Abstract
GNE myopathy is an adult-onset progressive myopathy, resulting from mutations in GNE, the key enzyme of sialic acid synthesis. The pathomechanism of GNE myopathy likely involves aberrant sialylation, since administration of sialic acid itself, or its precursor, N-acetylmannosamine (ManNAc), rescued hyposialylation of GNE myopathy mice. Recently, clinical trials for GNE myopathy patients were initiated. A robust, noninvasive biomarker is highly desirable for diagnosis of GNE myopathy and for evaluating response to therapy. Since muscle biopsies of patients with GNE myopathy demonstrated hyposialylation of predominantly O-linked glycans, we analyzed the O-linked glycome of patients’ plasma proteins using mass spectrometry. Most patients showed increased plasma levels of the core 1 O-linked glycan, Thomsen-Friedenreich (T)-antigen and/or decreased amounts of its sialylated form, ST-antigen. In addition, compared to unaffected individuals, all analyzed patients had a consistently increased ratio of T-antigen to ST-antigen. Importantly, the T/ST ratios were in the normal range in a GNE myopathy patient treated with intravenous immunoglobulins as a source of sialic acid, indicating response to therapy. Natural history and clinical trial data will reveal whether T/ST ratios can be correlated to muscle function. These findings not only highlight plasma T/ST ratios as a robust blood-based biomarker for GNE myopathy, but may also help explain the pathology and course of the disease.
Keywords: core 1 O-linked glycan, glycosylation, hereditary inclusion body myopathy, lectin, LC-MS/MS, N-acetylmannosamine (ManNAc), sialic acid, ST-antigen
Introduction
GNE myopathy, also called Hereditary Inclusion Body Myopathy (HIBM) or distal myopathy with rimmed vacuoles (DMRV), is a rare neuromuscular disorder, characterized by adult-onset, slowly progressive, distal and proximal myopathy that typically leaves patients wheelchair-bound 10–20 years after onset [1–4]. GNE myopathy is caused by biallelic mutations in the GNE gene, encoding the bifunctional enzyme UDP-N-acetylglucosamine 2-epimerase/N-acetylmanosamine kinase (GNE) [5]. GNE is the rate-limiting enzyme in the biosynthesis of 5-N-acetylneuraminic acid (Neu5Ac, Sia), the main mammalian sialic acid and precursor of most other sialic acids [6,7]. Sialic acids are terminal carbohydrate residues of most glycoconjugates, where they serve many functions, including cellular interactions and signaling [8,9].
GNE myopathy-associated GNE mutations are predominantly missense, resulting in reduced, but not absent, enzyme activities [3,10,11]. GNE null mutations have never been identified on both alleles of a patient; this would most likely be lethal since Gne ‘knock-out’ mice do not survive past the embryonic stage [12]. The exact pathology of GNE myopathy remains unknown; symptoms seem to occur due to hyposialylation of a select group of (sialo-) glycans [10,13–17]. More evidence that hyposialylation is a key factor in the pathomechanism came from mouse models, in which hyposialylation and pathology could be prevented by treatment with sialic acid metabolites [18,19].
Based on the hypothesis that certain molecules could maintain or restore the structure and function of aberrantly sialylated muscle glycoproteins in GNE myopathy patients, several clinical treatment protocols were recently developed [20–22] (http://clinicaltrials.gov/ identifiers: NCT01236898, NCT01359319, NCT01517880, NCT01634750). For these trials, informative, noninvasive biomarkers would be invaluable. In addition, such markers will foster early diagnosis of GNE myopathy, since many patients now experience a significant diagnostic delay [4].
Possible markers that aid in diagnosis of GNE myopathy have previously been suggested. Most of these markers require an invasive muscle biopsy, including analysis of glycosylation/sialylation status of muscle alpha-dystroglycan [14], neural crest cell adhesion molecule (NCAM) [23], neprilysin [24], or other O-linked glycans [13]. No robust blood-based biomarkers have been identified for GNE, although serum sialylation of NCAM was suggested [25]. The historically accepted blood-based tests to identify disorders of glycosylation/sialylation, isoelectric focusing of serum transferrin for N-linked glycosylation defects and Apolipoprotein C-III for O-linked glycosylation defects, show normal results in GNE myopathy patients [26,27].
In the current study, we explored blood-based glycans as possible markers for GNE myopathy. Through O-linked glycan profiling of plasma glycoproteins using mass spectrometry, we demonstrate that the ratio of the core 1 O-glycan species, Thomsen-Friedenreich (T)-antigen (Gal-GalNAc-) to its sialylated form, the ST-antigen (core 1 Sia-Gal-GalNAc-), provides an informative, reproducible plasma biomarker for diagnosis and, potentially, response to therapy for GNE myopathy.
Materials & Methods
Patients
GNE myopathy patients were enrolled in either clinical protocol NCT01417533, ‘A Natural History Study of Patients With Hereditary Inclusion Body Myopathy’, or protocol NCT00369421, ‘Diagnosis and Treatment of Inborn Errors of Metabolism and Other Genetic Disorders’, approved by the Institutional Review Board of the National Human Genome Research Institute. All patients provided written informed consent. Peripheral blood samples were obtained and used for serum or plasma preparations. Genomic DNA was isolated from white blood cell pellets, and used for GNE mutation analysis for molecular validation of the GNE myopathy diagnosis (Table S1). Peripheral blood from healthy donors without clinical complaints at the time of donation were obtained from the NIH Clinical Center blood bank or from the normal serum or plasma collection at the Emory Biochemical Genetics Laboratory.
Whole blood sample preparations
Serum (non-gel serum separator tube, clot activator) and plasma (K2EDTA-anticoagulant) were isolated from whole blood using standard protocols, followed by albumin and IgG depletion using a Qproteome Albumin/IgG depletion kit (Qiagen). Protein purification and concentration was performed with micron Ultra-0.5 mL Centrifugal Filters (EMD Millipore, Billerica, MA). Selected control samples were desialylated by incubation with 1 µl (50U) neuraminidase for 1 hour at a 37°C (P0720, New England Biolabs, Ipswich, MA). This neuraminidase (cloned from Clostridium perfringens and overexpressed in E. coli) catalyzes the hydrolysis of α2–3, α2–6, and α2–8 linked N-acetyl-neuraminic acid residues from glycoconjugates.
Immunoblotting
Serum (10–40µg) proteins were boiled at 95°C for 5 min in Laemmli Sample buffer (Bio-Rad Laboratories) and electrophoresed on 4–12% Tris-Glycine gels (Invitrogen), followed by electroblotting onto nitrocellulose membranes (Invitrogen). The membranes were either probed with primary antibodies against NCAM or with different lectins. Two antibodies against NCAM were evaluated H-300 (sc-10735) and RNL-1 (sc-53007) (Santa Cruz Biotechnology, Santa Cruz, CA), whose binding was visualized by IRDye 800CW conjugated secondary anti-mouse (for RNL-1) or anti-rabbit (for H-300) antibodies (Li-Cor Biosciences, Lincoln, NE, USA). The antigen-antibody complexes were visualized with the Li-Cor Odyssey Infrared imaging system (Li-Cor Biosciences). For lectin probing (Supplemental Figure 1), biotinylated SNA (Sambucus Nigra Agglutinin) and WGA (Wheat Germ Agglutinin) were purchased from Vector Laboratories (Burlingame, CA), and biotinylated VVA (Vicia Villosa Agglutinin) was purchased from EY Laboratories (San Mateo, CA). IRDye 680Streptavidin (Li-Cor Biosciences, Lincoln, NE) was used to bind to biotin-labeled proteins and visualized with a Li-Cor Odyssey Infrared imaging system (Li-Cor Biosciences).
Muscle lectin histochemistry
Paraffin embedded sections (5 µm) were obtained from control biceps muscle (National Disease Research Interchange (NDRI), Philadelphia, PA), right gastrocnemius muscle from patient GNE-21 (carrying GNE mutations D378Y and A631V), and left biceps muscle from patient GNE-28 (carrying GNE mutations R129X and V696M). The sections were deparaffinized in Hemo-De (Scientific Safety Solvents, Keller, TX), rehydrated in a series of ethanol solutions, followed by antigen retrieval (by microwaving in 0.01M Sodium Citrate, pH 6.4) and blocking in Carbo-Free Blocking solution (Vector Laboratories, Burlingame, CA). The slides were incubated at 4°C overnight with each fluorescein isothiocyanate (FITC)-labeled lectin aliquoted (5 µg/mL) in Carbo-Free Blocking solution. The FITC-labeled lectins VVA and WGA were purchased from purchased from EY Laboratories (San Mateo, CA) and SNA was purchased from Vector Laboratories (Burlingame, CA). After overnight incubation, washes were performed with 0.1% Triton-X-100 in 1× Tris-buffered saline (TBS). The lectin-stained slides were incubated in 0.3% Sudan Black in 70% ethanol solution to reduce autofluorescence. Slides were mounted with Vectashield containing the nuclear dye DAPI (Vector Laboratories) and digitally imaged with a Zeiss LSM 510 META confocal laser-scanning microscope (Carl Zeiss, Microimaging Inc., Thornwood, NY). Images were acquired using a Plan-Apochromat 40× oil DIC objective. All images are 3D projections of confocal Z-stacks.
Preparation and permethylation of plasma O-linked glycan species
O-linked glycan species were released from total plasma or serum glycoproteins by β-elimination, essentially as described [28–31]. Briefly, 10 µL of plasma was mixed with raffinose (1250 pmol in 5 µL) internal standard and 65 µl water for a final volume of 100 µL. To denature the plasma proteins and release the O-linked glycan species, the sample was mixed with 100 µL 2 M sodium borate in 0.1 M sodium hydroxide (freshly prepared) and incubated at 45 °C for 16 hours. Next, 1.6 mL of 0.25 M acetic acid-methanol solution was drop wise added to neutralize the reaction, followed by O-glycan extraction with methanol. The extracted glycans were desalted through ion-exchange AG 50W-X8 resin (Bio-Rad, Hercules, CA) and lyophilized overnight.
For permethylation, four NaOH pellets (approximately 375 mg) were crushed in 10 mL anhydrous dimethyl sulfoxide (DMSO) with 0.5 µL water; 0.5 mL of this slurry and 0.2 mL CH3I were added to the dried glycans and the mixture was shaken vigorously for 1 hour, followed by five sequential chloroform/water (600 µL/200 µL) extractions from which the chloroform fractions were pooled. These combined chloroform phases were dried for 30 min under nitrogen (in chemical hood) and the permethylated O-glycan species were resuspended in 50 µL of 50% methanol and further purified through a C18 Stage Tip (Thermo Scientific, West Palm Beach, FL) as described [32].
Quantitation of O-linked glycans by HPLC-MALDI-TOF/TOF (LC-MS/MS)
High performance liquid chromatography (HPLC) separation of 10 µl of each sample of permethylated O-glycan species was performed on a Shimadzu Prominence 20 AD LC and a Thermo gold 3-µm C18 column (2 × 100 mm). The binary method used buffer A (acetonitrile:formic acid: water; 1:0.1:99 (v:v:v)) and buffer B (acetonitrile:formic acid: water; 99:0.1:1 (v:v:v)) with a flow rate at 0.25 mL/min under the following gradient conditions: 0–20 min, 50% to 80% buffer B; 20–28 min, 98% buffer B; 28–39 min, 50% buffer B. The permethylated O-glycans were subsequently analyzed by matrix-assisted laser desorption-ionization (MALDI) time-of-flight (TOF) mass spectrometry on an Applied Biosystems MALDI-TOF/TOF 4800 Plus (Applied Biosystems, Foster City, CA) as described [31].
Results
NCAM immunoblotting
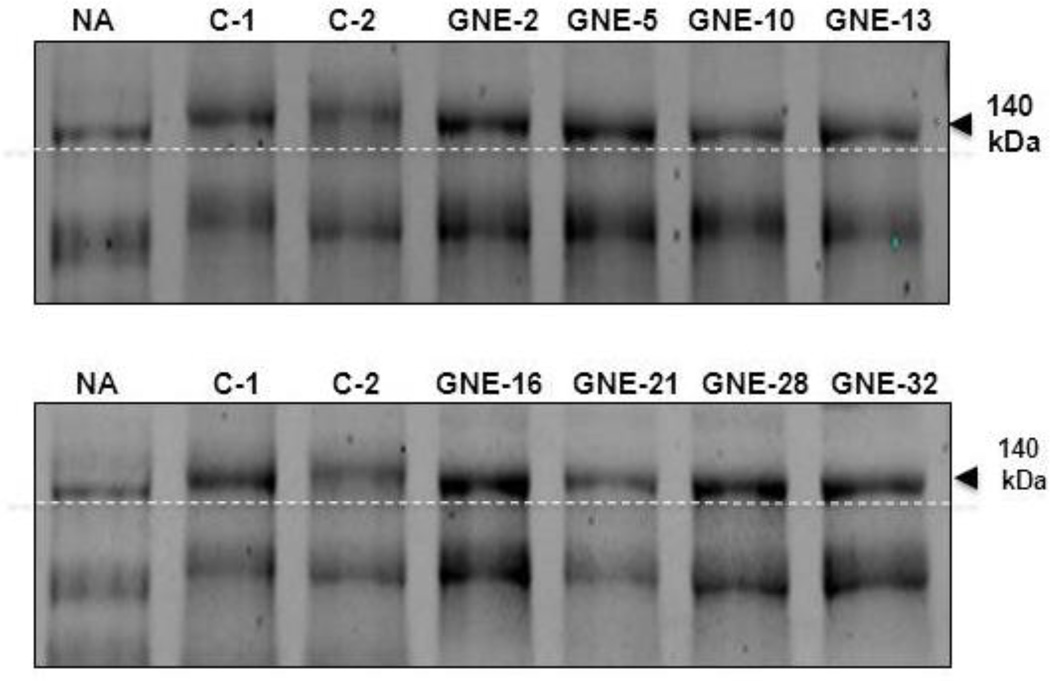
Aberrantly sialylated NCAM, detected by immunoblotting of patients’ serum, is the only previously suggested blood-based marker for GNE myopathy [25]. We performed immunoblotting of GNE myopathy serum using the same conditions and NCAM (RNL-1; Santa Cruz Biotechnology) antibodies as previously employed [25], but we were unable to observe an immunoresponsive band (Supplemental Figure S1). This may have been due to different sample handling or processing, or a different batch of the antibody than that used in the previous study. However, a different antibody to NCAM (H-300; Santa Cruz Biotechnology) showed a slight downshift of the 140kDa cytoplasmic soluble isoform of NCAM [33,34] in a desialylated (by neuraminidase treatment) control sample compared to normal serum (Figure 1A). Importantly, all GNE myopathy serum samples showed a similar slight downshift of this NCAM isoform, suggesting hyposialylation of a soluble NCAM isoform in GNE myopathy serum. This downshift likely resulted from different electrophoretic mobility due to hyposialylation. A similar downshift of NCAM was reported in muscle extracts of GNE myopathy patients [23].
Figure 1. NCAM (H-300) immunoblotting of serum glycoproteins.
Serum samples (20 µg) from neuraminidase treated control (NA), control (C-1, C-2), and GNE myopathy patients (GNE-2, -5, -10 and -13; see Table 1 for details) were immunoblotted with NCAM antibodies (H-300; sc-10735). Compared to control, serum from GNE myopathy patients showed a slight downshift of the 140 kDa NCAM isoform (arrow). A similar downshift was present in neuraminidase treated control serum (NA).Dotted line is to aid in discerning migration.
Lectin histochemistry and lectin blotting
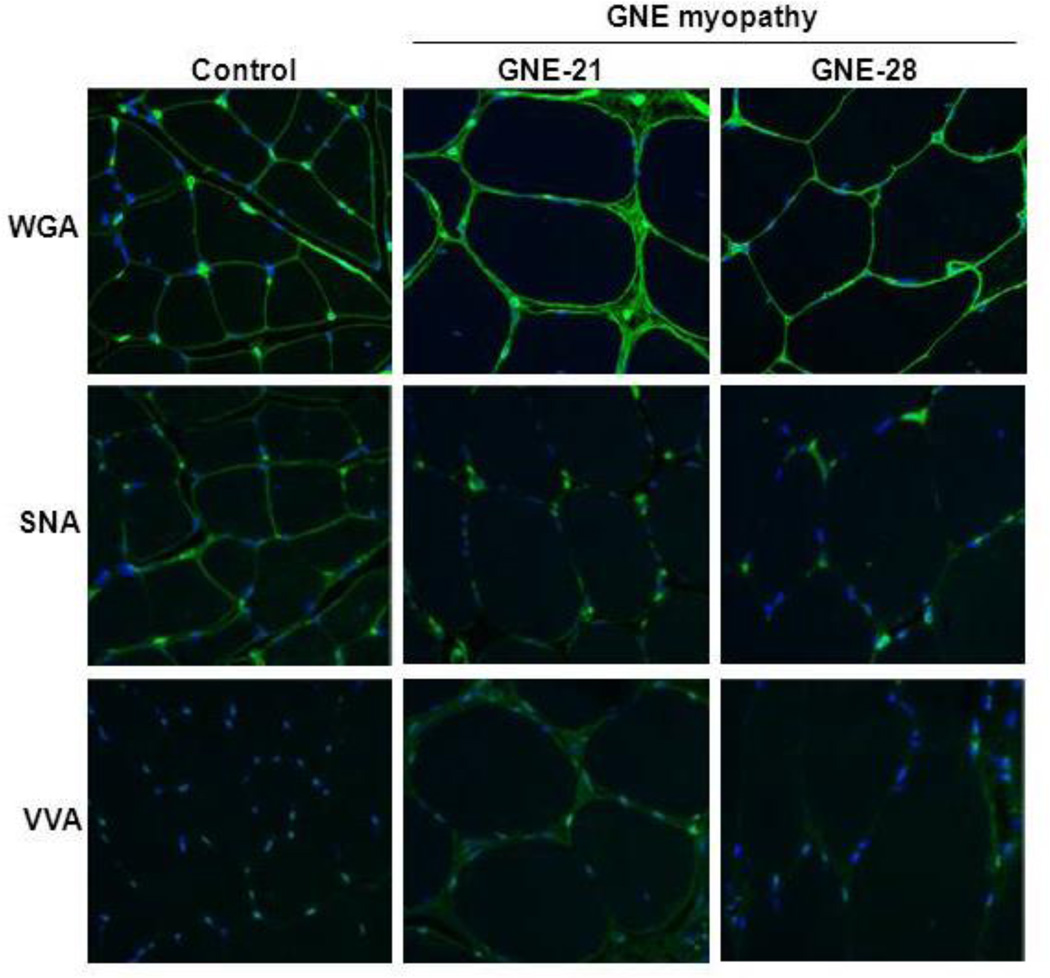
Staining with lectins (i.e., sugar-binding proteins with ligand specificities for defined carbohydrate sequences [35]) was performed on normal and GNE myopathy muscle slides to examine the sialylation status. WGA (wheat germ agglutinin from Triticum vulgaris) predominantly recognizes terminal sialic acid (Sia) and N-acetylglucosamine (GlcNAc) on glycans [35–37], SNA (elderberry bark agglutinin from Sambucus nigra) predominantly recognizes terminal sialic acid (Sia) in an α(2,6)-linkage with either galactose (prevalent in N-linked glycans) or with N-acetylgalactosamine (GalNAc) (found in O-linked glycans) [36,38]. VVA (hairy vetch agglutinin from Vicia villosa) predominantly binds GalNAc O-linked to serine or threonine residues of proteins [36,39].
GNE myopathy muscle, stained with WGA (recognizing most terminal sialic acids), showed a similar staining pattern as normal muscle (Figure 2). However, staining with SNA (binding only α(2,6)-linked sialic acid) showed a markedly decreased signal in patients’ muscle slides compared to normal, indicating that only specific sialylglycans are hyposialylated in GNE myopathy. VVA staining was almost absent in normal muscle since most glycans are sialylated, while GNE myopathy muscle showed a significant increase in staining compared to normal, indicating hyposialylation of O-linked glycans (Figure 2).
Figure 2. Muscle lectin histochemistry.
Paraffin-embedded muscle sections from biceps (control and GNE-28) and gastrocnemius (GNE-21) were stained with three lectins (green) informative for sialylation status and co-stained with the nuclear dye DAPI (blue). GNE myopathy muscle specimens show selective hyposialylation compared to control muscle, demonstrated by apparent normal staining of WGA (binding to most sialic acid groups), but decreased staining of SNA (predominantly binding terminal α(2,6)-linked sialic acid on all glycans). In addition, staining of VVA (predominantly binding terminal GalNAc, without sialic acid attached, O-linked to serine or threonine residues of glycoproteins) was increased in GNE myopathy muscle specimen compared to control, indicating hyposialylation of O-linked glycans.
We performed Western blots of controls, neuraminidase treated controls, and GNE myopathy serum proteins, and probed the blots with WGA, SNA or VVA (Supplementary Figure S2). While the neuraminidase treated control samples showed the expected reduction (for WGA and SNA) or increase (for VVA) in lectin binding, no significant differences in lectin binding could be identified in GNE myopathy patients’ serum compared to control serum (Supplementary Figure 1).
T/ST ratios in GNE myopathy patients
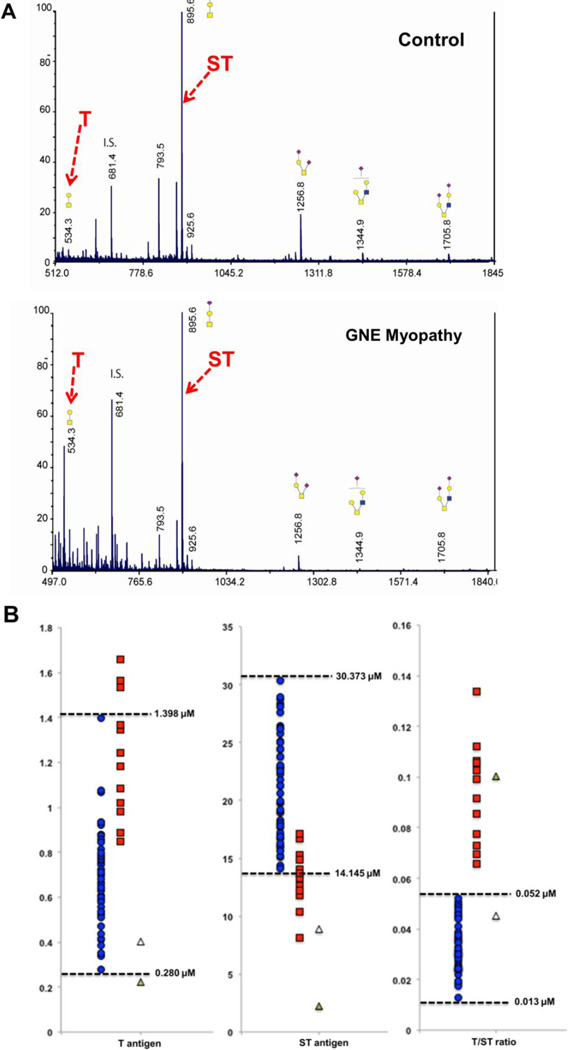
Plasma O-glycan species in control and GNE myopathy patients were analyzed by LC-MS/MS. Five abundant peaks were observed, at m/z 534, 895, 1256, 1344, and 1706 (Figure 3A). The two major peaks in GNE myopathy patients represent the core 1 O-glycan species T-antigen (m/z 534; Gal-GalNAc-) and the ST-antigen (m/z 895; Sia-Gal-GalNAc-) [30,31]. The relative quantities of T and ST antigens were calculated by comparing their intensities to the internal standard raffinose at m/z 681 (Table 1, Supplemental Table S1), as well as using purified T-antigen as external standard to further validate T-antigen quantities. Purified sialylated T-antigen was not available to be used as external standards. To evaluate the sialylation of core 1 O-glycan species per patient, the ratio between T- and ST-antigen was obtained. Fifty control samples were measured to establish a normal range for both T-antigen (0.280–1.398 µM), ST antigen (14.145–30.373 µM) and the T/ST ratio (< 0.052), similar ranges as recently described [31]. In GNE myopathy plasma, one of the absolute values of either T- or ST-antigen often appeared the normal range, but the T/ST ratio was consistently abnormal (> 0.052) in all analyzed samples from untreated patients (Figure 3B; Table 1; Supplemental Table S1). Importantly, the T/ST ratio of one of our untreated GNE myopathy patients was abnormal (GNE-914a; T/ST = 0.100), but shifted to the normal range 24 hours after intravenous immunoglobulin (IVIG) therapy on two consecutive days (GNE914b; T/ST = 0.0454).
Figure 3. Plasma O-glycan MALDI-TOF profiles and quantitative comparison of T and ST antigens of control and GNE myopathy patients.
A. Human control and GNE myopathy plasma O-glycan species were released by β-elimination and permethylated before HPLC-MS/MS analysis. Measured m/z and % intensity compared to the internal standard (I.S.) raffinose of the major detected small O-glycan species are shown as well as their structures (yellow squares, GalNAc; yellow circles, Gal; purple diamonds, Sia; blue squares, GlcNAc). The absolute quantity of T-antigen was also evaluated by the calculation from external standards using purified T-antigen [31].
B. Comparison of concentrations of T-antigen, monosialylated T-antigen (ST) and their ratio T/ST in plasma from 50 healthy controls (blue circles) and different GNE myopathy patients (red squares). Plasma values of a GNE myopathy patient before (green solid triangle) and after (open triangle) IVIG therapy are indicated. Dashed lines represent cutoffs to establish the normal range [~2 × standard deviation (SD) of the mean (0.033)]. For additional information see Tables 1 and S1.
Table 1.
Mutations and plasma T and ST values of GNE myopathy patients.
| Patient | GNE allele 1 |
GNE allele 2 |
T/ST | T (uM) |
ST (uM) |
|---|---|---|---|---|---|
| < 0.052 | Normal Range 0.280–1.14 | 14.1–30.3 | |||
| GNE-2 | c.1909+5G>A | p.V696M | 0.107 | 1.34 | 12.6 |
| GNE-5 | p.M712T | p.M712T | 0.073 | 1.02 | 14.0 |
| GNE-10 | p.D213V | p.V696M | 0.105 | 1.24 | 11.8 |
| GNE-13 | p.M712T | p.M712T | 0.134 | 1.09 | 8.10 |
| GNE-14 | p.V216A | p.A631V | 0.069 | 0.85 | 12.2 |
| GNE-16 | p.M712T | p.M712T | 0.099 | 1.66 | 16.7 |
| GNE-20 | p.W513X | p.A631V | 0.077 | 1.18 | 15.3 |
| GNE-21 | p.D378Y | p.A631V | 0.085 | 0.89 | 10.4 |
| GNE-28 | p.R129X | p.V696M | 0.091 | 1.56 | 17.1 |
| GNE-32 | p.M712T | p.M712T | 0.065 | 0.98 | 14.9 |
| GNE-980 | p.M712T | p.M712T | 0.102 | 1.37 | 13.3 |
| GNE-981 | p.M712T | p.M712T | 0.112 | 1.53 | 13.7 |
| GNE-914a1 | p.M712T | p.M712T | 0.100 | 0.22 | 2.20 |
| GNE-914b2 | p.M712T | p.M712T | 0.045* | 0.40 | 8.85 |
Gray highlight: Abnormal value
NE-914a = plasma value before administration of IVIG
GNE-914b = plasma value 24h after IVIG therapy
Discussion
Major barriers to the diagnosis of GNE myopathy have been the rarity of the disease and the lack of an inexpensive and noninvasive diagnostic test. Most GNE myopathy patients escape diagnosis, with a typical diagnostic delay of approximately 10 years after onset of symptoms [4]. This leads to anxiety and unnecessary testing, often involving an invasive muscle biopsy [10,13–16]. As an alternative, we explored blood-based markers to aid in diagnosis and monitoring response to therapy.
Sialylation on NCAM detected by immunochemistry was suggested as a muscle- [23] and blood-based marker for GNE myopathy patients [25], but results may vary with the antibodies used, since NCAM has many membrane bound and soluble tissue-specific isoforms. Our application of a reported informative NCAM antibody (RNL-1, [25]) on GNE myopathy serum samples did not show reproducible data (Supplemental Figure S1), possibly related to differences in sample processing or antibody batch. However, our tests with another NCAM antibody (H-300) showed a slight shift of immunoreactive bands in patients’ sera, indicating a possible difference of sialylation on NCAM, resulting in different gel mobility in GNE myopathy patients (Figure 1). The subtle shifts in serum NCAM may not allow for the desired specificity and sensitivity of a robust blood-based biomarker for GNE myopathy patients.
Based on the presence of predominantly hyposialylated O-linked glycans in GNE myopathy [13,14,22,40], we explored plasma analysis of O-linked glycan structures by a recently developed method that determines the ratio of the T- and ST-antigens (T/ST) [31]. Using this method, we demonstrated mild undersialylation of plasma O-linked glycan species in all tested GNE myopathy patients, resulting in abnormally high T/ST ratios (>0.052; Table 1). Determining the T/ST ratios in GNE myopathy proved robust and superior to solely quantifying and comparing only the individual T- and ST-antigen values; while individual T- and ST-antigen values can be in the normal range in some GNE myopathy patients (Table 1), the T/ST ratio was abnormal (>0.052) in all untreated patients. Serum samples from selected GNE myopathy patients showed similar T/ST ratios (results not shown) to the corresponding plasma samples, indicating that either serum or plasma can be used for this assay.
The fact that some GNE myopathy patients have normal values of T- or ST-antigen indicates that their undersialylation of O-linked glycan species is likely mild. It is credible that due to defects in GNE enzyme activities [10,11], a gradual defect in de novo sialic acid production occurs in GNE myopathy patients. Some glycans may be preferentially (under)sialylated, perhaps based on (tissue-specific) substrate affinity, protein-specific transport pathways through the Golgi-complex for sialylation, expression of certain sialyltransferases or neuraminidases, or other mechanisms [41–43]. The gradual shortage of tissue-, protein, or sialyl linkage-specific sialylation of predominantly O-linked glycans may play a role in the adult onset and muscle specific symptoms of GNE myopathy. Proteins with significant O-linked glycosylation, most of which remain to be identified, may largely be affected and contribute to the phenotype. In our cohort of GNE myopathy patients, there was no direct correlation of T/ST plasma ratios to severity and onset of the disease, nor to GNE gene mutations (Table 1). Testing more patients and analysis of natural history data will reveal whether T/ST ratios can be correlated to muscle function. Unfortunately, it is difficult to identify GNE myopathy patients before the onset of symptoms, but the evaluation of T/ST ratios in such non-symptomatic patients may indicate the usefulness of T/ST ratios as an early diagnostic tool for the disease.
Abnormal plasma T/ST values are not unique to GNE myopathy patients. Historically, the presence of T-antigen, Tn-anigen and STn-antigens are utilized as markers for certain cancers. Because absolute T-, ST-, Tn-, and STn-antigen values are often significantly altered in different forms or stages of cancers [44–47], their ratios (including T/ST) are rarely used in cancer research. For some other recently reported disorders, T/ST ratios were informative, including abnormal T/ST values in patients with classic galactosemia (galactose-1-phosphate uridylyltransferase (GALT)-deficiency [28]), deficiency in Conserved Oligomeric Golgi complex 4 (COG4) or COG7 [31,48,49], Transmembrane Protein 165 (TMEM165) [31,50], or phosphoglucomutase 1 (PGM1) [31,51]. Most such glycosylation disorders present with severe congenital clinical phenotypes, much different from adult onset GNE myopathy. Early clinical symptoms of GNE myopathy (waddling gait, foot drop) are non-specific features of various neurological/muscular disorders and contribute to the delayed diagnosis of patients. Such early symptoms in combination with abnormal plasma T/ST ratios may be future indicators for GNE mutation testing, which will ultimately confirm the diagnosis of GNE myopathy.
These findings beg the question whether sialylation-increasing therapies could normalize the plasma T/ST ratios in GNE myopathy patients, and possibly indicate response to therapy. Unfortunately, no therapies are currently approved for GNE myopathy. We acquired plasma samples from one GNE myopathy patient who was part of a previously conducted pilot clinical trial of intravenous supplementation of sialylated compounds in the form of immune globulins (IVIG; (http://clinicaltrials.gov/ identifier: NCT00195637) [20]. The sialic acid residues on IgG (~8 µmol of sialic acid/g) could presumably be recycled to sialylate other glycans. While this study showed improvement in strength of different muscle groups and notable subjective improvement reported by the patients, no biochemically relevant evidence of re-sialylation could be detected at that time [20]. Plasma from the patient before therapy had an abnormal T/ST value (0.100), while a plasma sample acquired 24h after 1g/kg IVIG loading on two consecutive days showed a normalized T/ST ratio (0.045). These findings offer prospects for exploring plasma T/ST ratios for response to therapy in GNE myopathy patients.
Other substrate replacement therapies for GNE myopathy patients are currently in exploratory stages, and include oral supplementation of sialic acid itself (http://clinicaltrials.gov/ identifiers: NCT01634750, NCT01236898, and NCT01517880) and oral supplementation of the sialic acid precursor N-acetylmannosamine (ManNAc) (http://clinicaltrials.gov/ identifier: NCT01634750). Once patients’ plasma samples from these trials become available, it would be of great interest to analyze their T/ST ratios to verify whether this ratio is informative for gauging response to therapy.
Conclusion and Future Prospective
In this study we demonstrate that the ratio of the Thomsen-Friedenreich (T)-antigen to its sialylated form, ST-antigen, detected by LC-MS/MS, is a robust blood-based (serum or plasma) biomarker informative for diagnosis and possibly for response to therapy for GNE myopathy. In addition, the specific hyposialylation of core 1 O-linked glycan species may aid in further elucidating the pathology and adult onset clinical symptoms of GNE myopathy.
Supplementary Material
Executive Summary.
Background
GNE myopathy is a recessive inherited, adult onset, rare neuromuscular disorder.
Mutations in GNE, encoding the key enzyme of sialic acid synthesis, are associated with GNE myopathy.
Clinical trials for sialylation-increasing therapies for GNE myopathy are currently ongoing.
A non-invasive biomarker for GNE myopathy diagnosis and response to therapy is highly desirable.
Patients & Methods
Blood samples and selected muscle biopsies from GNE myopathy patients enrolled in clinical protocols at the National Institutes of Health (Bethesda, MD, USA) were investigated.
Lectin histochemistry, western blotting and mass spectrometry-based glycan profiling studies were performed to assess sialylation status in plasma, serum or muscle from control individuals and GNE myopathy patients.
Results
Immunoblotting of serum glycoproteins with NCAM antibodies (H-300, Santa CruzBiotechnology, Santa Cruz, CA) showed a slight downshift of the 140 kDa NCAM isoform in all GNE myopathy patients’ samples compared to control samples.
Lectin histochemistry on paraffin-embedded slides from GNE myopathy patients’ muscle biopsies demonstrated hyposialylation of predominantly O-linked muscle glycans.
Plasma O-glycan MALDI-TOF mass spectrometry analysis demonstrated that the ratio of the Thomsen-Friedenreich (T)-antigen (Gal-GalNAc-) to its sialylated form, ST-antigen (Sia-Gal-GalNAc-) is abnormal high (>0.052) in all tested, untreated GNE myopathy patients when compared to 50 unaffected control samples (normal range: 0.013–0.052).
Plasma T/ST values of one untreated GNE myopathy patient was abnormal high (T/ST=0.100), but shifted to the normal range (T/ST = 0.0454) after sialylation-increasing therapy in the form of intravenous immunoglobulins (IVIG).
Conclusion
Plasma T/ST values, measured by LC-MS\MS provide an informative, reproducible, blood-based biomarker for diagnosis, and, potentially, response to therapy for GNE myopathy.
Hyposialylation of core 1 O-linked glycan species (such as the T-antigen) may aid in elucidating the still obscure pathomechanism of GNE myopathy.
Acknowledgements
We greatly appreciate the expert laboratory work of Terren Niethamer, Obi Okafor, Heidi Dorward and Andrew Cullinane. We thank Lea Latham, Chevalia Robinson and David Draper for excellent patient care. This work was performed in partial fulfillment of the requirements for a PhD degree of T.Y., Sackler Faculty of Medicine, Tel Aviv University, Israel.
Financial & competing interests disclosure
This study was supported by the Intramural Research Program of the National Human Genome Research Institute (NHGRI) and the Therapeutics for Rare and Neglected Diseases (TRND) Program of the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health, Bethesda, Maryland, United States. Some of the authors (X.L., R.J., W.A.G., N.C-C., M.H., M.H.) are co-inventors on U.S. Provisional Application Serial No. 61/785,094 “Biomarker for Hyposialylation Disorders”. No writing assistance was utilized in the production of this manuscript.
Footnotes
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
References
* Of interest
** Of considerable interest
- 1.Argov Z, Yarom R. "Rimmed vacuole myopathy" sparing the quadriceps. A unique disorder in Iranian Jews. J. Neurol. Sci. 1984;64(1):33–43. doi: 10.1016/0022-510x(84)90053-4. [DOI] [PubMed] [Google Scholar]
- 2.Argov Z, Mitrani-Rosenbaum S. The hereditary inclusion body myopathy enigma and its future therapy. Neurotherapeutics. 2008;5(4):633–637. doi: 10.1016/j.nurt.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huizing M, Krasnewich DM. Hereditary inclusion body myopathy: a decade of progress. Biochim. Biophys. Acta. 2009;1792(9):881–887. doi: 10.1016/j.bbadis.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huizing M, Malicdan M, Krasnewich D, Manoli I, Carrillo-Carrasco N. GNE Myopathy. Scriver's Online Metabolic and Molecular Bases of Inherited Disease. 2013;(258) http://www.ommbid.com. * Extensive, most recent review of clinical, genetic, biochemical, cellular and therapeutic aspects of GNE myopathy.
- 5. Eisenberg I, Avidan N, Potikha T, et al. The UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase gene is mutated in recessive hereditary inclusion body myopathy. Nat. Genet. 2001;29(1):83–87. doi: 10.1038/ng718. * The first report demonstrating that mutations in the GNE gene underlay GNE myopathy (Hereditary Inclusion Body Myopathy).
- 6.Hinderlich S, Stasche R, Zeitler R, Reutter W. A bifunctional enzyme catalyzes the first two steps in N-acetylneuraminic acid biosynthesis of rat liver. Purification and characterization of UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase. J. Biol. Chem. 1997;272(39):24313–24318. doi: 10.1074/jbc.272.39.24313. [DOI] [PubMed] [Google Scholar]
- 7.Varki A. Diversity in the sialic acids. Glycobiology. 1992;2(1):25–40. doi: 10.1093/glycob/2.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keppler OT, Hinderlich S, Langner J, Schwartz-Albiez R, Reutter W, Pawlita M. UDPGlcNAc 2-epimerase: a regulator of cell surface sialylation. Science. 1999;284(5418):1372–1376. doi: 10.1126/science.284.5418.1372. [DOI] [PubMed] [Google Scholar]
- 9.Varki A. Sialic acids in human health and disease. Trends Mol. Med. 2008;14(8):351–360. doi: 10.1016/j.molmed.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Noguchi S, Keira Y, Murayama K, et al. Reduction of UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase activity and sialylation in distal myopathy with rimmed vacuoles. J. Biol. Chem. 2004;279(12):11402–11407. doi: 10.1074/jbc.M313171200. ** Reporting biochemical and cellular consequences of GNE mutations that underly GNE myopathy, including GNE enzyme activity measurements, assessment of sialylation status, and lectin stainings.
- 11.Sparks SE, Ciccone C, Lalor M, et al. Use of a cell-free system to determine UDP-N-acetylglucosamine 2-epimerase and N-acetylmannosamine kinase activities in human hereditary inclusion body myopathy. Glycobiology. 2005;15(11):1102–1110. doi: 10.1093/glycob/cwi100. [DOI] [PubMed] [Google Scholar]
- 12.Schwarzkopf M, Knobeloch KP, Rohde E, et al. Sialylation is essential for early development in mice. Proc. Natl. Acad. Sci. U.S.A. 2002;99(8):5267–5270. doi: 10.1073/pnas.072066199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tajima Y, Uyama E, Go S, et al. Distal myopathy with rimmed vacuoles: impaired O-glycan formation in muscular glycoproteins. Am. J. Pathol. 2005;166(4):1121–1130. doi: 10.1016/S0002-9440(10)62332-2. * Reporting hyposialylation of predominantly O-linked glycans in GNE myopathy muscle tissues.
- 14.Huizing M, Rakocevic G, Sparks SE, et al. Hypoglycosylation of alpha-dystroglycan in patients with hereditary IBM due to GNE mutations. Mol. Genet. Metab. 2004;81(3):196–202. doi: 10.1016/j.ymgme.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 15.Broccolini A, Gidaro T, De Cristofaro R, et al. Hyposialylation of neprilysin possibly affects its expression and enzymatic activity in hereditary inclusion-body myopathy muscle. J. Neurochem. 2008;105(3):971–981. doi: 10.1111/j.1471-4159.2007.05208.x. [DOI] [PubMed] [Google Scholar]
- 16.Ricci E, Broccolini A, Gidaro T, et al. NCAM is hyposialylated in hereditary inclusion body myopathy due to GNE mutations. Neurology. 2006;66(5):755–758. doi: 10.1212/01.wnl.0000200956.76449.3f. [DOI] [PubMed] [Google Scholar]
- 17.Paccalet T, Coulombe Z, Tremblay JP. Ganglioside GM3 levels are altered in a mouse model of HIBM: GM3 as a cellular marker of the disease. PLoS One. 2010;5(4):e10055. doi: 10.1371/journal.pone.0010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Galeano B, Klootwijk R, Manoli I, et al. Mutation in the key enzyme of sialic acid biosynthesis causes severe glomerular proteinuria and is rescued by N-acetylmannosamine. J. Clin. Invest. 2007;117(6):1585–1594. doi: 10.1172/JCI30954. * Presenting preclinical evidence of utilizing the sialic acid precursor ManNAc as substrate replacement therapy in a mouse model of GNE myopathy.
- 19. Malicdan MC, Noguchi S, Hayashi YK, Nonaka I, Nishino I. Prophylactic treatment with sialic acid metabolites precludes the development of the myopathic phenotype in the DMRV-hIBM mouse model. Nat. Med. 2009;15(6):690–695. doi: 10.1038/nm.1956. * Presenting preclinical evidence of applying sialic acid metabolite therapy in a mouse model of GNE myopathy.
- 20.Sparks S, Rakocevic G, Joe G, et al. Intravenous immune globulin in hereditary inclusion body myopathy: a pilot study. BMC Neurol. 2007;7:3. doi: 10.1186/1471-2377-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nemunaitis G, Maples PB, Jay C, et al. Hereditary inclusion body myopathy: single patient response to GNE gene Lipoplex therapy. J. Gene Med. 2010;12(5):403–412. doi: 10.1002/jgm.1450. [DOI] [PubMed] [Google Scholar]
- 22.Nemunaitis G, Jay CM, Maples PB, et al. Hereditary inclusion body myopathy: single patient response to intravenous dosing of GNE gene lipoplex. Hum. Gene Ther. 2011;22(11):1331–1341. doi: 10.1089/hum.2010.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Broccolini A, Gidaro T, Tasca G, et al. Analysis of NCAM helps identify unusual phenotypes of hereditary inclusion-body myopathy. Neurology. 2010;75(3):265–272. doi: 10.1212/WNL.0b013e3181e8e8f1. [DOI] [PubMed] [Google Scholar]
- 24.Broccolini A, Gidaro T, Morosetti R, et al. Neprilysin participates in skeletal muscle regeneration and is accumulated in abnormal muscle fibres of inclusion body myositis. J. Neurochem. 2006;96(3):777–789. doi: 10.1111/j.1471-4159.2005.03584.x. [DOI] [PubMed] [Google Scholar]
- 25.Valles-Ayoub Y, Esfandiarifard S, Sinai P, et al. Serum neural cell adhesion molecule is hyposialylated in hereditary inclusion body myopathy. Genet. Test. Mol. Biomarkers. 2012;16(5):313–317. doi: 10.1089/gtmb.2011.0146. [DOI] [PubMed] [Google Scholar]
- 26.Savelkoul PJ, Manoli I, Sparks SE, et al. Normal sialylation of serum N-linked and O-GalNAc-linked glycans in hereditary inclusion-body myopathy. Mol. Genet. Metab. 2006;88(4):389–390. doi: 10.1016/j.ymgme.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 27.Wopereis S, Grunewald S, Huijben KM, et al. Transferrin and apolipoprotein C-III isofocusing are complementary in the diagnosis of N- and O-glycan biosynthesis defects. Clin. Chem. 2007;53(2):180–187. doi: 10.1373/clinchem.2006.073940. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y, Xia B, Gleason TJ, et al. N- and O-linked glycosylation of total plasma glycoproteins in galactosemia. Mol. Genet. Metab. 2012;106(4):442–454. doi: 10.1016/j.ymgme.2012.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carlson DM. Structures and immunochemical properties of oligosaccharides isolated from pig submaxillary mucins. J. Biol. Chem. 1968;243(3):616–626. [PubMed] [Google Scholar]
- 30.Faid V, Chirat F, Seta N, Foulquier F, Morelle W. A rapid mass spectrometric strategy for the characterization of N- and O-glycan chains in the diagnosis of defects in glycan biosynthesis. Proteomics. 2007;7(11):1800–1813. doi: 10.1002/pmic.200600977. [DOI] [PubMed] [Google Scholar]
- 31. Xia B, Zhang W, Li X, et al. Serum N-glycan and O-glycan analysis by mass spectrometry for diagnosis of congenital disorders of glycosylation. Anal. Biochem. 2013 doi: 10.1016/j.ab.2013.07.037. ** Describing the full methods, sensitivity and specificity and applicability of determining T/ST ratios in plasma or serum by LC-MS/MS.
- 32.Guillard M, Morava E, Van Delft FL, et al. Plasma N-glycan profiling by mass spectrometry for congenital disorders of glycosylation type II. Clin. Chem. 2011;57(4):593–602. doi: 10.1373/clinchem.2010.153635. [DOI] [PubMed] [Google Scholar]
- 33.Small SJ, Shull GE, Santoni MJ, Akeson R. Identification of a cDNA clone that contains the complete coding sequence for a 140-kD rat NCAM polypeptide. J. Cell. Biol. 1987;105(5):2335–2345. doi: 10.1083/jcb.105.5.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tur MK, Etschmann B, Benz A, et al. The 140-kD isoform of CD56 (NCAM1) directs the molecular pathogenesis of ischemic cardiomyopathy. Am. J. Pathol. 2013;182(4):1205–1218. doi: 10.1016/j.ajpath.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 35.Sharon N. Lectins: carbohydrate-specific reagents and biological recognition molecules. J. Biol. Chem. 2007;282(5):2753–2764. doi: 10.1074/jbc.X600004200. [DOI] [PubMed] [Google Scholar]
- 36.Iskratsch T, Braun A, Paschinger K, Wilson IB. Specificity analysis of lectins and antibodies using remodeled glycoproteins. Anal. Biochem. 2009;386(2):133–146. doi: 10.1016/j.ab.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 37.Kronis KA, Carver JP. Specificity of isolectins of wheat germ agglutinin for sialyloligosaccharides: a 360-MHz proton nuclear magnetic resonance binding study. Biochemistry. 1982;21(13):3050–3057. doi: 10.1021/bi00256a003. [DOI] [PubMed] [Google Scholar]
- 38.Shibuya N, Goldstein IJ, Broekaert WF, Nsimba-Lubaki M, Peeters B, Peumans WJ. The elderberry (Sambucus nigra L.) bark lectin recognizes the Neu5Ac(alpha 2–6)Gal/GalNAc sequence. J. Biol. Chem. 1987;262(4):1596–1601. [PubMed] [Google Scholar]
- 39.Puri KD, Gopalakrishnan B, Surolia A. Carbohydrate binding specificity of the Tn-antigen binding lectin from Vicia villosa seeds (VVLB4) FEBS Lett. 1992;312(2–3):208–212. doi: 10.1016/0014-5793(92)80937-c. [DOI] [PubMed] [Google Scholar]
- 40.Niethamer TK, Yardeni T, Leoyklang P, et al. Oral monosaccharide therapies to reverse renal and muscle hyposialylation in a mouse model of GNE myopathy. Mol. Genet. Metab. 2012;107(4):748–755. doi: 10.1016/j.ymgme.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harduin-Lepers A, Vallejo-Ruiz V, Krzewinski-Recchi MA, Samyn-Petit B, Julien S, Delannoy P. The human sialyltransferase family. Biochimie. 2001;83(8):727–737. doi: 10.1016/s0300-9084(01)01301-3. [DOI] [PubMed] [Google Scholar]
- 42.Giacopuzzi E, Bresciani R, Schauer R, Monti E, Borsani G. New insights on the sialidase protein family revealed by a phylogenetic analysis in metazoa. PLoS One. 2012;7(8):e44193. doi: 10.1371/journal.pone.0044193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pshezhetsky AV, Ashmarina LI. Desialylation of surface receptors as a new dimension in cell signaling. Biochemistry (Mosc) 2013;78(7):736–745. doi: 10.1134/S0006297913070067. [DOI] [PubMed] [Google Scholar]
- 44.Springer GF. Immunoreactive T and Tn epitopes in cancer diagnosis, prognosis, and immunotherapy. J. Mol. Med. (Berl) 1997;75(8):594–602. doi: 10.1007/s001090050144. [DOI] [PubMed] [Google Scholar]
- 45.Cao Y, Karsten UR, Liebrich W, Haensch W, Springer GF, Schlag PM. Expression of Thomsen-Friedenreich-related antigens in primary and metastatic colorectal carcinomas. A reevaluation. Cancer. 1995;76(10):1700–1708. doi: 10.1002/1097-0142(19951115)76:10<1700::aid-cncr2820761005>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 46.Goletz S, Cao Y, Danielczyk A, Ravn P, Schoeber U, Karsten U. Thomsen-Friedenreich antigen: the "hidden" tumor antigen. Adv. Exp. Med. Biol. 2003;535:147–162. doi: 10.1007/978-1-4615-0065-0_10. [DOI] [PubMed] [Google Scholar]
- 47.Imai J, Ghazizadeh M, Naito Z, Asano G. Immunohistochemical expression of T, Tn and sialyl-Tn antigens and clinical outcome in human breast carcinoma. Anticancer Res. 2001;21(2B):1327–1334. [PubMed] [Google Scholar]
- 48.Ng BG, Sharma V, Sun L, et al. Identification of the first COG-CDG patient of Indian origin. Mol. Genet. Metab. 2011;102(3):364–367. doi: 10.1016/j.ymgme.2010.11.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu X, Steet RA, Bohorov O, et al. Mutation of the COG complex subunit gene COG7 causes a lethal congenital disorder. Nat. Med. 2004;10(5):518–523. doi: 10.1038/nm1041. [DOI] [PubMed] [Google Scholar]
- 50.Foulquier F, Amyere M, Jaeken J, et al. TMEM165 deficiency causes a congenital disorder of glycosylation. Am. J. Hum. Genet. 2012;91(1):15–26. doi: 10.1016/j.ajhg.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Perez B, Medrano C, Ecay MJ, et al. A novel congenital disorder of glycosylation type without central nervous system involvement caused by mutations in the phosphoglucomutase 1 gene. J. Inherit. Metab. Dis. 2013;36(3):535–542. doi: 10.1007/s10545-012-9525-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.