Abstract
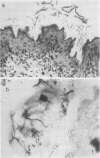
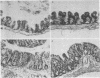
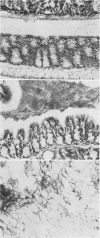
Strictly anaerobic and facultatively anaerobic bacteria were isolated from the intestinal tract of normal mice. Germ-free mice were associated with mixtures of varying complexity of pure cultures of these bacteria. The development of normal features in these animals was then determined on the basis of the following criteria: (i) size of the cecum, (ii) size of the Escherichia coli population in the cecum, (iii) histology of the intestinal tract, and (iv) development of a mucosa-associated flora in stomach and large intestine. Germ-free mice contaminated with cecal contents from conventional mice were used as controls to establish normal values for these parameters. Some strictly anaerobic bacteria could be implanted into germ-free mice only after prior implantation of an E. coli strain. E. coli was found in large numbers in stomach and cecum of mice monocontaminated with this organism. Use of restraining devices indicated that the E. coli population in the stomach was maintained by coprophagy and did not contribute significantly to the size of the cecal population. A mixture of 50 strictly anaerobic bacteria plus 80 facultative anaerobes rendered recipient animals normal with respect to the criteria tested. Other, less complex bacterial mixtures reduced the cecal size and the intestinal E. coli population to levels intermediate between those found in normal and germ-free mice. With all bacterial mixtures tested, the intestinal E. coli population decreased, if at all, within a period of about 10 days after introduction of other bacteria, and remained stable thereafter. This suggests that the intestinal E. coli population is controlled by a mechanism which reduces population size without affecting the growth rate.
Full text
PDF


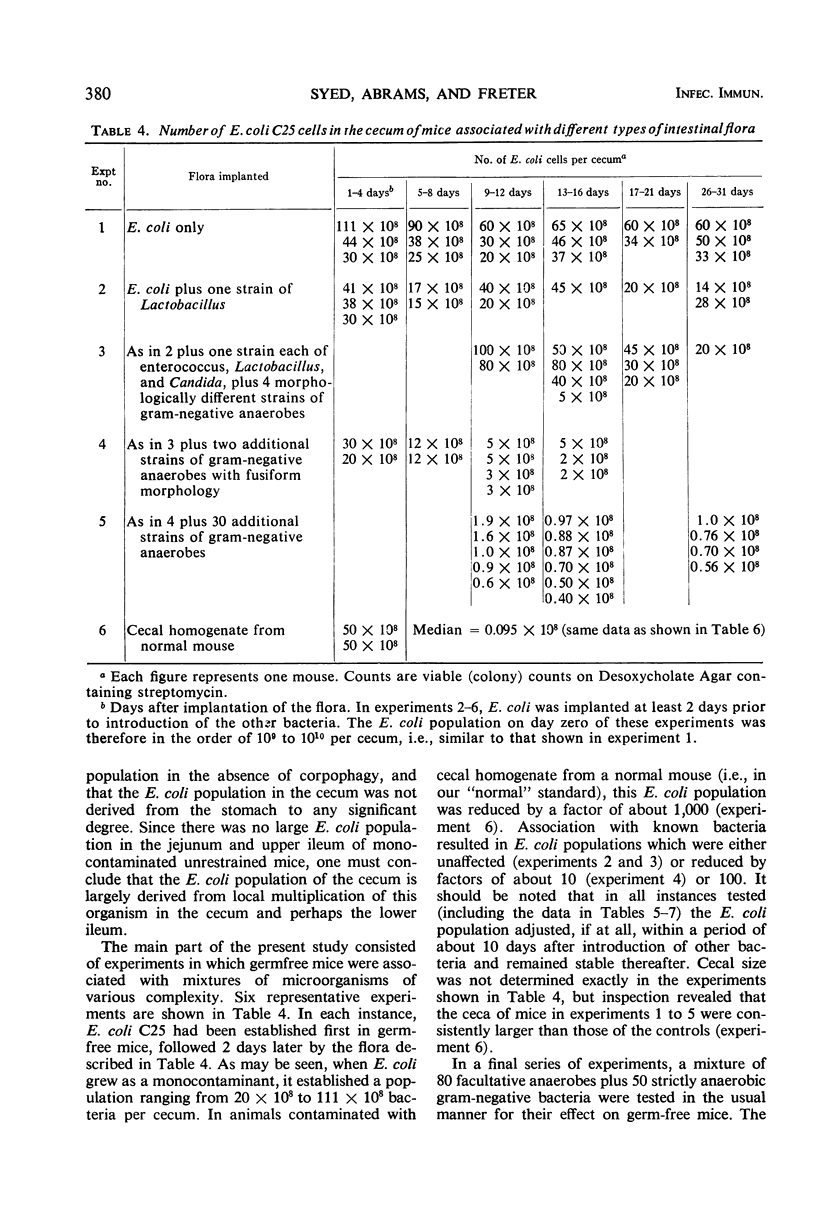
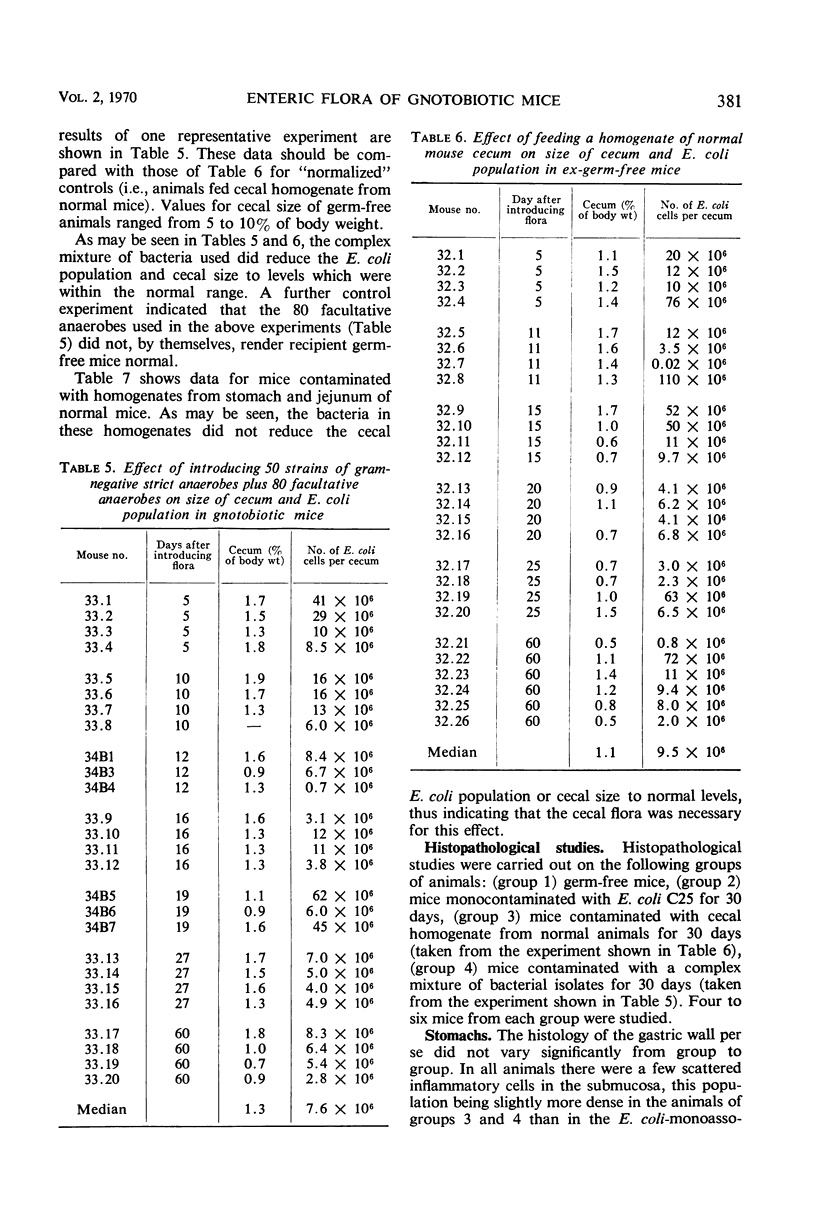
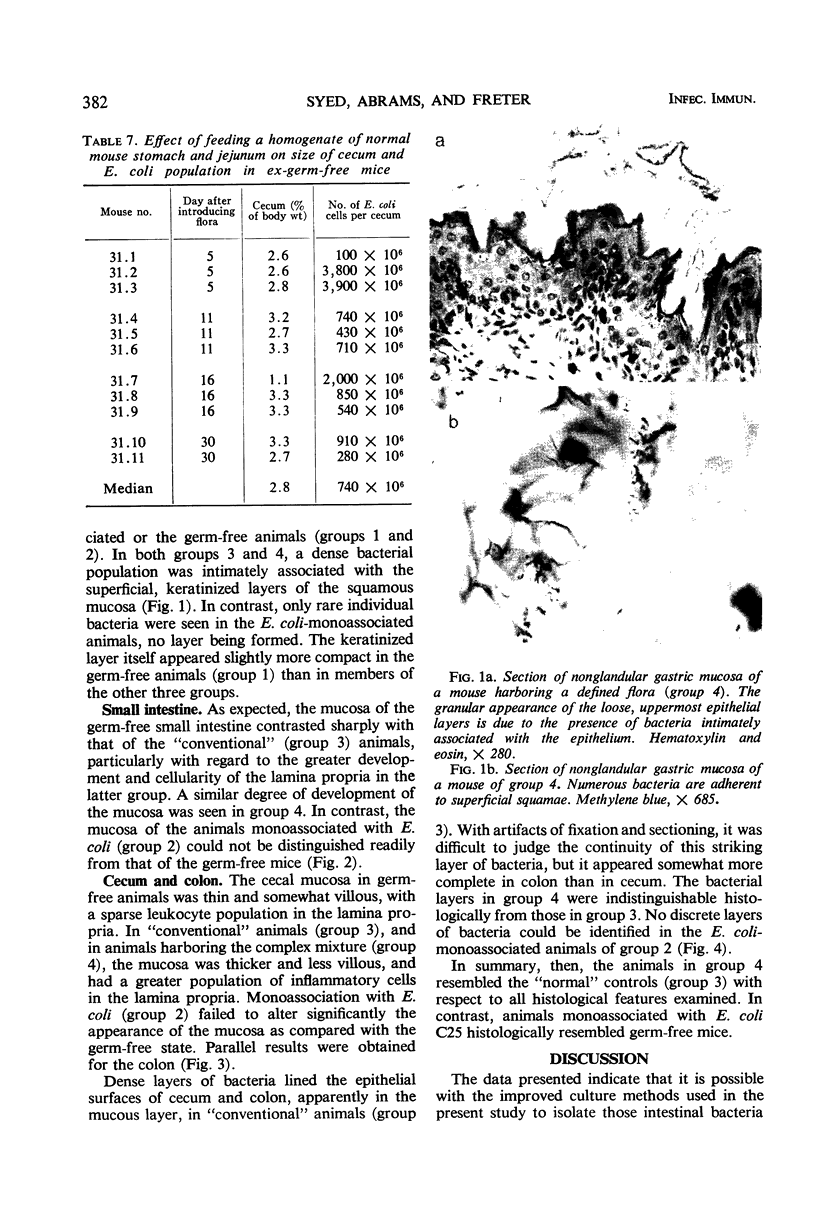
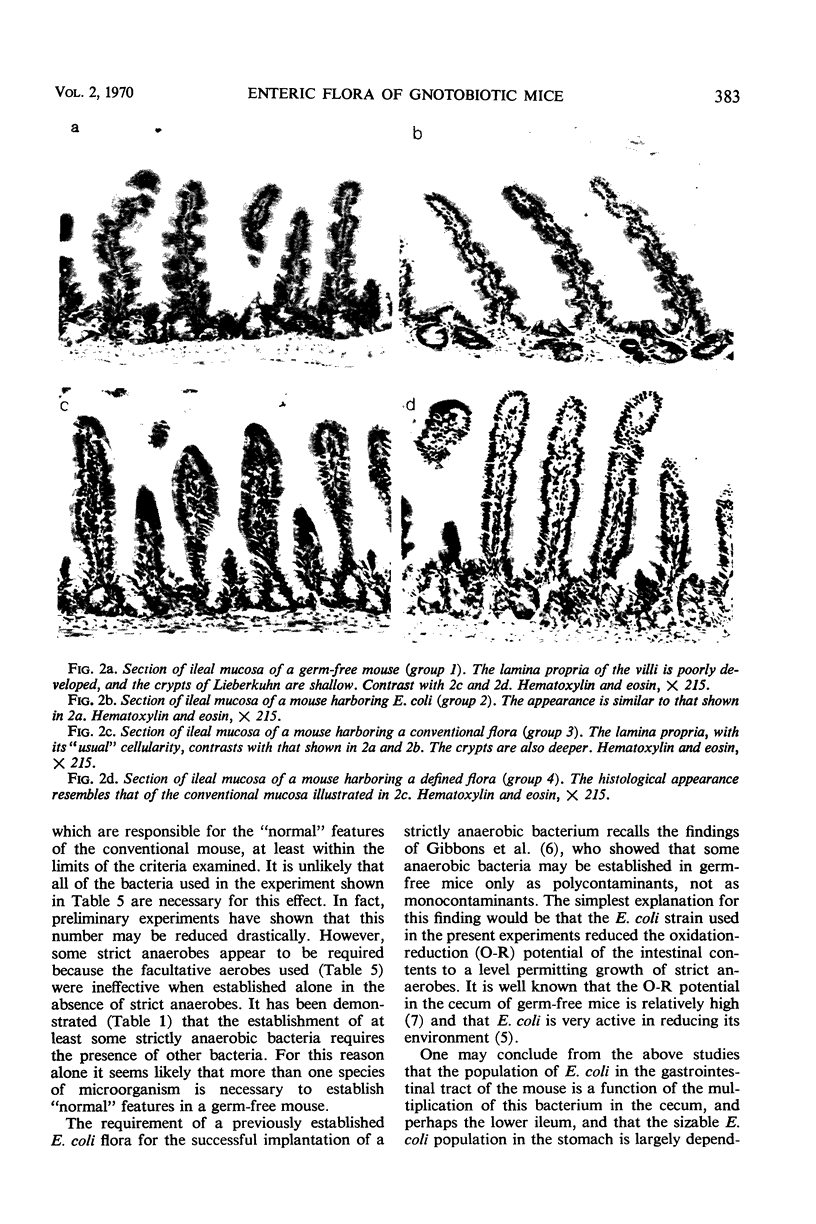


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams G. D., Bishop J. E. Effect of the normal microbial flora on gastrointestinal motility. Proc Soc Exp Biol Med. 1967 Oct;126(1):301–304. doi: 10.3181/00379727-126-32430. [DOI] [PubMed] [Google Scholar]
- Arank A., Syed S. A., Kenney E. B., Freter R. Isolation of anaerobic bacteria from human gingiva and mouse cecum by means of a simplified glove box procedure. Appl Microbiol. 1969 Apr;17(4):568–576. doi: 10.1128/am.17.4.568-576.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DONALDSON R. M., Jr NORMAL BACTERIAL POPULATIONS OF THE INTESTINE AND THEIR RELATION TO INTESTINAL FUNCTION. N Engl J Med. 1964 Apr 30;270:938–CONTD. doi: 10.1056/NEJM196404302701806. [DOI] [PubMed] [Google Scholar]
- DUBOS R., SCHAEDLER R. W., COSTELLO R., HOET P. INDIGENOUS, NORMAL, AND AUTOCHTHONOUS FLORA OF THE GASTROINTESTINAL TRACT. J Exp Med. 1965 Jul 1;122:67–76. doi: 10.1084/jem.122.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRETER R. In vivo and in vitro antagonism of intestinal bacteria against Shigellaflexneri. II. The inhibitory mechanism. J Infect Dis. 1962 Jan-Feb;110:38–46. doi: 10.1093/infdis/110.1.38. [DOI] [PubMed] [Google Scholar]
- GIBBONS R. J., SOCRANSKY S. S., KAPSIMALIS B. ESTABLISHMENT OF HUMAN INDIGENOUS BACTERIA IN GERM-FREE MICE. J Bacteriol. 1964 Nov;88:1316–1323. doi: 10.1128/jb.88.5.1316-1323.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEYNELL G. G. Antibacterial mechanisms of the mouse gut. II. The role of Eh and volatile fatty acids in the normal gut. Br J Exp Pathol. 1963 Apr;44:209–219. [PMC free article] [PubMed] [Google Scholar]
- MILLER C. P., BOHNHOFF M. CHANGES IN THE MOUSE'S ENTERIC MICROFLORA ASSOCIATED WITH ENHANCED SUSCEPTIBILITY TO SALMONELLA INFECTION FOLLOWING STREPTOMYCIN TREATMENT. J Infect Dis. 1963 Jul-Aug;113:59–66. doi: 10.1093/infdis/113.1.59. [DOI] [PubMed] [Google Scholar]
- Plaut A. G., Gorbach S. L., Nahas L., Weinstein L., Spanknebel G., Levitan R. Studies of intestinal microflora. 3. The microbial flora of human small intestinal mucosa and fluids. Gastroenterology. 1967 Dec;53(6):868–873. [PubMed] [Google Scholar]
- Savage D. C., Dubos R., Schaedler R. W. The gastrointestinal epithelium and its autochthonous bacterial flora. J Exp Med. 1968 Jan 1;127(1):67–76. doi: 10.1084/jem.127.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spears R. W., Freter R. Improved isolation of anaerobic bacteria from the mouse cecum by maintaining continuous strict anaerobiosis. Proc Soc Exp Biol Med. 1967 Mar;124(3):903–909. doi: 10.3181/00379727-124-31882. [DOI] [PubMed] [Google Scholar]