Abstract
In this study, we compared, for the first time, the release of a 432 kDa prostaglandin 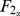 analogue drug, Latanoprost, from commercially available contact lenses using in vitro models with corneal epithelial cells. Conventional polyHEMA-based and silicone hydrogel soft contact lenses were soaked in drug solution (
analogue drug, Latanoprost, from commercially available contact lenses using in vitro models with corneal epithelial cells. Conventional polyHEMA-based and silicone hydrogel soft contact lenses were soaked in drug solution (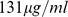 solution in phosphate buffered saline). The drug release from the contact lens material and its diffusion through three in vitro models was studied. The three in vitro models consisted of a polyethylene terephthalate (PET) membrane without corneal epithelial cells, a PET membrane with a monolayer of human corneal epithelial cells (HCEC), and a PET membrane with stratified HCEC. In the cell-based in vitro corneal epithelium models, a zero order release was obtained with the silicone hydrogel materials (linear for the duration of the experiment) whereby, after 48 hours, between 4 to 6
solution in phosphate buffered saline). The drug release from the contact lens material and its diffusion through three in vitro models was studied. The three in vitro models consisted of a polyethylene terephthalate (PET) membrane without corneal epithelial cells, a PET membrane with a monolayer of human corneal epithelial cells (HCEC), and a PET membrane with stratified HCEC. In the cell-based in vitro corneal epithelium models, a zero order release was obtained with the silicone hydrogel materials (linear for the duration of the experiment) whereby, after 48 hours, between 4 to 6 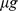 of latanoprost (an amount well within the range of the prescribed daily dose for glaucoma patients) was released. In the absence of cells, a significantly lower amount of drug, between 0.3 to 0.5
of latanoprost (an amount well within the range of the prescribed daily dose for glaucoma patients) was released. In the absence of cells, a significantly lower amount of drug, between 0.3 to 0.5 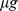 , was released, (
, was released, (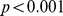 ). The difference observed in release from the hydrogel lens materials in the presence and absence of cells emphasizes the importance of using an in vitro corneal model that is more representative of the physiological conditions in the eye to more adequately characterize ophthalmic drug delivery materials. Our results demonstrate how in vitro models with corneal epithelial cells may allow better prediction of in vivo release. It also highlights the potential of drug-soaked silicone hydrogel contact lens materials for drug delivery purposes.
). The difference observed in release from the hydrogel lens materials in the presence and absence of cells emphasizes the importance of using an in vitro corneal model that is more representative of the physiological conditions in the eye to more adequately characterize ophthalmic drug delivery materials. Our results demonstrate how in vitro models with corneal epithelial cells may allow better prediction of in vivo release. It also highlights the potential of drug-soaked silicone hydrogel contact lens materials for drug delivery purposes.
Introduction
Ocular drug delivery is either intended to target the ocular surface to manage superficial conditions such as dry eye, microbial keratitis and conjunctivitis, or to treat intraocular disorders such as glaucoma, and age-related macular degeneration. Eye-drops are still the most common drug delivery method, comprising 90% of ophthalmic medications, followed by ointments and gels [1]. Eye-drop medications are applied topically to the eye in the form of either a solution or suspension in water [2]. The aqueous eye-drop is rapidly diluted in the tear film and most of it is drained through the lacrimal system, therefore, requiring frequent applications [3].
Studies show that only about 1 to 5% of the applied dose penetrates the cornea [4] and that due to the relatively fast turnover rate of the aqueous layer of the tear film, the residence time of hydrophilic medications is around 2 to 5 minutes [5]. The relatively slow turnover rate of the tear film lipid layer results in their increased residence time for lipophilic drugs, which reside in this layer, and consequently results in an increased uptake into the eye. The purpose of topical ophthalmic drug delivery devices is to deliver an adequate amount of medication to the anterior segment of the eye, with accurate targeted dosing at a sustained and controlled rate to increase bioavailability of the drug. Several commercial ocular delivery devices are currently available, including surface-located inserts [6], degradable or non-degradable implants [7], and in situ forming gels [8]. Despite almost 50 years of research being conducted on the potential use of soft contact lenses to deliver topical ophthalmic drugs [9], no drug delivery contact lens has yet been commercialized [10].
It is accepted that simple “soaking” of a contact lens in a topical drug solution may be insufficient for adequate elution on the ocular surface; therefore, it is considered to have a low potential for success [11], [12]. Thus a variety of research efforts are attempting to increase the drug uptake and/or release rates. These have included prolonged (up to 2 weeks) soaking [13], soaking the lenses in super-critical drug solutions [14], soaking the dehydrated contact lenses in drug solutions [15], and using vitamin E as a barrier to decrease diffusion of the drugs [16]. However, these efforts have resulted in minimal to no effect on the elution time and release kinetics [11]. It has been documented that the hydrophobic interactions of the active agents (i.e., drugs or other compounds) with the contact lens material is the primary governing factor in the adsorption and subsequent release of these compounds [17].
For the most part, drug release has been studied in a fixed volume of deionized water (DI), Phosphate Buffered Saline (PBS) or artificial tear solutions [11]. In these studies, the drug-eluting contact lens material is placed in a vial with a fixed volume of the release solution, and samples are collected from the solution at various time points. In fixed volume release studies, parameters such as the release medium and its volume, as well as mixing condition, are critically important [11]. The amount of released drug and the elution time have been shown to be consistently smaller when tested using the in vitro fixed volume model compared to in vivo experiments [12], [14], [18]–[23]. In the fixed volume conditions, the drug release mechanism is governed by diffusion, where concentration gradients generate the driving force and the ratio of the concentration between the contact lens and the medium is dictated by the partition ratio. The fixed volume environment does not represent the ocular environment, where there is a limited amount of tear liquid with a relatively fast tear turnover. The composition of the release medium also plays an important role in release studies. While a contact lens material may present optimal release in DI-water, their performance might be reduced dramatically in the presence of ions or surfactants [24]. In the field of contact lens drug delivery, the inadequacy of current release models has limited progress, primarily by giving rise to a false estimates of the kinetics of release, where the reported behavior cannot be recreated in the physiologic environment. Recently, Byrne et al. introduced a microfluidic device with the purpose of generating physiological flow rates to study the release rate through ophthalmic materials, thus generating a more representative release environment [25]. This microfluidic device mimics tear flow rate and the limited tear volume in the eye.
Considering that the primary drug permeation route to the front of the eye is through the transcorneal pathways, it is also important to consider the role of the cornea in drug release studies [26]. The lipid bilayer cell membrane retards the permeation of hydrophilic compounds. Through expressing certain transporters as well as certain enzymes present in the epithelial cells, the cornea is involved in metabolism and transportation of prodrugs and their active metabolized form [27]–[31]. The corneal epithelium is considered to be the rate-limiting factor in the transcorneal permeation of most ophthalmic drugs [32], [33], especially for hydrophilic drugs [34], [35]. Thus, using an in vitro corneal epithelial model will allow replication of the relevant factors of the in vivo environment. Human corneal in vitro models offer a cost effective and more standardizable substitutes [36] for animal studies while allowing a higher throughput testing of biomaterial interaction and drug permeation [37]. Reconstructed corneal equivalents as well as cell culture models of the corneal epithelium have been successfully used to study ocular toxicity and permeability [37]–[41].
Pharmacokinetics of most prostaglandin 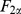 analogues has been extensively studied in vivo
[4], [42]. The contribution of the enzyme and transport activities such as the esterase activity of the corneal epithelium has been utilized in the design of ophthalmic prodrugs [43]. The lipophilicity, as a result of esterification or amidification of
analogues has been extensively studied in vivo
[4], [42]. The contribution of the enzyme and transport activities such as the esterase activity of the corneal epithelium has been utilized in the design of ophthalmic prodrugs [43]. The lipophilicity, as a result of esterification or amidification of 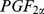 analogues, facilitates the penetration through the cornea. Prostaglandin analogues metabolism into the hydrophilic acid forms inside the epithelial cells allows permeation through the stroma [44] and thus, increases the bioavailability of the active substance in the interior of the eye [45]. We therefore hypothesize that the presence of corneal epithelial cells may have an impact when assessing drug-delivery materials in vitro. The objective of this study was to investigate the release of Latanoprost by commercially available contact lenses using in vitro models containing corneal epithelial cells.
analogues, facilitates the penetration through the cornea. Prostaglandin analogues metabolism into the hydrophilic acid forms inside the epithelial cells allows permeation through the stroma [44] and thus, increases the bioavailability of the active substance in the interior of the eye [45]. We therefore hypothesize that the presence of corneal epithelial cells may have an impact when assessing drug-delivery materials in vitro. The objective of this study was to investigate the release of Latanoprost by commercially available contact lenses using in vitro models containing corneal epithelial cells.
Materials and Methods
Preparation of Drug Doping Solutions
The lens doping solution was prepared by dissolving latanoprost and latanoprost free-acid (solution in methyl acetate, Cayman Chemical, Ann Arbor, MI) in PBS (Lonza, Walkersville, MD). The concentration of the stock drug solution was 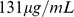 .
.
Preparation of Contact Lenses
Four commercially available contact lens materials, galyfilcon A, senofilcon A, omafilcon A, and balafilcon A were used. The properties of the four lens types are presented in table 1. All lenses had a back vertex power of -3.00 diopter. Lenses were incubated for 24 hours in PBS (Lonza, Allendale, New Jersey) to remove any remnants of their packaging solutions, before incubation in 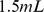 of the drug solution for 24 hours.
of the drug solution for 24 hours.
Table 1. Properties of the Contact Lens Hydrogel Materials [47].
| Commercial name | Acuvue Advance | Acuvue Oasys | ProClear | PureVision |
| (US adopted name) | Galyfilcon A | Senofilcon A | Omafilcon A | Balafilcon A |
| Manufacturer | Johnson & Johnson | Johnson & Johnson | Coopervision | Bausch & Lomb |
| Water content | 47 | 38 | 60 | 36 |
| Principal Monomer | mPDMS + DMA + HEMA+ siloxane macromer+ EGDMA + PVP | mPDMS + DMA + HEMA + siloxane macromer + TEGDMA + PVP | HEMA + PC | NVP + TPVC + NVA + PBVC |
| FDA group * | V(I) | V(I) | II | V(III) |
| Low water | Low water | High water | Low water | |
| Non-ionic | Non-ionic | Non-ionic | Ionic |
*FDA (Food and Drug Administration) categorizes all silicone hydrogel contact lenses as group V, however it is more practical to use groups for conventional hydrogels to better understand their material properties. HEMA, Hydroxyethyl Methacrylate; PC, Phosphotidylcholine; NVP, N-Vinylpyrrolidone; TPVC, Tris(trimethylsiloxysilyl) Propyvinyl Carbamate; NVA, N-Vinyl Aminobutyric Acid; PBVC, Poly(dimethysiloxy)di (silylbutanol) Bis(Vinyl Carbamate); mPDMS, monofunctional Polydimethylsiloxane; DMA, N, N-Dimethylacrylamide; EGDMA, Ethyleneglycol Dimethacrylate; PVP, Polyvinyl Pyrrolidone; TEGDMA, Tetra-Ethyleneglycol Dimethacrylate
In Vitro Cell Culture
HPV-immortalized human corneal epithelial cells, a generous gift from Dr. May Griffith (Integrative Regenerative Medicine (IGEN) Centre, Linköping University, Sweden) [38] were cultured in keratinocyte serum free medium (KSFM) supplemented with bovine pituitary extract, recombinant epidermal growth factor, and penicillin/streptomycin (Pen/Strep) (ScienCell, Carlsbad, California, USA) at 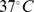 and 5% carbon dioxide (
and 5% carbon dioxide (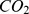 ). Fresh medium was added every other day and cells were grown to 90% confluency in tissue culture treated flasks. Adherent cells were removed using TryplExpress (Life Technologies, Burlington, Ontario, Canada) dissociation solution. Cells were routinely observed for any morphological changes and were used before their eleventh passage.
). Fresh medium was added every other day and cells were grown to 90% confluency in tissue culture treated flasks. Adherent cells were removed using TryplExpress (Life Technologies, Burlington, Ontario, Canada) dissociation solution. Cells were routinely observed for any morphological changes and were used before their eleventh passage.
In Vitro Drug Release Models
Three in vitro models were used to assess drug release from commercially available contact lenses in-cluding diffusion through a) a Polyethylene Terephthalate (PET) membrane (Millicell PET membrane with a 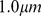 pore size, also referred to as culture inserts, Millipore, MA, USA) with no-cells; b) a PET membrane with a monolayer of human corneal epithelial cells (HCECs) and c) a PET membrane with a multilayer of HCECs (stratified culture). For the two latter models, the PET membranes were seeded with
pore size, also referred to as culture inserts, Millipore, MA, USA) with no-cells; b) a PET membrane with a monolayer of human corneal epithelial cells (HCECs) and c) a PET membrane with a multilayer of HCECs (stratified culture). For the two latter models, the PET membranes were seeded with 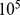 cells. The corneal epithelium models were fed with KSFM on each of the basal and apical sides of the cells layer for five days, with medium being exchanged every other day. After five days, for the multilayer models, cell differentiation was induced by exposing the monolayer to an air-liquid interface. Cells were fed only on the basal side with 2% fetal bovine serum (FBS, Invitrogen, Burlington, ON, Canada) in 1∶1 Dulbeccos minimum essential medium (DMEM, Invitrogen) in Hams F12 nutrient medium (DMEM/F12, Invitrogen); the medium was exchanged daily [41]. The cells grew under these conditions for seven days and were then ready for experimentation.
cells. The corneal epithelium models were fed with KSFM on each of the basal and apical sides of the cells layer for five days, with medium being exchanged every other day. After five days, for the multilayer models, cell differentiation was induced by exposing the monolayer to an air-liquid interface. Cells were fed only on the basal side with 2% fetal bovine serum (FBS, Invitrogen, Burlington, ON, Canada) in 1∶1 Dulbeccos minimum essential medium (DMEM, Invitrogen) in Hams F12 nutrient medium (DMEM/F12, Invitrogen); the medium was exchanged daily [41]. The cells grew under these conditions for seven days and were then ready for experimentation.
Measuring Drug Concentrations
Aliquots of 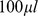 (10% of the total volume of the medium present in the bottom) were taken from the bottom of the in vitro models and replaced by fresh culture medium. For the latanaprost experiments, samples were taken at 1, 3, 6, 12, 18, 24 and 48 hours. For latanoprost free-acid experiments, samples were collected at 1, 3, 6, and 24 hours.
(10% of the total volume of the medium present in the bottom) were taken from the bottom of the in vitro models and replaced by fresh culture medium. For the latanaprost experiments, samples were taken at 1, 3, 6, 12, 18, 24 and 48 hours. For latanoprost free-acid experiments, samples were collected at 1, 3, 6, and 24 hours.
Collected samples were analyzed by an enzyme immuno-assay (EIA) for latanoprost (Cayman Chemical, Ann Arbor, MI, USA). Following the EIA kit instructions, each collected sample was analyzed in duplicate and at two different dilutions. To determine the uptake amount by the contact lenses, samples were also aliquoted from the original drug stock solution as well as the remaining drug solutions after soaking the lenses.
The release results represent the concentration of the drug on the other side of the PET membrane, meaning that the drug has been released from the contact lens material on top of the membrane, then diffused through the cells, if present, and the culture membrane. Note that the EIA kit does not distinguish between the free-acid form and ester form of the drug.
Drug Concentration Calculations
As mentioned above, to measure the amount of released drug, samples were taken from the bottom of the wells and replaced by fresh solution at each time point. Refreshing a fraction of the medium in the bottom at each time point affects measurements. Therefore, it is necessary to account for the dilution effect and adjust the measured concentrations to provide an accurate measure of the concentration without the dilution effect.
Assuming the fraction of total volume of medium in the bottom which is being aliquoted is “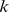 ”, the mass balance principle can be used to estimate for the actual concentration at each time point.
”, the mass balance principle can be used to estimate for the actual concentration at each time point.
| (1) |
| (2) |
In Eq.(1), 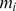 refers to the amount of drug at i-th time point (
refers to the amount of drug at i-th time point (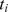 ),
), 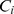 refers to the measured concentration at time
refers to the measured concentration at time 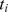 , and
, and 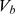 is the volume of the liquid in the bottom. An estimate of the actual amount of drug diffused through the insert adjusted for the dilution effect,
is the volume of the liquid in the bottom. An estimate of the actual amount of drug diffused through the insert adjusted for the dilution effect, 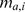 , can be calculated using Eq.(2). This equation can be obtained as below by calculating the accumulated drug amount in the medium by adding the removed amount in previous steps to the amount of the drug available in medium at each step.
, can be calculated using Eq.(2). This equation can be obtained as below by calculating the accumulated drug amount in the medium by adding the removed amount in previous steps to the amount of the drug available in medium at each step.
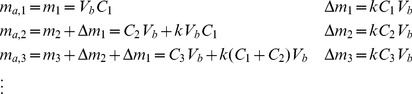 |
(3) |
The adjusted concentration, 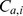 at i-th step can be found as below:
at i-th step can be found as below:
| (4) |
The proposed method to estimate adjusted concentrations neglects the effect that dilution might have on the diffusion rate. However, for small difference between calculated and measured concentrations, the change in diffusion rate will be insignificant.
Data Analysis
Results are presented as the mean of six experiments for latanoprost and three experiments for latanoprost free-acid  standard deviation. All experiments were performed on different days. To evaluate the significance of the differences between various contact lens materials, in vitro corneal models and time points, an analysis of variance (ANOVA) was performed, followed by multiple pair-wise comparisons using the Holm-Sidak test (SigmaPlot, San Jose, California, USA).
standard deviation. All experiments were performed on different days. To evaluate the significance of the differences between various contact lens materials, in vitro corneal models and time points, an analysis of variance (ANOVA) was performed, followed by multiple pair-wise comparisons using the Holm-Sidak test (SigmaPlot, San Jose, California, USA).
Results
Preliminary studies showed that there was no decay of latanoprost and latanoprost free-acid in the culture medium or buffered solution used in the current research (results not presented), thus enabling the use of the enzyme immuno-assay method to measure drug concentrations in both solutions for up to 48 hours. All the results presented have also been adjusted according to Eq. (4), to take into account the small dilution that may occur as samples are taken out and fresh medium is added.
The uptake analysis showed that 95% of the dissolved latanoprost was taken up by the galyfilcon A and senofilcon A silicone hydrogels and 98% by the balafilcon A (thus approximately 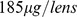 ) and nearly 25% of the latanoprost solution was taken up into omalfilcon A (
) and nearly 25% of the latanoprost solution was taken up into omalfilcon A (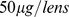 ).
).
Release in the absence of cells
In the no-cells model, release was first measured in KSFM to allow for comparisons between all in vitro models. As shown in Fig. 1, an initial burst in the first 6 hours was observed, followed by saturation, when no more drug was released, despite the available drug in the contact lens material.
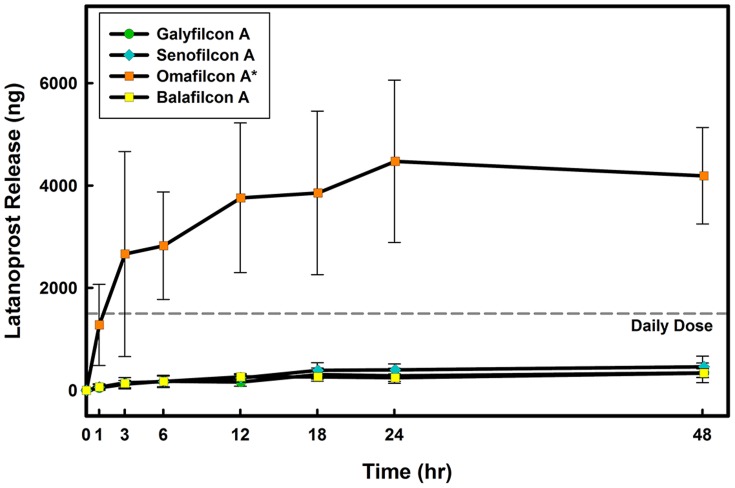
Figure 1. Time course of latanoprost release from four contact lens materials through the no-cells model.
Lenses were soaked for 24 hours in drug solution (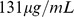 ) and then overlayed on the model for 24 hours. Aliquots were taken at specific times from the lower compartment and concentrations was measured using EIA. Daily dose line represents the amount of the administered latanoprost for a glaucoma patient [46]. *Significantly different from silicone hydrogel contact lens materials
) and then overlayed on the model for 24 hours. Aliquots were taken at specific times from the lower compartment and concentrations was measured using EIA. Daily dose line represents the amount of the administered latanoprost for a glaucoma patient [46]. *Significantly different from silicone hydrogel contact lens materials 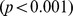 . (n = 6 Mean
. (n = 6 Mean  SD).
SD).
The effects of the release medium was also assessed with the three silicone hydrogel contact lens materials for 24 hours, where the cell culture medium (KSFM) was substituted with PBS. When compared to KSFM, the latanoprost release decreased significantly in the no-cell model in PBS 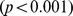 , Fig. 2.
, Fig. 2.
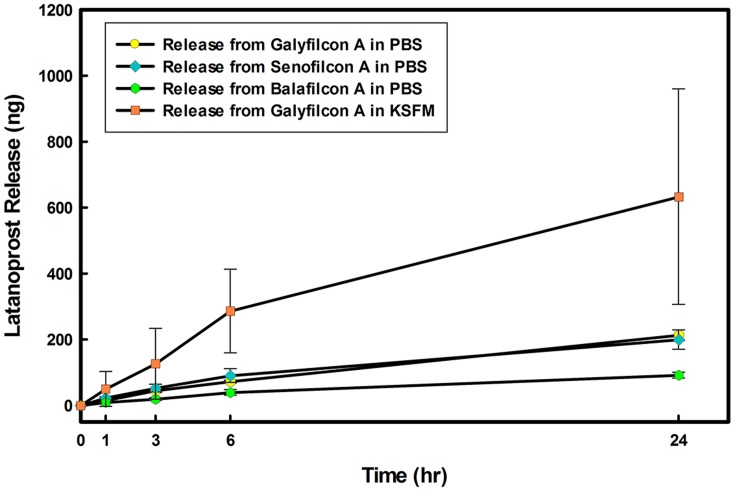
Figure 2. Comparison of latanoprost release from silicone hydrogels in no-cells model.
Release from three silicone hydrogel contact lens materials in PBS as well as release from galyfilcon A in KSFM (Keratinocyte Serum Free Medium) is shown. Lenses were soaked for 24 hours in drug solution (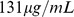 ) and then overlayed on the model for 24 hours. Aliquots were taken at specific times from the lower compartment and concentrations were measured using EIA. (n = 3 Mean
) and then overlayed on the model for 24 hours. Aliquots were taken at specific times from the lower compartment and concentrations were measured using EIA. (n = 3 Mean  SD).
SD).
Release in the presence of a monolayer or multilayer model
Performing the contact lens release experiments in the presence of corneal epithelial cells resulted in significant changes. As illustrated in Fig. 3, the amount of latanoprost released from senofilcon A was dependent on the presence of cells in the in vitro models; a significantly higher amount of latanoprost was released in the monolayer and multilayer models 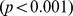 when compared to the no-cell model. Furthermore, while in the no-cell model, no significant difference in release was observed over time, for the monolayer and multilayer models, there was a significant increase in the amount released at 1, 3, 12, 18, 24 and 48 hrs
when compared to the no-cell model. Furthermore, while in the no-cell model, no significant difference in release was observed over time, for the monolayer and multilayer models, there was a significant increase in the amount released at 1, 3, 12, 18, 24 and 48 hrs 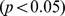 . For all contact lens materials studied, in the monolayer and multilayer in vitro corneal models, an extended release of drug was observed over time (Fig. 4). The improved release profiles from latanoprost-soaked contact lenses was similar between the monolayer and multilayer models
. For all contact lens materials studied, in the monolayer and multilayer in vitro corneal models, an extended release of drug was observed over time (Fig. 4). The improved release profiles from latanoprost-soaked contact lenses was similar between the monolayer and multilayer models 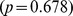 .
.
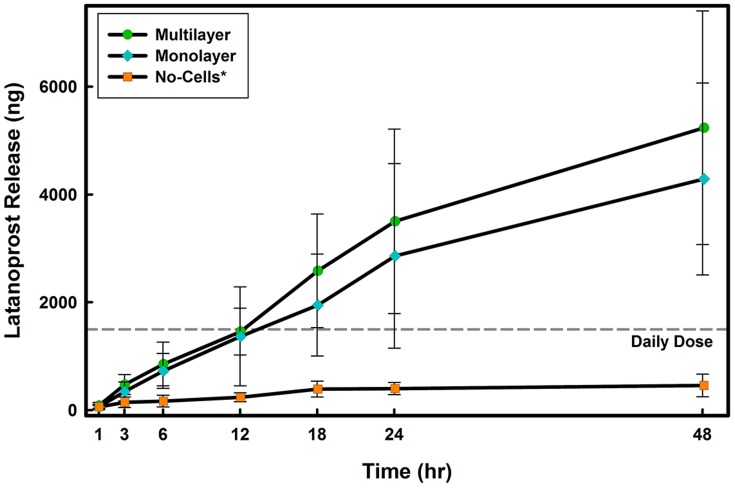
Figure 3. Time course of latanoprost release from senofilcon A in the three in vitro models.
Lenses were soaked for 24 hours in drug solution (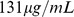 ) and then overlayed on the model for 24 hours. Aliquots were taken at specific times from the lower compartment and concentrations were measured using EIA. Daily dose line represents the amount of the administered latanoprost for a glaucoma patient [46]. No-Cell Model: Cell culture inserts (PET membrane) without cells, Monolayer Model: PET insert with a monolayer of human corneal epithelial cells, Multilayer Model: PET insert with a multilayer of human corneal epithelial cells (stratified culture). *Significantly different from in vitro models with cells
) and then overlayed on the model for 24 hours. Aliquots were taken at specific times from the lower compartment and concentrations were measured using EIA. Daily dose line represents the amount of the administered latanoprost for a glaucoma patient [46]. No-Cell Model: Cell culture inserts (PET membrane) without cells, Monolayer Model: PET insert with a monolayer of human corneal epithelial cells, Multilayer Model: PET insert with a multilayer of human corneal epithelial cells (stratified culture). *Significantly different from in vitro models with cells 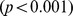 . (n = 6 Mean
. (n = 6 Mean  SD).
SD).
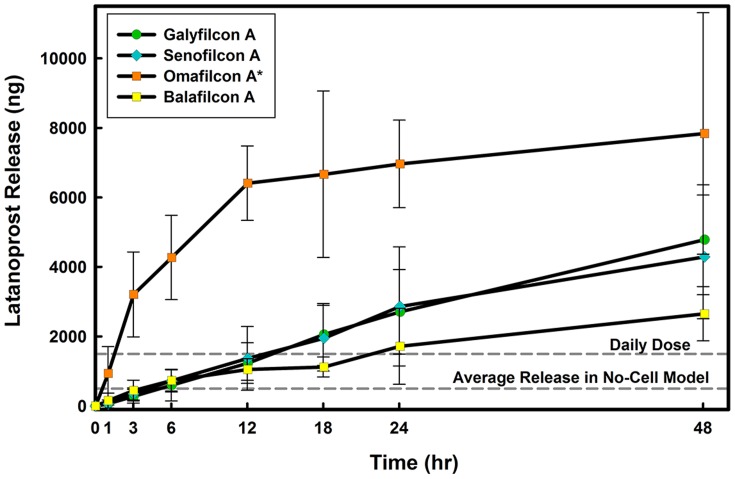
Figure 4. Time course of latanoprost release from four contact lens materials through the monolayer model.
Lenses were soaked for 24 hours in drug solution (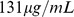 ) and then overlayed on the model for 24 hours. Aliquots were taken at specific times from the lower compartment and concentrations were measured using EIA. Daily dose line represents the amount of the administered latanoprost for a glaucoma patient [46]. No-Cell Model: Cell culture inserts (PET membrane) without cells, Monolayer Model: PET insert with a monolayer of human corneal epithelial cells, Multilayer Model: PET insert with a multilayer of human corneal epithelial cells (stratified culture). *Significantly different from in vitro models with cells
) and then overlayed on the model for 24 hours. Aliquots were taken at specific times from the lower compartment and concentrations were measured using EIA. Daily dose line represents the amount of the administered latanoprost for a glaucoma patient [46]. No-Cell Model: Cell culture inserts (PET membrane) without cells, Monolayer Model: PET insert with a monolayer of human corneal epithelial cells, Multilayer Model: PET insert with a multilayer of human corneal epithelial cells (stratified culture). *Significantly different from in vitro models with cells 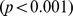 . (n = 6 Mean
. (n = 6 Mean  SD).
SD).
The release results for all tested commercial contact lenses are summarized in table 2. While the amount of drug released fell within potential therapeutic ranges, only 2% of the amount of the drug sorbed into silicone hydrogel contact lens material was released after 24 hours (Table 2). A significantly higher amount (between 10 to 17% depending on the model used) was released from the high water content hydrogel material, omafilcon A. The high release of latanoprost from omafilcon A (Fig. 4) is in spite of the lower drug uptake, which results in a significantly higher release percentage (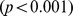 , Table 2). Latanoprost release from galyfilcon A and senofilcon A were not significantly different
, Table 2). Latanoprost release from galyfilcon A and senofilcon A were not significantly different 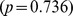 and neither were they different from the release observed with balafilcon A
and neither were they different from the release observed with balafilcon A 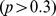 .
.
Table 2. Latanoprost Free-Acid Release from Tested Commercial Contact Lenses after 24 Hours.
| Contact Lens Material | No-Cells Model | Monolayer Model | Multilayer Model | |||
| Release | Percentage | Release | Percentage | Release | Percentage | |
|
of Release† (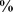 ) ) |
|
of Release† (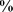 ) ) |
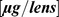
|
of Release† (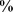 ) ) |
|
| Galyfilcon A |
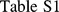
|
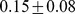
|
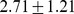 *
*
|
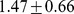 *
*
|
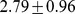 *
*
|
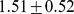 *
*
|
| Senofilcon A |
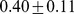
|
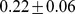
|
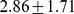 *
*
|
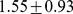 *
*
|
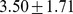 *
*
|
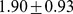 *
*
|
| Omafilcon A |
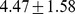 #
#
|
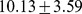 #
#
|
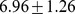 #
*
#
*
|
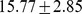 #
*
#
*
|
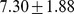 #
*
#
*
|
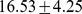 #
*
#
*
|
| Balafilcon A |
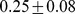
|
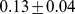
|
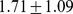 *
*
|
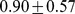 *
*
|
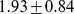 *
*
|
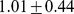 *
*
|
n = 6, Mean  Standard Deviation. Concentration of latanoprost were measured using EIA.
Standard Deviation. Concentration of latanoprost were measured using EIA.
No-Cell Model: Cell culture inserts (PET membrane) without cells, Monolayer Model: PET insert with a monolayer of human corneal epithelial cells, Multilayer Model: PET insert with a multilayer of human corneal epithelial cells (stratified culture).
The release as a percentage of uptake has been calculated based on the ratio of the released concentration over the sorbed amount.
Significantly different from other contact lens materials (silicone hydrogel) 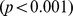 .
.
*Significantly different from the amount released by respective materials in the no-cells model 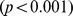 .
.
Release of Latanoprost Free-Acid
Since in the absence of cells, latanoprost cannot be metabolized to its free-acid form, the release of latanoprost free-acid from contact lens materials was studied to determine if latanoprost free-acid may be used as a substitute to latanoprost in a no-cell model. To allow for a more complete comparison between models and drug forms, release of latanoprost free-acid was also tested with the same in vitro models.
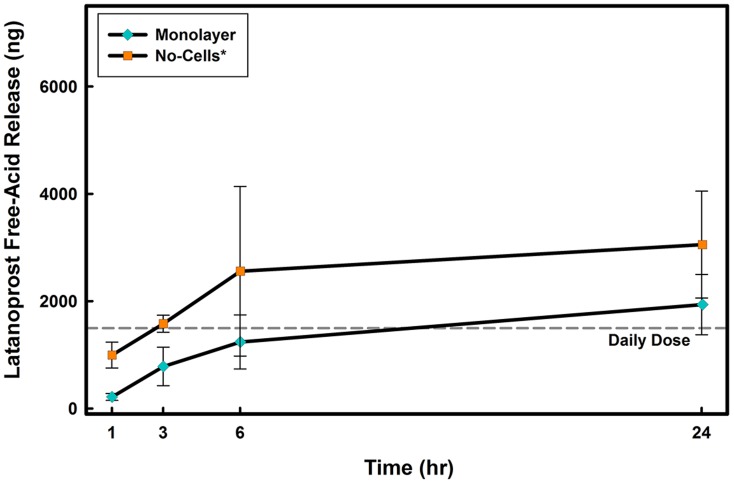
With latanoprost free-acid, contrary to what was observed with the ester form of the drug, a significantly lower release occurred in the presence of cells when compared to no-cells (Fig. 5). Table 3 presents the release of latanoprost free-acid from tested commercial contact lenses after 24 hours for each of the in vitro models. When comparing the amount of drug release at 24 hours in the monolayer model, the latanoprost free-acid results show a significant decrease (approximately 30%) in the amounts of the drug that has been released from galyfilcon A and senofilcon A silicone hydrogels (Table 2).
Figure 5. Time course of latanoprost free-acid release from senofilcon A in No-Cell and Monolayer in vitro models.
Lenses were soaked for 24 hours in drug solution (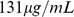 ) and then overlayed on the model for 24 hours. Aliquots were taken at specific times from the lower compartment and concentrations were measured using EIA. No-Cell Model: Cell culture inserts (PET membrane) without cells, Monolayer Model: PET insert with a monolayer of human corneal epithelial cells. Daily dose line represents the amount of the administered latanoprost for a glaucoma patient [46]. *Significantly different from in vitro models with cells
) and then overlayed on the model for 24 hours. Aliquots were taken at specific times from the lower compartment and concentrations were measured using EIA. No-Cell Model: Cell culture inserts (PET membrane) without cells, Monolayer Model: PET insert with a monolayer of human corneal epithelial cells. Daily dose line represents the amount of the administered latanoprost for a glaucoma patient [46]. *Significantly different from in vitro models with cells 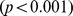 . (n = 3 Mean
. (n = 3 Mean  SD).
SD).
Table 3. Latanoprost Free-Acid Release from Tested Commercial Contact Lenses after 24 Hours.
| Contact Lens Material | Release Model 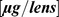
|
||
| No-Cells | Monolayer | Multilayer | |
| Galyfilcon A |
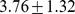
|
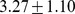
|
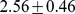
|
| Senofilcon A |
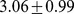
|
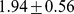
|
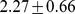
|
| Omafilcon A |
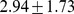
|
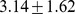
|
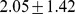
|
| Balafilcon A |
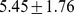 $
$
|
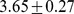 $
$
|
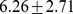 $
$
|
n = 3, Mean  Standard Deviation. Concentration of latanoprost free-acid were measured using EIA.
Standard Deviation. Concentration of latanoprost free-acid were measured using EIA.
No-Cell Model: Cell culture inserts (PET membrane) without cells, Monolayer Model: PET insert with a monolayer of human corneal epithelial cells, Multilayer Model: PET insert with a multilayer of human corneal epithelial cells (stratified culture).
Significantly different from other lens materials 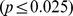 .
.
The Role of Live Cells
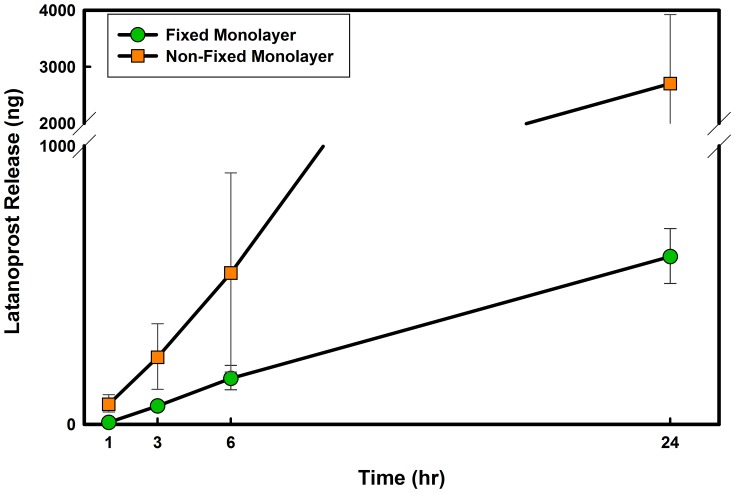
To study the importance of metabolically active cells, which not only provide a physical barrier to drug permeation, but also are able to transfer and metabolize the drug, a set of experiments was designed to compare latanoprost release from the galyfilcon A silicone hydrogel material through a fixed and a live monolayer corneal model. In the fixed monolayer, cells are dead and thus metabolism of the drug cannot occur.
As shown in Fig. 6, in the presence of fixed (dead) cells, the amount of latanoprost that was released from the soaked galyfilcon A lens and diffused through the monolayer was lower in the presence of paraformaldehyde-fixed cells when compared to metabolically-active cells. These results clearly highlight the importance of the metabolism and transportation in in vitro model of drug releasing materials.
Figure 6. Time course of latanoprost release from galyfilcon A contact lens through live and dead monolayer models.
Cells were killed by fixing in Paraformaldehyde. Lenses were soaked for 24 hours in drug solution (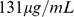 ) and then overlayed on the model for 24 hours. Release experiments through fixed monolayer were conducted in two separate dates (n = 2, Mean
) and then overlayed on the model for 24 hours. Release experiments through fixed monolayer were conducted in two separate dates (n = 2, Mean  SD). The results were compared to release through monolayer models, (n = 6 Mean
SD). The results were compared to release through monolayer models, (n = 6 Mean  SD).
SD).
Discussion
This study was undertaken to determine the impact of the presence of cells in in vitro models of drug releasing materials. The cells used in these experiments, HPV-immortalized corneal epithelial cells have been used previously by Griffith et. al. for corneal constructs and have been shown to exhibit key physiological functions and biochemical marker expression of corneal epithelial cells [38].
Our initial release experiments with drug soaked contact lens material in the absence of cells provided results similar to many others, ([11], [12], [18], [20], [22]) showing a mechanism of a first order release. The limited amount of drug that was released in our fixed volume model is likely the result of the high partition ratios of the latanoprost between the contact lens material and the aqueous solutions. Furthermore, our results from the no-cells model suggest that latanoprost has a lower affinity toward PBS compared to KSFM. The better solubility of latanoprost in KSFM compared to PBS is likely due to the difference in composition, such as the presence of growth factors and other ionic compounds in the culture medium which are absent in the buffered saline solution. While, in our experiments, the nature of the medium was found to have a statistically significant impact on release in the no-cell model, the actual improvement in drug release is actually insignificant when compared to the in vitro models with cells.
Significantly higher drug release and diffusion were observed in the presence of cells. Due to their hydrophobicity, ester prostaglandin analogues, such as the latanoprost prodrug, have a greater chance of diffusion through the hydrophobic corneal epithelium [43]. Furthermore, metabolism will also play a role in the presence of live (metabolically active) cells, since the latanoprost prodrug is expected to be metabolized by cells [27], [29], [30], [46] before diffusion through the cell layer. The metabolized product, the latanoprost free-acid, has a smaller partition ratio and is more water soluble when compared to latanoprost. Therefore, the presence of cells will improve the drug diffusion rate. A layer of cells will also improve drug release from the contact lens material by maintaining the concentration gradient between the lens and the solution above the cells through metabolism of the latanoprost.
A recent study showed that the nonmetabolized (ester) form of latanoprost contributed to only 4% of the total drug diffused through an in vitro corneal model and that no detectable amount of ester form of the latanoprost was observed in an ex vivo model [26]. We may thus assume that the majority of the diffused drug through the in vitro corneal models with cells is the free-acid form.
Different latanoprost release profiles were observed among the hydrogel contact lens materials tested. Compared to the silicone hydrogel materials, the high release of latanoprost from omafilcon A in spite of the lower drug uptake may be explained by the low affinity of the latanoprost (an hydrophobic compound) toward the omafilcon A contact lens material, which is a high water content hydrogel. The large partition ratio results in a low uptake by this material when soaking in aqueous solution of hydrophobic drugs, as well as relatively fast release rates in the release solution.
Using latanoprost esterified form, i.e. the active drug compound, latanoprost free-acid, affected results in all models. Higher water solubility of the latanoprost free-acid led to higher amounts of drug being released from the silicone hydrogel lens materials in the no-cell model. As a more polar molecule, latanoprost free-acid has a lower partition ratio between the hydrophobic silicone hydrogel contact lens materials and the aqueous solution when compared to latanoprost. While higher amounts of latanoprost free-acid were released in the no-cell model, lower releases were observed in the presence of cells. With latanoprost free-acid, epithelial cells now act as a barrier against the diffusion of the latanoprost free-acid, and hence limit the diffusion of the hydrophilic drug.
As one compares the latanoprost and latanoprost free-acid release results, it becomes evident that similar drug release profiles cannot be obtained by replacing the prodrug with the drug, even in the no-cell model. Not only are the amounts released significantly different by an order of magnitude, but while all silicone hydrogel materials released similar amounts of latanoprost, balafilcon A released significantly more latanoprost free-acid compared to the other two silicone hydrogels. The balafilcon A/latanoprost free-acid results are likely due to the fact that balafilcon A material has an overall net negative charge due to the incorporation of some acidic material components [46] and its surface charge increases the hydrodynamic attributes of the material [47], therefore increasing the role of adsorption of the hydrophilic drug on the surface of the contact lens during the uptake process and its subsequent release in solution. Nevertheless, taken together, our latanoprost free-acid results highlight the relevance of using in vitro models with cells when studying release of a prodrug that requires to be metabolized before diffusion through the tissue to the site of treatment.
Due to the lack of previous in vitro studies on prostaglandin analogues, our results can only be compared to the release of drugs from the contact lens materials with similar size and hydrophobicity. Previous in vivo studies have shown a prolonged release of relatively hydrophobic drugs such as ketotifen [21] and lomefloxacin [23], however such release profiles could not be replicated in vitro using a fixed volume release model [12], [22]. The extended release of latanoprost observed in the monolayer and multilayer in vitro models correlates well with the extended release profiles of the hydrophobic drugs observed in vivo [21], [23]. The release results of latanoprost in the no-cells model is also comparable to the release results of hydrophobic compounds in fixed volume solution [12], [22]. The significant role of cell metabolism and transport was further demonstrated using fixed (metabolically inactive) cells. Taken together, our results suggest that the absence of cells in in vitro models of drug release likely contributes to the contradiction between these in vitro and in vivo studies [12], [21]–[23].
Conclusion
Poor release results from commercially available contact lens materials soaked in hydrophobic compounds such as latanoprost have been obtained with fixed volume release models similar to the no-cells model used here. However, we have demonstrated, using drug-soaked silicone hydrogel materials, that the amount of drug diffusing through an in vitro corneal model is in the order of 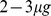 over a period of 24 hours, which is comparable to the
over a period of 24 hours, which is comparable to the 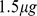 of drug in every drop of the commercial latanoprost. Our results emphasize the importance of the presence of cells when characterizing the release of drug-delivery materials and demonstrate how experimental in vitro models have a significant impact on the outcomes of testing ophthalmic drug delivery materials. Our in vitro study suggests that silicone hydrogels have the potential to deliver latanoprost effectively over an extended period of time.
of drug in every drop of the commercial latanoprost. Our results emphasize the importance of the presence of cells when characterizing the release of drug-delivery materials and demonstrate how experimental in vitro models have a significant impact on the outcomes of testing ophthalmic drug delivery materials. Our in vitro study suggests that silicone hydrogels have the potential to deliver latanoprost effectively over an extended period of time.
Acknowledgments
The authors would like to acknowledge Dr. H. Sheardown and Dr. Y-L. Cheng for fruitful discussions.
Funding Statement
The funding for this project was provided by a Collaborative Health Research Project grant (jointly funded by NSERC and CIHR). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Conway BR (2008) Recent patents on ocular drug delivery systems. Recent Patents on Drug Delivery & Formulation 2: 1–8. [DOI] [PubMed] [Google Scholar]
- 2. Ciolino JB, Dohlman CH, Kohane DS (2009) Contact lenses for drug delivery. Seminars in Ophthalmology 24: 156–60. [DOI] [PubMed] [Google Scholar]
- 3. Booth BA, Vidal Denham L, Bouhanik S, Jacob JT, Hill JM (2007) Sustained-release ophthalmic drug delivery systems for treatment of macular disorders: present and future applications. Drugs Aging 24: 581–602. [DOI] [PubMed] [Google Scholar]
- 4. Sjöquist B, Basu S, Byding P (1998) The pharmacokinetics of a new antiglaucoma drug, latanoprost, in the rabbit. Drug Metabolism and Disposition 26: 745–54. [PubMed] [Google Scholar]
- 5.Järvinen K, Järvinen T, Urtti A (1995) Ocular absorption following topical delivery. Advanced Drug Delivery Reviews: 3–19.
- 6. Wander AH (2011) Long-term use of hydroxypropyl cellulose ophthalmic insert to relieve symptoms of dry eye in a contact lens wearer: case-based experience. Eye & Contact Lens 37: 39–44. [DOI] [PubMed] [Google Scholar]
- 7. Choonara Y, Pillay V (2010) A review of implantable intravitreal drug delivery technologies for the treatment of posterior segment eye diseases. Journal of Pharmaceutical Sciences 99: 2219–2239. [DOI] [PubMed] [Google Scholar]
- 8. Lavik E, Kuehn MH, Kwon YH (2011) Novel drug delivery systems for glaucoma. Eye 25: 578–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sedlacek J (1965) Possibilities of application of ophthalmic drugs with the aid of gel-contact lenses. Nature 21: 509–512. [PubMed] [Google Scholar]
- 10. Chauhan A (2012) Ophthalmic drug delivery through contact lenses. Contact Lens Spectrum 27: 23–29. [Google Scholar]
- 11. White CJ, Tieppo A, Byrne ME (2011) Controlled drug release from contact lenses: a comprehensive review from 1965-present. Journal of Drug Delivery Science and Technology 21: 368–384. [Google Scholar]
- 12. Karlgard C, Wong N, Jones L, Moresoli C (2003) In vitro uptake and release studies of ocular pharmaceutical agents by silicon-containing and p-HEMA hydrogel contact lens materials. International Journal of Pharmaceutics 257: 141–151. [DOI] [PubMed] [Google Scholar]
- 13. Peng CC, Chauhan A (2011) Extended cyclosporine delivery by silicone-hydrogel contact lenses. Journal of Controlled Release 154: 267–74. [DOI] [PubMed] [Google Scholar]
- 14. Costa VP, Braga ME, Guerra JP, Duarte AR, Duarte CM, et al. (2010) Development of therapeutic contact lenses using a supercritical solvent impregnation method. The Journal of Supercritical Fluids 52: 306–316. [Google Scholar]
- 15. Hillman JS, Marsters JB, Broad A (1975) Pilocarpine delivery by hydrophilic lens in the management of acute glaucoma. Transactions of the Ophthalmological Societies of the United Kingdom 95: 79–84. [PubMed] [Google Scholar]
- 16. Kim J, Peng CC, Chauhan A (2010) Extended release of dexamethasone from silicone-hydrogel contact lenses containing vitamin E. Journal of Controlled Release 148: 110–6. [DOI] [PubMed] [Google Scholar]
- 17. Tabuchi N, Watanabe T, Hattori M, Sakai K, Sakai H, et al. (2009) Adsorption of actives in ophthalmological drugs for over-the-counter on soft contact lens surfaces. Journal of Oleo Science 58: 43–52. [DOI] [PubMed] [Google Scholar]
- 18. Friedman Z, Allen RC, Raph SM (1985) Topical acetazolamide and methazolamide delivered by contact lenses. Archives of Ophthalmology 103: 963–6. [DOI] [PubMed] [Google Scholar]
- 19. Hehl EM, Beck R, Luthard K, Guthoff R, Drewelow B (1999) Improved penetration of aminoglycosides and fluorozuinolones into the aqueous humour of patients by means of Acuvue contact lenses. European journal of clinical pharmacology 55: 317–23. [DOI] [PubMed] [Google Scholar]
- 20. Jain MR (1988) Drug delivery through soft contact lenses. The British Journal of Ophthalmology 72: 150–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xu J, Li X, Sun F (2011) In vitro and in vivo evaluation of ketotifen fumarate-loaded silicone hydrogel contact lenses for ocular drug delivery. Drug Delivery 18: 150–8. [DOI] [PubMed] [Google Scholar]
- 22. Tian X, Iwatsu M, Kanai A (2001) Disposable 1-Day Acuvue (R) contact lenses for the delivery of Lomefloxacin to rabbits' eyes. Eye & Contact Lens 27: 212–5. [PubMed] [Google Scholar]
- 23. Tian X, Iwatsu M, Sado K, Kanai A (2001) Studies on the uptake and release of fluoroquinolones by disposable contact lenses. Contact Lens Association of Ophthalmologists 27: 216–20. [PubMed] [Google Scholar]
- 24. Vaughan A, Zhang J, Byrne M (2010) Enhancing therapeutic loading and delaying transport via molecular imprinting and living/controlled polymerization. AIChE Journal 56: 268–79. [Google Scholar]
- 25. White CJ, McBride MK, Pate KM, Tieppo A, Byrne ME (2011) Extended release of high molecular weight hydroxypropyl methylcellulose from molecularly imprinted, extended wear silicone hydrogel contact lenses. Biomaterials 32: 5698–705. [DOI] [PubMed] [Google Scholar]
- 26. Xiang C, Batugo M, Gale D, Zhang T (2009) Characterization of human corneal epithelial cell model as a surrogate for corneal permeability assessment: metabolism and transport. Drug Metabolism and Disposition 37: 992–998. [DOI] [PubMed] [Google Scholar]
- 27. Kraft ME, Glaeser H, Mandery K, König J, Auge D, et al. (2010) The prostaglandin transporter OATP2A1 is expressed in human ocular tissues and transports the antiglaucoma prostanoid latanoprost. Investigative ophthalmology and visual science 51: 2504–11. [DOI] [PubMed] [Google Scholar]
- 28. Zhang T, Xiang C, Gale D, Carreiro S (2008) Drug transporter and cytochrome P450 mRNA expression in human ocular barriers: implications for ocular drug disposition. Drug Metabolism and Disposition 36: 1300–1307. [DOI] [PubMed] [Google Scholar]
- 29. Mannermaa E, Vellonen KS, Urtti A (2006) Drug transport in corneal epithelium and blood-retina barrier: emerging role of transporters in ocular pharmacokinetics. Advanced Drug Delivery Reviews 58: 1136–63. [DOI] [PubMed] [Google Scholar]
- 30. Duvvuri S, Majumdar S, Mitra AK (2004) Role of metabolism in ocular drug delivery. Current Drug Metabolism 5: 507–15. [DOI] [PubMed] [Google Scholar]
- 31. Hariharan S, Minocha M, Mishra GP, Pal D, Krishna R, et al. (2009) Interaction of ocular hypotensive agents (PGF2 alpha analogs-bimatoprost, latanoprost, and travoprost) with MDR efflux pumps on the rabbit cornea. Journal of Ocular Pharmacology and Therapeutics 25: 487–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Araie M, Maurice D (1987) The rate of diffusion of fluorophores through the corneal epithelium and stroma. Experimental eye research: 73–87. [DOI] [PubMed]
- 33. Maurice D, Mishima S (1984) Ocular pharmacokinetics. Handbook of Experimental Pharmacology 69: 19–116. [Google Scholar]
- 34. Huang HS, Schoenwald RD, Lach JL (1983) Corneal penetration behavior of beta-blocking agents II: Assessment of barrier contributions. Journal of Pharmaceutical Sciences 72: 1272–9. [DOI] [PubMed] [Google Scholar]
- 35. Huang HS, Schoenwald RD, Lach JL (1983) Corneal penetration behaviour of beta-blocking agents III: in vitro-in vivo correlations. Journal of Pharmaceutical Sciences 72: 1279–1281. [DOI] [PubMed] [Google Scholar]
- 36. Reichl S, Kölln C, Hahne M, Verstraelen J (2011) In vitro cell culture models to study the corneal drug absorption. Expert opinion on Drug Metabolism & Toxicology 7: 559–78. [DOI] [PubMed] [Google Scholar]
- 37. Hornof M, Toropainen E, Urtti A (2005) Cell culture models of the ocular barriers. European journal of pharmaceutics and biopharmaceutics 60: 207–225. [DOI] [PubMed] [Google Scholar]
- 38. Griffith M, Osborne R, Munger R, Xiong X, Doillon CJ, et al. (1999) Functional Human Corneal Equivalents Constructed from Cell Lines. Science 286: 2169–2172. [DOI] [PubMed] [Google Scholar]
- 39. Reichl S, Bednarz J, Mueller-Goymann CC (2004) Human corneal equivalent as cell culture model for in vitro drug permeation studies. British Journal of Ophthalmology 88: 560–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zieske J, Mason V, Wasson M (1994) Basement membrane assembly and differentiation of cultured corneal cells: importance of culture environment and endothelial cell interaction. Experimental cell Research 214: 621–633. [DOI] [PubMed] [Google Scholar]
- 41. Postnikoff CK, Pintwala R, Williams S, Wright AM, Hileeto D, et al. (2014) Development of a curved, stratified, in vitro model to assess ocular biocompatibility. PloS one 9: e96448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sjöquist B, Stjernschantz J (2002) Ocular and systemic pharmacokinetics of latanoprost in humans. Survey of Ophthalmology 47 Suppl 1 S6–12. [DOI] [PubMed] [Google Scholar]
- 43. Shirasaki Y (2008) Molecular design for enhancement of ocular penetration. Journal of Pharmaceutical Sciences 97: 2462–2496. [DOI] [PubMed] [Google Scholar]
- 44. Sachdev M (2011) Pharmacokinetics of antiglaucoma medications. Journal of Current Glaucoma Practice 5: 21–26. [Google Scholar]
- 45. Basu S, Sjöquist B, Stjernschantz J, Resul B (1994) Corneal permeability to and ocular metabolism of phenyl substituted prostaglandin esters in vitro. Prostaglandins, Leukotrienes and Essential Fatty Acids 50: 161–168. [DOI] [PubMed] [Google Scholar]
- 46. Shah B, Arora V, Wadhwani M, Mishra SK (2011) Prostaglandin analogs. Journal of Current Glaucoma Practice 5: 15–20. [Google Scholar]
- 47. Soluri A, Hui A, Jones L (2012) Delivery of ketotifen fumarate by commercial contact lens materials. Optometry and Vision Science 89: 1140–1149. [DOI] [PubMed] [Google Scholar]