Abstract
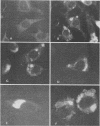
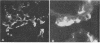
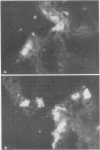
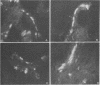
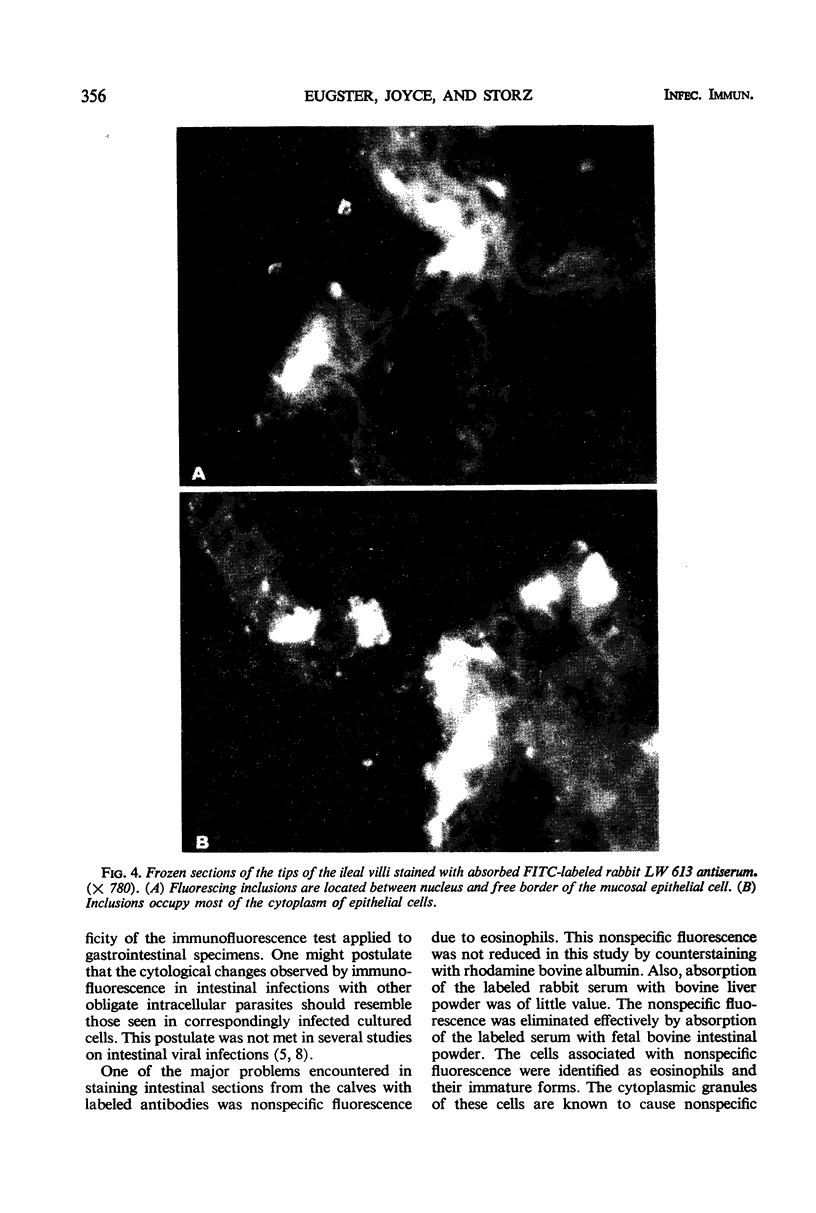
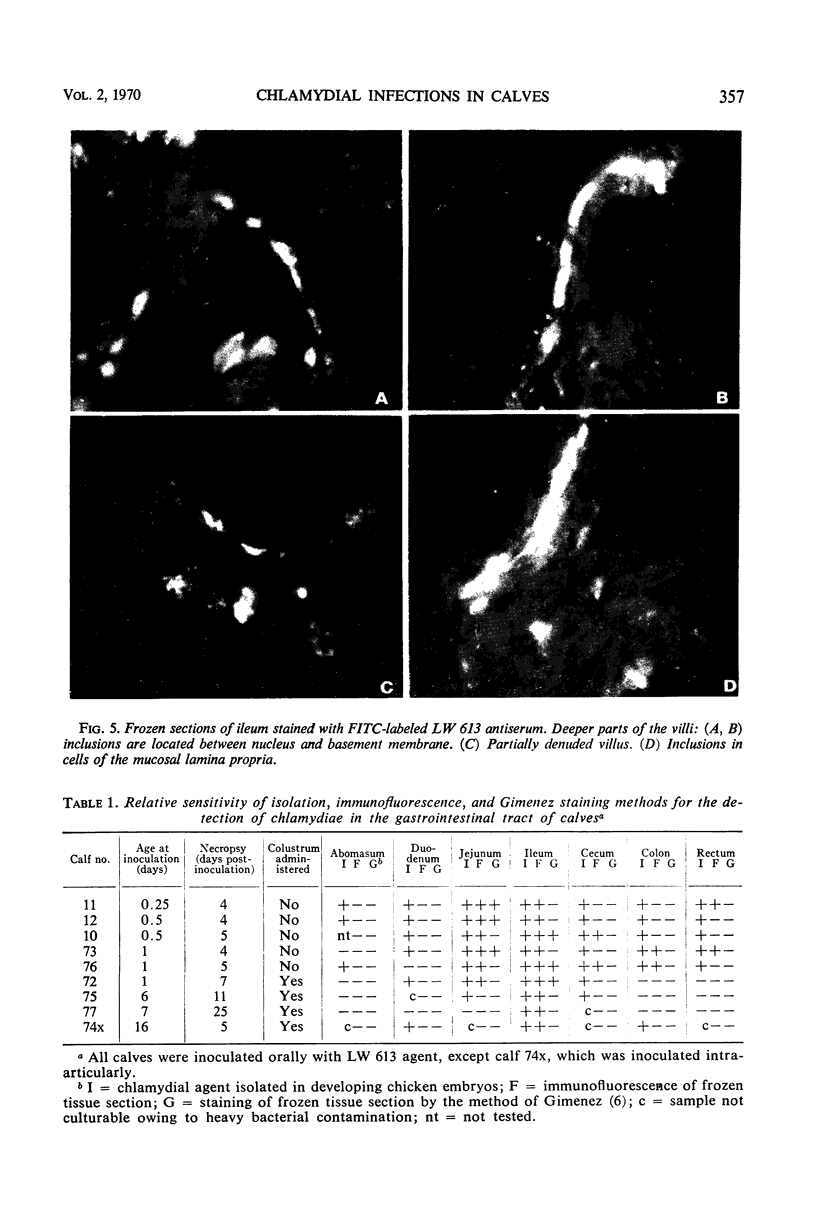
Newborn calves were exposed orally to the chlamydial agent of bovine polyarthritis. The chlamydial infection in the gastrointestinal tract was traced by reisolation of the agent and by fluorescent-antibody techniques. Absorption of the fluorescein isothiocyanate (FITC)-labeled antiserum with bovine fetal intestinal tissue powder eliminated effectively the nonspecific fluorescence of eosinophilic granules in intestinal tissue sections. Cells of the eosinophilic series were observed in great numbers in the gastrointestinal tract of inoculated and normal calves. Although chlamydial agents could be reisolated from mucosal scrapings of abomasum and duodenum for 5 days after inoculation, specific fluorescence was not observed in these gastrointestinal portions. As the chlamydial infection progressed, it localized in the mucosal epithelial cells of the jejunum and ileum. Fluorescing chlamydial inclusions were observed most consistently in the cytoplasm of mucosal epithelial cells on the tips of the jejunal and ileal villi. The inclusions were located between the nucleus and the free border of the epithelial cells. In the deeper parts of the villi, the inclusions in the epithelial cells were situated frequently between the nucleus and the basement membrane of the mucosal lamina propria. In calves examined 7 days after inoculation, fluorescing chlamydial inclusions were seen in the cells of the crypts and the mucosal lamina propria of the lower portions of the small intestine. Chlamydial infection of cells in the intestinal interstitium reflected a process of systemic invasion.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAKER J. A., YORK C. J. Miyagawanella bovis infection in calves. Ann N Y Acad Sci. 1956 Aug 10;66(1):210–214. doi: 10.1111/j.1749-6632.1956.tb40123.x. [DOI] [PubMed] [Google Scholar]
- BUCKLEY S. M., WHITNEY E., RAPP F. Identification by fluorescent antibody of developmental forms of psittacosis virus in tissue culture. Proc Soc Exp Biol Med. 1955 Oct;90(1):226–230. doi: 10.3181/00379727-90-21990. [DOI] [PubMed] [Google Scholar]
- CLEMMER D. L. EXPERIMENTAL ENTERIC INFECTION OF CHICKENS WITH AN AVIAN ADENO-VIRUS (STRAIN 93). Proc Soc Exp Biol Med. 1965 Apr;118:943–948. doi: 10.3181/00379727-118-30013. [DOI] [PubMed] [Google Scholar]
- Colgrove G. S., Haelterman E. O., Coggins L. Pathogenesis of African swine fever in young pigs. Am J Vet Res. 1969 Aug;30(8):1343–1359. [PubMed] [Google Scholar]
- Fernelius A. L., Lambert G. Detection of bovine viral diarrhea virus and antigen in tissues of experimentally infected calves by cell inoculation and fluorescent antibody techniques. Am J Vet Res. 1969 Sep;30(9):1551–1559. [PubMed] [Google Scholar]
- GIMENEZ D. F. STAINING RICKETTSIAE IN YOLK-SAC CULTURES. Stain Technol. 1964 May;39:135–140. doi: 10.3109/10520296409061219. [DOI] [PubMed] [Google Scholar]
- Hanna L., Dawson C. R., Briones O., Thygeson P., Jawetz E. Latency in human infections with TRIC agents. J Immunol. 1968 Jul;101(1):43–50. [PubMed] [Google Scholar]
- Kanamitsu M., Kasamaki A., Ogawa M., Kasahara S., Imamura M. Immunofluorescent study on the pathogenesis of oral infection of poliovirus in monkeys. Jpn J Med Sci Biol. 1967 Apr;20(2):175–194. doi: 10.7883/yoken1952.20.175. [DOI] [PubMed] [Google Scholar]
- MATUMOTO M., OMORI T., MORIMOTO T., HARADA K., INABA Y., ISHII S. Studies on the disease of cattle caused by bovine PL virus, a psittacosis-lymphogranuloma group virus (Miyagawanella). VIII. Sites for virus to leave the infected host. Jpn J Exp Med. 1955 Dec;25(6):223–245. [PubMed] [Google Scholar]
- NICHOLS R. L., McCO MB D. E., HADDAD N., MURRAY E. S. Studies on trachoma. II. Comparison of fluorescent antibody, giemsa, and egg isolation methods for detection of trachoma virus in human conjunctival scrapings. Am J Trop Med Hyg. 1963 Mar;12:223–229. [PubMed] [Google Scholar]
- NICHOLS R. L., McCOMB D. E. Immunofluorescent studies with trachoma and related antigens. J Immunol. 1962 Oct;89:545–554. [PubMed] [Google Scholar]
- Pensaert M. B., Burnstein T., Haelterman E. O. Cell culture-adapted SH strain of transmissible gastroenteritis virus of pigs: in vivo and in vitro studies. Am J Vet Res. 1970 Apr;31(4):771–781. [PubMed] [Google Scholar]
- Peter C. P., Gratzek J. B., Ramsey F. K. Isolation and characterization of a strain of infectious bovine rhinotracheitis virus associated with enteritis in cattle: pathogenesis studies by fluorescent antibody tracing. Am J Vet Res. 1966 Nov;27(121):1583–1590. [PubMed] [Google Scholar]
- Spendlove R. S. Optimal labeling of antibody with fluorescein isothiocyanate. Proc Soc Exp Biol Med. 1966 Jun;122(2):580–583. doi: 10.3181/00379727-122-31196. [DOI] [PubMed] [Google Scholar]
- Storz J., Smart R. A., Marriott M. E., Davis R. V. Polyarthritis of calves: isolation of psittacosis agents from affected joints. Am J Vet Res. 1966 May;27(118):633–641. [PubMed] [Google Scholar]
- Storz J., Thornley W. R. Serologische und aetiologische Studien über die intestinale Psittakose-Lymphogranuloma-infektion der Schafe. Zentralbl Veterinarmed B. 1966 Feb;13(1):14–24. [PubMed] [Google Scholar]
- TUOMI J., KANGAS J. A FLUORESCENT ANTIBODY TECHNIQUE FOR STUDIES OF MINK VIRUS ENTERITIS. (BRIEF REPORT). Arch Gesamte Virusforsch. 1963 May 20;13:430–434. doi: 10.1007/BF01244616. [DOI] [PubMed] [Google Scholar]
- YORK C. J., BAKER J. A. A new member of the psittacosis-lymphogranuloma group of viruses that causes infection in calves. J Exp Med. 1951 Jun;93(6):587–604. doi: 10.1084/jem.93.6.587. [DOI] [PMC free article] [PubMed] [Google Scholar]