Abstract
This article provides an overview of health-related quality of life (HRQoL) assessments in pediatric hematopoietic stem cell transplants, focusing on the relationship between child and parent proxy ratings of the child’s HRQoL and how measurement of HRQoL may be incorporated into clinical decision-making. Parent and child ratings of the child’s health may be affected differently by unequal access to and incongruent understanding of available information, as well as the effect of age difference on interpretation. In particular, parents and children may experience the impact of clinical events on HRQoL very differently. The recent US Federal emphasis on ‘patient-centeredness’ has helped fuel the development and application of more clinically functional and low-burden HRQoL measures. Future work in pediatric hematopoietic stem cell transplants must seek to capture the experiences and perceptions of all those involved.
Keywords: Child Health Ratings Inventories, health-related quality of life, hematopoietic stem cell transplant, parent–child difference, Patient-Reported Outcomes Measurement Information System™
For more than 20 years, researchers have grappled with the impact of hematopoietic stem cell transplantation (HSCT) on recipients’ health-related quality of life (HRQoL). HRQoL in this context refers to a multidimensional construct, incorporating the individual’s subjective appraisal of his/her functioning and well-being. The ‘health-relatedness’ refers to those aspects of overall quality of life that are influenced by the individual’s health and are within the purview or influence of the healthcare sector [1].
HSCT provides a compelling context within which to consider HRQoL, given its toll on the physical and emotional health of the recipient. The gravity of the underlying condition, the intensity of the treatment, and the potential for early and delayed sequelae all can adversely alter HRQoL. Specifically, HSCT offers potentially life-saving treatment for advanced malignancies and otherwise incurable diseases of hematologic, immunologic or metabolic origin. The treatment itself, however, beginning with a multi-day, and often multimodal, preparative regimen is intense, putting the recipient at risk for end organ toxicity, infection and bleeding. Following the initial transplant period, patients remain vulnerable to infection and, for recipients of allogeneic HSCT, to emergence or re-emergence of acute or chronic graft-versus-host disease (GVHD). In the several months following HSCT, patients are kept in protective isolation, which further taxes their ability to participate in typical social and role activities, such as school.
In this article, the authors will review the evolving field of HRQoL in pediatric HSCT with particular focus on two broad areas: the impact of child versus parent rater of the child’s HRQoL, and the future directions with regard to assessment and incorporation into clinical decision-making. In 2012, the authors summarized the state of science of HRQoL after pediatric HSCT with the added focus of functional and neurocognitive outcomes, provided by distinguished colleagues [2]. The reader is directed to that review for general descriptions of the HRQoL trajectory over the first 1–2 years post-transplant.
Barriers to early research on HRQoL in children/ adolescents
Research about pediatric HRQoL in general has lagged behind that in adults in two important ways. First, prior to the early 1990s, there were very few pediatric instruments that addressed the full range of HRQoL as a multidimensional construct. Instead, earlier instruments focused largely on a single aspect of functioning, such as psychosocial or physical functioning. Secondly, it was widely believed that children, particularly those of school age (i.e., latency), lacked the capacity to reflect on their own HRQoL. Historically, parents or other proxies (e.g., providers and teachers) were preferred raters.
Approaches to measurement of HRQoL
Over the past 15 years, there has been a burgeoning of pediatric HRQoL instruments, which have been used in clinical sub-populations, healthy children and epidemiologic surveys [3,4], and an increasing appreciation for the capacity of child raters to report on their own HRQoL.
Within the pediatric HSCT population, several studies have addressed the impact of HSCT on HRQoL. As recently reported [2], many of the initial studies employed generic HRQoL measures, such as the Pediatric Quality of Life (PedsQL™) 4.0 General Core Scales [5], the Child Health Questionnaire (CHQ) [6], the Child Health Ratings Inventories (CHRIs)-General Health Module (CHRIs-General) [7–9] and the Health Utilities Index 2/3 [10]. With the exception of the CHRIs-General, which was developed for use with children with chronic disease, the other instruments have been used successfully in healthy children as well as those with chronic conditions. Most of these generic measures are health profile measures, generating summary scores for each of the domains (or dimensions) of HRQoL, including physical, emotional and role functioning. Some of the measures, notably the PedsQL and the CHQ, also provide scores for physical and psychosocial subscales; the PedsQL generates a total score of all 23 items. Scores are scaled from 0 to 100, in which higher scores reflect better HRQoL. In contrast, the Health Utilities Index 2/3 is a preference-based measure, generating utility weights that can be used in quality-adjusted survival or cost–effectiveness analyses. All of the instruments have rater-specific versions for child/adolescent self-report as well as a parent-proxy report. The PedsQL and the CHRIs-General also offer age-specific versions for younger children (5–12 years) and adolescents (13 years of age and over). The CHQ has a single self-report measure for children 8–18 years.
As the field of HRQoL was evolving, early studies of HRQoL were cross-sectional in design; more recently, longitudinal studies have been conducted to describe the HRQoL trajectory over the first 1–2 years following HSCT [11]. All of the measures listed above have demonstrated adequate measurement properties in either kind of study – cross-sectional or longitudinal – including responsiveness to change over time, a required characteristic of measures used in longitudinal analyses. In addition to our 2012 review, which was prepared as part of the NCI, NHLBI, Pediatric Blood and Marrow Transplant Consortium First International Conference on Late Effects after Pediatric Hematopoietic Cell Transplantation, this literature and descriptions of the measures are summarized in the 2008 systematic review by Clarke et al. [12] and the 2005 critical review of the literature by Tsimicalis et al. [13].
In addition to validated generic instruments, two other tools have been validated for use in the pediatric HSCT populations: the Behavioral, Affective and Somatic Experiences Scale, designed to assess aspects of HRQoL in patients undergoing active, intensive therapy (such as HSCT) [14–16], and the CHRIs-HSCT, the only HSCT-specific HRQoL instrument for children. The CHRIs-HSCT is a 10-item measure, yielding three domain scores (i.e., hassles, body image and worry/distress). The questions themselves (‘item content’) address specific aspects of the HSCT experience, as summarized in Table 1. The CHRIs-HSCT can be used with a generic, multidimensional HRQoL core measure, as a ‘plug-in’ module. The preliminary psychometric properties of the CHRIs-General and CHRIs-HSCT were described in a cross-sectional sample of 122 pediatric HSCT recipients aged 5–18 years and their parents [8], and then subsequently in three separate longitudinal evaluations [9,17].
Table 1.
Item content of Child Health Ratings Inventories-hematopoietic stem cell transplantation module.
| Domain | Item concept |
|---|---|
| Body image | Having puffy cheeks and a puffy body because of medicines taken Having lost hair |
| Hassles | Having to wear a mask in public Avoiding foods that should not be eaten because of the transplant Taking medicine the way it is supposed to be taken Explaining the transplant to friends |
| Distress | Worrying about having to go back to the hospital Worrying about getting an infection Worrying about the disease coming back Time spent thinking about the transplant |
The principal HSCT-specific instrument for adults to capture the acute HSCT experience is the Functional Assessment of Cancer Therapy–Bone Marrow Transplant (FACT–BMT) [18]. Huang et al. adapted the FACT–BMT for adult survivors of HSCT, which is referred to as the FACT–BMT Survivor scale [19]. Studies are underway to evaluate the performance of this instrument in survivors of pediatric HSCT.
While the availability of several generic and HSCT-specific measures has allowed researchers to overcome previous barriers to HRQoL investigation, in some sense, this proliferation of measures has hampered direct comparisons between studies.
The use of standardized measures can overcome this limitation. In this context, standardization refers to the use of the same measure across studies within the same population. Standardization within the population is best illustrated by the 2004 NIH Consensus Conference, which was convened to establish standardized criteria to advance clinical trials in chronic GVHD [20]. Chronic GVHD, a devastating complication of HSCT, has been found to be associated with altered HRQoL and physical performance, particularly among adults [21,22], as well as long-term survivors of HSCT [23], and pediatric recipients [24,25]. In addition to end organ evaluation metrics, the NIH criteria included recommendations for standardized HRQoL assessment tools for adults and children, calling for the use of both generic and transplant-specific measures [26]. The panel selected the ShortForm (SF)-36 [27] and FACT–BMT [18] for the adult population and the CHRIs (General and HSCT) and the Activity Scale for Kids [28], the latter of which assesses physical disability in children.
In the absence of standard measures across studies, the use of standardized scores (e.g., mean: 50 [standard deviation: 10]) and/or the use of a cutoff value (i.e., at risk cutoff) [4] facilitate interpretation of study findings. The 2011 report by Brice et al. [29] is an excellent example of the second approach (see accompanying editorial by Kline [30]). The 2012 study by Oberg et al., reporting on the 2-year trajectory of HRQoL of 80 children cared for in a US-based transplant center, leveraged the availability of normative data from the PedsQL to compare HRQoL scores of HSCT recipients with age-based norms [31].
Inter-rater comparisons
The availability of instruments for both child and parent raters with parallel content has allowed researchers to explore levels of agreement between self and proxy ratings across a wide range of health states and age groups. Previous research on inter-rater comparisons was limited by the inclusion of different items for each rater, complicating the interpretation of the often limited to modest agreement observed between raters. In a 2008 review article by Upton et al., the authors noted that there was limited information in published studies about the variables contributing to levels of parent–child (P–C) agreement [32], which represented an important gap in our current knowledge base. The authors provided a cogent summary of methodological reporting of inter-rater agreement to be used in future studies, including information about the reliability of the instrument in the sample, details about mode of administration for each rater and the setting of data collection and analyses of inter-rater concordance at both the individual and the group level [32].
Several factors inform rater selection and influence their reports. While the data suggest that many children are capable of providing self-report, there are subgroups that may not be capable, such as children who are too sick, too young or those who have a cognitive impairment as a result of the underlying disease and/or treatment. Proxy ratings are likely to be more informative than the data being missing altogether.
The perceptions of a proxy rater may differ from those of the child about him/herself in two distinct ways. First, the proxy rater may not have the same access to the information that the child might. For example, the parent proxy does not typically see the child engage in school activities and forms his/her perceptions of the child’s school functioning from various sources of information (e.g., discussions with teachers, the child’s daily reports and periodic grade reports) that may be different from the information the child relies on to make his/her determination of school functioning. This is called information variance. One of the unique aspects of the acute HSCT period, stemming from the initial hospitalization and early recovery period, is that children are generally in protective isolation, not attending school or participating in age-appropriate activities with peers. Consequently, children and parents are thrust together, sharing the same routine and daily reality, much more than they would normally be if the child were in school.
In the clinical setting, however, information variance may also be manifested by differential access to and/or understanding of clinical events. Children may be less aware of the implications of a change in clinical status, particularly if that change is not associated with discrete symptoms or altered functioning.
The child and parent may weigh the available information differently. Parents may compare current functioning to prior functioning or anticipated future functioning, or compare one child’s functioning with that of another sibling or friend. This is referred to as criterion variance. In a fascinating report by Davis et al., 15 parent versus child pairs underwent cognitive interviewing, using the ‘think aloud’ technique as they completed a generic HRQoL instrument [33]. The qualitative analysis of these interviews, performed individually with each rater, revealed that the P–C discordance may be the result of different reasoning and response styles, rather than divergence in the interpretation of the individual items [33]. Both of these forms of variance may explain why reports from parents and children are not the same.
Several studies have explored P–C comparisons in ratings of the child’s HRQoL, principally utilizing methods to characterize the extent of agreement, such as Pearson or inter-class correlations, percentage agreement or Bland–Altman plots [34]. Levels of agreement vary by study sample from poor to strong and appear to be affected by factors including health status (healthy versus ill population), severity of disease, child age, level of emotional distress of parent and/or child/adolescent, the particular aspect of HRQoL and, possibly, the instrument(s) used [32].
Establishing the link between the HSCT clinical course & HRQoL
Several authors have explored demographic and family factors associated with HRQoL scores, such as child age, race/ethnicity and family factors, including parental emotional functioning (EF) and family cohesion. Barrera et al. highlighted the importance of pretransplant family cohesion on subsequent HRQoL [35]. The role of parental EF and adjustment on children’s HRQoL over time has also been described [9,35,36]. In contrast, few studies have identified the impact of baseline clinical characteristics on HRQoL or definitively established the link between the well-characterized clinical outcomes of HSCT, including organ toxicity, risk of infection and potential for acute and/or chronic GVHD, and HRQoL. This may be explained, at least in part, by the relative infrequency of clinical events, particularly in their more severe form (e.g., extensive chronic GVHD) in children.
Our group initially evaluated the association between physician-rated clinical severity and children’s self-reported HRQoL in a cross-sectional study of 82 children [37], finding that HRQoL was lower for children with increased clinical severity. The authors later explored the relationship between discrete clinical outcomes and each of the domains of general and HSCT-specific HRQoL in a longitudinal evaluation of 160 parent-proxy raters of children’s HRQoL [9], relying on pattern-mixture models to account for nonignorable missing data. Overall, the authors found stronger association between clinical events and HSCT-specific domains. Specifically, chronic GVHD was significantly associated with the ‘hassles’ domain (β: 27.5 [3.3]; p < 0.001) and the body image domain (β: 23.2 [6.2]; p < 0.001), but not significantly associated with generic HRQoL domains of physical, emotional or role functioning.
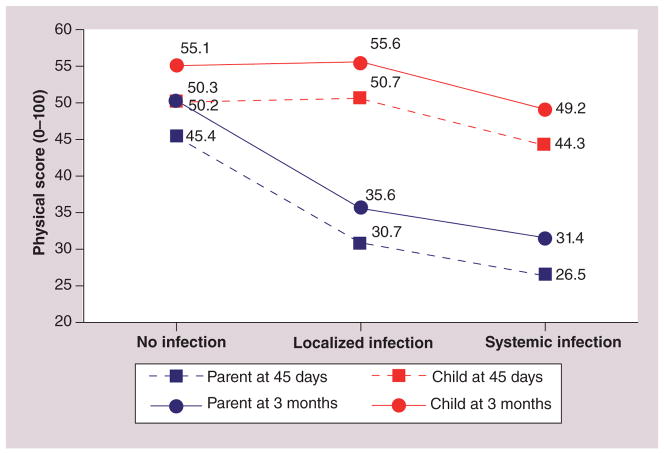
Subsequently, in a longitudinal study of 165 P–C pairs (children’s age: 5–18 years), the authors found that parents’ ratings of their children’s physical functioning were more sensitive to grade of infection [38], as measured by trained research staff, using the Common Toxicity Criteria of Adverse Events version 3.0 [101]. Infections were categorized as absent, localized or systemic. Children’s physical functioning scores were essentially identical for those in the absent or localized group, whereas those in the systemic infection group had physical functioning scores of approximately ten points lower than the others. In contrast, parent rating of the children’s physical functioning was approximately 12 points lower for each successive severity level. The interaction of rater and infection grade was statistically significant (p = 0.003) (Figure 1). The authors do not know if parents and children were told different things about the clinical significance of localized infection in the setting of an immunocompromised state, or if they assigned a different meaning to that state on their own.
Figure 1.
Child Health Ratings Inventories physical functioning scores for child and parent rater by degree of infection severity.
Based on our observation of rater–grade interaction, the authors then explored clinical factors that may explain differences between parent and child reports [36]. The authors created models to explain parent-minus-child differences for each domain of generic HRQoL (i.e., physical, emotional and role functioning) using the CHRIs-General, which was collected prior to transplant at day 45 and at 3, 6 and 12 months following HSCT. In addition to an overall model that took into account the full 12-month trajectory, the authors also created separate models for clinical variables, pertaining to specific time points or populations (e.g., acute GVHD in recipients of allogeneic transplant only over 3 months). The authors again utilized pattern mixture models to account for nonignorable missing data, this time creating strata based on the reason for a missed assessment [39].
The results were striking. Overall, parents rated children’s HRQoL lower than children did. In each of the models, child age and parent EF at baseline were associated with P–C differences. Duration of illness was significantly associated with rater differences in role and physical functioning, but not in children’s EF. Higher baseline child age was associated with a better agreement (as the difference in P–C gets closer to zero). Similarly, higher baseline parent EF was associated with more agreement between parent and child. Emotional and physical functioning move from greater disagreement to greater agreement over time; this pattern is attenuated with role functioning (Figure 2).
Figure 2.
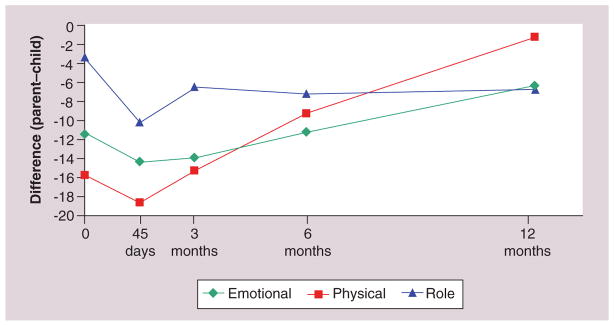
Parent–child differences across health-related quality of life domains and time.
However, in separate models by clinical events, the differences between P–C reports was even more pronounced. Clinical complications had greater impact on parental ratings than child ratings across all domains, increasing adjusted P–C differences. To illustrate this, for each clinical complication, parental EF was set at the mean baseline value, child age was set at the mean value of 10.5 years and log duration of illness was set at 1 year. Among children with severe (i.e., grade 3 or 4) acute GVHD, P–C differences in physical functioning were 22 points further apart (95% CI: −39 to −4; p = 0.015) further apart when compared with children without acute GVHD. Similarly, P–C differences in EF were eight points further apart (95% CI: −15 to −1; p = 0.034) further apart when the child had extensive chronic GVHD (Figure 3) as compared with limited chronic GVHD or none. Similarly, for role functioning, systemic infection and end-organ toxicity produced differences of 14 (95% CI: −22 to −6; p = 0.001) and 21 points (95 % CI: −40 to −1; p = 0.037), respectively. One might imagine that divergence of P–C perceptions about the child’s HRQoL could be an additional source of stress for both raters, particularly in the setting of severe clinical complications.
Figure 3. Parent–child differences in emotional functioning by degree of chronic graft-versus-host disease.
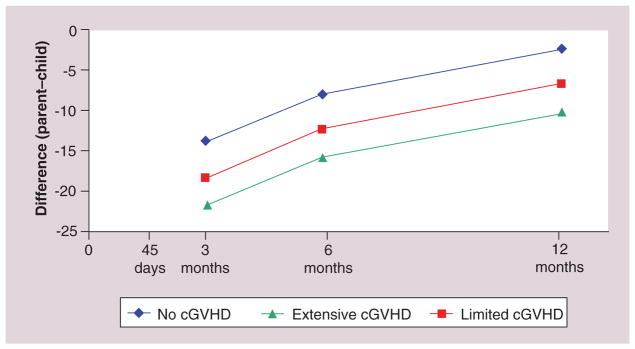
cGVHD: Chronic graft-versus-host disease.
These results highlight the complexity of P–C ratings. They suggest, as Upton proposed, that qualitative methods be employed to understand more fully “the processes through which parents and children make decisions when rating child HRQoL” [32].
Clinical applications of HRQoL information
HRQoL information has many applications in the clinical setting. It can help the patient and provider monitor the effects of the underlying disease and its treatment, and spur a discussion that may help divulge areas of importance to the individual patient and guide clinical decision-making.
Although routine HRQoL assessment has been used in adult-based care far more often than pediatric-based care, the overt benefit of this assessment in changing outcomes has been mixed. Studies have shown that while HRQoL assessment may facilitate communication between patients and providers [40,41], it has not been shown to result in significant changes in clinical management, improvement in patient satisfaction or changes in HRQoL [40–42].
Providers often report not understanding what the HRQoL scores mean or what to do with the information. Several studies have pointed to the need for management suggestions to accompany HRQoL information in the clinical setting. Moreover, formal evaluation of presentation formats is needed to determine ease and accuracy of interpretation. Several Internet-based HRQoL assessment systems now include brief reports for patients and providers.
The other fear providers raise about the incorporation of HRQoL assessments in clinical practice is increased encounter time. Engelen et al. recently reported the results of a sequential cohort study of 193 children, who were enrolled shortly after the completion of treatment for cancer (or status post-HSCT) [43]. The study was designed to evaluate the impact of giving patient HRQoL scores to providers on patient–provider communication about HRQoL domains and the identification of HRQoL problems. Strikingly, the intervention did result in increased discussion of EF and psychosocial functioning, while not increasing the duration of the visit. However, the intervention did not result in increased referrals, change satisfaction levels or lead to an overall improvement in HRQoL [43]. This is one of the first formal intervention studies in pediatric oncology/ transplantation. Future studies, conducted during the treatment phase, are needed to evaluate if availability of HRQoL scores can alter outcomes during active treatment.
Expert commentary & five-year view
It is exciting to consider what will unfold over the next 5 years in the field of HRQoL assessment for pediatric HSCT recipients. The availability of validated instruments, the use of electronic data capture systems and the gradual incorporation of HRQoL scores into clinical assessment all bode well for the future.
Several additional factors will undoubtedly shape the future of HRQoL assessment. The first is the emerging emphasis in the USA on ‘patient-centeredness,’ as evidenced by the 2010 creation of the Patient-Centered Outcomes Institute, a nonprofit corporation via Public Law 111–148. With increased patient-centeredness, the dialog could be expanded to include what is important to the patient (and his/her family), including those aspect of HRQoL threatened by disease and/or treatment, and the identification of issues that need to be addressed to enhance recovery and optimize functioning. In the arena of HRQoL assessment, one might envision expanding the standard assessment to a more individualized assessment, as proposed by Frick et al. with the Schedule for the Evaluation of Individual Quality of Life [44]. In this measurement approach, previously used with adults prior to autologous transplant, the individual nominates the five most important domains of quality of life without direct cueing to health or treatment. This information would elucidate for providers what is really important to the individual patient and may enhance adjustment and, ultimately, recovery.
Another potential ‘game changer’ is the Patient-Reported Outcomes Measurement Information System (PROMIS®), a trans-NIH initiative established in 2004, which has resulted in the creation of rich item banks in multiple areas of HRQoL for both adults and children [102]. Item banks can be administered in short forms (4–10 items) or through computer adaptive testing (with 3–7 items), which are available for parent-proxy report or child self-report. In addition to domains of physical, mental and social well-being, PROMIS instruments are also available to assess domains such as pain (and pain interference), fatigue and sexuality, all of which are commonly altered during or following cancer treatment. A 10-item global health scale has been created to assess adult quality of life, which has successfully been mapped to the EuroQoL-5 Dimension, a preference-based instrument, yielding utility weights that can be used in comparative–effectiveness studies [45,46]. A version of this scale is currently under development for pediatric use [Tucker C, Pers. Comm.]. The brevity, yet comprehensiveness, of the global scales may allow for more frequent assessment with minimal responder burden and the ability to target further measurement based on scoring patterns on global items. This, too, will reduce burden for people who do not need further assessment, but will ensure that among those who do, more in-depth probing is undertaken.
While clinical validation studies of PROMIS scales in HSCT populations are still underway, the public availability of PROMIS scales, common metrics and multiple modes of administration address many of the measurement gaps in the field of HRQoL assessment. PROMIS tools can be used alone or in combination with HRQoL legacy tools to expand existing constructs. The availability of PROMIS scales will facilitate the inclusion of HRQoL end points into clinical trials through groups such as the Children’s Oncology Group and the Pediatric Blood and Marrow Transplant Consortium [47].
Despite the opportunities, the field of pediatric HRQoL assessment also faces several challenges that will need to be addressed over the next 5 years. First, we must strive to expand the capture of family members that we routinely assess to include fathers as well as mothers, languages other than English or Spanish, various education levels and different racial, ethnic and disease groups; the latter is reflective of the expanding indications for HSCT. Second, further research is needed to understand the impact of emerging trends in care (e.g., progenitor sources, reduced intensity conditioning regimens, approach to acute GVHD prevention) on HRQoL. In addressing both of these challenges, we must ensure that the studies are adequately statistically powered to evaluate the HRQoL of each of these personal and clinical factors. Third, we must also expand the time trajectory beyond the first year to understand the long-term HRQoL implications of HSCT on survivors, not restricting this inquiry to the subset that might continue to receive HSCT care in the HSCT center. Remote data capture, using Internet-based scales will facilitate this enormously. Related topics include understanding the extent to which the first year is clinical and HRQoL course is predictive of later functioning and identifying the factors that influence longer term HRQoL. Fourth, further research is needed to guide researchers and clinicians about how to approach HRQoL measurement as children ‘grow out’ of pediatric instruments to adult-based scales. Finally, we need to address the best way to capture the full experience of survivorship within a medical focus and within a ‘life’ focus, using qualitative approaches, as needed, to supplement the information gleaned from questionnaires.
HRQoL assessment after pediatric HSCT, like HSCT itself, has undergone considerable changes over the past 20 years. We have moved from fledgling instrumentation and small cross-sectional studies to robust measurement with validated instruments and large multicenter studies. Researchers have called for standardization of reporting studies to facilitate comparisons across them, as well as the use of appropriate statistical methods to handle thorny topics, such as nonignorable missing data, multiple outcomes, dual raters and myriad clinical complications.
In closing, clarity of purpose is needed in future HRQoL research. Over the past two decades, we have demonstrated that we can measure HRQoL following HSCT – often from children and adolescents themselves. Going forward, we must ask ourselves, what are we trying to understand about this complex phenomenon? What aspects must be obtained through patient (or proxy report) and what can be answered elsewhere? How can we best communicate HRQoL results so that they accurately reflect the experience of the patient (and family) receiving care and the providers who deliver it?
Key issues.
Pediatric health-related quality of life (HRQoL) assessment has greatly increased in the past 15 years in a variety of populations, including children undergoing hematopoietic stem cell transplant (HSCT).
Few studies report on the relationship between the well-characterized clinical outcomes of HSCT and parent or child ratings of pediatric HRQoL in general and HSCT-specific domains.
Inter-rater comparisons reveal differences in the perceptions of child and parent proxy raters that may affect their evaluation of pediatric HRQoL.
Research has demonstrated that parent/child agreement is influenced by child age, time post-transplant, parent emotional functioning and the presence of clinical complications.
Clinical complications resulting from pediatric HSCT have a much greater influence on parent-proxy ratings of the child’s HRQoL than the child’s ratings, reflecting potential variability in access to and understanding of clinical information.
Although HRQoL assessment may strengthen the patient–provider relationship, it has not been definitively shown to promote significant improvement in clinical management, patient satisfaction or patient HRQoL. Furthermore, providers report not understanding what to do with HRQoL scores and increased encounter time to administer measures as barriers.
The current ‘patient-centered’ climate has increased emphasis on the importance of collecting and utilizing HRQoL assessments, highlighted by efforts to provide individualized assessments and create standardized, lower-burden measurement tools.
Many challenges still need to be addressed in pediatric HRQoL assessment, particularly the longitudinal interplay between changing trends in clinical care and HRQoL ratings of children and parent proxies.
Footnotes
For reprint orders, please contact reprints@expert-reviews.com
Financial & competing interests disclosure
Funding was provided in part from the American Cancer Society (RSGPB-02-186-01-PBP, SK Parsons) and the National Cancer Institute (R01 CA 119196, SK Parsons). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Wilson IB, Cleary PD. Linking clinical variables with health-related quality of life. A conceptual model of patient outcomes. JAMA. 1995;273(1):59–65. [PubMed] [Google Scholar]
- 2.Parsons SK, Phipps S, Sung L, Baker KS, Pulsipher MA, Ness KK. NCI, NHLBI/ PBMTC First International Conference on Late Effects after Pediatric Hematopoietic Cell Transplantation: health-related quality of life, functional, and neurocognitive outcomes. Biol Blood Marrow Transplant. 2012;18(2):162–171. doi: 10.1016/j.bbmt.2011.12.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3•.Ravens-Sieberer U, Erhart M, Wille N, Wetzel R, Nickel J, Bullinger M. Generic health-related quality-of-life assessment in children and adolescents: methodological considerations. Pharmacoeconomics. 2006;24(12):1199–1220. doi: 10.2165/00019053-200624120-00005. Provides an excellent overview of the conceptualization and measurement issues in children’s health-related quality of life (HRQoL) assessment, building on the seminal work of Wilson and Cleary (see [1]) [DOI] [PubMed] [Google Scholar]
- 4.Varni JW, Burwinkle TM, Seid M, Skarr D. The PedsQL 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambul Pediatr. 2003;3(6):329–341. doi: 10.1367/1539-4409(2003)003<0329:tpaapp>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 5.Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care. 2001;39(8):800–812. doi: 10.1097/00005650-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Landgraf JM, Abetz L, Ware JE., Jr . The CHQ: A User’s Manual. 1. The Health Institute; MA, USA: 1996. [Google Scholar]
- 7.Kaplan SH, Barlow S, Spetter D, et al. Assessing functional status and health-related quality of life among school-aged children: Reliability and validity of a new self-reported measure. Qual Life Res. 1995;4(5):444–445. [Google Scholar]
- 8.Parsons SK, Shih MC, Mayer DK, et al. Preliminary psychometric evaluation of the Child Health Ratings Inventory (CHRIs) and Disease-Specific Impairment Inventory–Hematopoietic Stem Cell Transplantation (DSII–HSCT) in parents and children. Qual Life Res. 2005;14(6):1613–1625. doi: 10.1007/s11136-005-1004-2. [DOI] [PubMed] [Google Scholar]
- 9•.Parsons SK, Shih MC, Duhamel KN, et al. Maternal perspectives on children’s health-related quality of life during the first year after pediatric hematopoietic stem cell transplant. J Pediatr Psychol. 2006;31(10):1100–1115. doi: 10.1093/jpepsy/jsj078. One of the earliest studies to describe the longitudinal HRQoL trajectory of children after a hematopoetic stem cell transplant (HSCT). It describes nonignorable missing data and methodological approaches to account for it. [DOI] [PubMed] [Google Scholar]
- 10.Feeny D, Furlong W, Barr RD, Torrance GW, Rosenbaum P, Weitzman S. A comprehensive multiattribute system for classifying the health status of survivors of childhood cancer. J Clin Oncol. 1992;10(6):923–928. doi: 10.1200/JCO.1992.10.6.923. [DOI] [PubMed] [Google Scholar]
- 11.Tanzi EM. Health-related quality of life of hematopoietic stem cell transplant childhood survivors: state of the science. J Pediatr Oncol Nurs. 2011;28(4):191–202. doi: 10.1177/1043454211408100. [DOI] [PubMed] [Google Scholar]
- 12.Clarke SA, Eiser C, Skinner R. Health-related quality of life in survivors of BMT for paediatric malignancy: a systematic review of the literature. Bone Marrow Transplant. 2008;42(2):73–82. doi: 10.1038/bmt.2008.156. [DOI] [PubMed] [Google Scholar]
- 13.Tsimicalis A, Stinson J, Stevens B. Quality of life of children following bone marrow transplantation: critical review of the research literature. Eur J Oncol Nurs. 2005;9(3):218–238. doi: 10.1016/j.ejon.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 14.Phipps S, Dunavant M, Jayawardene D, Srivastiva DK. Assessment of health-related quality of life in acute in-patient settings: use of the BASES instrument in children undergoing bone marrow transplantation. Int J Cancer Suppl. 1999;12:18–24. doi: 10.1002/(sici)1097-0215(1999)83:12+<18::aid-ijc5>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 15.Phipps S, Dunavant M, Garvie PA, Lensing S, Rai SN. Acute health-related quality of life in children undergoing stem cell transplant: I. Descriptive outcomes. Bone Marrow Transplant. 2002;29(5):425–434. doi: 10.1038/sj.bmt.1703377. [DOI] [PubMed] [Google Scholar]
- 16.Phipps S, Dunavant M, Lensing S, Rai SN. Acute health-related quality of life in children undergoing stem cell transplant: II. Medical and demographic determinants. Bone Marrow Transplant. 2002;29(5):435–442. doi: 10.1038/sj.bmt.1703376. [DOI] [PubMed] [Google Scholar]
- 17.Kelly MJ, Pennarola BW, Rodday AM, Parsons SK. Journeys to Recovery Study; HSCT-CHESS™ Study. Health-related quality of life (HRQL) in children with sickle cell disease and thalassemia following hematopoietic stem cell transplant (HSCT) Pediatr Blood Cancer. 2012;59(4):725–731. doi: 10.1002/pbc.24036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McQuellon RP, Russell GB, Cella DF, et al. Quality of life measurement in bone marrow transplantation: development of the Functional Assessment of Cancer Therapy–Bone Marrow Transplant (FACT–BMT) scale. Bone Marrow Transplant. 1997;19(4):357–368. doi: 10.1038/sj.bmt.1700672. [DOI] [PubMed] [Google Scholar]
- 19.Huang I, Bishop M, Cella D, et al. Development of the Functional Assessment of Cancer Therapy–Bone Marrow Transplant Survivor (FACT–BMTS) scale. 2009 International Society for Quality of Life Research meeting abstracts. QLR J. 2009;38:Abstract 1378. [Google Scholar]
- 20.Pavletic SZ, Lee SJ, Socie G, Vogelsang G. Chronic graft-versus-host disease: implications of the National Institutes of Health consensus development project on criteria for clinical trials. Bone Marrow Transplant. 2006;38(10):645–651. doi: 10.1038/sj.bmt.1705490. [DOI] [PubMed] [Google Scholar]
- 21.Fraser CJ, Bhatia S, Ness K, et al. Impact of chronic graft-versus-host disease on the health status of hematopoietic cell transplantation survivors: a report from the Bone Marrow Transplant Survivor Study. Blood. 2006;108(8):2867–2873. doi: 10.1182/blood-2006-02-003954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pidala J, Kurland B, Chai X, et al. Patient-reported quality of life is associated with severity of chronic graft-versus-host disease as measured by NIH criteria: report on baseline data from the Chronic GVHD Consortium. Blood. 2011;117(17):4651–4657. doi: 10.1182/blood-2010-11-319509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23•.Sanders JE, Hoffmeister PA, Storer BE, Appelbaum FR, Storb RF, Syrjala KL. The quality of life of adult survivors of childhood hematopoietic cell transplant. Bone Marrow Transplant. 2010;45(4):746–754. doi: 10.1038/bmt.2009.224. Despite being a single institutional experience, this is one of the few studies describing the HRQoL of long-term survivors of pediatric HSCT, describing factors associated with a worse HRQoL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matthes-Martin S, Lamche M, Ladenstein R, et al. Organ toxicity and quality of life after allogeneic bone marrow transplantation in pediatric patients: a single centre retrospective analysis. Bone Marrow Transplant. 1999;23(10):1049–1053. doi: 10.1038/sj.bmt.1701754. [DOI] [PubMed] [Google Scholar]
- 25.Forinder U, Löf C, Winiarski J. Quality of life following allogeneic stem cell transplantation, comparing parents’ and children’s perspective. Pediatr Transplant. 2006;10(4):491–496. doi: 10.1111/j.1399-3046.2006.00507.x. [DOI] [PubMed] [Google Scholar]
- 26.Pavletic SZ, Martin P, Lee SJ, et al. Response Criteria Working Group. Measuring therapeutic response in chronic graft-versus-host disease: National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: IV. Response Criteria Working Group report. Biol Blood Marrow Transplant. 2006;12(3):252–266. doi: 10.1016/j.bbmt.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 27.McHorney CA, Ware JE, Jr, Raczek AE. The MOS 36-item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31(3):247–263. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Young NL, Williams JI, Yoshida KK, Wright JG. Measurement properties of the activities scale for kids. J Clin Epidemiol. 2000;53(2):125–137. doi: 10.1016/s0895-4356(99)00113-4. [DOI] [PubMed] [Google Scholar]
- 29.Brice L, Weiss R, Wei Y, et al. Health-related quality of life (HRQoL): the impact of medical and demographic variables upon pediatric recipients of hematopoietic stem cell transplantation. Pediatr Blood Cancer. 2011;57(7):1179–1185. doi: 10.1002/pbc.23133. [DOI] [PubMed] [Google Scholar]
- 30.Kline RM. ‘Risk adapted’ assessments of health related quality of life in HSCT recipients. Pediatr Blood Cancer. 2011;57(7):1095–1096. doi: 10.1002/pbc.23212. [DOI] [PubMed] [Google Scholar]
- 31.Oberg JA, Bender JG, Morris E, et al. Pediatric allo-SCT for malignant and non-malignant diseases: impact on health-related quality of life outcomes. Bone Marrow Transplant. 2012 doi: 10.1038/bmt.2012.217. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 32••.Upton P, Lawford J, Eiser C. Parent–child agreement across child health-related quality of life instruments: a review of the literature. Qual Life Res. 2008;17(6):895–913. doi: 10.1007/s11136-008-9350-5. This ‘must-read’ review of the literature on inter-rater comparisons contains a call for standardization of reporting and suggests the role of qualitative methods to enhance our collection understanding of the topic. [DOI] [PubMed] [Google Scholar]
- 33••.Davis E, Nicolas C, Waters E, et al. Parent-proxy and child self-reported health-related quality of life: using qualitative methods to explain the discordance. Qual Life Res. 2007;16(5):863–871. doi: 10.1007/s11136-007-9187-3. Includes fascinating results gleaned from qualitative methods, that were used to explore parent–child differences. [DOI] [PubMed] [Google Scholar]
- 34.Lal SD, McDonagh J, Baildam E, et al. Agreement between proxy and adolescent assessment of disability, pain, and well-being in juvenile idiopathic arthritis. J Pediatr. 2011;158(2):307–312. doi: 10.1016/j.jpeds.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barrera M, Boyd-Pringle LA, Sumbler K, Saunders F. Quality of life and behavioral adjustment after pediatric bone marrow transplantation. Bone Marrow Transplant. 2000;26(4):427–435. doi: 10.1038/sj.bmt.1702527. [DOI] [PubMed] [Google Scholar]
- 36.Parsons S, Terrin N, Ratichek S, et al. Trajectories of HRQL following pediatric hematopoietic stem cell transplantation (HSCT) QLR J. 2009;38:Abstract 1420. [Google Scholar]
- 37.Parsons SK, Barlow SE, Levy SL, Supran SE, Kaplan SH. Health-related quality of life in pediatric bone marrow transplant survivors: according to whom? Int J Cancer Suppl. 1999;12:46–51. doi: 10.1002/(sici)1097-0215(1999)83:12+<46::aid-ijc9>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 38.Parsons S, Terrin N, Ratichek S, Tighiouart H, Chang G. The influence of clinical events after HSCT on parent and child assessments of HRQL. International Society for Quality of Life Research meeting abstracts. QLR J. 2007;38:1371–1372. [Google Scholar]
- 39.Hedeker D, Gibbons R. Longitudinal Data Analysis. John Wiley & Sons Inc; NJ, USA: 2006. [Google Scholar]
- 40.Detmar SB, Muller MJ, Schornagel JH, Wever LD, Aaronson NK. Health-related quality-of-life assessments and patient-physician communication: a randomized controlled trial. JAMA. 2002;288(23):3027–3034. doi: 10.1001/jama.288.23.3027. [DOI] [PubMed] [Google Scholar]
- 41.Velikova G, Booth L, Smith AB, et al. Measuring quality of life in routine oncology practice improves communication and patient well-being: a randomized controlled trial. J Clin Oncol. 2004;22(4):714–724. doi: 10.1200/JCO.2004.06.078. [DOI] [PubMed] [Google Scholar]
- 42.Rosenbloom SK, Victorson DE, Hahn EA, Peterman AH, Cella D. Assessment is not enough: a randomized controlled trial of the effects of HRQL assessment on quality of life and satisfaction in oncology clinical practice. Psychooncology. 2007;16(12):1069–1079. doi: 10.1002/pon.1184. [DOI] [PubMed] [Google Scholar]
- 43••.Engelen V, Detmar S, Koopman H, et al. Reporting health-related quality of life scores to physicians during routine follow-up visits of pediatric oncology patients: is it effective? Pediatr Blood Cancer. 2012;58(5):766–774. doi: 10.1002/pbc.23158. Among the first intervention studies of its kind in pediatric oncology, this paper demonstrates that provision of HRQoL to providers resulted in increased discussion of emotional functioning without increasing the duration of the visit. Of note, no improvements were detected in overall HRQoL or in satisfaction levels, and there was no change in referrals. [DOI] [PubMed] [Google Scholar]
- 44.Frick E, Borasio GD, Zehentner H, Fischer N, Bumeder I. Individual quality of life of patients undergoing autologous peripheral blood stem cell transplantation. Psychooncology. 2004;13(2):116–124. doi: 10.1002/pon.730. [DOI] [PubMed] [Google Scholar]
- 45.Hays RD, Bjorner JB, Revicki DA, Spritzer KL, Cella D. Development of physical and mental health summary scores from the Patient-Reported Outcomes Measurement Information System (PROMIS) global items. Qual Life Res. 2009;18(7):873–880. doi: 10.1007/s11136-009-9496-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46••.Revicki DA, Kawata AK, Harnam N, Chen WH, Hays RD, Cella D. Predicting EuroQol (EQ-5D) scores from the Patient-Reported Outcomes Measurement Information System (PROMIS) global items and domain item banks in a United States sample. Qual Life Res. 2009;18(6):783–791. doi: 10.1007/s11136-009-9489-8. Reports on the ‘cross-walk’ between health profile-based and preference-based measurements. The derived utility weights generated by the cross-walk can be used in comparative–effectiveness and cost–effectiveness analyses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47•.Pulsipher MA, Horwitz EM, Haight AE, et al. Advancement of pediatric blood and marrow transplantation research in North America: priorities of the Pediatric Blood and Marrow Transplant Consortium. Biol Blood Marrow Transplant. 2010;16(9):1212–1221. doi: 10.1016/j.bbmt.2009.12.536. A timely and well-written update on the progress that has been made in pediatric HSCT, reflecting the current state of the science and future directions. [DOI] [PMC free article] [PubMed] [Google Scholar]
Websites
- 101.National Cancer Institute. [Accessed on 25 July 25 2011];Common Terminology Criteria for Adverse Events version 3.0 (CTCAE) http://ctep.cancer.gov.
- 102. [Accessed 30 May 2012];NIH PROMIS®. www.nihpromis.org.