Abstract
Long term therapeutic drug monitoring and assessment of renal function are required in renal transplant recipients on immunosuppressant therapy such as tacrolimus. Dry blood spots (DBS) have been used successfully in the clinic for many years and offers a convenient, simple and non-invasive method for repeated blood tests. We developed and performed a preliminary validation of a method for the analysis of tacrolimus and creatinine from a single DBS using liquid chromatography-tandem mass spectrometric (LC–MS/MS). Tacrolimus and creatinine were extracted from a 6 mm punch with a mixture of methanol/acetonitrile containing ascomycin and deuterated creatinine as internal standards. A 10 μl aliquot of the extract was analyzed directly after dilution for creatinine with normal phase high performance liquid chromatography and multiple reaction monitoring. The remainder of the extract was processed and analyzed for tacrolimus. The lower limit of quantification for tacrolimus was 1 ng/ml with accuracy of 0.34% bias and precision (CV) of 11.1%. The precision ranged from 1.33% to 7.68% and accuracy from −4.44% to 11.6% bias for the intra- and inter-day analysis. The lower limit of quantification of creatinine was 0.01 mg/dL with precision of 7.94%. Accuracy was based on recovery of additional creatinine spiked into whole blood samples and ranged from −2.45% bias at 5 mg/dL to 3.75% bias at 0.5 mg/dL. Intra- and inter-day precision was from 3.48 to 4.11%. The assay was further validated with DBS prepared from pediatric renal transplant recipients. There was excellent correlation between the levels of tacrolimus and creatinine obtained from the clinical laboratory and the DBS method developed. After additional validation, this assay may have a significant impact on compliance with medication intake as well as potentially lowering the cost associated with intravenous blood draws in clinical laboratories.
Keywords: Creatinine, Tacrolimus, Therapeutic drug monitoring, Liquid chromatography tandem mass, spectrometry (LC–MS/MS), Dried blood spot (DBS)
1. Introduction
Childhood solid organ transplant recipients form a unique population with a life-long requirement for immunosuppression therapy. The calcineurin inhibitors, such as cyclosporine and tacrolimus (TAC), are the cornerstone of any pediatric immunosuppression protocol. Variable TAC levels are a primary indicator of medication non-adherence, which is common with adolescent and young adult transplant patients [1–4]. The 10-year kidney allograft survival for adolescents ages 12–18 years and young adults 19–24 is the lowest among all age groups, averaging 40.1% for deceased donors and 52.9% for living donor recipient [5]. Furthermore, non-adherence in adolescent renal transplant recipients can negatively impact the successful transition to adult care and affect long term graft outcome. A high rate of graft loss of up to 35% was reported within 3 years of transitioning the adolescents to adult care [6]. A high rate of graft loss of up to 35% was reported within 3 years of transitioning the adolescents to adult care [7]. Non adherence with immunosuppressant medications is also common among adult kidney transplant recipients leading to allograft rejection and loss [8]. A meta analysis reported a rate of non adherence of 36 cases per 100 persons per year [9], while another reported a rate of 27% [10]. The economic cost and societal implications of graft loss due to non-adherence in renal transplant recipients is yet to be fully recognized, but a number of reports confirm the general belief that renal transplantation is indeed a more cost effective modality for managing renal failure than dialysis [11,12]. Optimizing drug therapy through close monitoring may have a positive impact in improving adherence and ultimately graft survival while reducing the unwanted side effects of such therapy such as renal toxicity, viral infections and malignancies.
Tacrolimus levels exhibit very high inter- and intra-individual variability. Pharmacokinetic processes, mainly clearance, is considered to be the main causal factor of the high variability observed in blood levels [13,14]. Together with increased rates of non-adherence to drug regiments, the variable clearance necessitates routine therapeutic drug monitoring and is thus essential for the success of renal transplantation. In the past several years there has been a significant increase in the use of dried blood spots (DBS) for the collection and analysis of drug levels for pharmacokinetic studies and for therapeutic drug monitoring as a result of the development of methods using liquid chromatography with tandem mass spectrometry (LC–MS/MS) [15–21]. The use of DBS for the analysis of TAC was reported by several groups [22–28] and has been applied to TAC monitoring using a limited sampling strategy and abbreviated area under the curve estimation [29]. The analysis of TAC from salivary samples has also been reported with poor correlation to blood TAC levels [30].
Even with the current less-invasive DBS methods, the narrow therapeutic window and subsequent renal toxicity requires that a blood sample must also be obtained simultaneously to assess renal function by monitoring plasma creatinine. Plasma creatinine is routinely measured in clinical laboratories using an automated colorimetric Jaffe reaction [31–33]. Other methods reported include high pressure liquid chromatography (HPLC) with ultraviolet detection, gas chromatography–mass spectrometry (GC/MS), and LC–MS/MS [34–40].
It is essential to make therapeutic drug monitoring as simple and as non-invasive as possible to increase compliance. As a result, we sought to develop an assay that took advantage of DBS methods that would permit patients to take their own samples at home as well as permit the simultaneous analysis of both TAC and creatinine for assessing drug therapy compliance and kidney function, respectively. To this end we developed an LC–MS/MS assay for both TAC and creatinine from the same dried blood spot sample. This assay can have a significant impact both on compliance as well as potentially decreasing the costs associated with current methods for obtaining multiple blood samples for both the patients and the medical providers.
2. Experimental
2.1. Chemicals and reagents
Creatinine hydrochloride, ascomycin, ammonium acetate, ammonium hydroxide (28% ammonia in water), creatinine standard solutions and tacrolimus (FK 506) were from Sigma–Aldrich (St. Louis, MO). (Methyl-d3) creatinine was from CDN Isotopes (Quebec, Canada). The FTA-DMPK-A cards were from GE Healthcare Bio-Science (Piscataway, NJ). Methanol and water (GC–MS grade) were obtained from Burdick and Jackson (Muskegon, MI). Acetonitrile (GC–MS grade), and hydrochloric acid were from Fisher Scientific (Fairlawn, NJ). Formic acid was J.T. Baker brand (Phillipsburg, NJ). Sample vials were obtained from Sun Sri (a subdivision of Fisher Scientific, Rockwood, TN) and the Oasis HLB 1cc, 10 mg extraction cartridges were obtained from Waters (Taunton, MA) The standard 6 mm steel hole punch was obtained from McGill Inc. (Marengo, IL). The MassTrak® Immunosuppressants Kit was purchased from Waters Corporation (Milford, MA).
2.2. Human subjects
Informed consent and child assent were obtained from pediatric kidney transplant patients and patient study protocols were approved by the Oregon Health & Science University Institutional Review Board. Blood samples were obtained from pediatric transplant patients at the same time as their standard laboratory samples were taken during routine clinic visits using an Accu-check Single Use Lancet from Safe-T-Pro Plus, 23 gauge, 0.65 mm, used on the low setting of 1.3 mm. Table 1 describes the patient population used. In addition, Oregon Health & Science University Institutional Review Board approved our use of surplus blood samples that were obtained from OHSU’s clinical laboratories prior to disposal. These samples were used for the preparation of TAC calibrators and for development and validation of the creatinine assay.
Table 1.
Patient demographic and clinical laboratory data.a
| Mean ± S.D. (range) | |
|---|---|
| Age (years) | 14.0 ± 4.6 (6–20) |
| Gender | 12 male; 9 female |
| Weight (kg) | 47.0 ± 19.1 (15.6–87.3) |
| BSA (M2) | 1.00 ± 0.40 (0.66–2.06) |
| Plasma creatinine (mg/dL) | 1.34 ± 0.90 (0.42–3.84) |
| Whole blood TAC trough concentration (ng/ml) | 5.0 ± 1.5 (3.0–8.8) |
| Hemoglobin (g/dL) | 12.0 ± 1.1 (9.7–14.0) |
| Hematocrit (%) | 36.0 ± 3.4 (28.4–41.7) |
All participants enrolled in the study during their routine clinic visits to the pediatric kidney transplant clinic at Oregon Health and Science University (OHSU). The study was approved by the Institutional Review Board at OHSU (N = 21 patients).
2.3. Blood sample collection
FTA DMPK-A filter paper cards were used that contain 4 marked circles for blood spots. A phlebotomist was trained in proper technique to obtain a DBS. A blood drop obtained after fingerstick with a lancet was applied to two circles on the left-hand side of each card, completely filling the circle and taking care not to touch the card directly. Phlebotomy was performed at the same time to obtain venous blood by syringe for routine blood tests in the clinical laboratory. A blood drop from the syringe was applied to each of the remaining two circles on the right-hand side of the card. The cards were air dried on a horizontal rack for 12 h and placed in a desiccator at room temperature until analysis.
2.4. Preparation of calibrators
Creatinine stock solutions were prepared in water at a concentration of 0.1 and 1.0 mg/ml and stored at −20 °C. The stock was diluted the day of use in phosphate buffered saline (PBS) to prepare calibrators that ranged from 0 to 60 μg/ml (0 to 6.0 mg/dL). The internal standard, (methyl-d3) creatinine was dissolved in water at a concentration of 2.0 mg/ml, stored at −20 °C and diluted to a working stock of 3.0 μg/ml for sample preparation each day. Stocks of creatinine were compared to commercially available standard solutions and did not vary by more than 5%.
TAC stock solutions were prepared in methanol at a concentration of 1.0 mg/ml and stored at −20 °C. The stock was diluted in methanol the day of use to working stocks of 1 and 10 ng/μl when calibrator blood samples were prepared. The internal standard, ascomycin was dissolved in methanol at a concentration of 500 ng/ml and stored at −20 °C. It was diluted into methanol/acetonitrile (80:20) fresh to a working stock of 4.0 ng/ml each day. TAC calibrators were prepared by spiking whole blood with concentrations of tacrolimus in clinically relevant concentrations ranging from 1 to 50 ng/ml. A 30 μl aliquot was then applied to the cards in each of the four circles. The cards were dried at room temperature as with the blood samples and stored in a desiccator until used as calibrators for unknown samples. The assumption was made that all blood samples were saturating the card and therefore the same blood volume was obtained with a 6 mm punch obtained manually using a standard 6 mm steel hole punch from McGill, Inc (Marengo, IL) from the calibrators as well as the unknown samples.
2.5. Preparation of TAC and creatinine samples
Tacrolimus was determined in whole blood using LC–MS/MS as described by Bogusz et al. [19]. This was accomplished using the MassTrack Immunosuppressants Kit® from Waters Corporation with ascomycin as the internal standard. The kit was used as directed with 50 μl of whole blood. Briefly, the blood sample was treated with 200 μl of 0.1 M zinc sulfate and vortex mixed for 4 s. Then 500 μl of acetonitrile containing the internal standard, ascomycin, at a final concentration of 2 ng/ml was added and vortex mixed for 20 s. Precipitated protein was removed by centrifugation for 5 min at 14,000 × g. The supernatant was transferred to an autosampler vial and analyzed by LC–MS/MS as described below.
The DBS method of Hoogtanders et al. [23] for TAC determination showed excellent correlation with the whole blood assay and was used for a limited sampling strategy to monitor TAC [22,23]. They used an on line solid phase extraction system so we adapted this to manual solid phase extraction columns after elution from the DBS. Briefly, a 6 mm punch was removed from the DMPK-A blood spot card spotted with 30–50 μl of whole blood containing from 1 to 50 ng/ml of TAC. The punch was placed in a glass test tube and 250 μl of extraction solution (methanol/acetonitrile (80:20) containing 4 ng/ml of the internal standard, ascomycin) was added. The tubes were capped, vortex mixed briefly to ensure the punch was at the bottom of the tube and placed into a New Brunswick Scientific C24 Incubator shaker at 25–27 °C at 100 rpm for 60 min. After dilution with 750 μl of water, samples were processed with a 10 mg Oasis HLB extraction cartridge. The cartridge was pre-equilibrated with 1 ml of methanol and then 1 ml of water and the sample applied by gravity feed. After washing the column with 1 ml of 30% methanol, the column was dried for 10 min under maximum house vacuum (between 5 mm Hg to 15 mm Hg). The analytes were eluted with 500 μl of 0.1% formic acid in acetonitrile. The eluate was dried under reduced pressure using a Savant speed vac. The residue was dissolved in 50 μl of acetonitrile and 10 μl was analyzed by LC–MS/MS as described below.
We initially developed an LC–MS/MS assay for creatinine from whole blood using methyl-d3-creatinine as the internal standard. A 15 μl portion of whole blood was added to a 1.5 ml microcentrifuge tube and treated with 250 μl of the same extraction buffer used for TAC analysis (methanol/acetonitrile (80:20) containing the internal standard, methyl-d3-creatinine at a concentration of 3 μg/ml). After vortex mixing for 30 sec and centrifugation at 14,000 × g for 5 min to remove particulates, a 10 μl aliquot of the supernatant was removed and added to 390 μl of acetonitrile containing 0.4 mM HCl. A 5 μl aliquot was analyzed by LC–MS/MS as described below.
For the DBS analysis of creatinine, 250 μl of extraction solution (methanol/acetonitrile, 80:20) containing 4 ng/ml of ascomycin and 3 μg/ml methyl-d3-creatinine was added to the DBS. For creatinine only determination, ascomycin was omitted. The tubes were capped, vortex mixed briefly to ensure the punch was at the bottom of the tube and placed into a New Brunswick Scientific C24 Incubator shaker at 25–27 °C at 100 rpm for 60 min. After extraction, a 10 μl aliquot was removed added to 390 μl of acetonitrile containing 0.4 mM HCl, and a 5 μl aliquot was analyzed by LC–MS/MS as with the whole blood assay. When both creatinine and TAC were determined from the same DBS, the remainder of the supernatant was diluted with water and prepared for TAC analysis as described above.
2.6. LC–MS/MS analysis
The HPLC system for the analysis of the calibrators, whole blood and DBS samples for both analytes was a Shimadzu (Columbia, MD) SIL-20AC XR auto-sampler, a CBM-20A system controller, two LC-20AD XR LC pumps, a DGU-20 A5 in-line solvent degasser and a CTO-20A column oven.
Creatinine (5 μl injection), was resolved on an HYPERSIL Silica (50 mm × 2.1 mm, 3 μm) column with a Betasil Silica 100 Javelin pre-column maintained at 40 °C. An isocratic mobile phase composed of acetonitrile:methanol:water:ammonium hydroxide (28% ammonia in water) (1000:50:50:2) was delivered at a flow rate of 0.4 ml/min as described by Ziemniak et al. [40]. The run time was 5 min. Column integrity and resolution was maintained by including a wash step of 100% methanol for 1 h after every 20 samples and at the end of sample analysis. For the analysis of creatinine the LC system was interfaced to an Applied Biosystems/MDS SCIEX 4000 QTRAP triple-quadrupole hybrid linear ion trap mass spectrometer (Foster City, CA) and was used in triple quadrupole mode with multiple reaction monitoring (MRM). It was equipped with a TurboIonSpray® ESI source operated in the positive mode with the following settings: source voltage 3.5 kV, nebulizer gas (GS1) 40 psi, heater gas (GS2) 50 psi, curtain gas (CUR) 40 psi, source temperature (TEM) 450 °C and collision associated dissociate gas (CAD) MED. Gases were 99.999% nitrogen. The MRM transitions monitored were m/z 114.1 → 86.2, and m/z 114.1 → 72.1 for creatinine, and m/z 117.1 → 89.2, and m/z 117.1 → 75.1 for d3-creatinine. Optimal parameters for the four MRM transitions were: collision energy (CE; V): 17, 23, 27 and 27, respectively; the declustering potential (DP; V) 41, 41, 56, and 46, respectively, the collision cell exit potential (CXP; V): 6, 12, 16, and 16 respectively; the exit potential (EP) was 10 V. The MRM transitions of m/z 114.1 → 86.2 and m/z 117.1 → 89.2 were used for quantification. Dwell times were 150 ms and Q1 and Q3 were operated at unit resolution.
TAC and ascomycin were resolved on a Waters Nova-Pak C18 column (10 mm × 2.1 mm, 60 Å, 4 μm, part no. 186003523) maintained at 35 °C. The gradient mobile phase was delivered at a flow rate of 0.6 ml/min, and consisted of two solvents: A, 2 mm ammonium acetate with 0.1% formic acid in water and B, 2 mm ammonium acetate with 0.1% formic acid in methanol. The gradient was 50% Solvent B for 0.1 min; increase to 100% solvent B in 0.3 min; held for 1.5 min at 100% solvent B; returned to 50% solvent B in 0.1 min; re-equilibrated at 50% solvent B for 2.4 min. The LC system was interfaced to an Applied Biosystems/MDS SCIEX 5500 QTRAP triple-quadrupole hybrid linear ion trap (Foster City, CA) and was used in triple quadrupole mode with multiple reaction monitoring. It was equipped with a TurboIonSpray® ESI source operated in the positive mode with the following settings: source voltage 4.5 kV, GS1 50 psi, GS2 40 psi, CUR 30 psi, TEM 450 °C and CAD gas MED. Gases were 99.999% nitrogen. The MRM transitions monitored were m/z 821 → 768.2, and m/z 821 → 786.2 for TAC, and m/z 809 → 756.2, and m/z 809 → 564.1 for ascomycin [21]. The DP was 86 V for TAC, and 81 V for ascomycin. The dwell time was 150 ms, and Q1 and Q3 were operated at unit resolution. The optimal instrument parameters for the four MRM transitions were: EP, 10 V; CE (V), 29, 23, 29, and 33, respectively; CXP (V), 4, 4, 4 and 8, respectively. The MRM transitions used for quantification were m/z 821 → 768.2 for TAC and m/z 809 → 756.2 for ascomycin. Instrument control, data acquisition, and analysis were done with Analyst® Software (Version 1.5).
2.7. Assay performance, validation and statistical analysis
Standard and quality control samples were prepared by processing whole blood from healthy subjects spiked with TAC or creatinine. Standard curves were constructed by calculating the ratio of the analyte peak area to that of the internal standard and plotting the ratio versus the theoretical concentration. Data was fit using weighted (1/x) least squares regression analysis. Standard curves were acceptable if the correlation coefficient was >0.99 and calculated accuracies of the standards were within 20% of the theoretical value for the lower limit of quantification and <15% of the theoretical values for the remaining standards. The lower limit of quantification (LLOQ) was determined experimentally to be the lowest concentration within the linear range of the standard curve having an accuracy within 20% of the theoretical value and a coefficient of variation of ≤20%. Intra-day accuracy and precision were calculated by comparing the theoretical and experimentally determined concentrations in six replicates of QC samples prepared at three different concentrations that low, middle and high GC samples within the linear range of the standard curves. Inter-day accuracy and precision was determined from triplicate QC samples prepared on three different days.
Statistical analysis was done using SigmaPlot Version 12.3 from Systat Software, Inc. (San Jose, CA). The parallel line test was used to test for the equality of slopes and intercepts. Different analytical methods were compared using the method of Bland–Altman [41].
3. Results
3.1. TAC determination and validation from DBS cards
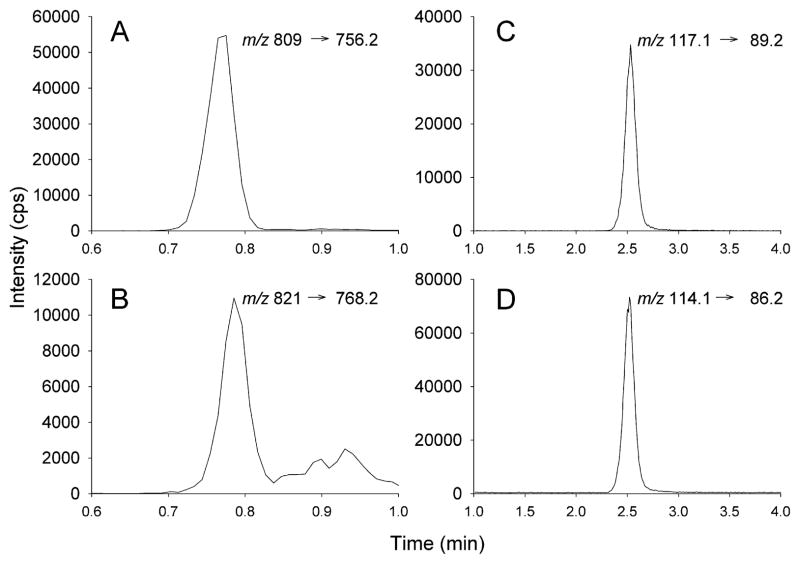
Hoogtanders et al. [23] described a DBS assay for TAC that involved on line solid phase extraction using a Waters Oasis HLB cartridge column. We adapted this strategy using standard Oasis HLB solid phase extraction cartridges after elution of the DBS as described by Hoogtanders et al. [21]. Prior to preparing calibrator blood spot cards we established the concentration of TAC in whole blood using the validated kit, MassTrack Immunosuppressants Kit®, from Waters Corporation as directed. This method is based on the method described by Bogusz et al. [13]. The accuracy of determining the quality controls included in the kit was 11.1% at 2.2 ng/ml TAC and 6.6% at 27.2 ng/ml TAC (N = 3 for both concentrations). Whole blood spiked with increasing concentrations of TAC characterized in this way was then used to prepare DBS standard for quantification. A typical extracted ion chromatogram (XIC) of TAC and the internal standard, ascomycin, are shown in Fig. 1A and B.
Fig. 1.
Extracted ion chromatograms (XIC) for the analysis of TAC (left hand panels) and creatinine (right hand panels). DBS were processed as described in the text. The MRM transitions monitored are indicated in each panel. The on-column levels of the compounds were: ascomycin, 200 pg; TAC, 9 pg; methyl-d3-creatinine, 0.375 ng; creatinine 0.188 ng.
Standard curves were prepared from cards stored over a period of 4 weeks dessicated at room temperature and the mean slope was 0.0091 with a SD of 0.0007 (N = 5; CV = 7.74%). Beyond 4 weeks, the slope decreased significantly. The reason for this change when stored dessicated at room temperature was not explored further and all samples were analyzed within one month of collection and DBS calibrator cards were prepared monthly. Additional studies are required to optimize storage for longer times at different temperatures although samples prepared for TDM are usually processed within a month. Samples of whole blood were spiked with TAC from 1 ng/ml to 50 ng/ml and analyzed. The intra-day variability at 1 ng/ml, the lower limit of quantification, was 11.1% and the accuracy was 0.34% and at 50 ng/ml the variability was 6.5% with an accuracy of 0.62% (N = 4 at each concentration). A representative standard curve is shown in supplementary data, Fig. 1S.
The intra- and inter-day variability of three different quality control concentrations of TAC, low (3 ng/ml), middle (10 ng/ml) and high (30 ng/ml), was assessed over a three day period. The results are presented in Table 2. As reported by Hoogtanders et al. [23] the analysis from DBS was very sensitive with excellent accuracy and precision at all three concentrations.
Table 2.
Summary of intra-day and inter-day variability of TAC quality control sample results from three day validation runs.
| LQC (3 ng/ml) | MQC (10 ng/ml) | HQC (30 ng/ml) | |
|---|---|---|---|
| Intra-day mean Day 1 | 3.13 | 9.85 | 31.51 |
| Intra-day SD | 0.10 | 0.20 | 0.42 |
| Intra-day % CV | 3.09 | 2.01 | 1.33 |
| Intra-day % Bias | −4.44 | 1.51 | −5.02 |
| N | 6 | 6 | 6 |
| Intra-day mean Day 2 | 2.86 | 8.84 | 28.42 |
| Intra-day SD | 0.09 | 0.13 | 0.91 |
| Intra-day % CV | 3.18 | 1.52 | 3.21 |
| Intra-day % Bias | 4.46 | 11.55 | 5.24 |
| N | 3 | 3 | 3 |
| Intra-day mean Day 3 | 2.93 | 9.68 | 30.76 |
| Intra-day SD | 0.22 | 0.28 | 1.36 |
| Intra-day % CV | 7.68 | 2.86 | 4.43 |
| Intra-day % Bias | 2.34 | 3.17 | −2.53 |
| N | 3 | 3 | 3 |
| Overall mean | 3.01 | 9.56 | 30.55 |
| Inter-day SD | 0.18 | 0.47 | 1.52 |
| Inter-day % CV | 5.85 | 4.95 | 4.97 |
| Inter-run % Bias | −0.47 | 4.44 | −1.83 |
| N | 12 | 12 | 12 |
3.2. Creatinine determination and validation from DBS cards
The goal of the current study was to develop methodology that allowed the determination of both TAC and creatinine from a single DBS. As a result, we analyzed a sample of the initial DBS extract for TAC prior to solid phase extraction for creatinine using chromatographic conditions described by Ziemniak et al. [40]. This normal phase chromatographic method permitted the direct injection of the extract without further processing. We found that with reverse phase methods it was necessary to remove all organic solvent, a time consuming step in the protocol. Typical XIC’s for creatinine and the deuterated internal standard are shown in Fig. 1, Panels C and D. Excellent chromatographic peak shape was obtained with isocratic elution.
Since creatinine is an endogenous compound it is impossible to obtain “blank” blood for the preparation of standard curves from calibrator samples. Attempts to generate “blank” washed red blood cells by pelleting the red cells and then resuspending in volumes of PBS equivalent to the plasma removed were not successful. After 5 washes creatinine was still present. As a result, we initially generated calibration curves with samples prepared in PBS and whole blood. Additionally, the same whole blood was spotted on DBS cards and all samples were processed for creatinine determination. As expected, the regression analysis of the standards prepared in PBS yielded near zero intercept and those from whole blood and the DBS card are positive and significantly different than zero (P < 0.005), as a result of the presence of endogenous creatinine (supplementary data, Fig. 2S). Importantly, the slopes of all three curves are not significantly different from each other (P < 0.1639). As a result, standard curves for the DBS analysis could be generated in PBS and used for quantification of the DBS samples. The volume of the PBS standards used (15 μl) was selected based on an estimate of the volume of blood associated with the 6 mm punch of 14.7 μl determined from the area of the spots of different volumes. We more accurately assessed the volume in a 6 mm punch using the endogenous concentration of creatinine and the area ratio of the internal standard, methyl-d3 creatinine. A series of volumes of the same whole blood sample (2, 5, 10, 15, 20, and 30 μl) were spotted on the card and the entire blood spot was cut out and processed along with a 6 mm punch obtained at the same time from the same blood sample. The results were plotted as the blood volume spotted versus the peak area ratio (supplementary data, Fig. 3S). The peak area ratio of the 6 mm punch was obtained and corresponded to a volume of 12.1 μl. This value was then used to correct for the volume taken for the standard curve from PBS for subsequent samples.
We assessed the accuracy of the whole blood analysis by determining the recovery of 0.20, 1.0 and 3.0 mg/dL spiked into a whole blood sample. The results are shown in Table 3. The accuracy was well within acceptable guidelines with a bias of less than 5% and precision at all levels less than 10%.
Table 3.
Recovery of creatinine from whole blood spiked with 3 different creatinine concentrations.
| Spiked amount (mg/dL) | ||||
|---|---|---|---|---|
| Whole blood | 0 | 0.5 | 1.0 | 5.0 |
| Expected total | 0.80 | 1.30 | 5.30 | |
| Recovered mean | 0.30 | 0.82 | 1.25 | 5.43 |
| SD | 0.03 | 0.07 | 0.12 | 0.68 |
| % CV | 8.86 | 7.06 | 9.53 | 5.12 |
| % Bias | – | 3.75 | 3.85 | −2.45 |
| N | 6 | 6 | 6 | 6 |
The lower limit of quantification of creatinine from the DBS was 0.01 mg/dL with a CV of 7.94% (N = 6) and a signal to noise of >20. The intra- and inter-day variability of the analysis from a DBS was determined on cards spotted with a single blood sample that was not spiked with additional creatinine and then sampled over three days. The results are shown in Table 4. As with the analysis of TAC, the assay exhibited excellent precision at all three concentrations exhibiting values less than 5% in all cases.
Table 4.
Summary of intra-day and inter-day variability of DBS creatinine determination from an un-spiked blood sample.
| (mg/dL) | |
|---|---|
| Intra-day mean Day 1 | 0.82 |
| Intra-day SD | 0.03 |
| Intra-day % CV | 3.53 |
| N | 6 |
| Intra-day mean Day 2 | 0.80 |
| Intra-day SD | 0.03 |
| Intra-day % CV | 3.48 |
| N | 6 |
| Intra-day mean Day 3 | 0.79 |
| Intra-day SD | 0.03 |
| Intra-day % CV | 4.11 |
| N | 6 |
| Overall mean | 0.80 |
| Inter-day SD | 0.03 |
| Inter-day % CV | 3.79 |
| N | 18 |
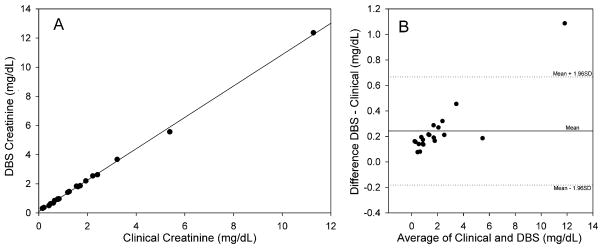
The clinical laboratory at OHSU determines creatinine from plasma samples using a Synchron CX® System with CREA® reagent by a modified Jaffẻ reaction with alkaline sodium picrate [31–33]. In order to determine if the whole blood from the DBS card would be reflective of these values we obtained blood samples from the clinical laboratory that had creatinine determinations and prepared DBS cards from the whole blood and analyzed them for creatinine. The results are shown in Fig. 2A and B. There is a significant correlation (P < 0.001) between the two parameters with a near zero intercept and a slope of 1.070, slightly greater than one, Panel A. The two methods were further compared using the method of Bland Altman [41] as shown in Panel B. There is positive bias of the mean difference suggesting the DBS method is measuring greater values than the clinical laboratory. The plot also suggests that there is a proportional error that is greater at higher creatinine concentrations. Both of these most likely reflects the fact that the clinical laboratory is determining creatinine on plasma from the same sample that we use is whole blood. As a result, creatinine present in red cells is not taken into account. Creatinine readily passes through red cell membranes [42] and it has been reported that there is some bound creatinine in red cells [43] consistent with a higher level in the DBS samples. All but the highest value fall within 1.96 SD, acceptable results to substitute one method for the other. Additional samples at higher creatinine levels need to be determined to confirm the proportional error. We were only able to acquire sufficient numbers of blood spots from 9 of the 21 patients to compare the DBS from the finger prick and from the venous blood sample spotted separately. There was excellent agreement with the 9 samples ranging in creatinine levels from 0.63 mg/dL differing by 0.01 mg/dL (1.6%) and 3.89 mg/dL differing by 0.12 mg/dL, 3.1%. We did not have sufficient samples to do this comparison with TAC.
Fig. 2.
Panel A: Correlation between creatinine determined from a general patient population in the clinical laboratory and a DBS card. Blood samples were obtained from the clinical laboratory and DBS was prepared by spotting 30 μl as described in Methods. The creatinine values obtained in the clinical laboratory using the Jaffe method are plotted versus the levels obtained using LC–MS/MS from the DBS. The line is from linear regression analysis with slope = 1.077, intercept = 0.093, R2 = 0.999, P < 0.001, N = 20. Panel B: Bland–Altman relation showing the difference in creatinine concentrations from the data plotted in Panel A. The central horizontal line represents the mean difference or bias. The two other lines represent the expected distribution of 95% of the measured points as determined by the combined total variation of each individual method.
As with the TAC, we found that creatinine levels were stable on DBS cards for up to one month when stored dessicated at room temperature, 7.91 mg/dL ± 0.14 (N = 3; CV = 1.8%) when measured weekly.
3.3. Application of methods with clinical samples
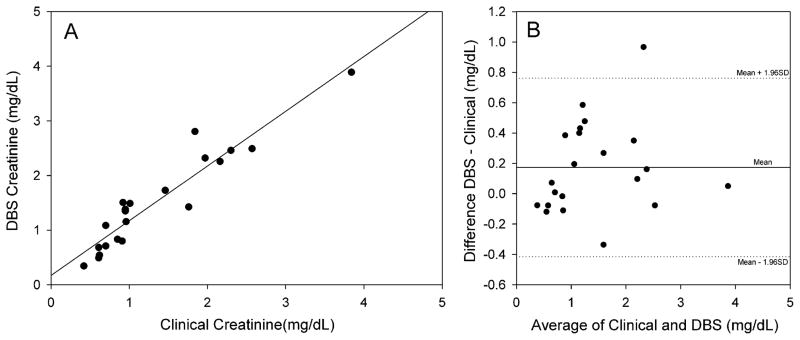
We used the assays to compare the values of TAC and creatinine with the clinical values from 21 patients seen in the OHSU clinic after kidney transplantation. Two of the patients refused to have the finger stick. DBS were prepared from the index finger and the venous blood draw with assistance from clinic staff at the same time a venous blood sample was taken for the determination in the clinical laboratory of TAC by radioimmunoassay and creatinine using the automated Jaffe picric acid colorimetric method. The results for these comparisons are shown in Fig. 3, Panel A and B for creatinine. The correlation coefficient (R2) was 0.890, P < 0.001 with a slope of 1.002 and an intercept of 0.170. The Bland–Altman [41] analysis shows a similar bias as observed with the samples prepared in the laboratory, about 0.2 mg/dL. All but one sample was within acceptable limits suggesting the methods could be used interchangeably for analysis.
Fig. 3.
Comparison of the creatinine concentration determined from DBS and the clinical laboratory from pediatric transplant patients. Panel A: During a clinic visit a DBS card was prepared and analyzed by LC–MS/MS as described. At the same time, a plasma sample was submitted to the clinical laboratory for analysis using the modified Jaffe method. The correlation coefficient (R2) was 0.890, P < 0.001 with a slope of 1.002 and an intercept of 0.170 (N = 21).
Panel B: Bland–Altman relation showing the difference in creatinine concentrations from the data plotted in Panel A. The central horizontal line represents the mean difference or bias. The two other lines represent the expected distribution of 95% of the measured points as determined by the combined total variation of each individual method.
The results obtained for TAC are shown in Fig. 4A and B. There were only 18 samples that could be compared for TAC since adequate DBS were not obtained from 2 patients and one patient had a clinical value reported as <2 ng/ml the lower limit of quantification for the RIA used in the clinical lab. For that particular patient the DBS had no detectable level of TAC. There is an excellent correlation between the two methods. The correlation coefficient (R2) was 0.742, P < 0.001 with a slope of 0.969 and an intercept of 0.272. The Bland Altman plot shows that there is no significant bias and all values are within 2 S.D. from the mean difference, acceptable for clinical analysis.
Fig. 4.
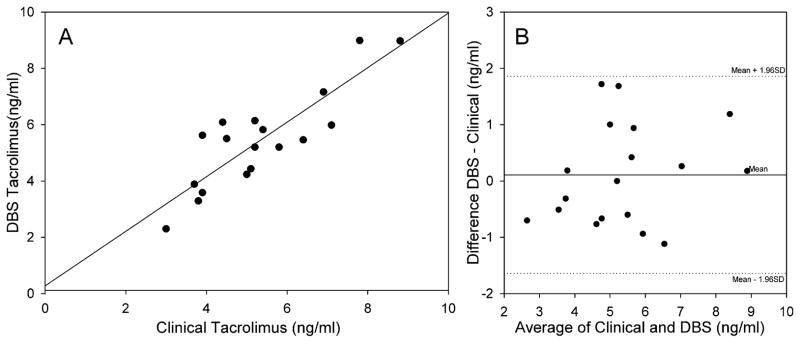
Comparison of the TAC concentration determined from DBS using the method outlined in the text with the values obtained from the clinical laboratory using radioimmunoassay. The correlation coefficient (R2) was 0.742, P < 0.001 with a slope of 0.969 and an intercept of 0.272 (N = 18).
Panel B: Bland–Altman relation showing the difference in TAC concentrations for the data plotted in Panel A. The central horizontal line represents the mean difference or bias. The two other lines represent the expected distribution of 95% of the measured points as determined by the combined total variation of each individual method.
4. Discussion
Neonatal screening has utilized DBS sampling for many years since it combines the advantage of requiring a small amount of blood with the technical ease of sample collection [44,45]. As analytical methods utilizing LC–MS/MS became more sensitive, DBS methods have been developed for many clinical applications including diagnosis and clinical management of patients [26,46], and for pharmacokinetics and pharmacogenomics [21,27,47].
The use of DBS has been reported for cyclosporine as well as TAC. Yonan et al. [48] reported the use of DBS with samples prepared at home with DBS kits to measure the 2 h cyclosporine level (C2) in 52 heart transplant recipients. The authors reported that only 6% of the samples were unsuitable for analysis due to of faulty collection techniques [44]. In a similar study for TAC, Hoogtanders et al. [22] reported the use of DBS and LC–MS/MS in 26 stable kidney transplant patients and found excellent correlation between the DBS and standard laboratory tests. In addition, they found that when duplicate spots prepared with and without nursing assistance were compared there was excellent agreement suggesting it is reasonable to assume the preparation of DBS in the home is realistic and possible.
While having the ability to prepare a DBS at home is extremely useful for being able to monitor immunosuppressant drug levels, the narrow therapeutic window and subsequent renal toxicity also requires the evaluation of plasma creatinine. Thus for DBS to become a valuable in-home method it is essential that creatinine be determined at the same time. Keevil et al. [49] reported the analysis of creatinine and cyclosporine A from whole blood prick collected into Microtainer® tubes with EDTA from a finger prick. However, since this assay required whole blood it would make transport difficult if collection were possible at home. Our assay is the first to report coupling the analysis of both of these analytes from a single DBS. Thus the most critical values followed in any transplant could be readily obtained from samples obtained at home and easily mailed to the analytical laboratory. Abnormal levels in either of these levels would then trigger a response by the medical provider to evaluate the health of the allograft. We found that the levels of creatinine were higher from the DBS than those obtained from plasma. Additional planned repeated comparisons of DBS and plasma creatinine in several hundred individuals would permit us to derive a correction factor for the DBS values. Despite this difference the excellent correlation between the DBS and plasma creatinine suggests that the DBS values could be used in repeated measurements from a single individual longitudinally to detect changes over time.
In conclusion, we have established a method to determine TAC and creatinine levels from a single DBS on filter paper. This dual assay requires additional validation for parameters such as the effect of shipping temperatures, blood spot volume, blood hematocrit, matrix effects and potential interference from other analytes. When completed, it can have potential important clinical implications for allograft monitoring in all transplant recipients including decreased cost for both patients and medical providers, and the potential to monitor blood levels more frequently. In addition, it has the potential to be used to monitor renal function alone, to monitor potential idiosyncratic metabolism and drug/drug interactions using a complete PK analysis from DBS. Since the DBS cards used have room for 4 spots, it is also feasible that if needed, repeat analysis of abnormally high and/or low values could be obtained and genomic analysis could be conducted if genetic polymorphisms are suspected. While DBS methods are simple and accurate, the use might still have limitations. It was a surprise that two patients refused to participate in the DBS part of the study citing more pain with a finger stick method than with intravenous blood collection. This is a clear indication that personal preference in some patients cannot be ignored. The utility of in-home DBS preparation and subsequent analysis as described in this work can only be determined in a large scale clinical trial.
Supplementary Material
Acknowledgments
We acknowledge the expert assistance of Jenny Luo in establishing the analysis of TAC and the University Shared Resource Program at OHSU for support of the Bioanalytical Shared Resource and Pharmacokinetics Core Facility. Support for this study was provided through a grant from the Center for Pediatric Pharmacology and Therapeutics at Doernebecher Children’s Hospital by the Doernbecher Foundation.
Abbreviations
- TAC
tacrolimus
- DBS
dried blood spot
- LC–MS/MS
liquid chromatography tandem mass spectrometry
- XIC
extracted ion current
- ESI
electrospray ionization
- CV
coefficient of variation
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jchromb.2013.02.035.
References
- 1.Chisholm-Burns MA, Spivey CA, Rehfeld R, Zawaideh M, Roe DJ, Gruessner R. Am J Transplant. 2009;9:2497. doi: 10.1111/j.1600-6143.2009.02793.x. [DOI] [PubMed] [Google Scholar]
- 2.Pollock-Barziv SM, Finkelstein Y, Manlhiot C, Dipchand AI, Hebert D, Ng VL, Solomon M, McCrindle BW, Grant D. Pediatr Transplant. 2010;14:968. doi: 10.1111/j.1399-3046.2010.01409.x. [DOI] [PubMed] [Google Scholar]
- 3.Shemesh E, Shneider BL, Savitzky JK, Arnott L, Gondolesi GE, Krieger NR, Kerkar N, Magid MS, Stuber ML, Schmeidler J, Yehuda R, Emre S. Pediatrics. 2004;113:825. doi: 10.1542/peds.113.4.825. [DOI] [PubMed] [Google Scholar]
- 4.Stuber ML, Shemesh E, Seacord D, Washington J, Hellemann G, III, McDiarmid S. Pediatr Transplant. 2008;12:284. doi: 10.1111/j.1399-3046.2008.00923.x. [DOI] [PubMed] [Google Scholar]
- 5.US Department of Health and Human Services. Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplanation; Rockville, MD: 2009. [Google Scholar]
- 6.Annunziato RA, Emre S, Shneider B, Barton C, Dugan CA, Shemesh E. Pediatr Transplant. 2007;11:608. doi: 10.1111/j.1399-3046.2007.00689.x. [DOI] [PubMed] [Google Scholar]
- 7.Vlaminck H, Maes B, Evers G, Verbeke G, Lerut E, Van DB, Vanrenterghem Y. Am J Transplant. 2004;4:1509. doi: 10.1111/j.1600-6143.2004.00537.x. [DOI] [PubMed] [Google Scholar]
- 8.Morrissey PE, Flynn ML, Lin S. Drugs. 2007;67:1463. doi: 10.2165/00003495-200767100-00007. [DOI] [PubMed] [Google Scholar]
- 9.Dew MA, DiMartini AF, De Vito DA, Myaskovsky L, Steel J, Unruh M, Switzer GE, Zomak R, Kormos RL, Greenhouse JB. Transplantation. 2007;83:858. doi: 10.1097/01.tp.0000258599.65257.a6. [DOI] [PubMed] [Google Scholar]
- 10.Dharancy S, Giral M, Tetaz R, Fatras M, Dubel L, Pageaux GP. Clin Transplant. 2012;26:E293–E299. doi: 10.1111/j.1399-0012.2012.01652.x. [DOI] [PubMed] [Google Scholar]
- 11.de Wit GA, Ramsteijn PG, de Charro FT. Health Policy. 1998;44:215. doi: 10.1016/s0168-8510(98)00017-7. [DOI] [PubMed] [Google Scholar]
- 12.Laupacis A, Keown P, Pus N, Krueger H, Ferguson B, Wong C, Muirhead N. Kidne Int. 1996;50:235. doi: 10.1038/ki.1996.307. [DOI] [PubMed] [Google Scholar]
- 13.Press RR, Ploeger BA, den HJ, van der Straaten T, van PJ, Danhof M, de Fijter JW, Guchelaar HJ. Ther Drug Monit. 2009;31:187. doi: 10.1097/FTD.0b013e31819c3d6d. [DOI] [PubMed] [Google Scholar]
- 14.Borra LC, Roodnat JI, Kal JA, Mathot RA, Weimar W, van GT. Nephrol Dial Transplant. 2010;25:2757. doi: 10.1093/ndt/gfq096. [DOI] [PubMed] [Google Scholar]
- 15.Keevil BG. Clin Biochem. 2011;44:110. doi: 10.1016/j.clinbiochem.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 16.Jabor VA, Coelho EB, Dos Santos NA, Bonato PS, Lanchote VL. J Chromatogr B: Analyt Technol Biomed Life Sci. 2005;822:27. doi: 10.1016/j.jchromb.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 17.Beaudette P, Bateman KP. J Chromatogr B: Analyt Technol Biomed Life Sci. 2004;809:153. doi: 10.1016/j.jchromb.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 18.Adaway JE, Keevil BG. J Chromatogr B: Analyt Technol Biomed Life Sci. 2011 doi: 10.1016/j.jchromb.2011.09.041. [DOI] [PubMed] [Google Scholar]
- 19.Bogusz MJ, Enazi EA, Hassan H, bdel-Jawaad J, Ruwaily JA, Tufail MA. J Chromatogr B: Analyt Technol Biomed Life Sci. 2007;850:471. doi: 10.1016/j.jchromb.2006.12.048. [DOI] [PubMed] [Google Scholar]
- 20.Li W, Tse FL. Biomed Chromatogr. 2010;24:49. doi: 10.1002/bmc.1367. [DOI] [PubMed] [Google Scholar]
- 21.Rowland M, Emmons GT. AAPS J. 2010;12:290. doi: 10.1208/s12248-010-9188-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoogtanders K, van der Heijden J, Christiaans M, van de Plas A, van HJ, Stolk L. Transplantation. 2007;83:237. doi: 10.1097/01.tp.0000250730.30715.63. [DOI] [PubMed] [Google Scholar]
- 23.Hoogtanders K, van der HJ, Christiaans M, Edelbroek P, van Hooff JP, Stolk LM. J Pharm Biomed Anal. 2007;44:658. doi: 10.1016/j.jpba.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 24.Hinchliffe E, Adaway JE, Keevil BG. J Chromatogr B: Analyt Technol Biomed Life Sci. 2011 doi: 10.1016/j.jchromb.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 25.Hooper PF, Dreesen TD, Keevil BG, Florman S. Nat Clin Pract Nephrol. 2009;5:E1. doi: 10.1038/ncpneph1054. [DOI] [PubMed] [Google Scholar]
- 26.Taylor PJ, Franklin ME, Tai CH, Pillans PI. J Chromatogr B: Analyt Technol Biomed Life Sci. 2012;883–884:108. doi: 10.1016/j.jchromb.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 27.Taylor PJ, Tai CH, Franklin ME, Pillans PI. Clin Biochem. 2011;44:14. doi: 10.1016/j.clinbiochem.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 28.Webb NJ, Roberts D, Preziosi R, Keevil BG. Pediatr Transplant. 2005;9:729. doi: 10.1111/j.1399-3046.2005.00367.x. [DOI] [PubMed] [Google Scholar]
- 29.Cheung CY, van der HJ, Hoogtanders K, Christiaans M, Liu YL, Chan YH, Choi KS, van de PA, Shek CC, Chau KF, Li CS, van HJ, Stolk L. Transpl Int. 2008;21:140. doi: 10.1111/j.1432-2277.2007.00584.x. [DOI] [PubMed] [Google Scholar]
- 30.Belostotsky V, Adaway J, Keevil BG, Cohen DR, Webb NJ. Pediatr Nephrol. 2011;26:133. doi: 10.1007/s00467-010-1670-3. [DOI] [PubMed] [Google Scholar]
- 31.Jaffe M. Z Physiol Chem. 1886;10:391. [Google Scholar]
- 32.Heinegard D, Tiderstrom G. Clin Chim Acta. 1973;43:305. doi: 10.1016/0009-8981(73)90466-x. [DOI] [PubMed] [Google Scholar]
- 33.Vasiliades J. Clin Chem. 1976;22:1664. [PubMed] [Google Scholar]
- 34.Tsikas D, Wolf A, Mitschke A, Gutzki FM, Will W, Bader M. J Chromatogr B: Analyt Technol Biomed Life Sci. 2010;878:2582. doi: 10.1016/j.jchromb.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 35.Hetu PO, Gingras ME, Vinet B. Clin Biochem. 2010;43:1158. doi: 10.1016/j.clinbiochem.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 36.Johns BA, Broten T, Stranieri MT, Holahan MA, Cook JJ. J Chromatogr B: Biomed Sci Appl. 2001;759:343. doi: 10.1016/s0378-4347(01)00229-8. [DOI] [PubMed] [Google Scholar]
- 37.Smith-Palmer T. J Chromatogr B: Analyt Technol Biomed Life Sci. 2002;781:93. doi: 10.1016/s1570-0232(02)00617-7. [DOI] [PubMed] [Google Scholar]
- 38.Spencer K. Ann Clin Biochem. 1986;23(Pt 1):1. doi: 10.1177/000456328602300101. [DOI] [PubMed] [Google Scholar]
- 39.Stokes P, O’Connor G. J Chromatogr B: Analyt Technol Biomed Life Sci. 2003;794:125. doi: 10.1016/s1570-0232(03)00424-0. [DOI] [PubMed] [Google Scholar]
- 40.Ziemniak JA, Chiarmonte DA, Schentag JJ. Clin Chem. 1981;27:272. [PubMed] [Google Scholar]
- 41.Bland JM, Altman DG. Lancet. 1986;1:307. [PubMed] [Google Scholar]
- 42.Ku CP, Passow H. Biochim Biophys Acta. 1980;600:212. doi: 10.1016/0005-2736(80)90426-5. [DOI] [PubMed] [Google Scholar]
- 43.Nolph K, Felts J, Moore R, Van SJ. Int Urol Nephrol. 1978;10:59. doi: 10.1007/BF02082794. [DOI] [PubMed] [Google Scholar]
- 44.Corso G, Apolito OD, Gelzo M, Paglia G, Dello RA. Bioanalysis. 2010;2:1883. doi: 10.4155/bio.10.149. [DOI] [PubMed] [Google Scholar]
- 45.Fernhoff PM. Pediatr Clin North Am. 2009;56:505. doi: 10.1016/j.pcl.2009.03.002. Table. [DOI] [PubMed] [Google Scholar]
- 46.Koal T, Burhenne H, Romling R, Svoboda M, Resch K, Kaever V. Rapid Commun Mass Spectrom. 2005;19:2995. doi: 10.1002/rcm.2158. [DOI] [PubMed] [Google Scholar]
- 47.Wijnen PA, Op den Buijsch RA, Cheung SC, van der HJ, Hoogtanders K, Stolk LM, van Dieijen-Visser MP, Neef C, Drent M, Bekers O. Clin Chim Acta. 2008;388:189. doi: 10.1016/j.cca.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 48.Yonan N, Martyszczuk R, Machaal A, Baynes A, Keevil BG. Clin Transplant. 2006;20:221. doi: 10.1111/j.1399-0012.2005.00472.x. [DOI] [PubMed] [Google Scholar]
- 49.Keevil BG, Tierney DP, Cooper DP, Morris MR, Machaal A, Yonan N. Ther Drug Monit. 2002;24:757. doi: 10.1097/00007691-200212000-00013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.