Abstract
Crohn’s disease (CD) and irritable bowel syndrome (IBS) involve brain-gut dysfunctions where vagus nerve is an important component. The aim of this work was to study the association between vagal tone and markers of stress and inflammation in patients with CD or IBS compared to healthy subjects (controls). The study was performed in 73 subjects (26 controls, 21 CD in remission and 26 IBS patients). The day prior to the experiment, salivary cortisol was measured at 8∶00 AM and 10∶00 PM. The day of the experiment, subjects completed questionnaires for anxiety (STAI) and depressive symptoms (CES-D). After 30 min of rest, ECG was recorded for heart rate variability (HRV) analysis. Plasma cortisol, epinephrine, norepinephrine, TNF-alpha and IL-6 were measured in blood samples taken at the end of ECG recording. Compared with controls, CD and IBS patients had higher scores of state-anxiety and depressive symptomatology. A subgroup classification based on HRV-normalized high frequency band (HFnu) as a marker of vagal tone, showed that control subjects with high vagal tone had significantly lower evening salivary cortisol levels than subjects with low vagal tone. Such an effect was not observed in CD and IBS patients. Moreover, an inverse association (r = −0.48; p<0.05) was observed between the vagal tone and TNF-alpha level in CD patients exclusively. In contrast, in IBS patients, vagal tone was inversely correlated with plasma epinephrine (r = −0.39; p<0.05). No relationship was observed between vagal tone and IL-6, norepinephrine or negative affects (anxiety and depressive symptomatology) in any group. In conclusion, these data argue for an imbalance between the hypothalamus-pituitary-adrenal axis and the vagal tone in CD and IBS patients. Furthermore, they highlight the specific homeostatic link between vagal tone and TNF-alpha in CD and epinephrine in IBS and argue for the relevance of vagus nerve reinforcement interventions in those diseases.
Introduction
Crohn’s disease (CD) is an inflammatory bowel disease (IBD) characterized by a chronic abnormal mucosal immune response with periods of remission of unpredictable duration alternating with acute episodes of flare [1], [2]. Irritable bowel syndrome (IBS) is a highly prevalent functional gastrointestinal disorder characterized by abdominal pain and discomfort associated with altered bowel habits [3]. Both pathologies involve brain-gut interaction perturbations and are strongly influenced by narrow interactions between biological and psychosocial factors, and thus considered as bio-psychosocial diseases [4]–[8]. High perceived stress, negative affects such as anxiety, depression and an imbalanced autonomic nervous system (ANS) are common features in CD and IBS [7], [9], [10]. The neuroendocrine communication between the brain and the gut is mediated by the parasympathetic and sympathetic branches of the ANS, and by the hypothalamus-pituitary-adrenal (HPA) axis (Bonaz and Bernstein, 2013 for review). These regulatory systems, as a part of the allostatic network, are interrelated and functionally coupled to adapt physiological responses to external and/or internal challenges ensuring homeostasis and promoting health [11]–[13]. Specifically, the parasympathetic nervous system plays a major role in gastrointestinal homeostasis [14] and is involved in physiological and psychological flexibility in reaction to stress [15], [16], emotional regulation, and stress recovery [17], [18]. Furthermore, the parasympathetic nervous system, through the vagus nerve, modulates the production of pro-inflammatory cytokines such as TNF-alpha [19] through both vagal afferents and efferents activating respectively the HPA axis and the cholinergic anti-inflammatory pathway [9], [20], [21]. TNF-alpha is a key pro-inflammatory cytokine involved in CD and anti-TNF therapy is currently the gold standard in the treatment of IBD patients [22]. The vagus nerve is also combined with the HPA axis and under physiological conditions a balance is observed between the parasympathetic nervous system and the HPA axis [23]. This reflects an adapted homeostatic regulation by coupling high vagal tone to low cortisol level. However, in chronic diseases such as alcoholism, where the parasympathetic tone is dramatically blunted, this coupling is altered [24] reflecting an impaired inhibitory control of the HPA axis and an allostatic load as defined by McEwen [25]. An autonomic imbalance with a sympathetic dominance has been described in IBD and IBS [10], [26] and should logically have an impact on the HPA axis regulation and thus on catecholamines and pro-inflammatory cytokines levels such as TNF-alpha or IL-6. However, little is known about the nature of the relationship between the vagal tone and the HPA axis in these pathologies and even less with catecholamines and pro-inflammatory cytokines. This raises the question of the correlation, in CD or IBS patients, between the resting vagal tone, which could be considered as a functional parasympathetic fingerprint, on the one hand, and cortisol, catecholamines and pro-inflammatory cytokines levels on the other hand.
Consequently, the principal aim of this study was to examine this functional coupling. If the ANS and the HPA axis are functionally uncoupled in CD and IBS, then we should find no relation between vagal tone and cortisol levels in patients while a high vagal tone will be associated to a low cortisol level (and conversely) in controls. Furthermore, we hypothesized that negative affects (anxiety and depressive symptomatology), catecholamines and cytokines levels were dependent on vagal tone in CD and IBS patients but not in controls. For this purpose, heart rate variability (HRV), an index of the parasympathetic nervous system activity, was measured at rest in control healthy subjects, CD patients in remission and IBS patients. Then, a cluster analysis was performed in order to compare, between the low and high vagal tone subgroups, the levels of cortisol, TNF-alpha, IL-6, epinephrine, norepinephrine and negative affects.
Materials and Methods
Subjects and Ethics Statement
The study was performed in agreement with the Declaration of Helsinki and the guidelines of Good Clinical Practice and was approved by the Ethic Committee of the Grenoble Faculty of Medicine and Hospital (ref: 08-CHUG-23, ClinicalTrials.gov Identifier: NCT01095042). Written informed consent was obtained from each participant. White subjects, aged 18–60 years, were prospectively recruited between September 2009 and October 2011. CD and IBS patients were recruited in our Department of Gastroenterology while age and sex-matched healthy subjects were recruited by the Grenoble INSERM Clinical Investigation Centre (CIC).
Criteria for Inclusion
Crohn’s Disease (CD) patients
CD patients were selected according to their phenotype as defined by the Montreal classification [27]. CD patients with isolated ano-perineal or upper digestive tract lesions were not eligible. CD activity was evaluated by the Harvey–Bradshaw index (HBI) [28] and patients with an HBI<4 on inclusion were considered in clinical remission. The endoscopic, contrast-enhanced ultrasound and biologic explorations (CRP<5 mg/l) showed that all patients were under mucosal healing and/or parietal healing under their current treatment. Patients were included only if they had a stable dose of i) 5-aminosalicylates for at least 2 weeks, ii) immunosuppressives for at least 12 weeks, and iii) biological therapy (e.g., anti-TNF-alpha) for at least 8 weeks.
Irritable Bowel Syndrome (IBS) patients
Patients were selected according to Rome II criteria [29]: at least 12 weeks, not necessarily consecutive, in the preceding 12 months of abdominal discomfort or pain with two out of the three following features: 1) relieved with defecation; and/or 2) onset associated with a change in frequency of stool; and/or 3) onset associated with a change in form (appearance) of stool. The lack of organicity for patient’s symptoms was assumed through: i) a negative physical examination; ii) a normal colonoscopy performed within the last five years with normal biopsies (i.e., absence of microscopic colitis); iii) normal limited laboratory evaluations with a lack of inflammation (i.e., erythrocyte sedimentation rate, C-reactive protein), anaemia, infection (complete blood cell count) and endocrine or metabolic disturbances (i.e., thyroid stimulating hormone, chemical analysis) as well as the absence of IgA anti-transglutaminase (without IgA deficiency).
Criteria for Exclusion
Patients were excluded from the study if: (i) they had past or present medical conditions complicated by autonomic dysfunction (e.g., peripheral neuropathy, diabetes, vagotomy, dysthyroidism, amyloidosis, asthma, heart failure, renal insufficiency, alcoholism), (ii) they were under medication susceptible to modify the ANS (e.g., anticholinergics, antiarrhytmics, alpha or beta blocking agents, antibiotics). Patients with previous abdominal surgery, except appendectomy and/or cholecystectomy, were excluded from the study.
Experimental Design
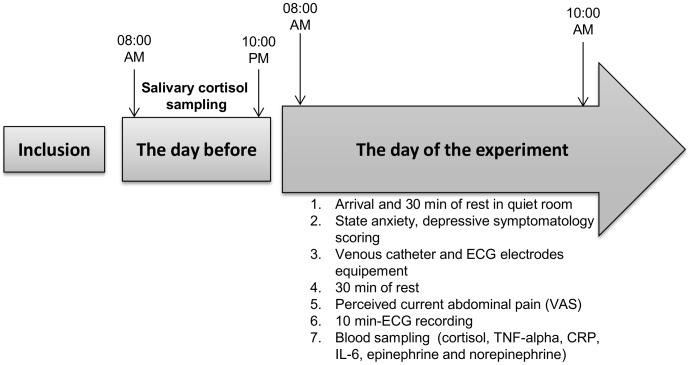
All patients underwent an interview concerning their history (disease duration, extent, extra-intestinal manifestations, course, current and past therapies, medications) and a physical examination to determine their inclusion in the study according to the inclusion-exclusion criteria. After information and consent, subjects were enrolled and an appointment was fixed. As shown in figure 1, the day before the experiment, salivary cortisol was measured at 08∶00 AM and 10∶00 PM at home. Participants were asked to have a light breakfast on the morning of their running session. On their arrival in our department (8∶00 AM), each participant was oriented to a quiet room to sit and relax during 30 min in a comfortable chair. After explanations on the running of the session, participants completed questionnaires for state-anxiety (State-Trait Anxiety Inventory; STAI) [30] and depressive symptomatology (Center for Epidemiologic Studies-Depression Scale; CES-D) [31], [32]. They were then equipped with electrodes for electrocardiogram (ECG) recording and a venous catheter for blood sampling. After a resting period of 30 min, participants were asked to evaluate their current visceral perception through a visual analogic scale (VAS) measuring the intensity of perceived abdominal pain (0: no perceived pain; 10: the maximum perceived pain), then the electrocardiogram (ECG) was recorded for 10 minutes. During this recording period, a technician carefully observed the optimal conditions to ensure that the recording was free of body movements, conversations and any subjective discomfort. Experimental sessions were always performed between 08.00 AM and 10.00 AM to avoid any influence of circadian variations. Catecholamines (epinephrine, norepinephrine), pro-inflammatory cytokines (TNF-alpha, IL-6), cortisol and C-reactive protein (CRP) were measured in blood samples collected at the end of the resting ECG.
Figure 1. The experimental design.
Parasympathetic Assessment: Power spectral analysis of Heart Rate Variability (HRV)
ANS activity was explored using HRV as a reliable and non-invasive method [33]. Initially described by Akselrod [34] to explore cardiovascular control, this tool is now commonly used in gastrointestinal physiology to assess autonomic imbalance related to digestive autonomic regulation [10], [35]. ECG signal was acquired through electrodes placed on each wrist. HRV analysis was performed using specific software (Heart Rhythm Scanner, Biocom Technologies, USA). First, QRS complexes were automatically classified. Ectopic or abnormal QRS complexes were visually detected in the software and removed. The signal was then carefully checked and remaining abnormalities were manually removed. Then, a standard spectral analysis was applied on inter-beat intervals using a Fast Fourier Transformation (FFT) according to the standards of measurement of the Task Force on HRV [36]. The following parameters were calculated: (i) Total Power (TP) corresponds to the spectral power density in the range of 0 to 0.40 Hz. It was considered as the net effect of all physiological mechanisms contributing to HRV; (ii) High Frequency power spectrum (HF, from 0.15 to 0.40 Hz, msec2) reflected for 90% parasympathetic tone fluctuations caused by respiratory sinus arrhythmia; [37], [38] (iii) Low Frequency power spectrum (LF, from 0.04 to 0.15 Hz, msec2) at rest in sitting position, is explained by parasympathetic tone for at least 50% and sympathetic tone for 25%. This HRV component is related to baroreflex modulation [38] (iv) Very Low Frequency power spectrum (VLF, from 0.0033 to 0.04 Hz, msec2) represented various negative emotions or worries in short time recording [39] and various long term endocrine regulations such as renin-angiotensin system and thermoregulation [36], [40]. LF and HF variables were also expressed in normalized units: normalized HF [HFnu = HF/(TP–VLF)] and normalized LF [LFnu = LF/(TP–VLF)], respectively. This calculation minimized the effect of changes in Very Low Frequency power on LF and HF power and emphasized the changes in sympathetic or parasympathetic regulation. (v) Lastly, LF/HF ratio was calculated as a global marker of the autonomic balance.
Cytokines Measurement
Interleukin-6 and TNF-alpha were evaluated by the Randox Biochip Array technology (Randox Laboratories, Roissy-en-France). This miniaturized ELISA-based technic allows simultaneous quantitative detection of multiple cytokines from a patient low volume single sample. The array used in this study is the Cytokine Array I, which is coated with antibodies against 12 cytokines. Briefly 100 µl of EDTA plasma or standards were added in each well of the biochip and were incubated for 1 hour at 37°C at 370 rpm. Biochip was quickly washed twice with 350 µl of wash buffer, and 4 more washings with a 2-minute soaking step were performed. Then 300 µl of HRP-conjugate antibodies were added and incubated for 1 hour at 37°C at 370 rpm. Washings were realized as previously described and the biochip was briefly air dried. The two components of the signal reagent, luminol and peroxide, were mixed in a ratio of 1∶1 and 250 µl were added per well. Signal reading was performed on the Randox Evidence Investigator device, after incubation of the biochip for 2 minutes in the dark. Captured RLU were converted into concentration of cytokines using the 9-point calibration curves run in parallel for each cytokine.
Salivary Cortisol Measurements
Saliva was collected on Salivette (Sarstedt, Marnay, France) the day before the experiment at 8∶00 AM and 10∶00 PM and stored at −20°C until analysis. Cortisol was evaluated by a commercial radioimmunoassay kit (Cisbio International; Gif-sur-Yvette, France). The principle of the assay is based on the competition between the labelled cortisol and cortisol contained in calibrators or samples to be assayed for a fixed and limited number of antibody binding sites bound to the solid phase (coated tubes). Briefly 150 µl of calibrators, controls or samples were dispensed into the labelled coated tubes and 500 µl of 125I-cortisol was added to each tube. After incubation for 30 minutes at 37°C, unbound tracer was removed by a washing step with 1 ml of distilled water. The remaining radioactivity bound to the tubes was measured with a gamma scintillation counter calibrated for 125 Iodine. The amount of labelled cortisol bound to the antibody was inversely related to the amount of unlabelled cortisol initially present in the sample. Concentration of cortisol in saliva was determined by referring to the radioactivity of the 8-point calibration curve. The range of reference values for the morning and evening salivary cortisol concentrations at the CHU of Grenoble are 6.2–38 nmol/l at 06∶00–08∶00 AM, 0.8–4.9 nmol/l at 06∶00–08∶00 PM and <3 nmol/l at 10∶00–00∶00 PM.
Catecholamines Measurement
Analysis of catecholamines (epinephrine and norepinephrine) was performed with a commercial kit according to the manufacturer’s specifications (Chromsystems, Munich, Germany). Briefly, according to Hue [41], catecholamines were purified from plasma through solid phase extraction by aluminium oxide and secondly measured by reversed phase HPLC on isocratic mode with electrochemical detection (ESA-CoulArray, Eurosep Instruments, Saint Chamond, France).
Psychological Assessments
Anxiety was assessed using the State-Trait Anxiety Inventory (STAI; [30], validated in French by Bruchon-Schweitzer and Paulhan [42] consisting of a scale with 20 items with a score varying from 20 to 80. A high score indicates high anxiety. In the present sample, the internal consistency was high (alpha = 0.91).
Depressive symptomatology was assessed by the Center for Epidemiologic Studies-Depression Scale (CES-D) [31], [32]. This brief scale of 20 items assesses symptoms or behaviours often associated with depression. The total score varies from 0 to 60, a high score signifying a high level of depressive symptomatology. An alpha coefficient for internal consistency of 0.85 has been reported in general population samples and 0.90 in psychiatric samples [43]. In the present sample, the alpha coefficient was 0.87.
Statistical Analyses
Statistical analysis was carried out with Statistica 7.1 (Statsoft, Maisons-Alfort, France) and StatExact 9 (Cytel, Paris, France). ANOVA was used to evaluate main effects of group on age, disease duration, visceral pain, state anxiety and depressive symptomatology. When a significant effect was observed, a Bonferroni post-hoc test was applied to determine the differences between each group. Since the high frequency component of HRV is explained for 90% by the parasympathetic activity as described above, the normalized unit of HF (HFnu) has been considered to be the most appropriate, among HRV components, to represent the resting parasympathetic tone. Thus, HFnu was used to categorize subjects in high or low parasympathetic tone using K-means clustering method based on observations. Two clusters of subjects were therefore identified. Non-parametric permutation tests for small samples were performed to make comparisons between the low and high vagal tone subgroups within each group. Spearman correlation coefficients were used to evaluate relationships among vagal tone and cytokines or catecholamines within each group (controls, IBS and CD). Data are expressed as means (± standard error of the mean, SEM). The alpha value for statistical significance was set at p<0.05.
Results
Participants
Patients and healthy controls demographics and psycho-immunological data are detailed in table 1. Seventy-three subjects were distributed as healthy volunteers (controls), IBS and CD patients in remission. The mean age of all the participants was 38±10 years old. There was no significant difference in the age (F(2,70) = 0.85, p = 0.43) between groups. Among the 26 IBS patients, 7 patients (6 women and 1 man) were diarrhea predominant, 1 patient (woman) constipation predominant and the other 18 patients with alternative diarrhea/constipation. The mean duration of the disease was not significantly different between patients groups (F(1,45) = 1.46, p = 0.23). CRP plasmatic level was normal (<5 mg/l) in all groups. There was a significant effect of the disease on the level of perceived visceral pain as evaluated on the day of the experiment (F(2,70) = 7.48, p = 0.001). IBS patients had the highest score of perceived visceral pain compared to controls (p<0.001). There was also a significant effect of the disease on the scores of state-anxiety (F(2,66) = 7.63, p = 0.001) and depressive symptomatology (F(2.66) = 14.28, p<0.001) with CD and IBS patients exhibiting the highest scores of state-anxiety (p<0.05 and p = 0.001 respectively) and depressive symptomatology (p = 0.07 and p<0.001 respectively) compared to controls. Moreover, the scores of depressive symptomatology were significantly (p<0.02) higher in IBS than CD patients.
Table 1. Socio-demographic and psycho-immunologic data of the healthy control subjects, Crohn’s disease (CD) and irritable bowel syndrome (IBS) patients who participated to the study.
| Controls | Crohn’sDisease (CD) | Irritable BowelSyndrome (IBS) | p value | |
| Total number of subjects | 26 | 21 | 26 | |
| Mean age, year ± SD | 36±10 | 40±11 | 38±11 | NS CD or IBS vs controls |
| Sex, M/F | 8/18 | 9/12 | 7/19 | |
| BMI (Kg/m2) | 23±3.5 | 22±4.3 | 22±5.2 | NS CD or IBS vs controls |
| Mean duration of disease,year (range) | - | 13.4 (1–28) | 10.3 (1–31) | |
| Localization of Crohn’s disease according to Montreal classification | Ileal: | |||
| L1B1: n = 3 | ||||
| L1B2: n = 3 | ||||
| B1pB3: n = 1 | ||||
| Colonic: | ||||
| L2B1: n = 6 | ||||
| L2B1pB3: n = 2 | ||||
| Ileocolonic: | ||||
| L3B1: n = 2 | ||||
| L3B2: n = 2 | ||||
| L3B2pB3: n = 2 | ||||
| Inflammatory markers (circulating levels) | ||||
| CRP level (mg/l) | <4 | <5 | <5 | NS CD or IBS vs controls |
| Perceived abdominal visceral pain | ||||
| VAS | 0.30±0.34 | 1.28±0.38 | 2.19±0.34 | IBS vs controls p<0.001 |
| Mood variables | ||||
| State-Anxiety | 31±1.90 | 39±2.15 | 41±1.91 | CD vs controls p<0.05; IBS vs controls p<0.001 |
| Depressive symptomatology | 8.94±1.39 | 13.68±1.58 | 19.51±1.40 | CD vs controls p = 0.07; IBS vs controls p<0.001; IBS vs CD p<0.05 |
Balance between resting vagal tone and cortisol, TNF-alpha, epinephrine and negative affects in CD and IBS patients
The parasympathetic fingerprint
The HRV variable HFnu was used to categorize subjects into low and high parasympathetic tone as a hallmark of the level of their vagal tone. Two clusters of subjects were therefore identified as high or low parasympathetic level within control, CD, and IBS groups. This subgroup classification revealed that about half of the subjects had a high resting parasympathetic tone (HFnu = 56±1.5, n = 35) and the other one a low resting parasympathetic tone (HFnu = 25±1.5; n = 38). Data reporting mean values of HRV variables in low and high subgroups in controls, CD and IBS patients are detailed in table 2.
Table 2. Data representing the sub-group categorization based on HFnu-HRV K-mean classification.
| Controls | Crohn’s Disease (CD) | Irritable Bowel Syndrome (IBS) | ||||
| Resting parasympathetic level | High (n = 15) | Low (n = 11) | High (n = 8) | Low (n = 13) | High(n = 12) | Low (n = 14) |
| HR (bpm) | 68±2 | 65±3 | 71±4 | 65±3 | 64±2 | 66±2 |
| RRI (ms) | 894±35 | 928±41 | 879±58 | 938±45 | 940±30 | 912±28 |
| Total Power (ms2) | 982±134 | 718±157 | 492±184 | 973±134* | 885±150 | 693±140 |
| VLF (ms2) | 323±65 | 275±76 | 202±89 | 493±70** | 387±73 | 311±67 |
| LFnu | 39±3 | 68±3*** | 36±6 | 63±4** | 34±3 | 66±3*** |
| HFnu | 57±2 | 27±3*** | 56±3 | 20±3*** | 57±2 | 28±2*** |
| LF/HF | 0.74±0.2 | 2.75±0.2*** | 0.71±0.8 | 3.89±0.6*** | 0.62±0.3 | 2.79±0.3*** |
Data are expressed as mean ± sem. Comparisons are made between low and high parasympathetic level using permutations test.
*p<0.05;
**p<0.01;
***p<0.001.
Interestingly, CD patients with low parasympathetic tone showed significantly higher levels in Total Power (p<0.02) and VLF (p<0.01) HRV variables compared to CD patients with high parasympathetic tone. VLF seemed to be related to visceral sensitivity since (i) CD patients with low parasympathetic tone reported higher scores of perceived abdominal pain than CD patients with high parasympathetic tone (1.76±0.4 and 0.50±0.5 respectively; p<0.05) and (ii) VLF was positively correlated with the score of perceived abdominal pain (r = 0.65; p<0.001). It is interesting to note that this correlation observed in CD was not found in controls (r = –0.29; p = 0.14) or IBS patients (r = 0.30; p = 0.13).
Vagal tone and evening salivary cortisol level (figure 2)
Figure 2. Relationship between the resting parasympathetic vagal tone and the evening salivary cortisol in controls, Crohn’s disease (CD) and Irritable bowel syndrome (IBS) patients.
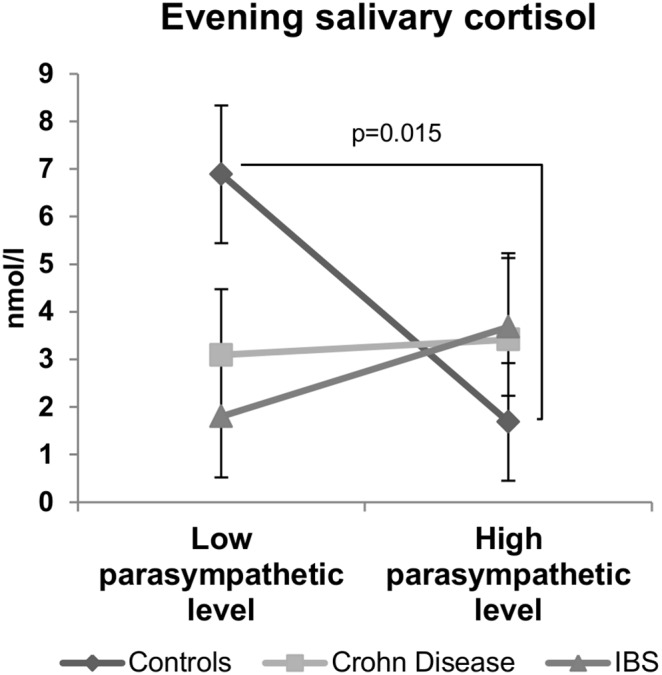
A balance was observed between the parasympathetic tone and the evening salivary cortisol in healthy subjects (control group) but not in CD and IBS patients. Data are expressed as mean ± sem. Comparisons are made between the high and low parasympathetic level subgroups using permutations test.
Controls with high parasympathetic level (HFnu = 57±2) exhibited significantly (p<0.05) lower evening salivary cortisol (1.69±1.30 nmol/l) than controls with low parasympathetic level (HFnu = 27±3; evening salivary cortisol = 6.89±1.30 nmol/l). Interestingly, this inverse balance between morning vagal tone and evening salivary cortisol level was observed neither in CD (3.41±1.81 nmol/l for high parasympathetic tone and 3.09±1.38 nmol/l for low parasympathetic tone subgroup; p = 0.16) nor in IBS patients (3.68±1.44 nmol/l for high parasympathetic tone and 1.80±1.28 nmol/l for low parasympathetic tone subgroups; p = 0.42). In another way, it is interesting to note that no significant difference was observed between the high and low parasympathetic vagal tone subgroups for the morning plasma and salivary cortisol levels in any group (table 3).
Table 3. Influence of the vagal tone on the plasma levels of the morning salivary and plasma cortisol, IL-6, norepinephrine concentrations, state-anxiety and depressive symptomatology scores in Controls, Crohn’s disease (CD) and Irritable Bowel syndrome (IBS) patients.
| Controls | Crohn’s Disease (CD) | Irritable Bowel Syndrome (IBS) | ||||
| Resting parasympathetic level | High (n = 15) | Low (n = 11) | High (n = 8) | Low (n = 13) | High (n = 12) | Low (n = 14) |
| Morning salivary cortisol (nmol/l) | 14.35±2.27 | 9.75±2.56 | 9.37±3.21 | 15.80±2.45 | 14.30±2.56 | 16.69±2.36 |
| Morning plasma cortisol (nmol/l) | 389.5±61.4 | 343±69.2 | 484.9±81.2 | 419.33±66.3 | 344.5±66.3 | 319.1±61.4 |
| IL-6 (ng/l) | 0.83±0.28 | 0.22±0.32 | 0.50±0.38 | 0.75±0.31 | 0.61±0.31 | 0.65±0.29 |
| Norepinephrine (pmol/l) | 1.8±0.18 | 1.6±0.22 | 2.3±0.24 | 2.05±0.2 | 2.01±0.20 | 2.38±0.19 |
| State-anxiety score | 33.06±2 | 29.1±3 | 37.7±4 | 40.2±2 | 41.1±3 | 41.3±2 |
| Depressive symptomatology score | 8.5±2 | 9.3±2 | 13.7±2 | 13.6±2 | 20.3±2 | 18.7±2 |
Data are expressed as mean ± sem. Comparisons are made between low and high parasympathetic level using permutations test.
Vagal tone and pro-inflammatory cytokines (figure 3)
Figure 3. Specific inverse relationship between the resting parasympathetic vagal tone and TNF-alpha plasma level in CD patients.
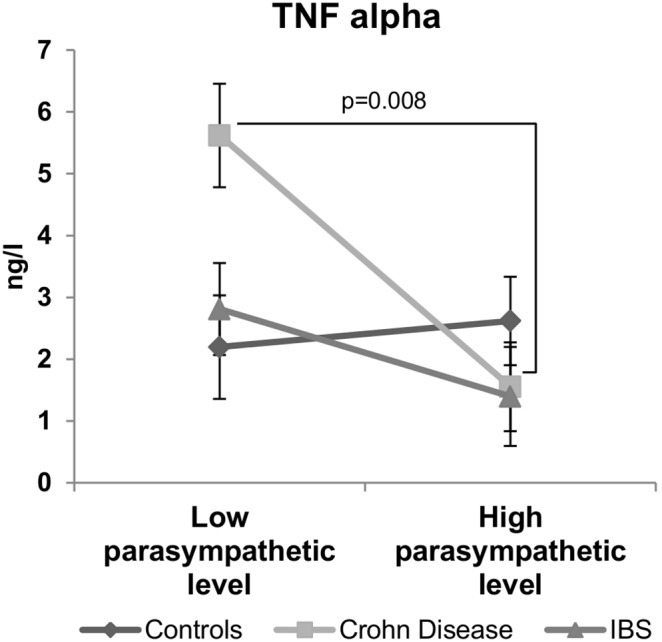
CD patients with low parasympathetic vagal tone exhibit a higher level of TNF-alpha than those with high parasympathetic vagal tone. This inverse relationship was not observed in controls or IBS patients. Data are expressed as mean ± sem. Comparisons are made between the high and low parasympathetic level subgroups using permutations test.
In CD patients, a significant inverse relationship (r = –0.48; p<0.05) was observed between the parasympathetic tone and TNF-alpha plasma concentration. Thus, CD patients exhibiting a high parasympathetic tone (HFnu = 56±3) had significantly (p<0.01) lower levels of TNF-alpha plasma concentration (1.55±0.98 ng/l) than those with low parasympathetic tone (HFnu = 20±3; TNF-alpha = 5.62±0.80 ng/l). Such a negative correlation was neither observed in IBS patients (r = –0.34; p = 0.09) nor in controls (r = 0.19; p = 0.33) where the TNF-alpha plasma levels did not differ according to the parasympathetic vagal tone. As presented in table 3, IL-6 plasma levels measured in controls, CD and IBS patients were not different between the low and high parasympathetic vagal tone subgroups.
Vagal tone and catecholamines (figure 4)
Figure 4. Specific inverse relationship between the resting parasympathetic vagal tone and epinephrine plasma level in IBS patients.
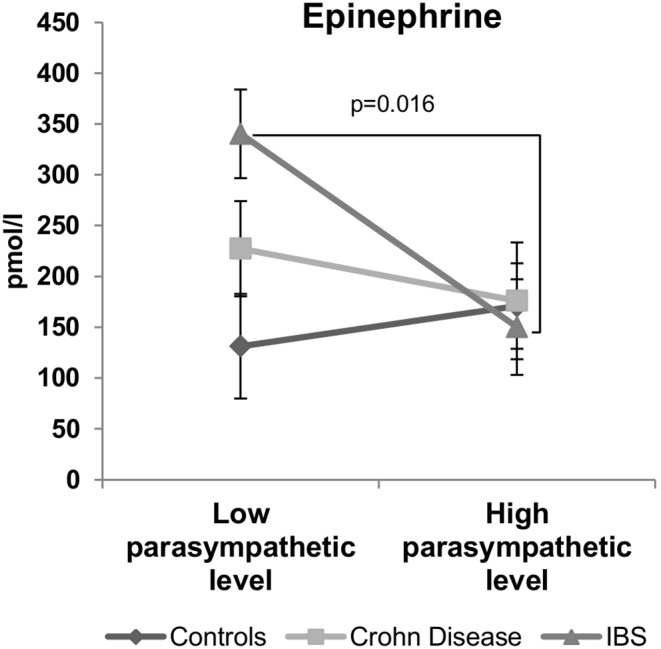
IBS patients with low parasympathetic vagal tone exhibit a higher level of plasma epinephrine at rest than those with high parasympathetic vagal tone. This inverse relationship was not observed in controls or CD patients. Data are expressed as mean ± sem. Comparisons are made between the high and low parasympathetic level subgroups using permutations test.
In IBS patients, a significant inverse relationship (r = –0.39; p<0.05) was observed between the parasympathetic tone and the epinephrine plasma concentration. IBS patients exhibiting a high parasympathetic tone (HFnu = 57±2) had significantly (p<0.05) lower levels of epinephrine plasma concentrations (150±47 pmol/l) than those with a low parasympathetic tone (HFnu = 28±2; epinephrine = 340±43 pmol/l). Such a negative correlation was neither observed in CD patients (r = –0.07; p = 0.75) nor in controls (r = –0.05; p = 0.82). Norepinephrine plasma levels did not present any significant difference between high and low parasympathetic tone subgroups in control, CD and IBS patients (table 3).
Vagal tone and negative affects
No significant difference was observed between low and high parasympathetic tone subgroups for state-anxiety and depressive symptomatology scores within any group (table 3). However, in CD group, there was a significant correlation between evening salivary cortisol level and state-anxiety score (r = 0.49; p<0.05) on the one hand, and depressive symptomatology (r = 0.69; p<0.001) on the other hand. Such an association was not observed in IBS group.
Discussion
The present study shows three important results highlighting the strong relationship between the vagal tone and markers of stress regulation and inflammation in CD and IBS patients. First, we observed that a high morning vagal tone is associated with a low evening cortisol level in healthy subjects but not in CD and IBS patients suggesting an uncoupling between vagal tone and cortisol level in those patients. Second, we found that TNF-alpha plasma level is negatively correlated to vagal tone in CD patients suggesting that the cholinergic anti-inflammatory pathway may be blunted in CD patients with low vagal tone. Third, we show that IBS patients with low vagal tone exhibit high plasma level of epinephrine as a mark of an unadapted high sympathetic activity. Finally, even if one limitation of our study may concern the possible impact of gender on the main effects that we observed, data reported here highlight the interest of measuring the resting vagal tone in IBS and CD patients as a marker of homeostatic imbalance that could predict a state of vulnerability to relapse.
The resting vagal tone as a marker of the central homeostatic balance
In the present study, we have categorized individuals according to their resting vagal tone based on the HRV high frequency component (HFnu). The resting vagal tone is strongly involved in the regulation of physiological systems that are important in health and disease and notably those concerning the HPA axis and inflammation [23]. HRV has been previously proposed as an endophenotype marker particularly as a mediator between physiology and behavior [44]. In the present study, we used vagal tone rather like a fingerprint reflecting the balance of the autonomic network. Indeed, Thayer and Lane [44] described a model of neurovisceral integration in which a set of neural structures involved in cognitive, affective, and autonomic regulation referred as the central autonomic network or CAN [45] are related to HRV; thus they proposed HRV as an indicator of CAN-ANS integration. In this integrative interplay, the functional coupling between low cortisol levels and high vagal tone at rest would reflect, at the peripheral level, the central top-down inhibition of the medial prefrontal cortex on subcortical sympatho-excitatory circuits such as the amygdala [23], [46]. The hypoactivity of the medial prefrontal cortex enhances amygdala activity and then induce a parasympathetic withdrawal and a sympathetic activation. Thus, according to this model, the lower the vagal tone, the less active the prefrontal cortex will be, reflecting a shift from a homeostatic state to a stress state. This must be associated with emotional and physiological outputs such as an increase in pro-inflammatory cytokines, epinephrine and anxiety. In the present study, we have observed a negative coupling between the vagal tone and cortisol level in healthy subjects. Individuals exhibiting high resting vagal tone in the morning will have the greater decrease in salivary cortisol levels in the evening. In contrast, this balance between cortisol and HRV (vagal tone) was no more observed in CD and IBS patients. This argues for an uncoupling between the HPA axis and the ANS in both diseases and suggests a breakdown of the functional connectivity between the prefrontal cortex and the amygdala as recently shown in depression and anxiety [47], [48]. These results are independent of the circadian cycle since the salivary level of cortisol is high in the morning and low in the evening in the three groups as normally observed [49], [50]. According to the McEwen model of stress [11], this uncoupling would be the sign of a costly allostatic regulation with reduced flexibility in the regulatory systems. Such a situation would make CD and IBS patients more reactive to stressful life events or other challenging situations and thus more probable to trigger symptoms. Indeed, a reduced vagal tone could not be in favor of a positive effect of the cholinergic anti-inflammatory pathway (CAP) and thus inflammation and/or pain could be enhanced.
Inverse relationship between vagal tone and TNF-alpha and perceived visceral pain in CD
The second important result reported in this study concerns the inverse relationship between HRV variables representative of the vagal tone and TNF-alpha in CD patients. TNF-alpha is a key pro-inflammatory cytokine in the pathogenesis of CD [51]. TNF-alpha is abundantly expressed in the gastrointestinal tracts of CD patients and contributes to intestinal mucosal inflammation [52]. Currently, the gold standard therapy aims at reducing the activity of TNF-alpha in IBD patients using anti-TNF therapies [53]. The vagus nerve is known to play a dual inhibitory control on inflammation. Its afferent fibers reach the brainstem and activate the HPA axis and cortisol release as an endpoint [4]. Further, more recently, vagal efferent fibers have been shown to exert an anti-inflammatory effect (i.e., the CAP) by inhibiting TNF-alpha production from macrophages [54]. Today, the CAP is a therapeutic target in chronic inflammatory diseases such as CD in which low frequency vagus nerve stimulation is used [55], [56]. Recent data, supporting our findings, have described an inverse relationship between HRV indices and TNF-alpha levels in heart failure [57] but also in healthy subjects under stressful situations [18]. In a recent review, Huston and Tracey supported the idea that HRV would be a relevant marker of excessive inflammation [58] in line with our findings. However, in our study, we did not found a significant relationship between HRV and TNF-alpha in healthy volunteers; a similar observation has also been recently reported in patients with chronic heart failure [59]. This is explained by the facts that healthy subjects (i) were under resting and not stressful conditions and (ii) were not under an inflammatory state.
The other interesting result of our study concerns the correlation between the spontaneous visceral abdominal pain perception and the VLF band of HRV. CD patients with low parasympathetic tone had higher levels of VLF than CD patients with high parasympathetic tone, and also reported higher scores of visceral perception. Such an observation has never been reported before. Although the physiological meaning of VLF oscillations has not been completely understood yet, the increase of this HRV variable is related to an important parasympathetic impairment with a loss of coherence between RR intervals and systolic blood pressure variability [60]. Furthermore, VLF power level is influenced by the renin-angiotensin system since the blockade of the angiotensin converting enzyme has been shown to decrease VLF [61]. In another way, VLF oscillations have been related to an increase in peripheral chemosensitivity in patients with congestive heart failure [62]. Angiotensin also acts as a modulator in the spinal transmission of nociceptive information [63]. Interestingly, a recent pilot study revealed an up-regulation of the renin-angiotensin system in inflammatory bowel disease patients [64]. Consequently, one can hypothesize that the increase of VLF oscillations observed in the low vagal tone CD patients, could be related to an impairment of the angiotensin system leading to the increase in visceral pain perception. This could enhance a shift toward hypersensitivity and IBS-like symptoms. If so, VLF oscillations would be a relevant marker of autonomic visceral sensitivity impairment that could be used in the patients’ follow-up. Further experiments are currently underway to deepen this question.
Inverse relationship between vagal tone and epinephrine in IBS
Another important finding of our study is the inverse specific relationship between HRV and plasma levels of epinephrine in IBS. Patients with IBS exhibit visceral hypersensitivity (VHS) [65]. In our study, IBS patients reported higher scores of perceived abdominal pain than CD patients or healthy subjects. Besides pain, IBS patients reported more depressive symptoms and anxiety than healthy subjects. Psychosocial factors are often found in IBS patients and IBS is considered as a biopsychosocial model disorder [5]. Indeed, about 20 to 50% of IBS patients have psychiatric disorders, such as major depression, anxiety and somatoform disorders [66]. High perceived stress, negative mood and autonomic imbalance also characterized IBS as reported in previous studies [10], [67]. In the present work, we found that IBS patients exhibit higher circulating levels of norepinephrine at rest than healthy subjects. These findings are corroborated by several studies revealing abnormal catecholamines levels in IBS [68], [69]. In addition, our study reveals, for the first time, that IBS patients with low vagal tone have higher plasma levels of epinephrine than those with high vagal tone. This inverse relationship in addition to the uncoupling between the vagal tone and cortisol argues for a hyperactivity of the amygdala and a hypo-activation of the prefrontal cortex underlying vulnerability to stress in this disease [46]. This is strengthened by the elevated scores of state-anxiety and depressive symptomatology observed in those patients even if we did not find a linear relationship between the parasympathetic vagal tone at rest and these psychological scores. These affects would be rather associated to the HPA axis and thus to the level of cortisol as previously shown [70] and more probably to the decrease in the evening cortisol as suggested by the results of our study in CD patients.
Conclusion
The fact that HRV is inversely related to TNF-alpha in CD patients and to norepinephrine in IBS, suggests that HRV would be a reliable marker of the allostatic load in such chronic diseases. This idea supports the fact that HRV that indexes vagal tone is a real marker of homeostasis and autonomic flexibility. In CD patients, the homeostasis of inflammation is imbalanced and a low vagal tone favors an overexpression of TNF-alpha. In IBS, a low vagal tone will be representative of a homeostatic imbalance of the sympatho-adrenergic axis. This is in agreement with the findings that in atherosclerosis, an inflammatory disease characterized by elevated levels of CRP and IL-6, a low vagal tone is inversely correlated with these inflammatory markers [71]. As we could see herein, among patients, only a part of them would require a vagal reinforcement that could be achieved by targeting the vagus nerve through electrical stimulation, pharmacology and/or complementary medicines such as hypnotherapy [9] or Mindfulness Based Stress Reduction a program which increases vagal tone [72]. These therapies would also improve visceral pain perception; reduce epinephrine and TNF-alpha levels allowing remission maintenance.
Acknowledgments
We aknowledge Patricia Raiewski, Nathalie Drivas, David Tartry and Françoise Bardin and Virginie Debard from the Clinique Universitaire d’Hépato-Gastroentérologie of the CHU de Grenoble, for their helpful technical support during the patients enrollment.
This work has been presented at the Digestive Disease Week, Orlando (May 21, 2013), in top ten of the poster presentation (Pellissier S. et al. Gastroenterology 2013; 144(5): S-930), and at the meeting of the International Society for Autonomic Neuroscience (ISAN; August 1st, 2013), Giessen, Germany (Pellissier S. et al. Autonomic Neuroscience 2013; 177(2): 315–316).
Data Availability
The authors confirm that, for approved reasons, some access restrictions apply to the data underlying the findings. Two additional papers related to this study have to be written and we wouldn’t like to make public right now all the details of the study while the data are not published yet. However, as far as the data of the current manuscript are concerned, data are from the 817-Protocol study whose authors may be contacted at sonia.pellissier@univ-savoie.fr.
Funding Statement
This work was supported by the Association François Aupetit (AFA), the Société Nationale Française de Gastroentérologie (SNFGE) and the Direction Hospitalière de Recherche Clinique (DHRC) of the Grenoble hospital. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
References
- 1. Strober W, Fuss I, Mannon P (2007) The fundamental basis of inflammatory bowel disease. J Clin Invest 117: 514–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Xie J, Itzkowitz SH (2008) Cancer in inflammatory bowel disease. World J Gastroenterol 14: 378–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Drossman DA, Dumitrascu DL (2006) Rome III: New standard for functional gastrointestinal disorders. J Gastrointestin Liver Dis 15: 237–241. [PubMed] [Google Scholar]
- 4. Bonaz B (2013) Inflammatory bowel diseases: a dysfunction of brain-gut interactions? Minerva Gastroenterol Dietol 59: 241–259. [PubMed] [Google Scholar]
- 5. Long MD, Drossman DA (2010) Inflammatory bowel disease, irritable bowel syndrome, or what?: A challenge to the functional-organic dichotomy. Am J Gastroenterol 105: 1796–1798. [DOI] [PubMed] [Google Scholar]
- 6. Grover M, Herfarth H, Drossman DA (2009) The functional-organic dichotomy: postinfectious irritable bowel syndrome and inflammatory bowel disease-irritable bowel syndrome. Clin Gastroenterol Hepatol 7: 48–53. [DOI] [PubMed] [Google Scholar]
- 7. Kovacs Z, Kovacs F (2007) Depressive and anxiety symptoms, dysfunctional attitudes and social aspects in irritable bowel syndrome and inflammatory bowel disease. Int J Psychiatry Med 37: 245–255. [DOI] [PubMed] [Google Scholar]
- 8. Bitton A, Dobkin PL, Edwardes MD, Sewitch MJ, Meddings JB, et al. (2008) Predicting relapse in Crohn’s disease: a biopsychosocial model. Gut 57: 1386–1392. [DOI] [PubMed] [Google Scholar]
- 9. Bonaz BL, Bernstein CN (2013) Brain-gut interactions in inflammatory bowel disease. Gastroenterology 144: 36–49. [DOI] [PubMed] [Google Scholar]
- 10. Pellissier S, Dantzer C, Canini F, Mathieu N, Bonaz B (2010) Psychological adjustment and autonomic disturbances in inflammatory bowel diseases and irritable bowel syndrome. Psychoneuroendocrinology 35: 653–662. [DOI] [PubMed] [Google Scholar]
- 11. Peters A, McEwen BS (2012) Introduction for the allostatic load special issue. Physiol Behav 106: 1–4. [DOI] [PubMed] [Google Scholar]
- 12. McEwen BS, Wingfield JC (2010) What is in a name? Integrating homeostasis, allostasis and stress. Horm Behav 57: 105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McEwen BS, Wingfield JC (2003) The concept of allostasis in biology and biomedicine. Horm Behav 43: 2–15. [DOI] [PubMed] [Google Scholar]
- 14. Holzer P, Schicho R, Holzer-Petsche U, Lippe IT (2001) The gut as a neurological organ. Wien Klin Wochenschr 113: 647–660. [PubMed] [Google Scholar]
- 15. Tran BW, Papoiu AD, Russoniello CV, Wang H, Patel TS, et al. (2010) Effect of itch, scratching and mental stress on autonomic nervous system function in atopic dermatitis. Acta Derm Venereol 90: 354–361. [DOI] [PubMed] [Google Scholar]
- 16. Porges SW (1992) Vagal tone: a physiologic marker of stress vulnerability. Pediatrics 90: 498–504. [PubMed] [Google Scholar]
- 17. Goehler LE, Lyte M, Gaykema RP (2007) Infection-induced viscerosensory signals from the gut enhance anxiety: implications for psychoneuroimmunology. Brain Behav Immun 21: 721–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Weber CS, Thayer JF, Rudat M, Wirtz PH, Zimmermann-Viehoff F, et al. (2010) Low vagal tone is associated with impaired post stress recovery of cardiovascular, endocrine, and immune markers. Eur J Appl Physiol 109: 201–211. [DOI] [PubMed] [Google Scholar]
- 19. Pavlov VA, Tracey KJ (2006) Controlling inflammation: the cholinergic anti-inflammatory pathway. Biochem Soc Trans 34: 1037–1040. [DOI] [PubMed] [Google Scholar]
- 20. Galvis G, Lips KS, Kummer W (2006) Expression of nicotinic acetylcholine receptors on murine alveolar macrophages. J Mol Neurosci 30: 107–108. [DOI] [PubMed] [Google Scholar]
- 21. Pavlov VA, Wang H, Czura CJ, Friedman SG, Tracey KJ (2003) The cholinergic anti-inflammatory pathway: a missing link in neuroimmunomodulation. Mol Med 9: 125–134. [PMC free article] [PubMed] [Google Scholar]
- 22. Lichtenstein GR (2013) Comprehensive review: antitumor necrosis factor agents in inflammatory bowel disease and factors implicated in treatment response. Therap Adv Gastroenterol 6: 269–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Thayer JF, Sternberg E (2006) Beyond heart rate variability: vagal regulation of allostatic systems. Ann N Y Acad Sci 1088: 361–372. [DOI] [PubMed] [Google Scholar]
- 24. Thayer JF, Hall M, Sollers JJ, Fischer JE (2006) Alcohol use, urinary cortisol, and heart rate variability in apparently healthy men: Evidence for impaired inhibitory control of the HPA axis in heavy drinkers. Int J Psychophysiol 59: 244–250. [DOI] [PubMed] [Google Scholar]
- 25. McEwen BS (1998) Stress, Adaptation, and Disease: Allostasis and Allostatic Load. Annals of the New York Academy of Sciences 840: 33–44. [DOI] [PubMed] [Google Scholar]
- 26. Boisse L, Chisholm SP, Lukewich MK, Lomax AE (2009) Clinical and experimental evidence of sympathetic neural dysfunction during inflammatory bowel disease. Clin Exp Pharmacol Physiol 36: 1026–1033. [DOI] [PubMed] [Google Scholar]
- 27. Satsangi J, Silverberg MS, Vermeire S, Colombel JF (2006) The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut 55: 749–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Harvey RF, Bradshaw JM (1980) A simple index of Crohn’s disease activity. Lancet 1: 514. [DOI] [PubMed] [Google Scholar]
- 29. Thompson WG, Longstreth GF, Drossman DA, Heaton KW, Irvine EJ, et al. (1999) Functional bowel disorders and functional abdominal pain. Gut 45: 43–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spielberger CD, Gorsuch RL, Lushene R, Vaag PR, Jacobs GA (1983) Manual for State-Trait Anxiety Inventory (STAI). Alto P, editor: Consulting Psychologists Press Inc.
- 31. Radloff LS (1977) The CES-D scale: a self-report depression scale for research in the general population. Applied Psychological Measurement 3: 385–401. [Google Scholar]
- 32. Fuhrer R, Rouillon F (1989) La version française de l’échelle CES-D (Center for Epidemiologic Studies-Depression Scale). Description et traduction de l’échelle d’autoévaluation Psychiatry and Psychobiology 4: 163–166. [Google Scholar]
- 33. Lombardi F, Malliani A, Pagani M, Cerutti S (1996) Heart rate variability and its sympatho-vagal modulation. Cardiovascular Research 32: 208–216. [DOI] [PubMed] [Google Scholar]
- 34. Akselrod S, Gordon D, Ubel FA, Shannon DC, Barger AC, et al. (1981) Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science 213: 220–222. [DOI] [PubMed] [Google Scholar]
- 35. Jarrett ME, Burr RL, Cain KC, Rothermel JD, Landis CA, et al. (2008) Autonomic nervous system function during sleep among women with irritable bowel syndrome. Dig Dis Sci 53: 694–703. [DOI] [PubMed] [Google Scholar]
- 36. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 93: 1043–1065. [PubMed] [Google Scholar]
- 37. Billman GE (2013) The effect of heart rate on the heart rate variability response to autonomic interventions. Front Physiol 4: 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Billman GE (2013) The LF/HF ratio does not accurately measure cardiac sympatho-vagal balance. Front Physiol 4: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yeragani VK, Sobolewski E, Igel G, Johnson C, Jampala VC, et al. (1998) Decreased heart-period variability in patients with panic disorder: a study of Holter ECG records. Psychiatry Research 78: 89–99. [DOI] [PubMed] [Google Scholar]
- 40. Reyes del Paso GA, Langewitz W, Mulder LJ, van Roon A, Duschek S (2013) The utility of low frequency heart rate variability as an index of sympathetic cardiac tone: a review with emphasis on a reanalysis of previous studies. Psychophysiology 50: 477–487. [DOI] [PubMed] [Google Scholar]
- 41. Hue O, Le Gallais D, Boussana A, Galy O, Chamari K, et al. (2000) Catecholamine, blood lactate and ventilatory responses to multi-cycle-run blocks. Med Sci Sports Exerc 32: 1582–1586. [DOI] [PubMed] [Google Scholar]
- 42.Bruchon-Schweitzer M, Paulhan I (1993) Manuel de l’inventaire d’anxiété état-trait forme Y (STAI-Y). Adapté par Bruchon-Schweitzer et Paulhan; ECPA, editor. Paris.
- 43.Nunnally JC (1978) Psychometric theory; Hill M, editor. New York.
- 44. Thayer JF, Lane RD (2009) Claude Bernard and the heart-brain connection: further elaboration of a model of neurovisceral integration. Neurosci Biobehav Rev 33: 81–88. [DOI] [PubMed] [Google Scholar]
- 45. Benarroch EE (1993) The central autonomic network: functional organization, dysfunction, and perspective. Mayo Clin Proc 68: 988–1001. [DOI] [PubMed] [Google Scholar]
- 46.Bonaz B, Pellissier S, Sinniger V, Clarençon D, Peinnequin A, et al.. (2012) The irritable bowel syndrome: how stress can affect the amygdala activity and the brain-gut axis. In: Ferry DB, editor. The Amygdala - A Discrete Multitasking Manager: InTech.
- 47. Kong L, Chen K, Tang Y, Wu F, Driesen N, et al. (2013) Functional connectivity between the amygdala and prefrontal cortex in medication-naive individuals with major depressive disorder. J Psychiatry Neurosci 38: 417–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Prater KE, Hosanagar A, Klumpp H, Angstadt M, Phan KL (2013) Aberrant amygdala-frontal cortex connectivity during perception of fearful faces and at rest in generalized social anxiety disorder. Depress Anxiety 30: 234–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Brown GL, McGarvey EL, Shirtcliff EA, Keller A, Granger DA, et al. (2008) Salivary cortisol, dehydroepiandrosterone, and testosterone interrelationships in healthy young males: a pilot study with implications for studies of aggressive behavior. Psychiatry Res 159: 67–76. [DOI] [PubMed] [Google Scholar]
- 50. Shinkai S, Watanabe S, Kurokawa Y, Torii J (1993) Salivary cortisol for monitoring circadian rhythm variation in adrenal activity during shiftwork. Int Arch Occup Environ Health 64: 499–502. [DOI] [PubMed] [Google Scholar]
- 51. Bosani M, Ardizzone S, Porro GB (2009) Biologic targeting in the treatment of inflammatory bowel diseases. Biologics 3: 77–97. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 52. Kmiec Z (1998) Cytokines in inflammatory bowel disease. Arch Immunol Ther Exp (Warsz) 46: 143–155. [PubMed] [Google Scholar]
- 53. Patil SA, Rustgi A, Langenberg P, Cross RK (2013) Comparative effectiveness of anti-TNF agents for Crohn’s disease in a tertiary referral IBD practice. Dig Dis Sci 58: 209–215. [DOI] [PubMed] [Google Scholar]
- 54. Altavilla D, Guarini S, Bitto A, Mioni C, Giuliani D, et al. (2006) Activation of the cholinergic anti-inflammatory pathway reduces NF-kappab activation, blunts TNF-alpha production, and protects againts splanchic artery occlusion shock. Shock 25: 500–506. [DOI] [PubMed] [Google Scholar]
- 55. Bonaz B, Picq C, Sinniger V, Mayol JF, Clarencon D (2013) Vagus nerve stimulation: from epilepsy to the cholinergic anti-inflammatory pathway. Neurogastroenterol Motil 25: 208–221. [DOI] [PubMed] [Google Scholar]
- 56. Meregnani J, Clarencon D, Vivier M, Peinnequin A, Mouret C, et al. (2011) Anti-inflammatory effect of vagus nerve stimulation in a rat model of inflammatory bowel disease. Auton Neurosci 160: 82–89. [DOI] [PubMed] [Google Scholar]
- 57. Nikolic VN, Jevtovic-Stoimenov T, Stokanovic D, Milovanovic M, Velickovic-Radovanovic R, et al. (2013) An inverse correlation between TNF alpha serum levels and heart rate variability in patients with heart failure. J Cardiol 62: 37–43. [DOI] [PubMed] [Google Scholar]
- 58. Huston JM, Tracey KJ (2011) The pulse of inflammation: heart rate variability, the cholinergic anti-inflammatory pathway and implications for therapy. J Intern Med 269: 45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Papaioannou V, Pneumatikos I, Maglaveras N (2013) Association of heart rate variability and inflammatory response in patients with cardiovascular diseases: current strengths and limitations. Front Physiol 4: 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Saul JP, Arai Y, Berger RD, Lilly LS, Colucci WS, et al. (1988) Assessment of autonomic regulation in chronic congestive heart failure by heart rate spectral analysis. Am J Cardiol 61: 1292–1299. [DOI] [PubMed] [Google Scholar]
- 61. Taylor JA, Carr DL, Myers CW, Eckberg DL (1998) Mechanisms underlying very-low-frequency RR-interval oscillations in humans. Circulation 98: 547–555. [DOI] [PubMed] [Google Scholar]
- 62. Ponikowski P, Chua TP, Amadi AA, Piepoli M, Harrington D, et al. (1996) Detection and significance of a discrete very low frequency rhythm in RR interval variability in chronic congestive heart failure. Am J Cardiol 77: 1320–1326. [DOI] [PubMed] [Google Scholar]
- 63. Nemoto W, Nakagawasai O, Yaoita F, Kanno SI, Yomogida S, et al. (2013) Angiotensin II produces nociceptive behavior through spinal AT1 receptor-mediated p38 mitogen-activated protein kinase activation in mice. Mol Pain 9: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Garg M, Burrell LM, Velkoska E, Griggs K, Angus PW, et al.. (2014) Upregulation of circulating components of the alternative renin-angiotensin system in inflammatory bowel disease: A pilot study. J Renin Angiotensin Aldosterone Syst. [DOI] [PubMed]
- 65. Elsenbruch S, Rosenberger C, Enck P, Forsting M, Schedlowski M, et al. (2010) Affective disturbances modulate the neural processing of visceral pain stimuli in irritable bowel syndrome: an fMRI study. Gut 59: 10.1136/gut.2008.175000. [DOI] [PubMed] [Google Scholar]
- 66. Garakani A, Win T, Virk S, Gupta S, Kaplan D, et al. (2003) Comorbidity of irritable bowel syndrome in psychiatric patients: a review. Am J Ther 10: 61–67. [DOI] [PubMed] [Google Scholar]
- 67. Pellissier S, Dantzer C, Fichou C, Bonaz B (2007) Heart Rate Variability as a marker of stress in inflammatory bowel diseases AGA Institute abstracts. Gastroenterology 132: A–1-A-727. [Google Scholar]
- 68. FitzGerald LZ, Kehoe P, Sinha K (2009) Hypothalamic–pituitary– adrenal axis dysregulation in women with irritable bowel syndrome in response to acute physical stress. West J Nurs Res 31: 818–836. [DOI] [PubMed] [Google Scholar]
- 69. Burr RL, Jarrett ME, Cain KC, Jun SE, Heitkemper MM (2009) Catecholamine and cortisol levels during sleep in women with irritable bowel syndrome. Neurogastroenterol Motil 21: 1148–e1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Vedhara K, Miles J, Bennett P, Plummer S, Tallon D, et al. (2003) An investigation into the relationship between salivary cortisol, stress, anxiety and depression. Biol Psychol 62: 89–96. [DOI] [PubMed] [Google Scholar]
- 71. Richard PS, McCreath H, Tracey KJ, Sidney S, Liu K, et al. (2007) RR interval variability is inversely related to inflammatory markers: The CARDIA Study. Mol Med 13: 178–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Joo HM, Lee SJ, Chung YG, Shin IY (2010) Effects of mindfulness based stress reduction program on depression, anxiety and stress in patients with aneurysmal subarachnoid hemorrhage. J Korean Neurosurg Soc 47: 345–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that, for approved reasons, some access restrictions apply to the data underlying the findings. Two additional papers related to this study have to be written and we wouldn’t like to make public right now all the details of the study while the data are not published yet. However, as far as the data of the current manuscript are concerned, data are from the 817-Protocol study whose authors may be contacted at sonia.pellissier@univ-savoie.fr.