Clin. Cancer Res. 2007;13(1):323–330. doi: 10.1158/1078-0432.CCR-06-1313. [DOI] [PubMed] [Google Scholar]
- 104.Knowles JA, Heath CH, Saini R, et al. Molecular targeting of ultrasonographic contrast agent for detection of head and neck squamous cell carcinoma. Arch. Otolaryngol. Head Neck Surg. 2012;138(7):662–668. doi: 10.1001/archoto.2012.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Patil AV, Rychak JJ, Klibanov AL, Hossack JA. Real-time technique for improving molecular imaging and guiding drug delivery in large blood vessels: in vitro and ex vivo results. Mol. Imaging. 2011;10(4):238–247. doi: 10.2310/7290.2011.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Frinking PJ, Tardy I, Théraulaz M, et al. Effects of acoustic radiation force on the binding efficiency of BR55, a VEGFR2-specific ultrasound contrast agent. Ultrasound Med. Biol. 2012;38(8):1460–1469. doi: 10.1016/j.ultrasmedbio.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 107.Unnikrishnan S, Klibanov AL. Microbubbles as ultrasound contrast agents for molecular imaging: preparation and application. AJR Am. J. Roentgenol. 2012;199(2):292–299. doi: 10.2214/AJR.12.8826. [DOI] [PubMed] [Google Scholar]
- 108.Marshall D, Pedley RB, Boden JA, Boden R, Melton RG, Begent RH. Polyethylene glycol modification of a galactosylated streptavidin clearing agent: effects on immunogenicity and clearance of a biotinylated anti-tumour antibody. Br. J. Cancer. 1996;73(5):565–572. doi: 10.1038/bjc.1996.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Klibanov AL. Preparation of targeted microbubbles: ultrasound contrast agents for molecular imaging. Med. Biol. Eng. Comput. 2009;47(8):875–882. doi: 10.1007/s11517-009-0498-0. [DOI] [PubMed] [Google Scholar]
- 110.Fokong S, Fragoso A, Rix A, et al. Ultrasound molecular imaging of E-selectin in tumor vessels using poly n-butyl cyanoacrylate microbubbles covalently coupled to a short targeting peptide. Invest. Radiol. 2013;48(12):843–850. doi: 10.1097/RLI.0b013e31829d03ec. [DOI] [PubMed] [Google Scholar]
- 111.Grouls C, Hatting M, Rix A, et al. Liver dysplasia: US molecular imaging with targeted contrast agent enables early assessment. Radiology. 2013;267(2):487–495. doi: 10.1148/radiol.13120220. [DOI] [PubMed] [Google Scholar]
- 112.Anderson CR, Rychak JJ, Backer M, Backer J, Ley K, Klibanov AL. scVEGF microbubble ultrasound contrast agents: a novel probe for ultrasound molecular imaging of tumor angiogenesis. Invest. Radiol. 2010;45(10):579–585. doi: 10.1097/RLI.0b013e3181efd581. [DOI] [PMC free article] [PubMed] [Google Scholar]