Abstract
Within the central nervous system (CNS) ciliary neurotrophic factor (CNTF) is expressed by astrocytes where it remains stored as an intracellular protein; its release and function as an extracellular ligand are thought to occur in the event of cellular injury. We find that overexpression of CNTF in transgenic mice recapitulates the glial response to CNS lesion, as does its injection into the uninjured brain. These results demonstrate that CNTF functions as an inducer of reactive gliosis, a condition associated with a number of neurological diseases of the CNS.
Full text
PDF

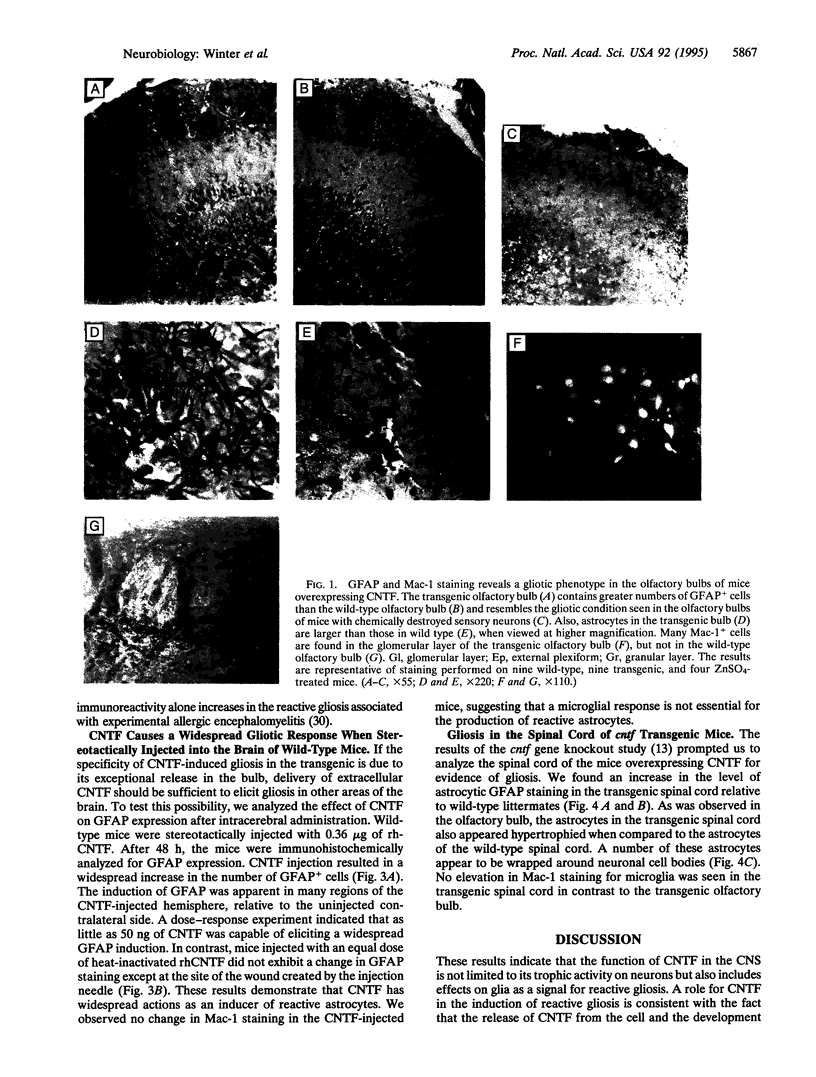
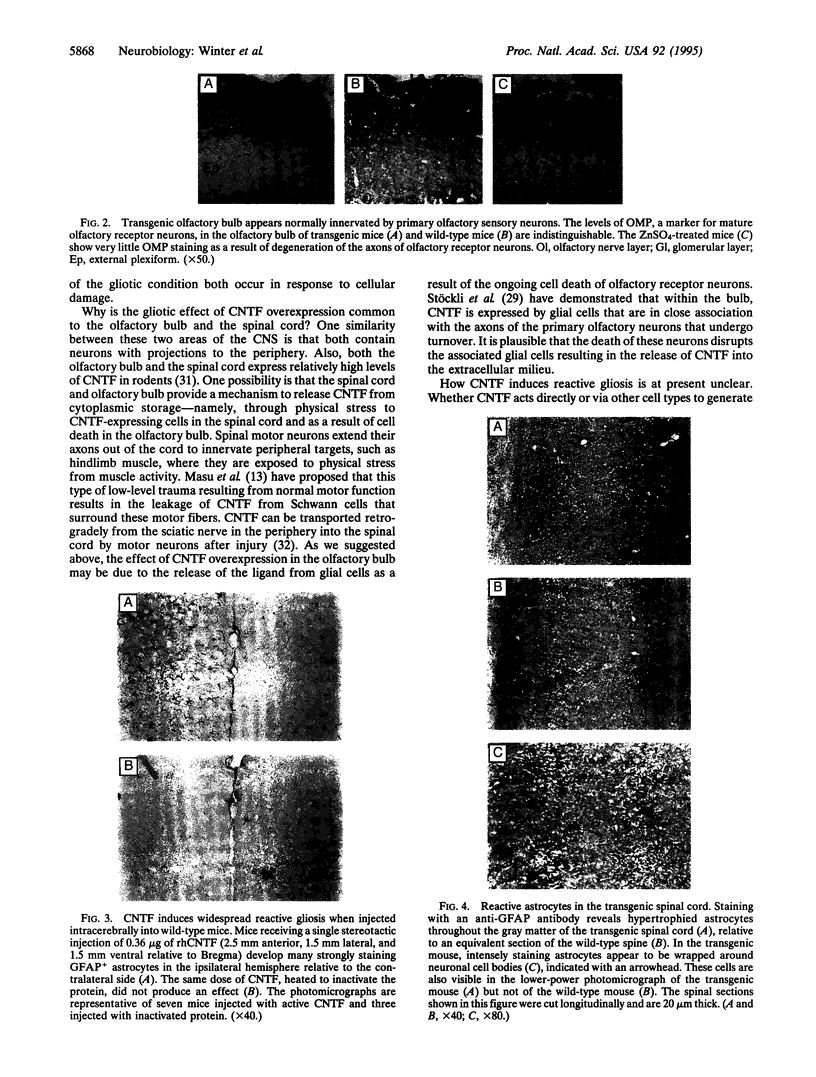

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arakawa Y., Sendtner M., Thoenen H. Survival effect of ciliary neurotrophic factor (CNTF) on chick embryonic motoneurons in culture: comparison with other neurotrophic factors and cytokines. J Neurosci. 1990 Nov;10(11):3507–3515. doi: 10.1523/JNEUROSCI.10-11-03507.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbin G., Manthorpe M., Varon S. Purification of the chick eye ciliary neuronotrophic factor. J Neurochem. 1984 Nov;43(5):1468–1478. doi: 10.1111/j.1471-4159.1984.tb05410.x. [DOI] [PubMed] [Google Scholar]
- Bignami A., Eng L. F., Dahl D., Uyeda C. T. Localization of the glial fibrillary acidic protein in astrocytes by immunofluorescence. Brain Res. 1972 Aug 25;43(2):429–435. doi: 10.1016/0006-8993(72)90398-8. [DOI] [PubMed] [Google Scholar]
- Cammer W., Tansey F., Abramovitz M., Ishigaki S., Listowsky I. Differential localization of glutathione-S-transferase Yp and Yb subunits in oligodendrocytes and astrocytes of rat brain. J Neurochem. 1989 Mar;52(3):876–883. doi: 10.1111/j.1471-4159.1989.tb02536.x. [DOI] [PubMed] [Google Scholar]
- Curtis R., Adryan K. M., Zhu Y., Harkness P. J., Lindsay R. M., DiStefano P. S. Retrograde axonal transport of ciliary neurotrophic factor is increased by peripheral nerve injury. Nature. 1993 Sep 16;365(6443):253–255. doi: 10.1038/365253a0. [DOI] [PubMed] [Google Scholar]
- Eddleston M., Mucke L. Molecular profile of reactive astrocytes--implications for their role in neurologic disease. Neuroscience. 1993 May;54(1):15–36. doi: 10.1016/0306-4522(93)90380-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genter M. B., Llorens J., O'Callaghan J. P., Peele D. B., Morgan K. T., Crofton K. M. Olfactory toxicity of beta,beta'-iminodipropionitrile in the rat. J Pharmacol Exp Ther. 1992 Dec;263(3):1432–1439. [PubMed] [Google Scholar]
- Giulian D. Ameboid microglia as effectors of inflammation in the central nervous system. J Neurosci Res. 1987;18(1):155-71, 132-3. doi: 10.1002/jnr.490180123. [DOI] [PubMed] [Google Scholar]
- Hagg T., Varon S. Ciliary neurotrophic factor prevents degeneration of adult rat substantia nigra dopaminergic neurons in vivo. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6315–6319. doi: 10.1073/pnas.90.13.6315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding J. W., Getchell T. V., Margolis F. L. Denervation of the primary olfactory pathway in mice. V. Long-term effect of intranasal ZnSO4 irrigation on behavior, biochemistry and morphology. Brain Res. 1978 Jan 27;140(2):271–285. doi: 10.1016/0006-8993(78)90460-2. [DOI] [PubMed] [Google Scholar]
- Ip N. Y., Li Y. P., van de Stadt I., Panayotatos N., Alderson R. F., Lindsay R. M. Ciliary neurotrophic factor enhances neuronal survival in embryonic rat hippocampal cultures. J Neurosci. 1991 Oct;11(10):3124–3134. doi: 10.1523/JNEUROSCI.11-10-03124.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip N. Y., McClain J., Barrezueta N. X., Aldrich T. H., Pan L., Li Y., Wiegand S. J., Friedman B., Davis S., Yancopoulos G. D. The alpha component of the CNTF receptor is required for signaling and defines potential CNTF targets in the adult and during development. Neuron. 1993 Jan;10(1):89–102. doi: 10.1016/0896-6273(93)90245-m. [DOI] [PubMed] [Google Scholar]
- Lillien L. E., Raff M. C. Differentiation signals in the CNS: type-2 astrocyte development in vitro as a model system. Neuron. 1990 Aug;5(2):111–119. doi: 10.1016/0896-6273(90)90301-u. [DOI] [PubMed] [Google Scholar]
- Lin L. F., Mismer D., Lile J. D., Armes L. G., Butler E. T., 3rd, Vannice J. L., Collins F. Purification, cloning, and expression of ciliary neurotrophic factor (CNTF). Science. 1989 Nov 24;246(4933):1023–1025. doi: 10.1126/science.2587985. [DOI] [PubMed] [Google Scholar]
- Louis J. C., Magal E., Takayama S., Varon S. CNTF protection of oligodendrocytes against natural and tumor necrosis factor-induced death. Science. 1993 Jan 29;259(5095):689–692. doi: 10.1126/science.8430320. [DOI] [PubMed] [Google Scholar]
- Magal E., Burnham P., Varon S. Effects of ciliary neuronotrophic factor on rat spinal cord neurons in vitro: survival and expression of choline acetyltransferase and low-affinity nerve growth factor receptors. Brain Res Dev Brain Res. 1991 Nov 19;63(1-2):141–150. doi: 10.1016/0165-3806(91)90074-s. [DOI] [PubMed] [Google Scholar]
- Margolis F. L. Carnosine in the primary olfactory pathway. Science. 1974 May 24;184(4139):909–911. doi: 10.1126/science.184.4139.909. [DOI] [PubMed] [Google Scholar]
- Masu Y., Wolf E., Holtmann B., Sendtner M., Brem G., Thoenen H. Disruption of the CNTF gene results in motor neuron degeneration. Nature. 1993 Sep 2;365(6441):27–32. doi: 10.1038/365027a0. [DOI] [PubMed] [Google Scholar]
- McDonald J. R., Ko C., Mismer D., Smith D. J., Collins F. Expression and characterization of recombinant human ciliary neurotrophic factor from Escherichia coli. Biochim Biophys Acta. 1991 Aug 27;1090(1):70–80. doi: 10.1016/0167-4781(91)90038-n. [DOI] [PubMed] [Google Scholar]
- Norton W. T., Aquino D. A., Hozumi I., Chiu F. C., Brosnan C. F. Quantitative aspects of reactive gliosis: a review. Neurochem Res. 1992 Sep;17(9):877–885. doi: 10.1007/BF00993263. [DOI] [PubMed] [Google Scholar]
- Perry V. H., Hume D. A., Gordon S. Immunohistochemical localization of macrophages and microglia in the adult and developing mouse brain. Neuroscience. 1985 Jun;15(2):313–326. doi: 10.1016/0306-4522(85)90215-5. [DOI] [PubMed] [Google Scholar]
- Rudge J. S., Li Y., Pasnikowski E. M., Mattsson K., Pan L., Yancopoulos G. D., Wiegand S. J., Lindsay R. M., Ip N. Y. Neurotrophic factor receptors and their signal transduction capabilities in rat astrocytes. Eur J Neurosci. 1994 May 1;6(5):693–705. doi: 10.1111/j.1460-9568.1994.tb00981.x. [DOI] [PubMed] [Google Scholar]
- Saotome Y., Winter C. G., Hirsh D. A widely expressed novel C2H2 zinc-finger protein with multiple consensus phosphorylation sites is conserved in mouse and man. Gene. 1995 Jan 23;152(2):233–238. doi: 10.1016/0378-1119(94)00717-7. [DOI] [PubMed] [Google Scholar]
- Sendtner M., Kreutzberg G. W., Thoenen H. Ciliary neurotrophic factor prevents the degeneration of motor neurons after axotomy. Nature. 1990 May 31;345(6274):440–441. doi: 10.1038/345440a0. [DOI] [PubMed] [Google Scholar]
- Sendtner M., Schmalbruch H., Stöckli K. A., Carroll P., Kreutzberg G. W., Thoenen H. Ciliary neurotrophic factor prevents degeneration of motor neurons in mouse mutant progressive motor neuronopathy. Nature. 1992 Aug 6;358(6386):502–504. doi: 10.1038/358502a0. [DOI] [PubMed] [Google Scholar]
- Sendtner M., Stöckli K. A., Thoenen H. Synthesis and localization of ciliary neurotrophic factor in the sciatic nerve of the adult rat after lesion and during regeneration. J Cell Biol. 1992 Jul;118(1):139–148. doi: 10.1083/jcb.118.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. E., Somera F. P., Eng L. F. Immunocytochemical staining for glial fibrillary acidic protein and the metabolism of cytoskeletal proteins in experimental allergic encephalomyelitis. Brain Res. 1983 Apr 4;264(2):241–253. doi: 10.1016/0006-8993(83)90822-3. [DOI] [PubMed] [Google Scholar]
- Stöckli K. A., Lillien L. E., Näher-Noé M., Breitfeld G., Hughes R. A., Raff M. C., Thoenen H., Sendtner M. Regional distribution, developmental changes, and cellular localization of CNTF-mRNA and protein in the rat brain. J Cell Biol. 1991 Oct;115(2):447–459. doi: 10.1083/jcb.115.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stöckli K. A., Lottspeich F., Sendtner M., Masiakowski P., Carroll P., Götz R., Lindholm D., Thoenen H. Molecular cloning, expression and regional distribution of rat ciliary neurotrophic factor. Nature. 1989 Dec 21;342(6252):920–923. doi: 10.1038/342920a0. [DOI] [PubMed] [Google Scholar]
- Verhaagen J., Oestreicher A. B., Grillo M., Khew-Goodall Y. S., Gispen W. H., Margolis F. L. Neuroplasticity in the olfactory system: differential effects of central and peripheral lesions of the primary olfactory pathway on the expression of B-50/GAP43 and the olfactory marker protein. J Neurosci Res. 1990 May;26(1):31–44. doi: 10.1002/jnr.490260105. [DOI] [PubMed] [Google Scholar]







