Abstract
Endometriosis is one of the most frequent benign gynecological disorders. Numerous studies have shown an association between GSTM1 and/or GSTT1 polymorphisms and endometriosis susceptibility. However, these associations remain inconclusive. To derive a more precise estimation, we conducted a comprehensive search to identify all existing studies and then performed a meta-analysis. Electronic literature searches of the PubMed, Chinese Biomedical, and China National Knowledge Infrastructure databases were performed up to December 2013. GSTM1-, GSTT1-, and dual-null genotypes were analyzed independently, and pooled odds ratios (ORs) with 95% confidence intervals (95% CIs) were calculated by comparing the null genotype with other genotypes using the random-effects or fixed-effects model. Twenty-five and 16 independent studies on GSTM1 and GSTT1 polymorphisms, respectively, and five GSTM1-GSTT1 interaction analyses were identified and included in this meta-analysis. Both GSTM1- and GSTT1-null genotypes increased risk of endometriosis (OR = 1.54, 95% CI: 1.30–1.83, P<0.001; OR = 1.41, 95% CI: 1.10–1.82, P = 0.007; respectively). Moreover, we found a significant positive association between the dual null genotype GSTM1-GSTT1 and endometriosis susceptibility (OR = 1.33, 95% CI: 1.03–1.72, P = 0.027). This meta-analysis provides evidence that null genotypes of GSTM1 and/or GSTT1 contribute to risk of endometriosis. Further investigations are required to confirm these findings.
Introduction
Endometriosis, a benign gynecological disease, is characterized by the presence of endometrial glands and stroma at extrauterine sites. Approximately 6–10% of women of reproductive age suffer from this condition [1], [2], which negatively impacts quality of life by causing pelvic pain, heavy menstrual flow, dysmenorrhea, and infertility [3], [4], making endometriosis a major public health threat.
The etiology and pathogenesis of endometriosis remain unclear. Endometriosis is commonly considered as a complex trait caused by the interaction between genetic and environmental factors [5]. Both genetic polymorphisms and environmental factors are considered risk factors for endometriosis [6], [7]. Even though no simple Mendelian inheritance was confirmed, geneticfactors increased the risk of endometriosis by 6% for near relatives [8].
Polymorphisms in the glutathione S-transferase (GST) system have long been recognized as a risk factor for endometriosis and have been extensively explored. Human GSTs are a multigene family of phase II metabolizing enzymes that are crucial in detoxification of xenobiotics such as carcinogens, environmental toxins and drugs [9]. Human cytosolic GSTs have been subdivided into eight distinct classes: alpha (GSTA), mu (GSTM), pi (GSTP), theta (GSTT), kappa (GSTK), zeta (GSTZ), omega (GSTO), and sigma (GSTS) [10]. Among the genes in the GST superfamily, GSTM1 (1p13.3, MIM 138350) [11] and GSTT1 (22q11.2, MIM 134660) [12] are the most extensively studied owing to their critical role in detoxification and the high-frequency of allelic variants. GSTM1 and GSTT1 polymorphisms have been suggested to be associated with endometriosis by many epidemiological studies [13]–[24]. However, findings on the direction of the association remain equivocal.
A previous meta-analysis conducted by Sun-Wei Guo [25] in 2005 indicated a significant association between the GSTT1-null genotype and endometriosis, however no such association was found between the GSTM1- or GSTM1-GSTT1-null genotypes and endometriosis risk. Since then, 12 relevant studies [5], [8], [19]–[24], [26]–[29] have further examined the associations between the two polymorphisms and endometriosis risk. We aimed to confirm the potential associations by conducting an updated meta-analysis, to provide insight into the pathophysiology of endometriosis.
Methods
Identification and eligibility of studies
Studies were identified by searching the PubMed, CBM (Chinese Biomedical), and CNKI (China National Knowledge Infrastructure) databases for relevant reports published prior to December 2013, using the key words “GSTM1”, “GSTT1”, “polymorphisms”, “endometriosis”, and combined phrases. Additional literature was collected from cross-references within both original and review articles. No restrictions on language, population, or sample size were set in this meta-analysis. Studies were required to comply with the following inclusion criteria: (1) original case-control or cohort studies; (2) studies investigated the association of GSTM1 or GSTT1 polymorphism with risk of endometriosis; (3) sufficient information to calculate odds ratios (ORs) with 95% confidence intervals (CIs); and (4) Chinese articles were published in Chinese core periodicals. Exclusion criteria were: (1) not case-control or cohort studies evaluating the association of the GSTM1 or GSTT1 polymorphism with endometriosis; (2) case reports, letters, reviews, editorials, or correspondence articles; (3) studies based on incomplete raw data; and (4) studies that contained overlapping data.
Data extraction
The data from eligible studies was independently checked and extracted according to the pre-specified selection criteria, and the discrepancies were resolved by discussion and agreement between all investigators. The following information was collected from each included study: name of the first author, publication year, study location, ethnicity, source of controls, sample-size of cases and controls, and genotype frequency in cases and controls. Different ethnicity descents were categorized as European and Asian. According to source of controls, all included studies were defined as population-based (PB) and hospital-based (HB).
Quality score assessment
Two investigators independently assessed the quality of included studies using the Newcastle–Ottawa Scale (NOS) [30]. The NOS ranges from zero to nine stars. Studies with a score of seven stars or greater were considered to be of high quality. Discrepancies were resolved as described above.
Statistical analysis
Meta-analyses were performed for polymorphisms investigated in at least three studies [31], [32]. The strength of the associations between GSTM1 and GSTT1 polymorphisms and endometriosis risk were measured by ORs and respective 95% CIs, which were calculated by comparing the null genotype with other genotypes. The significance of the pooled ORs was determined by the Z-test (P<0.05 was considered significant). Subgroup analyses were performed by ethnicity and source of controls.
Heterogeneity among studies in terms of degree of association was evaluated using χ2 tests. The I2 statistic was used to evaluate variations due to heterogeneity rather than chance. P<0.10 or I2>50% indicated the presence of between-study heterogeneity, and the random-effects model (DerSimonian-Laird method) [33] was used to calculate the pooled ORs; otherwise, the fixed- effects model (Mantel-Haenszel method) [34] was selected.
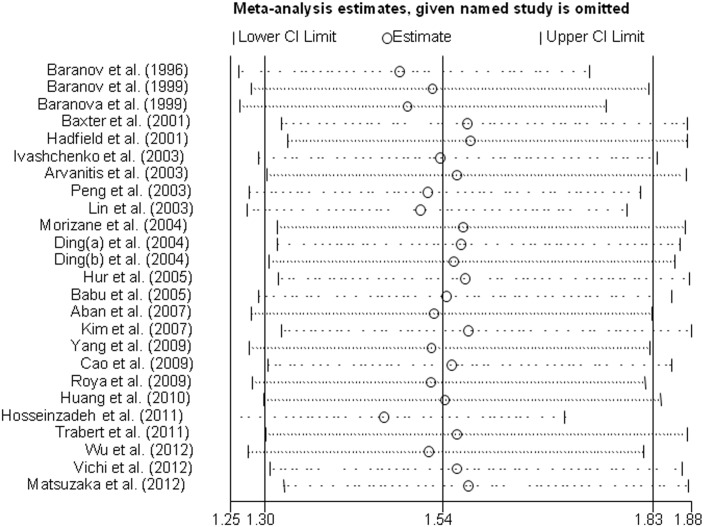
Sensitivity analyses were performed to evaluate the stability of the results of the meta-analysis. The influence of individual studies was evaluated by estimating the pooled ORs in the absence of each study [35]. Potential publication bias was investigated by visual inspection of Begg’s funnel plots. We also used the Begg’s [36] and Egger’s tests [37] to evaluate any possible publication bias (P<0.05 was treated as significant publication bias). All statistical analyses were performed using Stata statistical software, version 12.0 (Stata Corp., College Station, TX, USA).
Results
Study selection and characteristics
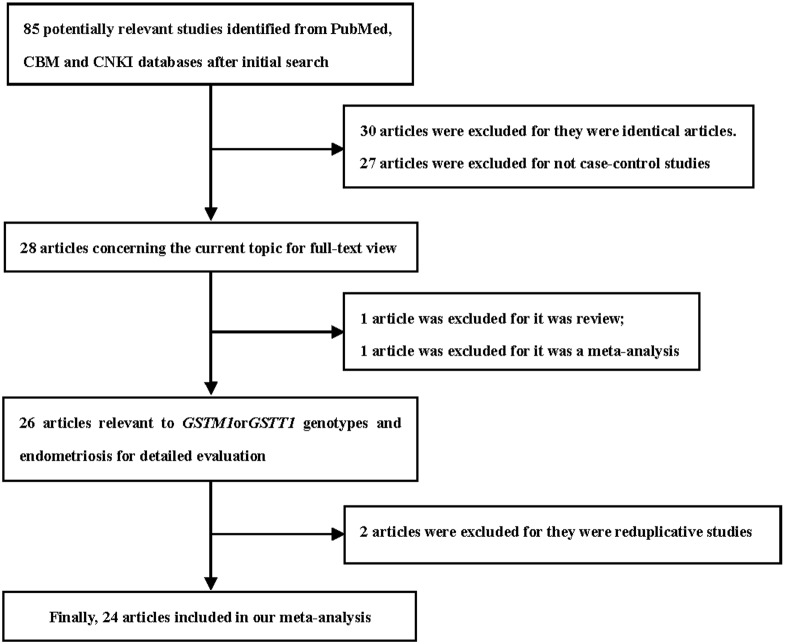
This meta-analysis is guided by the PRISMA statement ( Checklist S1 ). We initially identified 85 results relevant to the search terms in the selected databases. After reading the titles and abstracts, only 28 potentially eligible articles were identified for further detailed evaluation. Of these, two articles [38], [25] were excluded because one article was a review and the other was a meta-analysis. After further screening, two further articles [39], [40] were excluded for overlapping populations. Two ethnic populations (Hans and Uyghurs) were studied in the article by Ding et al [18], so we considered it as two studies in this analysis. In total, we included 24 articles [5], [8], [13]–[24], [26]–[29], [41]–[46], including 25 independent case-control studies ( Figure 1 ). Eighteen, four, and two of the articles were written in English, Chinese, and Russian, respectively. Twenty-five and 16 studies focused on GSTM1 and GSTT1 polymorphisms, respectively, and five were on GSTM1-GSTT1 interaction analysis. The characteristics of the included studies are described in Table 1 .
Figure 1. Flow diagram of the study selection process.
CBM: Chinese Biomedical; CNKI: China National Knowledge Infrastructure.
Table 1. Characteristics of studies included in the meta-analysis.
| First author[reference] | Year | Country | Ethnicity | Source ofControls | Case | Control | Null GSTM1Case Control | Null GSTT1Case Control | Dual nullCase Control | Qualityscore |
| Baranov et al. [13] | 1996 | Russia | European | PB | 42 | 67 | 34 26 | 0 0 | 0 0 | 7 |
| Baranov et al. [41] | 1999 | Russia | European | PB | 150 | 99 | 88 42 | 0 0 | 0 0 | 7 |
| Baranova et al. [14] | 1999 | France | European | HB | 65 | 72 | 50 33 | 13 7 | 0 0 | 7 |
| Baxter et al. [42] | 2001 | England | European | PB | 84 | 219 | 40 107 | 0 0 | 0 0 | 7 |
| Hadfield et al. [43] | 2001 | England | European | HB | 132 | 52 | 59 27 | 29 14 | 0 0 | 8 |
| Ivashchenko et al. [44] | 2003 | Russia | European | HB | 74 | 39 | 42 17 | 27 6 | 0 0 | 8 |
| Arvanitis et al. [15] | 2003 | Greece | European | HB | 275 | 346 | 161 181 | 24 31 | 11 16 | 8 |
| Peng et al. [16] | 2003 | China | Asian | HB | 76 | 80 | 50 37 | 0 0 | 0 0 | 6 |
| Lin et al. [17] | 2003 | China | Asian | HB | 68 | 28 | 49 12 | 53 9 | 0 0 | 7 |
| Morizane et al. [45] | 2004 | Japan | Asian | PB | 108 | 173 | 57 89 | 52 71 | 30 43 | 9 |
| Ding (a) et al. [18] | 2004 | China | Asian | HB | 41 | 107 | 21 57 | 15 32 | 0 0 | 8 |
| Ding (b) et al. [18] | 2004 | China | Asian | HB | 80 | 105 | 46 55 | 59 47 | 0 0 | 8 |
| Hur et al. [46] | 2005 | Korea | Asian | HB | 194 | 259 | 112 145 | 104 125 | 132 154 | 7 |
| Babu et al. [19] | 2005 | India | Asian | PB | 310 | 215 | 121 64 | 42 34 | 14 11 | 8 |
| Aban et al. [20] | 2007 | Turkey | European | HB | 150 | 150 | 88 65 | 59 44 | 0 0 | 8 |
| Kim et al. [26] | 2007 | Korea | Asian | HB | 316 | 256 | 183 146 | 178 124 | 0 0 | 7 |
| Yang et al. [21] | 2009 | China | Asian | HB | 216 | 216 | 134 100 | 0 0 | 0 0 | 7 |
| Cao et al. [27] | 2009 | China | Asian | HB | 51 | 102 | 33 61 | 22 39 | 0 0 | 7 |
| Roya et al. [22] | 2009 | India | Asian | HB | 97 | 102 | 26 15 | 0 0 | 0 0 | 6 |
| Huang et al. [28] | 2010 | China | Asian | HB | 28 | 29 | 12 10 | 0 0 | 0 0 | 7 |
| Hosseinzadeh et al. [23] | 2011 | Iran | Asian | HB | 120 | 200 | 87 80 | 0 0 | 0 0 | 5 |
| Trabert et al. [5] | 2011 | USA | European | PB | 254 | 567 | 137 268 | 0 0 | 0 0 | 9 |
| Wu et al. [24] | 2012 | China | Asian | HB | 121 | 171 | 57 52 | 40 33 | 23 15 | 7 |
| Vichi et al. [8] | 2012 | Italy | European | HB | 181 | 162 | 104 85 | 20 32 | 0 0 | 8 |
| Matsuzaka et al. [29] | 2012 | Japan | Asian | HB | 97 | 143 | 43 67 | 38 56 | 0 0 | 7 |
Abbreviations: PB, population-based; HB, hospital-based.
Quality assessment results
The scores of included studies were 5 to 9 ( Table 1 ).
Meta-analysis results
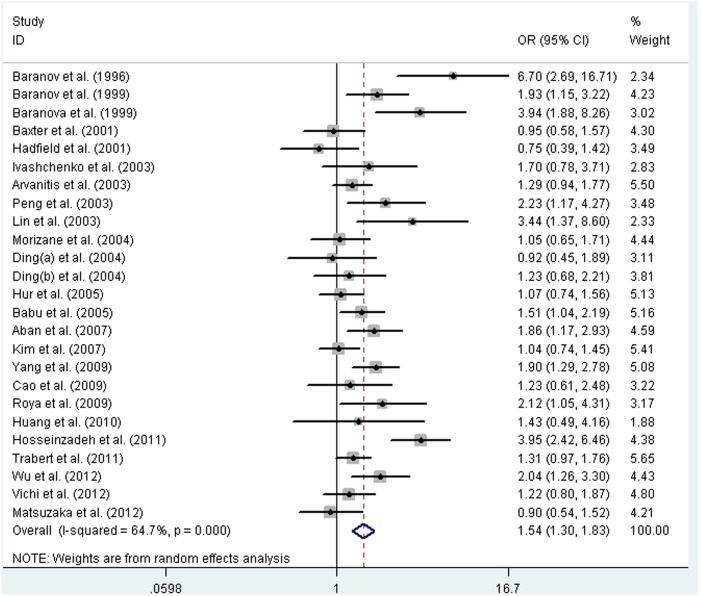
The association between GSTM1 polymorphism and endometriosis was investigated in 25 studies, which included a total of 3330 cases and 3959 controls. The heterogeneity was significant, so the random-effects model was selected. The result showed that the null genotype of GSTM1 was associated with an increased endometriosis risk (OR = 1.54, 95% CI: 1.30–1.83, P<0.001). A forest plot is shown in Figure 2 . Furthermore, we included 22 studies of high quality to validate the association, and the result again showed a strong association between this polymorphism and endometriosis risk (OR = 1.45, 95% CI: 1.23–1.71, P<0.001). Subgroup analysis by ethnicity was performed, and an increased risk of endometriosis was observed in Europeans and Asians (OR = 1.58, 95% CI: 1.19–2.09, P = 0.002; OR = 1.52, 95% CI: 1.21–1.91, P<0.001; respectively). In stratified analysis by source of controls, the results showed that source of controls did not affect the pooled results and a significantly increased risk of endometriosis was detected both in PB and HB studies (OR = 1.52, 95% CI: 1.08–2.16, P = 0.005; OR = 1.55, 95% CI: 1.26–1.91, P<0.001; respectively).
Figure 2. Forest plot of pooled OR with 95% CI for association between the null genotype of GSTM1 and endometriosis risk.
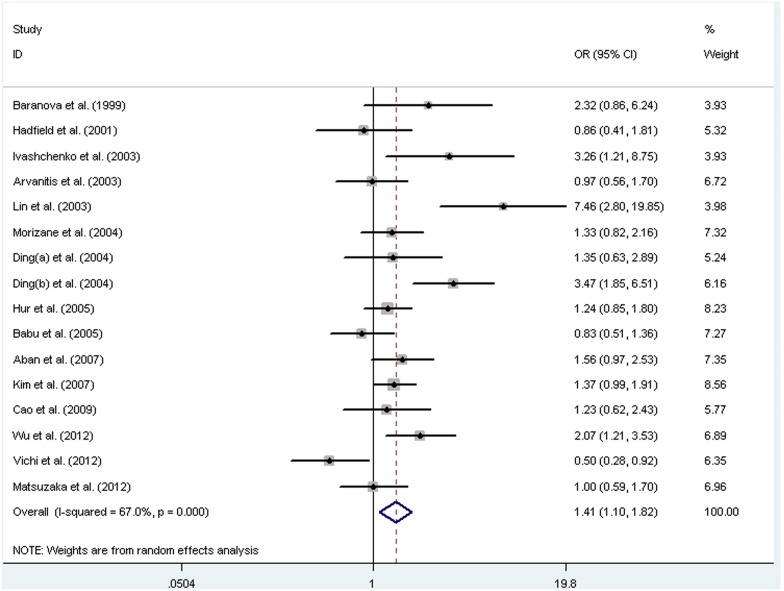
Sixteen independent studies, with a total of 2263 cases and 2380 controls, were included in the meta-analysis of GSTT1 polymorphism. We found significant heterogeneity between studies, so the random-effects model was used to pool the results. The results indicated a positive association between the null genotype of GSTT1 with endometriosis risk (OR = 1.41, 95% CI: 1.10–1.82, P = 0.007). A forest plot is shown in Figure 3 . Stratified analysis by ethnicity showed a significant association in Asians (OR = 1.53, 95% CI: 1.14–2.06, P = 0.005), but not in Europeans (OR = 1.21, 95% CI: 0.74–1.99, P = 0.45).
Figure 3. Forest plot of pooled OR with 95% CI for association between the null genotype of GSTT1 and endometriosis risk.
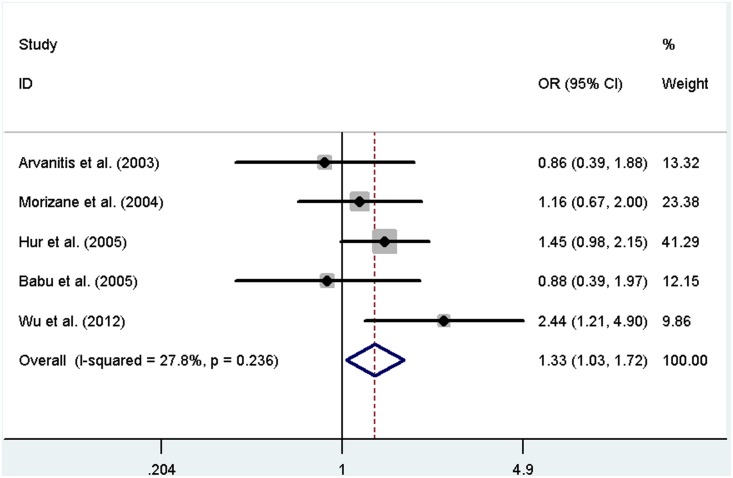
For GSTM1-GSTT1 interaction analysis, five independent studies, including 1008 cases and 1164 controls, were subjected to meta-analysis. The heterogeneity was not significant, so we selected the fixed-effects model. The results showed that the dual null genotype of GSTM1-GSTT1 was associated with an increased endometriosis risk (OR = 1.33, 95% CI: 1.03–1.72, P = 0.027). A forest plot is shown in Figure 4 . The results of this meta-analysis are summarized in Table 2 .
Figure 4. Forest plot of pooled OR with 95% CI for association between the dual null genotype of GSTM1-GSTT1 and endometriosis risk.
Table 2. Meta-analysis of associations between GSTM1, GSTT1, and GSTM1-GSTT1 polymorphisms and endometriosis.
| Genes | Comparisons | Sample size Case Control | No.ofstudies | Test of association OR (95% CI) p Model | Test of heterogeneity χ2 p I2 | ||||||
| GSTM1 | |||||||||||
| Overall | GSTM1(−) vs. GSTM1(+) | 3330 | 3959 | 25 | 1.54 | 1.30–1.83 | <0.001 | R | 0.115 | <0.001 | 64.7 |
| BS | GSTM1(−) vs. GSTM1(+) | 3037 | 3577 | 22 | 1.45 | 1.23–1.71 | <0.001 | R | 0.080 | <0.001 | 57.3 |
| Ethnicity | |||||||||||
| European | GSTM1(−) vs. GSTM1(+) | 1407 | 1773 | 10 | 1.58 | 1.19–2.09 | 0.002 | R | 0.130 | 0.001 | 68.5 |
| Asian | GSTM1(−) vs. GSTM1(+) | 1923 | 2186 | 15 | 1.52 | 1.21–1.91 | <0.001 | R | 0.121 | <0.001 | 64.5 |
| Source of Controls | |||||||||||
| PB | GSTM1(−) vs. GSTM1(+) | 948 | 1340 | 6 | 1.52 | 1.08–2.16 | 0.005 | R | 0.124 | 0.018 | 70.1 |
| HB | GSTM1(−) vs. GSTM1(+) | 2382 | 2619 | 19 | 1.55 | 1.26–1.91 | <0.001 | R | 0.125 | <0.001 | 64.8 |
| GSTT1 | |||||||||||
| Overall | GSTT1(−)vs. GSTT1(+) | 2263 | 2380 | 16 | 1.41 | 1.10–1.82 | 0.007 | R | 0.163 | <0.001 | 67.0 |
| Ethnicity | |||||||||||
| European | GSTT1(−) vs. GSTT1(+) | 877 | 821 | 6 | 1.21 | 0.74–1.99 | 0.450 | R | 0.253 | 0.007 | 68.4 |
| Asian | GSTT1(−) vs. GSTT1(+) | 1386 | 1559 | 10 | 1.53 | 1.14–2.06 | 0.005 | R | 0.143 | 0.001 | 67.5 |
| GSTM1-GSTT1 | GSTM1-GSTT1(−) vs.other genotypes | 1008 | 1164 | 5 | 1.33 | 1.03–1.72 | 0.027 | F | 0.037 | 0.236 | 27.8 |
Abbreviations: OR, odds ratio; CI, confidence interval; BS, based on score (studies with quality scores <7 were excluded); PB, population-based; HB, hospital-based; F, fixed-effects model; R, random-effects model.
Sensitivity analysis
Sensitivity analyses were performed after the sequential removal of each eligible study to assess the influence of each individual study on the pooled ORs. In the analysis of the GSTM1 polymorphism, the pooled ORs were not qualitatively changed when any single study was omitted, suggesting that no single study exhibited excessive influence, and that the results are reliable ( Figure 5 ). Other results were also relatively stable.
Figure 5. Sensitivity analysis for the meta-analysis regarding the association between GSTM1 polymorphism and endometriosis risk.
Publication Bias
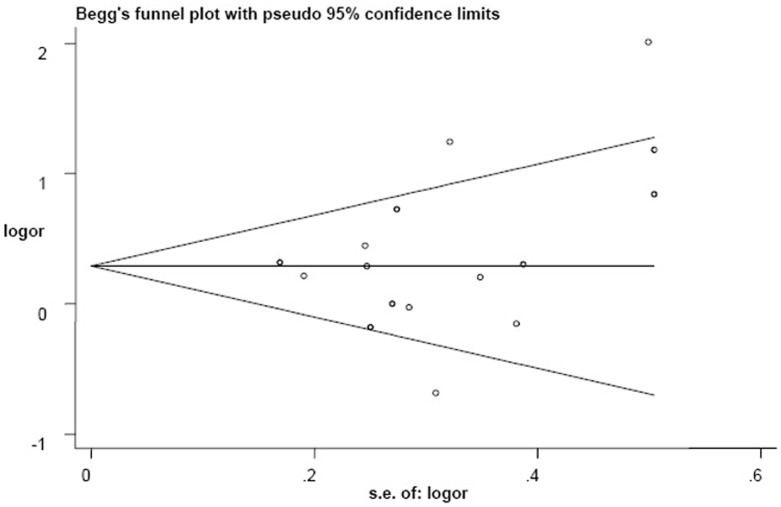
We conducted Begg’s and Egger’s tests to evaluate potential publication bias. There was no statistical evidence of publication bias regarding analysis of the GSTM1 polymorphism (GSTM1 (−) vs. GSTM1 (+): Begg, P = 0.129 and Egger, P = 0.079), and Begg’s funnel plots suggested no substantial asymmetry ( Figure 6 ). There was no publication bias for other results (GSTT1 (−) vs. GSTT1 (+): Begg, P = 0.344 and Egger, P = 0.207, and the shape of the funnel plot also did not reveal any evidence of obvious asymmetry ( Figure 7 ); GSTM1-GSTT1 (−) vs. other genotypes: Begg, P = 0.462 and Egger, P = 0.613).
Figure 6. Begg’s funnel plot for publication bias in selection of studies regarding the GSTM1 polymorphism.
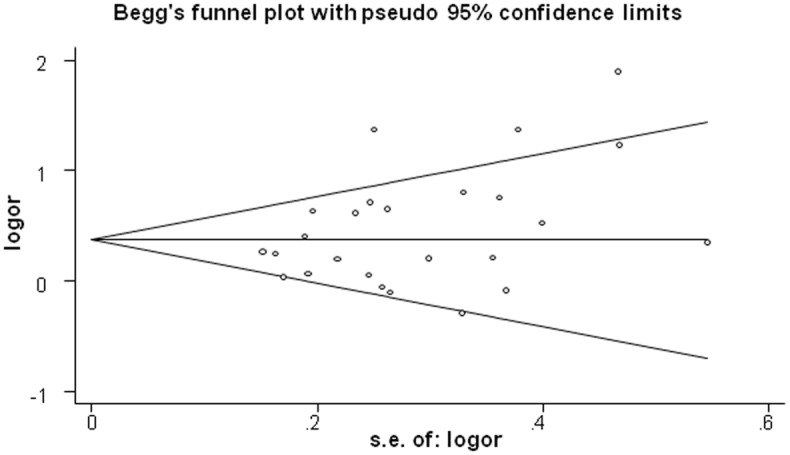
Figure 7. Begg’s funnel plot for publication bias in selection of studies regarding the GSTT1 polymorphism.
Discussion
As many GST genes are polymorphic, whether particular allelic variants in GST genes are correlated with altered risk of some kinds of diseases has provoked great interest [47]. The null genotypes of GSTM1 and GSTT1, two of the most widely-studied polymorphisms, are characterized by homozygous deletions of the respective genes [39]. The first study considering null genotype of GSTM1 as risk factors was conducted by Baranov et al. [13] in 1996. A preponderance of GSTT1-null subjects among endometriosis patients was detected in 1999, although it was not statistically significant [14]. Since then, many studies have investigated the associations between null genotypes of GSTM1 and GSTT1 and endometriosis susceptibility. However, the results are inconsistent and conflict, which compelled us to pay attention to the two polymorphisms at a meta-analytical level.
The current study, including 25 case-control studies with 3330 cases and 3959 controls, is the most comprehensive meta-analysis of the association of GSTM1- and GSTT1-null genotypes with endometriosis risk, which allows us to expand the discussion of possible implications and interpretations of the findings. Our meta-analysis suggested that there were significant associations between the null genotypes of GSTM1 and GSTT1 and endometriosis. Moreover, the combined GSTM1-GSTT1-null genotype also showed a positive association with endometriosis susceptibility.
A novel finding of the present study was the significant positive association between the null genotype of GSTM1 and endometriosis risk. The large number of studies and subjects included in this meta-analysis were substantial enough to resolve the issue of conflicting results obtained in individual studies, which was primarily caused by their small sample size. Furthermore, the results in subgroup analysis by ethnicity also indicated an association in both Europeans and Asians suggesting this association is reliable.
This meta-analysis showed a moderate positive association between the null genotype of GSTT1 and endometriosis risk, which is consistent with the result of a previous meta-analysis [25]. In the analysis stratified by ethnicity, a significant association was found in Asians, but no such association was detected among Europeans. There are several possible reasons for such a difference. First, the frequencies of the risk-associated homozygous null genotype vary between different races. The frequency of the GSTT1-null genotype is nearly 50% in the Chinese population [48], [49] 14.5–20.1% in Indians [50]–[52], and 11.0–37.9% in Europeans [53], [54]. Thus, the GSTT1 polymorphism may exert varying effects in different populations. Second, the different results could also be explained by study design or sample size. Other confounding factors, such as age and lifestyle may also be considered.
The GSTM1-GSTT1 interaction analysis indicated that women with double-null genotype had significantly increased endometriosis risk compared with those with other genotypes. If genetic susceptibility to endometriosis is, at least in part, mediated through polymorphisms of genes that encode enzymes responsible for detoxification, it is possible that the combination of GSTM1- and GSTT1-null genotypes may be more discriminating as a risk factor for endometriosis than a single null genotype.
The observation that the null genotypes of GSTM1, GSTT1, and GSTM1-GSTT1 increased the risk of developing endometriosis is biologically plausible. Environmental contaminants, such as polychlorinated dibenzo-p-dioxins, polychlorinated biphenyls, and polycyclic aromatic hydrocarbon, have been suggested to promote the occurrence and development of endometriosis by interfering with the estrogen signaling pathway and their immunosuppressive effects [55]. The GSTs plays a critical role in the detoxification of a broad range of environmental contaminants. As critical phase II metabolic enzymes, GSTs catalyze reactions between glutathione and all kinds of potentially lipophilic compounds, causing neutralization of the carcinogens, products of oxidative stress and toxic compounds [56]. Previous studies suggested that homozygous null deletions in GSTM1 and GSTT1 cause a complete loss of the activity of their encoded enzymes [57], [58]. The GSTM1 and GSTT1 deletions are detected in 42–60% and 13–26% of Caucasians, respectively [59]. Lack of GST enzyme activity resulting from the null genotypes may affect the detoxification of environmental toxins, and thus contribute to the pathogenesis of endometriosis.
Heterogeneity is an important issue in meta-analysis. Although we minimized the likelihood by performing a careful search for published studies, using the explicit criteria for study inclusion, statistically significant heterogeneity still existed in most comparisons. There are several explanations for the significant between-study heterogeneity, such as different study populations, genetic factors, and environmental factors. In particular, environmental contaminant exposure, a risk factor for endometriosis, is an important factor contributing to heterogeneity. The status of environmental contamination varies between countries, so the endometriosis incidence varies between populations. Publication bias is another important issue which should also be accounted for in meta-analyses. After evaluating the publication bias using Begg’s funnel plots we did not detect a publication bias, indicating the strength of the results.
Sun-Wei Guo [25] also evaluated the association between GSTM1 and GSTT1 polymorphisms and endometriosis risk by performing a meta-analysis including 14 studies with 1539 cases and 1805 controls. That study suggested that the endometriosis risk associated with the null genotype of GSTT1 was 29% higher than other genotypes, but it failed to find positive associations between the null genotype of GSTM1 or GSTM1-GSTT1 and the risk of endometriosis. There were some differences between that study and ours. First, our meta-analysis provided more comprehensive information on the relationships between the two polymorphisms and endometriosis by extracting data from more studies with more total cases and controls. Second, some issues that may affect the results of meta-analysis, such as publication bias, sensitivity analysis, and quality assessment of the included studies, were addressed in our study. Third, the current study also showed distinct findings, with the added statistical power.
This meta-analysis had several limitations that should be taken into account when considering its contributions. First, heterogeneity among studies existed in some comparisons of polymorphisms. Second, the results of our meta-analysis were applicable to only two ethnic groups, Europeans and Asians, as there were no relevant studies including data from African ethnic groups. Hence, to conduct a more precise analysis of the association between GSTM1- and GSTT1-null genotypes and endometriosis risk, additional studies with larger sample sizes and involving different ethnicities (especially African) are needed. Third, gene-environment interactions were not evaluated in this meta-analysis. Subgroup analyses based on environmental exposures were not performed because of insufficient data on such associations in all included studies.
In conclusion, this meta-analysis suggests that GSTM1- and GSTT1-null genotypes are associated with an increased risk of endometriosis. Null genotypes of GSTM1 and GSTT1 could act as biomarkers of endometriosis susceptibility. Larger and well-designed studies are needed to confirm these findings. Moreover, future studies should further evaluate potential gene-to-gene and gene-to-environment interactions to clarify the role of the GSTM1 and GSTT1 genes in endometriosis.
Supporting Information
PRISMA meta-analysis checklist.
(DOC)
Funding Statement
This study was supported by a grant from the National Natural Science Foundation of China (No. 81271729). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Bulun SE (2009) Endometriosis. N Engl J Med 360: 268–279. [DOI] [PubMed] [Google Scholar]
- 2. Giudice LC, Kao LC (2004) Endometriosis. Lancet 364: 1789–1799. [DOI] [PubMed] [Google Scholar]
- 3. Eskenazi B, Warner ML (1997) Epidemiology of endometriosis. Obstet Gynecol Clin North Am 24: 235–258. [DOI] [PubMed] [Google Scholar]
- 4. Melis GB, Ajossa S, Guerriero S, Paoletti AM, Angiolucci M, et al. (1994) Epidemiology and diagnosis of endometriosis. Ann N Y Acad Sci 743: 352–357. [DOI] [PubMed] [Google Scholar]
- 5. Trabert B, Schwartz SM, Peters U, De Roos AJ, Chen C, et al. (2011) Genetic variation in the sex hormone metabolic pathway and endometriosis risk: an evaluation of candidate genes. Fertil Steril 96: 1401–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stefansson H, Geirsson RT, Steinthorsdottir V, Jonsson H, Manolescu A, et al. (2002) Genetic factors contribute to the risk of developing endometriosis. Hum Reprod 17: 555–559. [DOI] [PubMed] [Google Scholar]
- 7. Pauwels A, Schepens PJ, D’Hooghe T, Delbeke L, Dhont M, et al. (2001) The risk of endometriosis and exposure to dioxins and polychlorinated biphenyls: a case-control study of infertile women. Hum Reprod 16: 2050–2055. [DOI] [PubMed] [Google Scholar]
- 8. Vichi S, Medda E, Ingelido AM, Ferro A, Resta S, et al. (2012) Glutathione transferase polymorphisms and risk of endometriosis associated with polychlorinated biphenylsexposure in Italian women: a gene-environment interaction. Fertil Steril 97: 1143–1151. [DOI] [PubMed] [Google Scholar]
- 9. Eaton DL, Bammler TK (1999) Concise review of the glutathione S-transferases and their significance to toxicology. Toxicol Sci 49: 156–164. [DOI] [PubMed] [Google Scholar]
- 10. Josephy PD (2010) Genetic variations in human glutathione transferase enzymes: significance for pharmacology and toxicology. Hum Genomics Proteomics 2010: 876–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pearson WR, Vorachek WR, Xu SJ, Berger R, Hart I, et al. (1993) Identification of class-mu glutathione transferase genes GSTM1-GSTM5 on human chromosome 1p13. Am J Hum Genet 53: 220–233. [PMC free article] [PubMed] [Google Scholar]
- 12. Webb G, Vaska V, Coggan M, Board P (1996) Chromosomal localization of the gene for the human theta class glutathione transferase (GSTT1). Genomics 33: 121–123. [DOI] [PubMed] [Google Scholar]
- 13. Baranov VS, Ivaschenko T, Bakay B, Aseev M, Belotserkovskaya R, et al. (1996) Proportion of the GSTM1 0/0 genotype in some Slavic populations and its correlation with cystic fibrosis and some multifactorial diseases. Hum Genet 97: 516–520. [DOI] [PubMed] [Google Scholar]
- 14. Baranova H, Bothorishvilli R, Canis M, Albuisson E, Perriot S, et al. (1999) Possible involvement of arylamine N-acetyltransferase 2, glutathione S-transferases M1 and T1genes in the development of endometriosis. Mol Hum Reprod 5: 636–641. [DOI] [PubMed] [Google Scholar]
- 15. Arvanitis DA, Koumantakis GE, Goumenou AG, Matalliotakis IM, Koumantakis EE, et al. (2003) CYP1A1, CYP19, and GSTM1 polymorphisms increase the risk of endometriosis. Fertil Steril 1: 702–709. [DOI] [PubMed] [Google Scholar]
- 16.Peng DX, He YL, Qiu LW, Yang F, Lin JM (2003) Association between glutathione S-transferase M1 gene deletion and genetic susceptibility to endometriosis. Di Yi Jun Yi Da Xue Xue Bao 23: 458–459, 462. [PubMed]
- 17. Lin J, Zhang X, Qian Y, Ye Y, Shi Y, et al. (2003) Glutathione S-transferase M1 and T1 genotypes and endometriosis risk: a case-controlled study. Chin Med J 116: 777–780. [PubMed] [Google Scholar]
- 18. Ding Y, Lin RY, Wang XF, Ding JB, Ai XZ (2004) Relationship between endometriosis and glutathione S-transferase M1, T1 genes of the Uyghurs and Hans in Xinjiang. Chinese Journal of Obstetrics and Gynecology 39: 101–104. [PubMed] [Google Scholar]
- 19. Babu KA, Reddy NG, Deendayal M, Kennedy S, Shivaji S (2005) GSTM1, GSTT1 and CYP1A1 detoxification gene polymorphisms and their relationship with advanced stages of endometriosis in South Indian women. Pharmacogenet Genomics 15: 167–172. [DOI] [PubMed] [Google Scholar]
- 20. Aban M, Ertunc D, Tok EC, Tamer L, Arslan M, et al. (2007) Modulating interaction of glutathione-S-transferase polymorphisms with smoking in endometriosis. J Reprod Med 52: 715–721. [PubMed] [Google Scholar]
- 21. Yang LP, An XF (2009) Analysis on the relationship between polymorphism of Exon7 situs in CYP 1 A 1 gene, GSTM 1 gene and susceptibility of endometriosis in women of Han nationality in Jilin city. Maternal and Child Health Care of China 24: 2556–2559. [Google Scholar]
- 22. Roya R, Baludu GS, Reddy BS (2009) Possible aggravating impact of gene polymorphism in women with endometriosis. Indian J Med Res 129: 395–400. [PubMed] [Google Scholar]
- 23. Hosseinzadeh Z, Mashayekhi F, Sorouri ZZ (2011) Association between GSTM1 gene polymorphism in Iranian patients with endometriosis. Gynecol Endocrinol 27: 185–189. [DOI] [PubMed] [Google Scholar]
- 24. Wu CH, Guo CY, Yang JG, Tsai HD, Chang YJ, et al. (2012) Polymorphisms of dioxin receptor complex components and detoxification-related genes jointly confersusceptibility to advanced-stage endometriosis in the taiwanese han population. Am J Reprod Immunol 67: 160–168. [DOI] [PubMed] [Google Scholar]
- 25. Guo SW (2005) Glutathione S-transferases M1/T1 gene polymorphisms and endometriosis: a meta-analysis of genetic association studies. Mol Hum Reprod 11: 729–743. [DOI] [PubMed] [Google Scholar]
- 26. Kim SH, Choi YM, Lee GH, Hong MA, Lee KS, et al. (2007) Association between susceptibility to advanced stage endometriosis and the genetic polymorphisms of aryl hydrocarbon receptor repressor and glutathione-S-transferase T1 genes. Hum Reprod 22: 1866–1870. [DOI] [PubMed] [Google Scholar]
- 27. Cao YH, Yao L, Wang D, Han P (2009) Relationship between endometriosis and glutathione S-transferase M1,T1 and P1 genetic polymorphism. Maternal and Child Health Care of China 24: 805–808. [Google Scholar]
- 28. Huang PC, Tsai EM, Li WF, Liao PC, Chung MC, et al. (2010) Association between phthalate exposure and glutathione S-transferase M1 polymorphism in adenomyosis, leiomyoma and endometriosis. Hum Reprod 25: 986–994. [DOI] [PubMed] [Google Scholar]
- 29. Matsuzaka Y, Kikuti YY, Goya K, Suzuki T, Cai LY, et al. (2012) Lack of an association human dioxin detoxification gene polymorphisms with endometriosis in Japanese women: results of a pilot study. Environ Health Prev Med 17: 512–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, et al. (2011) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Available at www.ohri.ca/programs/clinical epidemiology/oxford.asp.
- 31. Bertram L, McQueen MB, Mullin K, Blacker D, Tanzi RE (2007) Systematic meta-analyses of Alzheimer disease genetic association studies: the AlzGene database. Nat Genet Jan 39: 17–23. [DOI] [PubMed] [Google Scholar]
- 32. Qiu L1, Wang Z, Shi X, Wang Z (2008) Associations between XPC polymorphisms and risk of cancers: A meta-analysis. Eur J Cancer 44: 2241–2253. [DOI] [PubMed] [Google Scholar]
- 33. DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 34. Mantel N, Haenszel W (1959) Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 22: 719–748. [PubMed] [Google Scholar]
- 35. Tobias A (1999) Assessing the influence of a single study in the meta-analysis estimate. Stata Tech Bull 8: 15–17. [Google Scholar]
- 36. Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50: 1088–1101. [PubMed] [Google Scholar]
- 37. Egger MJ, Smith GD (1998) Bias in location and selection of studies. BMJ 316: 61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Guo SW (2006) The association of endometriosis risk and genetic polymorphisms involving dioxin detoxification enzymes: a systematic review. Eur J Obstet Gynecol Reprod Biol 124: 134–143. [DOI] [PubMed] [Google Scholar]
- 39. Baranova H, Bothorishvilli R, Canis M, Albuisson E, Perriot S, et al. (1997) Glutathione S-transferase M1 gene polymorphism and susceptibility to endometriosis in a French population. Mol Hum Reprod 3: 775–780. [DOI] [PubMed] [Google Scholar]
- 40. An XF, Yang LP, Liu SJ (2009) The correlation of GSTM1 gene polymorphism and endometriosis. Jilin Medical Journal 30: 1614–1615. [Google Scholar]
- 41. Baranov VS, Ivashchenko TE, Shved NIu, Iarmolinskata MI, Sel’kov SA, et al. (1999) Genetic factors of predisposition to endometriosis and response to its treatment. Genetika 35: 243–248. [PubMed] [Google Scholar]
- 42. Baxter SW, Thomas EJ, Campbell IG (2001) GSTM1 null polymorphism and susceptibility to endometriosis and ovarian cancer. Carcinogenesis 22: 63–65. [DOI] [PubMed] [Google Scholar]
- 43. Hadfield RM, Manek S, Weeks DE, Mardon HJ, Barlow DH, et al. (2001) Linkage and association studies of the relationship between endometriosis and genes encoding the detoxification enzymes GSTM1, GSTT1 and CYP1A1. Mol Hum Reprod 7: 1073–1078. [DOI] [PubMed] [Google Scholar]
- 44. Ivashchenko TE, Shved NIu, Kramareva NA, Ailamazian EK, Baranov VS (2003) Analysis of the polymorphic alleles of genes encoding phase 1 and phase 2 detoxication enzymes in patients with endometriosis. Genetika 39: 525–529. [PubMed] [Google Scholar]
- 45. Morizane M, Yoshida S, Nakago S, Hamana S, Maruo T, et al. (2004) No association of endometriosis with glutathione S-transferase M1 and T1 null mutations in a Japanese population. J Soc Gynecol Investig 11: 118–121. [DOI] [PubMed] [Google Scholar]
- 46. Hur SE, Lee JY, Moon HS, Chung HW (2005) Polymorphisms of the genes encoding the GSTM1, GSTT1 and GSTP1 in Korean women: no association with endometriosis. Mol Hum Reprod 11: 15–19. [DOI] [PubMed] [Google Scholar]
- 47. Strange RC, Spiteri MA, Ramachandran S, Fryer AA (2001) Glutathione-S-transferase family of enzymes. Mutat Res 482: 21–26. [DOI] [PubMed] [Google Scholar]
- 48. Wang G, Zhang L, Li Q (2006) Genetic polymorphisms of GSTT1, GSTM1, and NQO1 genes and diabetes mellitus risk in Chinese population. Biochem Biophys Res Commun 341: 310–313. [DOI] [PubMed] [Google Scholar]
- 49. Zhong SL, Zhou S, Chen X, Huang M (2006) Rapid determination of common mutations in glutathione S-transferase gene by PCR based methods in healthy Chinese. Clin Chim Acta 364: 205–208. [DOI] [PubMed] [Google Scholar]
- 50. Sobti RC, Al-Badran AI, Sharma S, Sharma SK, Krishan A, et al. (2005) Genetic polymorphisms of CYP2D6, GSTM1, and GSTT1 genes and bladder cancer risk in North India. Cancer Genet Cytogenet 156: 68–73. [DOI] [PubMed] [Google Scholar]
- 51. Mittal RD, Manchanda PK, Bid HK, Ghoshal UC (2007) Analysis of polymorphisms of tumor necrosis factor-alpha and polymorphic xenobiotic metabolizing enzymes in inflammatory, bowel disease: study from northern India. J Gastroenterol Hepatol 22: 920–924. [DOI] [PubMed] [Google Scholar]
- 52. Srivastava DS, Mandhani A, Mittal B, Mittal RD (2005) Genetic polymorphism of glutathione S-transferase genes (GSTM1, GSTT1 and GSTP1) and susceptibility to prostate cancer in Northern India. BJU Int 95: 170–173. [DOI] [PubMed] [Google Scholar]
- 53. Steinhoff C, Franke KH, Golka K, Thier R, Römer HC, et al. (2000) Glutathione transferase isozyme genotypes in patients with prostate and bladder carcinoma. Arch Toxicol 74: 521–526. [DOI] [PubMed] [Google Scholar]
- 54. Marinho C, Alho I, Arduíno D, Falcão LM, Brás-Nogueira J, et al. (2007) GSTM1/T1 and MTHFR polymorphisms as risk factors for hypertension. Biochem Biophys Res Commun 353: 344–350. [DOI] [PubMed] [Google Scholar]
- 55. De Felip E, Porpora MG, di Domenico A, Ingelido AM, Cardelli M, et al. (2004) Dioxin-like compounds and endometriosis: a study on Italian and Belgian women of reproductive age. Toxicol Lett 150: 203–209. [DOI] [PubMed] [Google Scholar]
- 56. Rushmore TH, Pickett CB (1993) GlutathioneS-transferases, structure, regulation, and therapeutic implications. J Biol Chem 268: 11475–11478. [PubMed] [Google Scholar]
- 57. Pemble S, Schroeder KR, Spencer SR, Meyer DJ, Hallier E, et al. (1994) Human glutathione S-transferase theta (GSTT1): cDNA cloning and the characterization of a genetic polymorphism. Biochem J 300: 271–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Josephy PD (2010) Genetic variations in human glutathione transferase enzymes: significance for pharmacology and toxicology. Hum Genomics Proteomics 2010: 876–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Garte S, Gaspari L, Alexandrie AK, Ambrosone C, Autrup H, et al. (2001) Metabolic gene polymorphism frequencies in control populations. Cancer Epidemiol Biomarkers Prev10: 1239–1248. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA meta-analysis checklist.
(DOC)