Abstract
IgM exists as both a monomer on the surface of B cells and a pentamer secreted by plasma cells. Both preimmune “natural” and antigen-induced “immune” IgM antibodies are important for protective immunity and for immune regulation of autoimmune processes by recognizing pathogens and self-antigens. Effector proteins interacting with the Fc portion of IgM, such as complement and complement receptors, have thus far been proposed but fail to fully account for the IgM-mediated protection and regulation. A major reason for this deficit in our understanding of IgM function seems to be lack of data on a long elusive Fc receptor for IgM (FcμR). We have recently identified a bona fide FcμR in both humans and mice. In this article we briefly review what we have learned so far about FcμR.
Keywords: IgM, Fc receptor, chronic lymphocytic leukemia, B cells, T cells
Introduction
Antibody is composed of a pair of heavy and light chains and has two biological activities: one is antigen binding via the N-terminal variable regions and the other is the effector functions mediated by the C-terminal constant regions of its heavy chains such as interaction with Fc receptors (FcRs). FcRs for IgG (FcγRI to FcγRIV), IgE (FcεRI) or IgA (FcαR) have been characterized extensively at both protein and DNA levels1-4, but FcR for IgM (FcμR) has been defied its genetic identification, although its existence on various cell types (B, T, NK and myeloid cells) was suggested in both humans and rodents more than four decades ago (see Ref. 5). In this article we describe FcμR, the newest member of the classical FcR family5, in terms of its brief history, structural aspects, cellular distribution including some new data, and potential functions. Human FcμR is mostly emphasized, but the recent findings on Fcmr-deficient mice6 are also briefly described.
FcμR cDNA Cloning
The initial series of studies on FcμR dealt with helper function of FcμR-bearing T cells, called Tμ cells, in pokeweed mitogen-induced polyclonal B cell responses in humans as reported by many different investigators in the 1970's7. When monoclonal antibodies (mAbs) specific for CD4 or CD8 became available, flow cytometric analysis of Tμ cells, which were separated by rosette formation with ox erythrocytes pre-coated with IgM antibodies, revealed that the Tμ cell fraction contained both CD4 T and CD8 T cell subsets8. The second wave of FcμR studies was the biochemical characterization of an activation antigen on B cells recognized by BAC-1 mAb of IgM isotype9-11. Sheila Sanders, then a post-doctoral fellow in the laboratory of Max Cooper, identified a sialoglycoprotein with a Mr of 60 kDa that precipitated with IgM mAbs irrespective of their antigen-binding specificity from lysates of surface iodinated, phorbol myristate acetate (PMA)-activated, but not resting, blood B cells. Since the 60 kDa glycoprotein was not precipitated by mAbs of other isotypes with the same antigen-binding specificity, it was suggested as an IgM-binding protein9. The 60 kDa IgM binding protein was also identified on freshly-prepared chronic lymphocytic leukemia (CLL) B cells and PMA-activated, human pre-B leukemia cell line 6979, 10. The IgM binding by PMA-activated, normal blood B or 697 pre-B cells as well as by CLL B cells could be demonstrated by flow cytometric analysis using highly purified IgM preparations9, 10. A similar 60 kDa IgM binding protein was also identified on T cells after short-term culture in IgM-free media with protease inhibitors11.
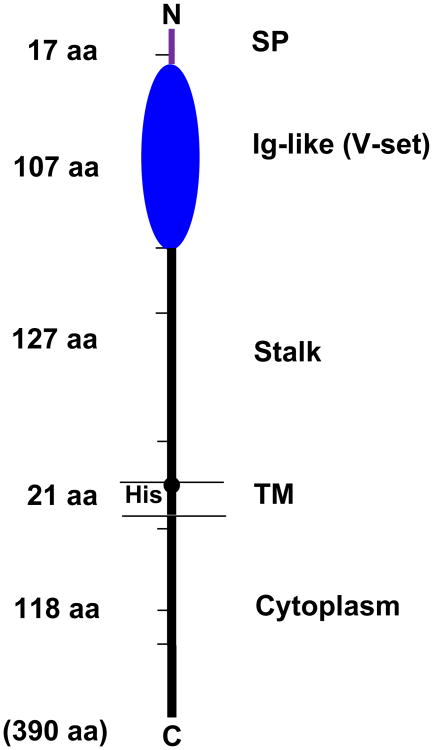
Given these precedents and the successful establishment of retroviral cDNA library-based functional cloning by Eiji Takayama in my laboratory, Ikuko Torii generated two cDNA libraries from human B-lineage cells, one from CLL B cells and the other from PMA-activated 697 pre-B cells, ligated them into a retroviral expression vector and introduced them into mouse thymoma line BW5147 which lacks IgM binding. Transduced cells exhibiting IgM binding in each library were enriched thrice by magnetic-activated cell sorting and then by fluorescence-activated cell sorting before subcloning by limiting dilution. Nucleotide sequence analyses of the insert cDNA responsible for IgM binding from both cDNA libraries revealed an identical 1,173-bp open reading frame which was predicted to encode a 390-aa type I membrane protein5. After cleavage of a 17-aa signal peptide, the predicted FcμR consists of a 107-aa V-set Ig-like domain responsible for IgM binding, an additional 127-aa extracellular region with no known domain structure (called “stalk” region in this paper), a 21-aa transmembrane segment containing a charged His residue, and a relatively long (118-aa) cytoplasmic tail (see Fig. 1). There are no N-linked glycosylation motifs in the extracellular region, consistent with our previous biochemical characterization of the FcμR10. Vire et al. have recently reported that there are several O-linked glycosylation sites in the stalk region and some of the potential Ser and Thr residues are indeed responsible for O-linked glycosylation as determined by point mutational analyses12. The core peptide is predicted to have a Mr of ∼41 kDa and an isoelectric point (pI) of ∼9.9. FCMR is a single copy gene located on chromosome 1q32.2, adjacent to two other IgM-binding receptor genes, polymeric Ig receptor (PIGR) and FcR for IgA and IgM (FCAMR)5 (Fig. 2).
Figure 1.
Predicted protein structure of human FcμR. The human FcμR cDNA encodes a type I transmembrane protein of 390 aa and with a peptide core of ∼41 kDa. Numbers indicate aa residues in each region: signal peptide (SP), a single Ig-like domain (V-set), remaining extracellular (stalk), transmembrane (TM; between the two lines) and cytoplasmic region. Hatch marks indicate exon boundaries in the FCMR gene. A small closed circle within the transmembrane segment indicates a charged histidine residue (His).
Figure 2.
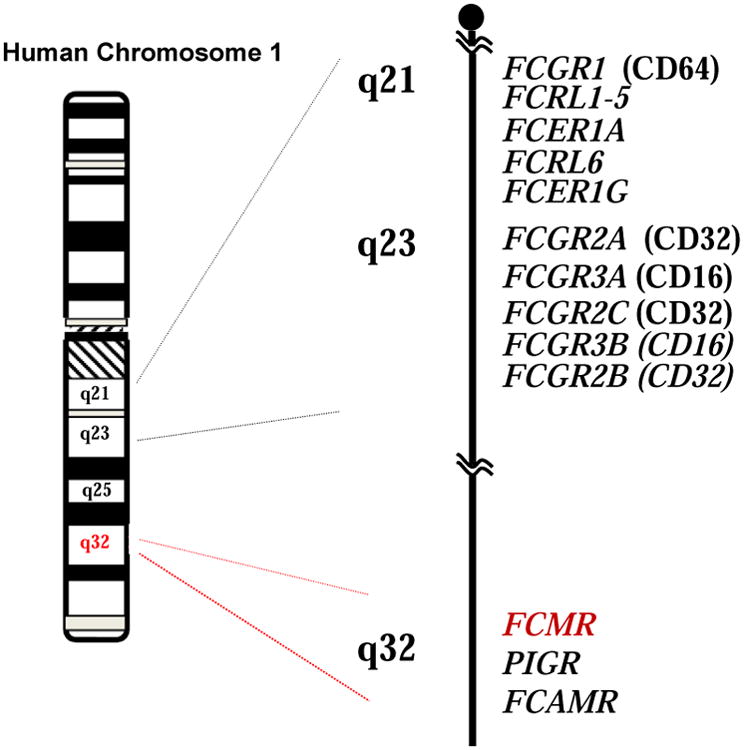
Schematic chromosomal localization of FCMR. Partial chromosome 1 linkage map showing a cluster of three IgM-binding receptors (FCMR, PIGR, FCAMR) in 1q32 in relation to other FcR genes in 1q21 – 1q23.
Structural Aspects
1) Ligand specificity
Satoshi Oka determined the Ig-binding specificity of FcμR cDNA-transduced cells by flow cytometric assays of the quantitative inhibition of various Ig isotypes and IgM fragments as inhibitors for biotin-labeled IgM. Pentameric IgM and its Fc5μ fragments consisting mostly of Cμ3/Cμ4 domains inhibited the IgM binding in a dose-dependent manner, whereas the Fabμ fragments and other Ig isotypes (IgG1-4, IgA1,2, IgD and IgE) did not, thereby confirming the Fcμ specificity of the FcμR5. The inability of FcμR to bind IgA polymers clearly indicates that FcμR is distinct from pIgR and Fcα/μR, both of which are shown to bind IgM and IgA polymers. IgM pentamers bound better to FcμR than IgM monomers. Curiously, mouse IgM bound better to the human FcμR than human IgM. The IgM binding was observed in the absence of Ca2+/Mg2+, distinct from the early observation with rosette formation. Pretreatment of FcμR+ cells with neuraminidase slightly enhanced IgM binding, suggesting a role of sialic acid in the ligand biding as suggested by others13. The avidity of IgM/FcμR binding was found to be strikingly high, ∼10 nM by Scatchard plot analysis with an assumption of a 1:1 stoichiometry of pentameric IgM ligand to FcμR5. Although Nguyen et al.14 have claimed the lack of IgM-ligand binding with their transductant transiently expressing Toso, an initial designation of FcμR15 (see below), the results from subsequent studies by us16 and others12, 17 clearly indicate that our isolated cDNA encodes an authentic FcμR with exclusive and high affinity binding specificity for the Fc portion of IgM.
2) Ig-like domain
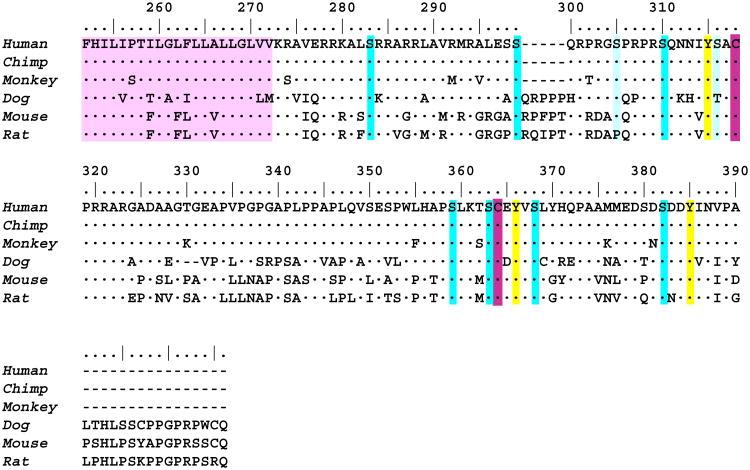
A comparison of the amino acid sequence of Ig-binding domains of three IgM-binding receptors (FcμR, pIgR, Fcα/μR) provided some potential insight into ligand binding when the pIgR structural data reported by Hamburger et al. were also integrated18. As shown in Fig. 3, in addition to a disulfide bond linking the two β sheets (B and F strands), a second disulfide bond linking the C and C′ strands is also conserved in all three receptors. Many other residues shown in yellow are also completely conserved, but several other residues shown in red are conserved in pIgR and Fcα/μR, and not in FcμR. A major difference between FcμR and the other two receptors is in the complementarity-determining region 1 (CDR1), which consists of 9 aa for pIgR and Fcα/μR, whereas FcμR has 5 aa and a non-charged residue (Met, Leu, or Thr) at the position corresponding to Arg that is predicted to interact directly with polymeric IgA with pIgR18. These findings suggest a structural basis for the distinct mode of IgM interaction with FcμR versus pIgR and Fcα/μR.
Figure 3.
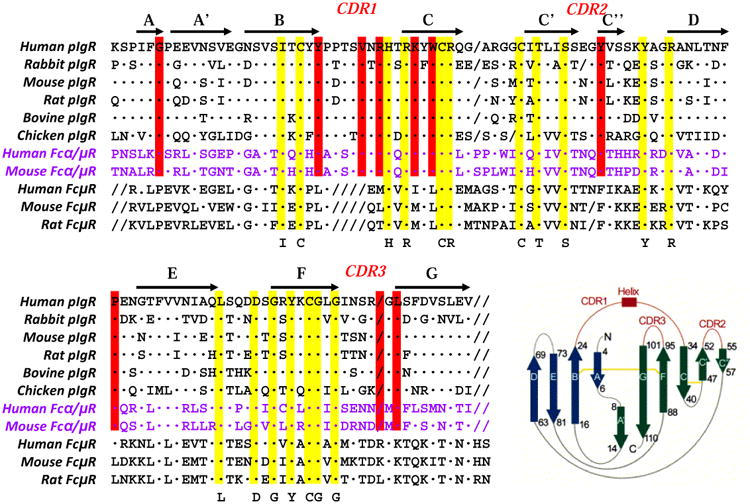
Amino acid sequence alignment of IgM-binding receptors. The Ig-binding domains of pIgR, Fcα/μR and FcμR from several species are aligned with each other. Amino acid identity is indicated by dots (·) and a deletion by slashes (/). Residues conserved in all three receptors and in pIgR and Fcα/μR are highlighted in yellow and red, respectively. Accession codes for these sequences are: pIgR of human (P01833), rabbit (P01832), mouse (070570), rat (P15083), bovine (P81265), and chicken (AAP69798); Fcα/μR of human (AAL51154) and mouse (NP_659209); and FcμR of human (NP_005440), mouse (NP_081252), and rat (Q5M871). Crystallographically determined secondary structure elements and the topology diagram of human pIgR, which are determined by Hamburger et al.18, are shown above the sequences and in a right lower corner, respectively. The β strands A, B, E, and D are shown in blue, β strands C″, C′, C, F, G, and A′ are in green, and three CDR loops (including the α helix within CDR1) are red. The permission to incorporate the structural data has been obtained from Dr. Pamela Bjorkman.
3) Biochemical nature
Yoshiki Kubagawa determined the biochemical natures of FcμR expressed on the surface of FcμR cDNA-transduced cells as well as PMA-activated 697 pre-B cells, CLL B cells and normal blood mononuclear cells (MNCs) using both receptor-specific mAbs and IgM ligands. Regardless of cell source, the surface FcμR was resolved as an ∼60 kDa sialoglycoprotein on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and was more efficiently identified by receptor-specific mAbs than IgM ligands5. Since the predicted core peptide is ∼41 kDa, one third of the Mr of FcμR is found to constitute carbohydrate moiety. While the pI is predicted as ∼9.9, the ∼60 kDa FcμR was resolved into a spot with a pI of ∼5 by two-dimensional gel electrophoresis, suggesting that mature FcμR contains many sialic acids, consistent with our previous findings10. An additional minor protein of ∼40 kDa (p40) was often co-precipitated with the FcμR, but it remains unclear whether p40 represents another membrane protein non-covalently associated with FcμR or an unglycosylated form of FcμR5. Sequential precipitation analysis with receptor-specific mAbs and IgM-ligands revealed that pre-incubation of membrane lysates of FcμR+ cells with mAbs completely removed the IgM-reactive 60 kDa protein, whereas the reverse did not efficiently remove the mAb-reactive 60 kDa protein, suggesting that mAbs are better than IgM ligands in the detection of FcμR.
In our earlier biochemical analysis, FcμR on PMA-activated 697 pre-B cells could be attached to the plasma membrane via a glycosylphosphatidylinositol (GPI) linkage, but the structure predicted by the cDNA is a transmembrane protein10. [Extensive search for a cDNA encoding a potential GPI-linked form of FcμR failed.] Satoshi Oka reexamined this issue using a highly purified GPI-specific phospholipase C (GPI-PLC) and found that after GPI-PLC treatment, the surface levels of FcμR and CD19 on PMA-activated 697 pre-B cells were unchanged, whereas the expression of GPI-anchored CD73 was reduced by ∼50%, indicating an authentic transmembrane protein of FcμR5. However, it is difficult to explain some biochemical data in our earlier studies where GPI-PLC was not used. Namely, when cell-surface iodinated, PMA-activated 697 pre-B cells were incubated at 37°C even without GPI-PLC, significant amounts of the ∼60 kDa IgM-binding protein was also released into the medium10. Furthermore, the ∼60 kDa IgM-binding protein was unequivocally precipitated from the culture supernatants of metabolically labeled, PMA-activated 697 pre-B cells by IgM-coupled beads10. Taken together, these findings highly suggest the possibility that FcμR is released as small vesicles or exsosomes from the plasma membrane upon activation.
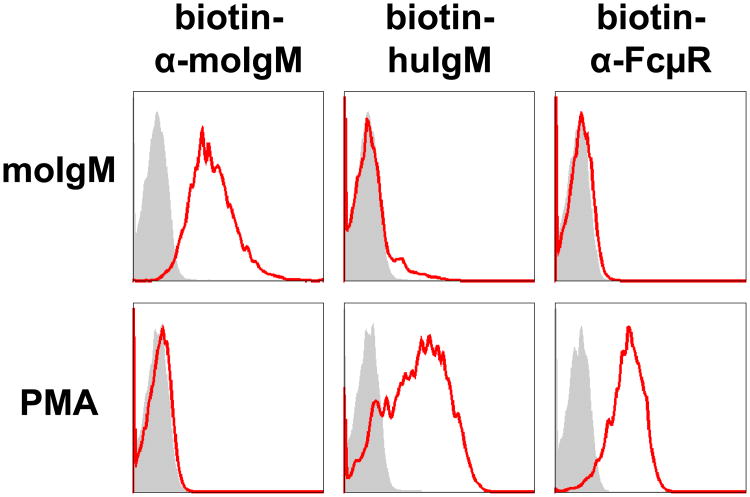
Another intriguing issue in FcR fields is the inducibility of FcRs by exposure to the corresponding Ig ligands, although such Ig-binding molecules have defied biochemical characterization. For example, IgA-binding capacity of mouse T cells was induced by exposure to IgA in vivo and in vitro19; however, this binding must be mediated by a non-FcαR/CD89 molecule, because mice lack the human FcαR/CD89 homolog gene20, 21. Similarly, both mouse and human IgM induced the IgM receptor on 697 pre-B cells in a dose-dependent manner without a plateau, and IgM receptor expression was maximal within 30 min after exposure, whereas longer exposure to PMA was required for maximal IgM binding10. The upregulation of IgM receptor was dependent on the continuous presence of the ligand, as removal of IgM from the culture resulted in a timedependent decline of IgM receptor expression by 697 pre-B cells. Yoshiki Kubagawa reexamined this dichotomy and concluded that such ligand-induced IgM receptor on 697 pre-B cells was not the FcμR as determined by receptor-specific mAbs (Fig. 4). It is thus suggested that another molecule such as certain lectins is responsible for the above IgM binding. In this regard, CD22 (siglec-2), a B cell membrane-bound lectin recognizing glycan ligands containing α2,6-linked sialic acid, was recently shown to interact with glycan ligands on soluble IgM/antigen complexes, thereby negatively regulating BCR signaling similar to FcγRIIB22, 23. Other IgM-binding proteins have also been demonstrated in other cell types. For example, tripartite motifcontaining protein 21 (TRIM21)/Ro52 binds antibody-opsonized pathogens and targets them to proteasomal degradation in phagocytes, and thus, the TRIM21/Ro52 behaves as a cytosolic Fc receptor for IgG and IgM24, 25 (see the article of Leo James in this volume). Apoptosis inhibitor of macrophage (AIM)/Spα, a member of the group B scavenger receptor cysteine-rich superfamily, is a soluble protein of ∼45 kDa produced by macrophages (Mφs) and is known to bind serum IgM but not IgG or IgA, in addition to support the survival of Mφs26-28. Recent data from analysis of Aim-deficient mice suggest that AIM plays an important role in obesityassociated natural IgM autoantibody process in Fcα/μR-bearing follicular dendritic cells (FDCs)29.
Figure 4.
Effect of IgM exposure and PMA treatment on the expression of IgM receptor by the 697 pre-B cell line. Cells were incubated for 16 h at 37°C with mouse IgM (0.3 mg/ml; top panel) or PMA (10 nM; bottom panel), washed, then assessed for IgM binding by flow cytometric analysis using biotin-labeled, rat anti-mouse μ mAb (left column), human IgM (middle column), mouse anti-human FcμR or isotype-matched control mAb (right column). The bound biotin-labeled reagents were detected by addition of phycoerythrin-labeled streptavidin (PE-SA). In the left two columns, the red lines are the reactivity of the indicated biotin reagents to cells preincubated with mouse IgM or PMA and the shaded histograms are that to cells preincubated with medium only as controls. In the right column, the red lines and shaded histograms are the reactivity of cells with anti-FcμR or isotype-matched control mAb, respectively. Note that mouse IgM-exposed 697 pre-B cells display already-bound mouse IgM and minimal binding of human IgM, but are negative for FcμR. By contrast, PMA-treated 697 pre-B cells clearly exhibit IgM binding and are positive for FcμR.
4) Conserved Tyr and Ser residues
The following common feature is observed with many paired receptors having a similar extracellular region but transmitting opposite signal potentials, such as FcγRs and NK cell receptors. One type has a short cytoplasmic tail but a charged aa in the transmembrane segment through which another transmembrane protein carrying immunoreceptor Tyr-based activation motifs (ITAMs) noncovalently associates with. The other type has a regular hydrophobic transmembrane and a long cytoplasmic tail containing immunoreceptor Tyr-based inhibitory motifs (ITIMs). In this regard, FcμR is unique, because it has a charged His residue in the transmembrane segment and a long cytoplasmic tail containing conserved Tyr and Ser residues, when compared with FcμRs from six different species (Fig. 5). This suggests that FcμR has a dual signaling ability: one from a potential adaptor protein non-covalently associating with FcμR via the His residue, similar to the association of FcR common γ chain with FcγRI1, and the other from its own Tyr and/or Ser residues in the cytoplasmic tail. While we have not yet identified a potential adaptor protein associated with the 60 kD ligand-binding chain of FcμR, Yoshiki Kubagawa found that FcμR ligation with preformed IgM immune complexes induced phosphorylation of both Tyr and Ser residues of the receptor5. Intriguingly, phosphorylated FcμR migrated faster on SDS-PAGE than unphosphorylated FcμR, suggesting that either phosphorylation-induced conformational changes or receptor ligation-induced proteolytic cleavage could be responsible for such migration behavior of the receptor. None of the Tyr residues correspond to an ITAM (D/Ex2Yx2L/Yx6-8Yx2L/I; x indicates any aa residues), ITIM (I/VxYx2L/V) or switch motif (TxYx2V/I)30-35, but the C-terminal Tyr matches the recently described Ig tail Tyr (ITT) motif (DYxN) in IgG and IgE isotypes36. The two carboxyl terminal Tyr residues were found to be involved in the FcμR-mediated endocytosis in CLL B cells12. It was also shown that FcμR ligation on NK cell transductants or primary NK cells with IgM immune complexes induced phosphorylation of PLCγ and ERK1/217.
Figure 5.
Amino acid sequence alignment of the transmembrane and cytoplasmic regions of FcμRs. The transmembrane and cytoplasmic regions of FcμR from six species are aligned with each other. Amino acid identity is indicated by dots (·) and a deletion by dashes (-). The predicted transmembrane region is colored in pink. Conserved Tyr, Ser and Cys residues are also highlighted in yellow, dark or light blue, and purple, respectively. Light blue indicate conservation of Ser residues in five species. The numbers indicate the aa position from the first Met residue of human FcμR.
Cellular Distribution of FcμR
Reverse transcription-polymerase chain reaction (RT-PCR) analysis of various tissues and a panel of representative cell lines revealed that FcμR transcripts were restricted to hematopoietic and lymphoid tissues, including the bone marrow, blood, spleen, tonsils and appendix5. FcμR transcripts were detected in both CD4+ and CD8+ T cells and in all subsets of tonsillar B cells, although the transcript levels appeared higher in the follicular and memory B cells than in the pre-germinal and germinal center B cells, consistent with data reported by others37. Gene array data suggested that CD4+ central memory (CD45RO+ CD27+ CCR7+) T cells expressed higher levels of FcμR transcripts than CD4+ effector memory (CD45RO+ CD27- CCR7-) T cells38. Among the cell lines, a couple of pro-B/pre-B cells lines and many B cell lines including Epstein Barr virus (EBV)-transformed lines also contained FcμR mRNA, but myeloid and erythroid lines did not. Interestingly, 697 pre-B cells expressed FcμR transcripts but did not constitutively express cell-surface FcμR proteins5.
Satoshi Oka examined the cell surface expression of FcμR by flow cytometric analysis using a panel of ten receptor specific mAbs and IgM ligands. Unlike earlier findings using rosette formation with IgM-coated erythrocytes, FcμR expression in humans is restricted to adaptive immune cells, both B and T lymphocytes, but not to innate immune cells5. The lack of FcμR expression by myeloid cells was also the case when they were activated with various stimuli including PMA/ionomycin, LPS, mitogens and several cytokines. Neither erythrocytes nor platelets expressed FcμR. NK cells were the only known exception for FcμR expression by cells other than lymphocytes in humans5, 17, but are now implicated to have features of both adaptive and innate cells39. FcμR is thus the only FcR constitutively expressed on T cells, which are generally negative for the expression of other FcRs. For B cells, FcμR is the only IgM-binding receptor expressed; although Fcα/μR was initially shown to be expressed by B cells40, our subsequent analyses revealed that the major cell type expressing Fcα/μR is a FDC in both humans and mice41. Clearly, mAb reactivity was a more sensitive assay for the detection of FcμR than ligand binding5. Notably, pre-incubation of blood MNCs with IgM-free media enhanced FcμR expression especially by T cells, consistent with our previous findings11. Intriguingly, this enhancement was more evident in cell preparations from tonsils and spleen than from blood. Freshly prepared tonsillar MNCs, including both B and T cells, had no reactivity with either anti-FcμR mAbs or IgM ligands, but after pre-incubation, there was clear-cut expression of FcμR on the surface of CD19+ B, CD4+ T and CD8+ T cells5. Since many other cell-surface antigens were easily detectable in those freshly isolated preparations, this in vivo down-modulation of FcμR was not due to an artifact of tissue manipulation, but rather to certain consequences. One possibility is that the IgM concentration in the interstitial spaces of such intact tissues, which has never been assessed, may be much higher than in blood (> ∼2 mg/ml). Alternatively, the tissue microenvironment (e.g., proteases) or cellular activation status may cleave the cell surface FcμR or may release FcμR-containing small membrane vesicles (or exosomes) from the plasma membrane.
In addition to the common classification of memory (CD27+) versus naïve (CD27-) blood B cells, the revised version has been suggested from the analysis of blood B cells in human immunodeficiency virus (HIV)-infected individuals. The increased levels of inhibitory receptors including FcR-like 4 are demonstrated on a unique memory B cell subset called tissue-like memory (CD20hi CD21lo CD27lo) in the blood from HIV-viremic individuals as compared with HIV-aviremic and -negative individuals and are thought to contribute to poor antibody responses against HIV in infected individuals42. Dewitt Jones and Kazuhito Honjo utilized this revised classification to determine the surface FcμR levels on various B cell subsets in the healthy adult blood and found the following hierarchy: naïve (CD20lo CD21+ CD27-) > resting memory (CD20lo CD21+ CD27+) > tissue-like memory (CD20hi CD21+ CD27lo/-) and activated memory (CD20hi CD21+ CD27+) (Fig. 6A). In tonsils from individuals with chronic tonsillitis, most follicular (IgD+ CD38-), memory (IgD- CD38-) and pregerminal center (IgD+ CD38+) B cells expressed FcμR, whereas only subpopulations of germinal center (GC; IgD- CD38+) B cells and plasma cells (CD38hi) expressed FcμR (Fig. 6B upper); the results are consistent with RT-PCR and microarray data by us and others5, 37. Among the memory B cell compartment, a similar trend of FcμR levels seen in the blood was also observed: resting > activated memory or tissue-like memory (Fig. 6B lower).
Figure 6.
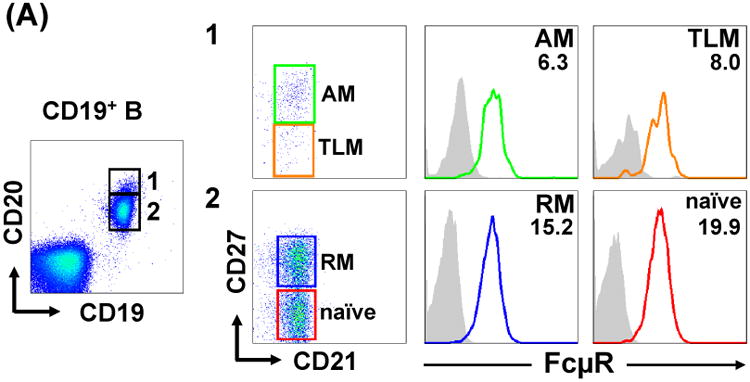
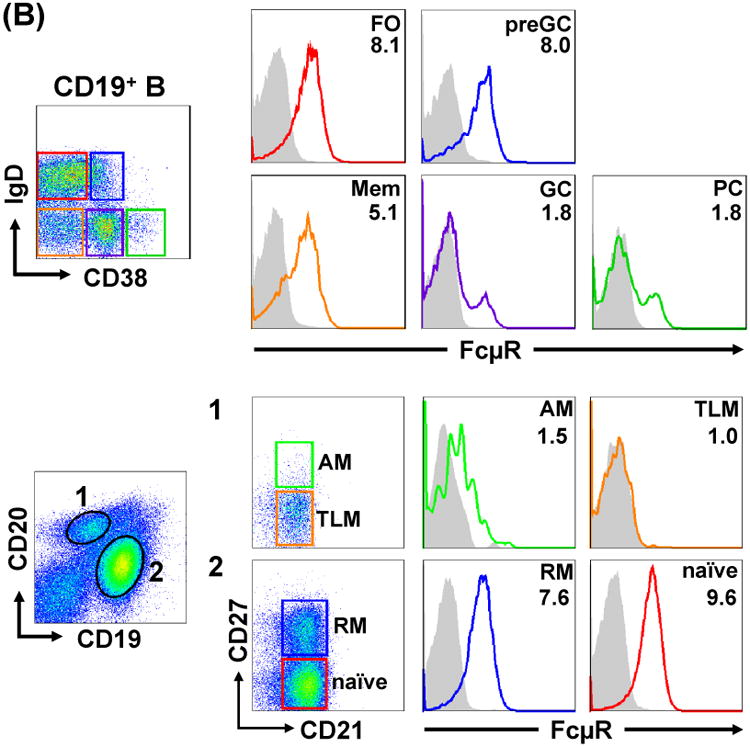
FcμR expression by B cell subsets in blood and tonsils. MNCs from adult blood (A) and tonsils (B) were first incubated with FcγR-blocking reagents and then with biotin-labeled, anti-FcμR (HM14; γ1κ) or isotype-matched control mAb, before developing with PE-SA. PE-stained cells were counterstained with fluorochrome-labeled mixture of four or three mAbs with specificity for: CD19, CD20, CD21 or CD27 (A and B lower panel) or CD19, IgD or CD38 (B upper panel), including fluorochrome-labeled, corresponding isotype-matched control mAbs for background setting. Stained cells were analyzed by BD LSR II (A and B lower) and Accuri C6 (B upper) flow cytometries. Cells in boxes with numbers or different color frames were examined for the reactivity with FcμR-specific (solid lines) or control (shaded histograms) mAb. Numbers indicate the mean fluorescence intensity (MFI) ratios defined as (MFI of anti-FcμR mAb ÷ MFI of control mAb). AM, activated memory; TLM, tissue-like memory; RM, resting memory; FO, follicular; preGC, pregerminal center; Mem, memory; GC, germinal center; PC, plasma cell.
For blood T cells, cell surface FcμR levels were slightly higher on α/β T cells than on γδ T cells and on CD4 T cells than on CD8 T cells. The hierarchy of FcμR levels on various T cell subsets was as follows: naïve (CD45RA+ CD27+ CCR7+) > central memory (CM; CD45RA- CD27+ CCR7+) > effector memory (EM; CD45RA-CD27+ CCR7lo/-) > effector (CD45RA- CD27- CCR7-) in both CD4 T and CD8 T cell subsets in the adult blood (Fig. 7A). In tonsils, the similar hierarchy of FcμR levels to that in the adult blood was also observed: naïve > CM > EM > effector (CD45RA- CD27- CCR7-) for CD4 T cells and naïve > CM > EMRA (CD45RA+ CD27+ CCR7-) > EM for CD8 T cells (Fig. 7B). As to FcμR expression during ontogeny, Naonori Nishida, Toshio Miyawaki and Kazuhito Honjo compared the expression of FcμR between cord and adult blood MNCs and found that FcμR levels on B cells were significantly lower in cord blood than adult blood, whereas FcμR levels on other cell types (CD4 T, CD8 T, Treg, and NK cells) were comparable.
Figure 7.
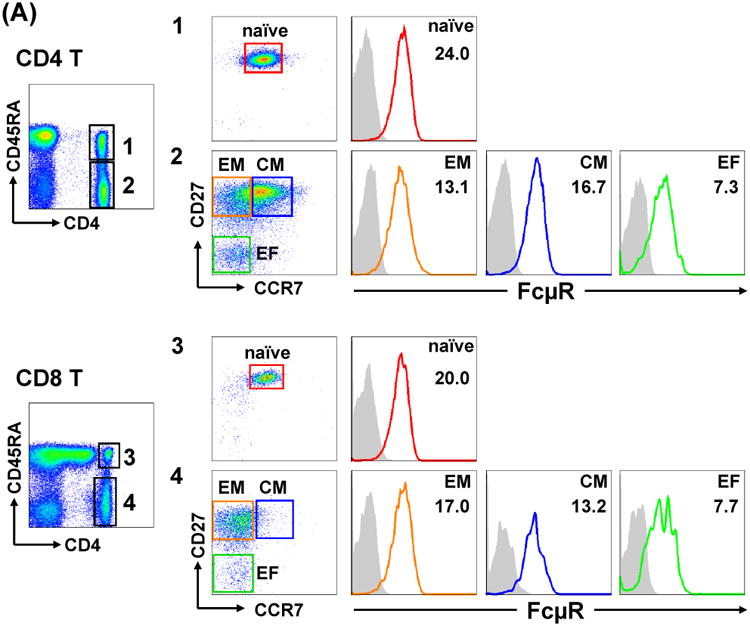
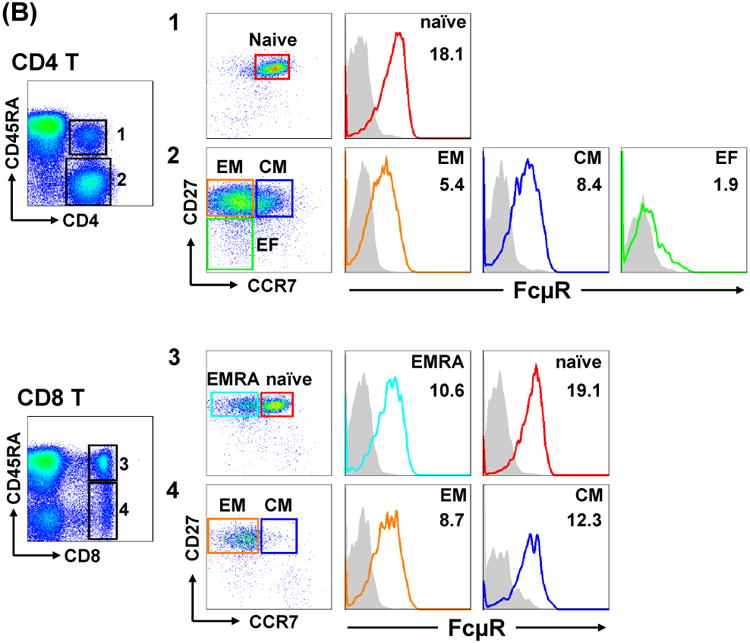
FcμR expression by T cell subsets in blood and tonsils. MNCs from blood (A) and tonsils (B) were similarly stained as in Fig. 6 for FcμR and counterstained with fluorochrome-labeled anti-CD4 (upper) or anti-CD8 (lower) mAb, along with other fluorochrome-labeled mAbs specific for CD45RA, CD27 or CCR7. Stained cells were similarly analyzed by BD LSR II flow cytometry as described in the Fig. 6 legend. EM, effector memory; CM, central memory; EF, effector; EMRA, effector memory CD45RA+.
In adult bone marrow, a small subpopulation (∼20%) of the pro-B/pre-B cells (CD19+ surface IgM-) expressed low levels of FcμR on their cell surface, whereas ∼40% of the B cells expressed slightly higher levels of FcμR, indicating that FcμR expression begins at the pro-B/pre-B cell stage in B-lineage differentiation. Collectively, these findings indicate that in striking contrast to FcRs for switched Ig isotypes, human FcμR is predominantly expressed by the adaptive immune cells and that the cell surface levels of FcμR are sensitive to IgM ligand concentration, tissue milieu and cellular activation status.
Enhanced Expression of FcμR in Patients with CLL
Consistent with previous findings from us and others5, 9, 10, 43-50, CLL B cells (CD5+ CD19+) clearly expressed increased levels of FcμR on their cell surface compared with B cells from normal individuals as determined by receptor-specific mAbs51. This enhanced expression was more evident in Ig heavy chain variable region (IGHV)-mutated, CD38- or early Rai-stage CLL than IGHV-unmutated, CD38+ or advanced Rai-stage CLL. Intriguingly, surface FcμR levels also were significantly elevated in the non-CLL B cells (CD5- CD19+) and T cells (CD5+ CD19-), especially in patients with IGHV-mutated CLL, when compared with the corresponding populations in normal individuals. The increase in FcμR expression on T cells in CLL patients is unique, because normal T cells activated ex vivo with anti-CD3 mAb or PMA down-modulate the surface expression of FcμR, whereas B cells activated with mitogenic anti-μ mAb or PMA up-regulate surface FcμR5. CLL patients also had high serum titers of FcμR compared with healthy donors as determined by sandwich ELISA using two different receptor-specific mAbs. Serum FcμR levels correlated well with circulating lymphocyte numbers but not with the IGHV mutation status or Rai stage. The serum FcμR was resolved as an ∼40 kDa protein, distinct from the cell surface FcμR of ∼60 kDa, was produced by both CLL B and non-CLL B cells and shown by proteomic analysis to be a soluble form of the receptor encoded by an alternatively spliced FcμR transcript. The molecular basis for such soluble FcμR production in CLL patients, however, remains unclear as does the clinical effect of such levels as a decoy receptor on the immune responses of CLL patients. These findings indicate enhanced levels of both membrane-bound and soluble forms of FcμR in CLL patients51.
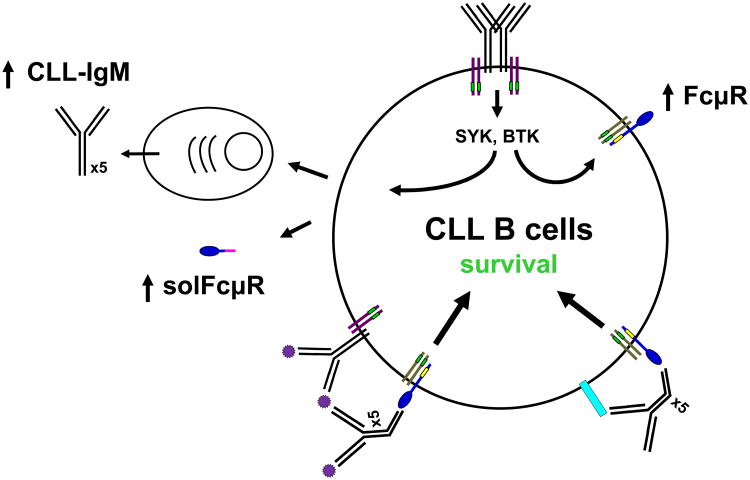
Recent seminal findings from the laboratory of Hassan Jumaa have suggested that in contrast to other B cell malignancies, CLL-derived BCR ligates each other via interactions between the Ig heavy chain CDR3 of one BCR and an intrinsic motif (WVRQxPG; bold fonts indicate critical aa residues) in the framework region 2 of another, thereby generating antigen-independent cell-autonomous signaling52. This antigen-independent self-ligation of BCR on CLL cells is consistent with the well-known findings of reduced levels of cell surface IgM and IgD on CLL cells and may induce up-regulation of cell surface FcμR as observed with ex vivo BCR cross-linkage with anti-μ mAbs. Many CLL patients contain significant amounts of CLL-derived IgM in their sera as determined by using CLL BCR idiotype-specific reagents53-55. As CLL-derived IgM often reacts with self-antigens56, the following proposed scenario would be conceivable. Subpopulations of CLL B cells undergo differentiation into plasma cells that secrete IgM. Secreted IgM in turn binds self-antigens of either soluble or membrane-associated form, and the resultant soluble IgM immune complexes co-ligate BCR and FcμR or the Fc portion of IgM bound to self-antigens on CLL cells binds FcμR in cis, thereby providing a survival signal via FcμR (Fig. 8).
Figure 8.
Hypothetical role of Fc μR in CLL. Our current working hypothesis of the role of FcμR in CLL is as follows. Antigen-independent self-ligation of BCR on CLL cells activates SYK and BTK tyrosine kinases and induces up-regulation of the cell surface expression of FcμR/adaptor protein complex. Subpopulations of CLL cells differentiate into plasma cells that secrete pentameric IgM antibodies. Secreted IgM antibodies recognize soluble (purple spiky small circles) or lymphocyte membrane (light blue rectangle) self-antigens. The colligation of FcμR and BCR by soluble IgM/self-antigen immune complexes or the cis interaction of FcμR and lymphocyte membrane self-antigen by secreted IgM provides a survival signal to CLL cells through FcμR. BCR is depicted as black Y shape heavy and light chain lines with Igα/β adaptor proteins (purple lines) carrying ITAM (small green rectangles). FcμR ligand-binding chain is depicted as a blue tennis racket shape with a small yellow rectangle indicating three conserved intracytoplasmic Tyr residues and associates with an unknown adaptor protein (gray lines) possibly carrying ITAM (small green rectangles). Soluble FcμR, an alternative splice variant, is also markedly elevated in CLL patients, but its biological function remains unknown.
Potential Functions
1) Toso/FAIM3 versus FcμR
When we isolated the FcμR cDNA from human B-lineage cDNA libraries by a functional cloning strategy and analyzed its nucleotide sequence with the basic local alignment search technique database, the FcμR cDNA was identical to that of human Fas apoptotic inhibitory molecule 3 (FAIM3), except for one nucleotide difference at a position reported as a synonymous single nucleotide polymorphism. [CLL- and PMA-activated 697 pre-B cell-derived FcμR cDNA and FAIM3 cDNA are available from GenBank/EMBL/DDB under accession nos. GQ160900, GQ160901 and NM_005449, respectively.] FAIM3 was also identified in a similar retroviral cDNA library-based functional assay as a potent inhibitor of Fas/CD95-induced apoptosis in Jurkat T cells and was originally designated as Toso, after a Japanese liquor drunk on New Year's day to celebrate long life and eternal youth15. However, this Toso/FAIM3 designation was incorrect, as the mouse IgM anti-Fas mAb (CH11) was used for induction of Fas-mediated apoptosis. In fact, Fas ligation with the CH11 IgM mAb induced robust apoptosis in control (FcμR-) Jurkat cells, but not in FcμR+ Jurkat cells, consistent with the previously reported anti-apoptotic activity of Toso/FAIM315. However, Fas ligation with an IgG3 mAb (2R2) or a recombinant Fas ligand induced apoptosis in both control and FcμR+ cells5, 16. Essentially identical results were also obtained with EBV-transformed B cell lines expressing both endogenous FcμR and Fas on their cell surface. Thus, Toso/FAIM3 per se has no intrinsic activity to inhibit Fas-mediated apoptosis and is an authentic IgM Fc-binding molecule, FcμR.
2) Mouse FcμR
Kazuhito Honjo determined the cellular distribution of mouse FcμR using a panel of five receptor-specific mAbs. Unlike humans, the expression of mouse FcμR was restricted to B-lineage cells only. The FcμR expression began at the early immature B cell stage in bone marrow and continued through to plasmablast stage of differentiation, accompanied by transient down-modulation during GC reactions6. Contrary to this, Lang et al. recently reported that Ly6G+ bone marrow granulocytes and Mφs expressed FcμR at extremely low density on their cell surface57. Strangely, Ly6G- bone marrow cells which should contain a significant number of FcμR-expressing B cells, were negative with their B68 mAb57. In this regard, we reexamined extensively and found that none of our mAbs reacted specifically with the cell surface of these phagocytes and that FcμR transcripts were clearly detectable in B-lineage cells but not in the double sorted Ly6G+ granulocytes or in Rag1-deficient splenocytes, which were devoid of B and T cells but contained abundant granulocytes and Mφs, even after 35 cycles of amplification6, 58. Thus, these findings conclusively demonstrate at both protein and RNA levels that FcμR is not expressed by myeloid cells. This conclusion is very important when we consider the role of FcμR in bacterial infection models as described in ref. 51.
3) FcμR-deficiency
Fcmr KO mice have been independently generated by two laboratories, our collaborator Hiroshi Ohno at RIKEN in Yokohama and Tak Mak at Ontario Cancer Institute in Toronto, and recently have been characterized by four different groups of investigators and there are clear differences in the reported phenotypes6, 57, 59, 60. Although the basis for these discrepancies requires further investigations, it might be due to different strategies for gene targeting [i.e., deletion of exon 2 to 46, 59 versus exon 2 to 857, 60 and the absence6, 59 versus presence of the Neo gene57, 60 in the mouse genome] or to other differences in mouse ages, environments including intestinal flora or reagents used. Nevertheless, the abnormal phenotypes commonly observed (in at least two laboratories) in Fcmr KO mice are: (i) increase in pre-immune serum IgM6, 59, (ii) alterations in B cell subpopulations6, 59, 54, (iii) dysregulation of humoral immune responses6, 59, 54, (iv) impairment of B cell proliferation upon ligation of BCR in vitro59, 60, and (v) predisposition to autoantibody production6, 59, 54.
Notably, many abnormalities in Fcmr KO mice mirror those observed in μs exon-deleted mice (μs-/-) that are unable to secrete IgM but are able to express surface IgM and other Ig isotypes on B cells and to secrete all other classes of Igs61. Studies with these μs-/- mice have reinforced the importance of both pre-immune “natural” and antigen-induce “immune” IgM antibodies in protection against infectious and autoimmune diseases62, 63. Such μs-/- mutant mice cannot control viral, bacterial or fungal infections, likely due to their unexpected inability to mount a protective IgG antibody response64-66. On the other hand, autoimmune pathology associated with IgG autoantibodies is exacerbated in these μs-/- mutant mice, possibly due to the absence of protective IgM natural antibodies, resulting in impaired clearance of autoantigen-containing apoptotic cells61, 67, 68. Secreted IgM can thus profoundly influence immune responses to pathogens and self-antigens and the role of FcμR in such effector functions has just begun to be explored. Although FCMR deficiency has not yet been identified in humans, it seems likely that the phenotype will be much more complex and profound than the Fcmr deficiency in mice, because human FcμR is expressed by additional cell types, namely T and NK cells.
Acknowledgments
The studies described in this article are accomplished based on the earlier studies on FcμR by Dr. Tatsuharu Ohno and Dr. Tetsuya Nakamura in the laboratory of Dr. Max Cooper and the authors wish to express our gratitude to them for their solid work and to Dr. Max Cooper for his great mentorship. This work was supported in part by National Institute of Health, National Institute of Allergy and Infectious Diseases Grants AI52243, R56AI82249, and R21AI94624 (to HK). Regretfully, Dr. Toshio Miyawaki, Professor Emeritus of Toyama University, was diseased on February 26, 2014, and we would like to offer our deepest sympathy.
Footnotes
Conflict of interest disclosure: The authors declare no conflicting financial interests
References
- 1.Ravetch JV, Kinet JP. Fc receptors. Annu Rev Immunol. 1991;9:457–92. doi: 10.1146/annurev.iy.09.040191.002325. [DOI] [PubMed] [Google Scholar]
- 2.Daëron M. Fc receptor biology. Annu Rev Immunol. 1997;15:203–34. doi: 10.1146/annurev.immunol.15.1.203. [DOI] [PubMed] [Google Scholar]
- 3.Monteiro RC, Van De Winkel JG. IgA Fc receptors. Annu Rev Immunol. 2003;21:177–204. doi: 10.1146/annurev.immunol.21.120601.141011. [DOI] [PubMed] [Google Scholar]
- 4.Nimmerjahn F, Bruhns P, Horiuchi K, Ravetch JV. FcγRIV: a novel FcR with distinct IgG subclass specificity. Immunity. 2005 Jul;23(1):41–51. doi: 10.1016/j.immuni.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 5.Kubagawa H, Oka S, Kubagawa Y, Torii I, Takayama E, Kang DW, et al. Identity of the elusive IgM Fc receptor (FcμR) in humans. J Exp Med. 2009 Nov 23;206(12):2779–93. doi: 10.1084/jem.20091107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Honjo K, Kubagawa Y, Jones DM, Dizon B, Zhu Z, Ohno H, et al. Altered Ig levels and antibody responses in mice deficient for the Fc receptor for IgM (FcμR) Proc Natl Acad Sci USA. 2012 Sep 25;109(39):15882–7. doi: 10.1073/pnas.1206567109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moretta L, Webb SR, Grossi CE, Lydyard PM, Cooper MD. Functional analysis of two human T-cell subpopulations: help and suppression of B-cell responses by T cells bearing receptors for IgM (Tμ) or IgG (Tγ) J Exp Med. 1977 Jul 1;146(1):184–200. doi: 10.1084/jem.146.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reinherz EL, Moretta L, Roper M, Breard JM, Mingari MC, Cooper MD, et al. Human T lymphocyte subpopulations defined by Fc receptors and monoclonal antibodies: a comparison. J Exp Med. 1980 Apr 1;151(4):969–74. doi: 10.1084/jem.151.4.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanders SK, Kubagawa H, Suzuki T, Butler JL, Cooper MD. IgM binding protein expressed by activated B cells. J Immunol. 1987 Jul 1;139(1):188–93. [PubMed] [Google Scholar]
- 10.Ohno T, Kubagawa H, Sanders SK, Cooper MD. Biochemical nature of an Fcμ receptor on human B-lineage cells. J Exp Med. 1990 Oct 1;172(4):1165–75. doi: 10.1084/jem.172.4.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakamura T, Kubagawa H, Ohno T, Cooper MD. Characterization of an IgM Fc-binding receptor on human T cells. J Immunol. 1993 Dec 15;151(12):6933–41. [PubMed] [Google Scholar]
- 12.Vire B, David A, Wiestner A. TOSO, the Fcμ receptor, is highly expressed on chronic lymphocytic leukemia B cells, internalizes upon IgM binding, shuttles to the lysosome, and is downregulated in response to TLR activation. J Immunol. 2011 Sep 9;187(8):4040–50. doi: 10.4049/jimmunol.1100532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pricop L, Rabinowich H, Morel PA, Sulica A, Whiteside TL, Herberman RB. Characterization of the Fcμ receptor on human natural killer cells. Interaction with its physiologic ligand, human normal IgM, specificity of binding, and functional effects. J Immunol. 1993 Sep 15;151(6):3018–29. [PubMed] [Google Scholar]
- 14.Nguyen XH, Lang PA, Lang KS, Adam D, Fattakhova G, Foger N, et al. Toso regulates the balance between apoptotic and nonapoptotic death receptor signaling by facilitating RIP1 ubiquitination. Blood. 2011 Jul 21;118(3):598–608. doi: 10.1182/blood-2010-10-313643. [DOI] [PubMed] [Google Scholar]
- 15.Hitoshi Y, Lorens J, Kitada SI, Fisher J, LaBarge M, Ring HZ, et al. Toso, a cell surface, specific regulator of Fas-induced apoptosis in T cells. Immunity. 1998 Apr 1;8(4):461–71. doi: 10.1016/s1074-7613(00)80551-8. [DOI] [PubMed] [Google Scholar]
- 16.Honjo K, Kubagawa Y, Kubagawa H. Is Toso an antiapoptotic protein or an Fc receptor for IgM? Blood. 2012 Feb 16;119(7):1789–90. doi: 10.1182/blood-2011-09-380782. [DOI] [PubMed] [Google Scholar]
- 17.Murakami Y, Narayanan S, Su S, Childs R, Krzewski K, Borrego F, et al. Toso, a functional IgM receptor, is regulated by IL-2 in T and NK cells. J Immunol. 2012 Jul 15;189(2):587–97. doi: 10.4049/jimmunol.1200840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamburger AE, West AP, Jr, Bjorkman PJ. Crystal structure of a polymeric immunoglobulin binding fragment of the human polymeric immunoglobulin receptor. Structure. 2004 Nov;12(11):1925–35. doi: 10.1016/j.str.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 19.Hoover RG, Dieckgraefe BK, Lynch RG. T cells with Fc receptors for IgA: induction of T alpha cells in vivo and in vitro by purified IgA. J Immunol. 1981 Oct;127(4):1560–3. [PubMed] [Google Scholar]
- 20.Reljic R. In search of the elusive mouse macrophage Fcα receptor. Immunol Lett. 2006 Sep 15;107(1):80–1. doi: 10.1016/j.imlet.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 21.Maruoka T, Nagata T, Kasahara M. Identification of the rat IgA Fc receptor encoded in the leukocyte receptor complex. Immunogenetics. 2004 Jan;55(10):712–6. doi: 10.1007/s00251-003-0626-1. [DOI] [PubMed] [Google Scholar]
- 22.Adachi T, Harumiya S, Takematsu H, Kozutsumi Y, Wabl M, Fujimoto M, et al. CD22 serves as a receptor for soluble IgM. Eur J Immunol. 2012 Jan;42(1):241–7. doi: 10.1002/eji.201141899. [DOI] [PubMed] [Google Scholar]
- 23.Poe JC, Tedder TF. CD22 and Siglec-G in B cell function and tolerance. Trends Immunol. 2012 Aug;33(8):413–20. doi: 10.1016/j.it.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McEwan WA, Tam JC, Watkinson RE, Bidgood SR, Mallery DL, James LC. Intracellular antibody-bound pathogens stimulate immune signaling via the Fc receptor TRIM21. Nat Immunol. 2013 Apr;14(4):327–36. doi: 10.1038/ni.2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Randow F, MacMicking JD, James LC. Cellular self-defense: how cell-autonomous immunity protects against pathogens. Science. 2013 May 10;340(6133):701–6. doi: 10.1126/science.1233028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tissot JD, Sanchez JC, Vuadens F, Scherl A, Schifferli JA, Hochstrasser DF, et al. IgM are associated to Sp alpha (CD5 antigen-like) Electrophoresis. 2002 Apr;23(7-8):1203–6. doi: 10.1002/1522-2683(200204)23:7/8<1203::AID-ELPS1203>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 27.Martinez VG, Moestrup SK, Holmskov U, Mollenhauer J, Lozano F. The conserved scavenger receptor cysteine-rich superfamily in therapy and diagnosis. Pharmacol Rev. 2011 Dec;63(4):967–1000. doi: 10.1124/pr.111.004523. [DOI] [PubMed] [Google Scholar]
- 28.Miyazaki T, Kurokawa J, Arai S. AIMing at metabolic syndrome. Towards the development of novel therapies for metabolic diseases via apoptosis inhibitor of macrophage (AIM) Circ J. 2011;75(11):2522–31. doi: 10.1253/circj.cj-11-0891. [DOI] [PubMed] [Google Scholar]
- 29.Arai S, Maehara N, Iwamura Y, Honda S, Nakashima K, Kai T, et al. Obesity-associated autoantibody production requires AIM to retain the immunoglobulin M immune complex on follicular dendritic cells. Cell Rep. 2013 Apr 25;3(4):1187–98. doi: 10.1016/j.celrep.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 30.Reth M. Antigen receptor tail clue. Nature. 1989 Mar 30;338(6214):383–4. doi: 10.1038/338383b0. [DOI] [PubMed] [Google Scholar]
- 31.Weiss A, Littman DR. Signal transduction by lymphocyte antigen receptors. Cell. 1994 Jan 28;76(2):263–74. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 32.Cambier JC. Antigen and Fc receptor signaling. The awesome power of the immunoreceptor tyrosine-based activation motif (ITAM) J Immunol. 1995 Oct 1;155(7):3281–5. [PubMed] [Google Scholar]
- 33.Vely F, Vivier E. Conservation of structural features reveals the existence of a large family of inhibitory cell surface receptors and noninhibitory/activatory counterparts. J Immunol. 1997 Sep 1;159(5):2075–7. [PubMed] [Google Scholar]
- 34.Cambier JC. Inhibitory receptors abound? Proc Natl Acad Sci U S A. 1997 Jun 10;94(12):5993–5. doi: 10.1073/pnas.94.12.5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sidorenko SP, Clark EA. The dual-function CD150 receptor subfamily: the viral attraction. Nat Immunol. 2003 Jan;4(1):19–24. doi: 10.1038/ni0103-19. [DOI] [PubMed] [Google Scholar]
- 36.Engels N, Konig LM, Heemann C, Lutz J, Tsubata T, Griep S, et al. Recruitment of the cytoplasmic adaptor Grb2 to surface IgG and IgE provides antigen receptor-intrinsic costimulation to class-switched B cells. Nat Immunol. 2009 Sep;10(9):1018–25. doi: 10.1038/ni.1764. [DOI] [PubMed] [Google Scholar]
- 37.Klein U, Tu Y, Stolovitzky GA, Keller JL, Haddad J, Jr, Miljkovic V, et al. Transcriptional analysis of the B cell germinal center reaction. Proc Natl Acad Sci U S A. 2003 Mar 4;100(5):2639–44. doi: 10.1073/pnas.0437996100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Riou C, Yassine-Diab B, van GJ, Somogyi R, Greller LD, Gagnon D, et al. Convergence of TCR and cytokine signaling leads to FOXO3a phosphorylation and drives the survival of CD4+ central memory T cells. J Exp Med. 2007 Jan 22;204(1):79–91. doi: 10.1084/jem.20061681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, et al. Innate or Adaptive Immunity? The Example of Natural Killer Cells. Science. 2011 Jan 7;331(6013):44–9. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shibuya A, Sakamoto N, Shimizu Y, Shibuya K, Osawa M, Hiroyama T, et al. Fcα/μ receptor mediates endocytosis of IgM-coated microbes. Nat Immunol. 2000 Nov;1(5):441–6. doi: 10.1038/80886. [DOI] [PubMed] [Google Scholar]
- 41.Kikuno K, Kang DW, Tahara K, Torii I, Kubagawa HM, Ho KJ, et al. Unusual biochemical features and follicular dendritic cell expression of human Fcα/μ receptor. Eur J Immunol. 2007 Dec;37(12):3540–50. doi: 10.1002/eji.200737655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moir S, Ho J, Malaspina A, Wang W, DiPoto AC, O'Shea MA, et al. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J Exp Med. 2008 Aug 4;205(8):1797–805. doi: 10.1084/jem.20072683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pichler WJ, Knapp W. Receptors for IgM-coated erythrocytes on chronic lymphatic leukemia cells. J Immunol. 1977 Mar;118(3):1010–5. [PubMed] [Google Scholar]
- 44.Ferrarini M, Hoffman T, Fu SM, Winchester R, Kunkel HG. Receptors for IgM on certain human B lymphocytes. J Immunol. 1977 Oct;119(4):1525–9. [PubMed] [Google Scholar]
- 45.Burns GF, Cawley JC, Barker CR. Characterization of the receptor for IgM present on human B lymphocytes. Immunology. 1979 Mar;36(3):569–77. [PMC free article] [PubMed] [Google Scholar]
- 46.Rudders RA, Andersen J, Howard JP, Fried R. The binding of IgM to B lymphocytes. I. Studies of the binding of aggregated IgM to normal and leukemic human B lymphocytes. Clin Immunol Immunopathol. 1980 Apr;15(4):673–86. doi: 10.1016/0090-1229(80)90012-4. [DOI] [PubMed] [Google Scholar]
- 47.Platsoucas CD, Kempin S, Karanas A, Clarkson B, Good RA, Gupta S. Receptors for immunoglobulin isotype on T and B lymphocytes from untreated patients with chronic lymphocytic leukaemia. Clin Exp Immunol. 1980 May;40(2):256–63. [PMC free article] [PubMed] [Google Scholar]
- 48.Proto-Siqueira R, Panepucci RA, Careta FP, Lee A, Clear A, Morris K, et al. SAGE analysis demonstrates increased expression of TOSO contributing to Fas-mediated resistance in CLL. Blood. 2008 Jul 15;112(2):394–7. doi: 10.1182/blood-2007-11-124065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pallasch CP, Schulz A, Kutsch N, Schwamb J, Hagist S, Kashkar H, et al. Overexpression of TOSO in CLL is triggered by B-cell receptor signaling and associated with progressive disease. Blood. 2008 Nov 15;112(10):4213–9. doi: 10.1182/blood-2008-05-157255. [DOI] [PubMed] [Google Scholar]
- 50.Pallasch CP, Wendtner CM. Overexpression of the Fas-inhibitory molecule TOSO: a novel antiapoptotic factor in chronic lymphocytic leukemia. Leuk Lymphoma. 2009 Mar;50(3):498–501. doi: 10.1080/10428190902763491. [DOI] [PubMed] [Google Scholar]
- 51.Li FJ, Kubagawa Y, McCollum MK, Wilson L, Motohashi T, Bertoli LF, et al. Enhanced levels of both membrane-bound and soluble forms of IgM Fc receptor (FcμR) in patients with chronic lymphocytic leukemia. Blood. 2011 Sep 9;118(18):4902–9. doi: 10.1182/blood-2011-04-350793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Duhren-von MM, Ubelhart R, Schneider D, Wossning T, Bach MP, Buchner M, et al. Chronic lymphocytic leukaemia is driven by antigen-independent cell-autonomous signalling. Nature. 2012 Sep 13;489(7415):309–12. doi: 10.1038/nature11309. [DOI] [PubMed] [Google Scholar]
- 53.Stevenson FK, Hamblin TJ, Stevenson GT, Tutt AL. Extracellular idiotypic immunoglobulin arising from human leukemic B lymphocytes. J Exp Med. 1980 Dec 1;152(6):1484–96. doi: 10.1084/jem.152.6.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Qian GX, Fu SM, Solanki DL, Rai KR. Circulating monoclonal IgM proteins in B cell chronic lymphocytic leukemia: their identification, characterization and relationship to membrane IgM. J Immunol. 1984 Dec;133(6):3396–400. [PubMed] [Google Scholar]
- 55.Bertoli LF, Kubagawa H, Borzillo GV, Mayumi M, Prchal JT, Kearney JF, et al. Analysis with antiidiotype antibody of a patient with chronic lymphocytic leukemia and a large cell lymphoma (Richter's syndrome) Blood. 1987 Jul;70(1):45–50. [PubMed] [Google Scholar]
- 56.Chiorazzi N, Rai KR, Ferrarini M. Chronic lymphocytic leukemia. N Engl J Med. 2005 Feb 24;352(8):804–15. doi: 10.1056/NEJMra041720. [DOI] [PubMed] [Google Scholar]
- 57.Lang KS, Lang PA, Meryk A, Pandyra AA, Boucher LM, Pozdeev VI, et al. Involvement of Toso in activation of monocytes, macrophages, and granulocytes. Proc Natl Acad Sci USA. 2013 Feb 12;110(7):2593–8. doi: 10.1073/pnas.1222264110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Honjo K, Kubagawa Y, Kubagawa H. Is Toso/IgM Fc receptor (FcμR) expressed by innate immune cells? Proc Natl Acad Sci U S A. 2013 Jul 9;110(28):E2540–E2541. doi: 10.1073/pnas.1304904110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ouchida R, Mori H, Hase K, Takatsu H, Kurosaki T, Tokuhisa T, et al. Critical role of the IgM Fc receptor in IgM homeostasis, B-cell survival, and humoral immune responses. Proc Natl Acad Sci USA. 2012 Sep;17:E2699–E2706. doi: 10.1073/pnas.1210706109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Choi SC, Wang H, Tian L, Murakami Y, Shin DM, Borrego F, et al. Mouse IgM Fc receptor, FCMR, promotes B cell development and modulates antigen-driven immune responses. J Immunol. 2013 Feb 1;190(3):987–96. doi: 10.4049/jimmunol.1202227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ehrenstein MR, Notley CA. The importance of natural IgM: scavenger, protector and regulator. Nat Rev Immunol. 2010 Nov;10(11):778–86. doi: 10.1038/nri2849. [DOI] [PubMed] [Google Scholar]
- 62.Boes M, Esau C, Fischer MB, Schmidt T, Carroll M, Chen J. Enhanced B-1 cell development, but impaired IgG antibody responses in mice deficient in secreted IgM. J Immunol. 1998 May 15;160(10):4776–87. [PubMed] [Google Scholar]
- 63.Ehrenstein MR, O'Keefe TL, Davies SL, Neuberger MS. Targeted gene disruption reveals a role for natural secretory IgM in the maturation of the primary immune response. Proc Natl Acad Sci USA. 1998 Aug 18;95(17):10089–93. doi: 10.1073/pnas.95.17.10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boes M, Prodeus AP, Schmidt T, Carroll MC, Chen J. A critical role of natural immunoglobulin M in immediate defense against systemic bacterial infection. J Exp Med. 1998 Dec 21;188(12):2381–6. doi: 10.1084/jem.188.12.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baumgarth N, Herman OC, Jager GC, Brown LE, Herzenberg LA, Chen J. B-1 and B-2 cell-derived immunoglobulin M antibodies are nonredundant components of the protective response to influenza virus infection. J Exp Med. 2000 Jul 17;192(2):271–80. doi: 10.1084/jem.192.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Subramaniam KS, Datta K, Quintero E, Manix C, Marks MS, Pirofski La. The absence of serum IgM enhances the susceptibility of mice to pulmonary challenge with Cryptococcus neoformans. J Immunol. 2010 May 15;184(10):5755–67. doi: 10.4049/jimmunol.0901638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Boes M, Schmidt T, Linkemann K, Beaudette BC, Marshak-Rothstein A, Chen J. Accelerated development of IgG autoantibodies and autoimmune disease in the absence of secreted IgM. Proc Natl Acad Sci USA. 2000 Feb 1;97(3):1184–9. doi: 10.1073/pnas.97.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ehrenstein MR, Cook HT, Neuberger MS. Deficiency in serum immunoglobulin (Ig)M predisposes to development of IgG autoantibodies. J Exp Med. 2000 Apr 3;191(7):1253–8. doi: 10.1084/jem.191.7.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]