Abstract
Background
Anemia refers to low hemoglobin (Hb) levels, represents a common symptom and complication in cancer patients and was reported to negatively influence survival in patients with various malignancies. In the present study, we aimed to explore the prognostic impact of pre-operative Hb levels on clinical outcome in a large cohort of soft tissue sarcoma (STS) patients after curative surgery.
Methods
Retrospective data from 367 STS patients, which were operated between 1998 and 2013, were included in the study. Cut-off levels for anemia were defined as Hb<13 g/dl in males and Hb<12 g/dl in females according to the current WHO guidelines. The impact of pre-operative Hb levels on cancer-specific survival (CSS) and overall survival (OS) was assessed using Kaplan-Meier curves. Additionally, Hb levels were compared for the prognostic influence on CSS and OS applying univariate and multivariate Cox proportional models.
Results
Hb level was associated with established prognostic factors, including age, tumor grade, size and depth (p<0.05). Kaplan-Meier curves showed that low Hb levels were significantly associated with decreased CSS and OS in STS patients (p<0.001 for both endpoints, log-rank test). In multivariate analysis, we found an independent association between low Hb levels and poor CSS and OS (HR = 0.46, Cl 95% = 0.25–0.85, p = 0.012; HR = 0.34, Cl 95% = 0.23–0.51, p<0.001).
Conclusion
The present data underline a negative prognostic impact of low pre-operative Hb levels on clinical outcome in STS patients. Thus, Hb levels may provide an additional and cost-effective tool to discriminate between STS patients that are at high risk of mortality.
Introduction
Soft tissue sarcomas (STSs) are a relatively rare and heterogenous group of malignancies. They include a diverse group of non-epithelial extraskeletal tumors that show the histological differentiation of muscle, fat, nerves, and fibrous tissue. Their variable cellular appearance results in categorization of over 100 histologic sarcoma subtypes that differ in biology and behavior and make diagnosis and treatment challenging for clinicians [1]. Accordingly, the overall 5-year survival rate is only 50%, and especially STS patients that present with high grade tumors are at significant risk of disease recurrence [2]. The most important prognostic factors for local recurrence and distant metastasis are tumor size, tumor grade, histologic subtype, tumor depth and site and age at diagnosis [3]. However, predicting the outcome of STS patients remains sub-optimal and lacks predictive accuracy. Thus, there is an urgent need to identify additional prognostic markers. Blood-based biomarkers seem to be especially attractive for prognosis purposes, which might help clinicians to adopt preventive and therapeutic strategies for high risk patients [4]–[6].
Anemia is the most common hematologic abnormality in cancer patients. Although anemia incidence varies with types of malignancies and disease stage, it has been assumed that over 40% of all cancer patients are anemic at the time of diagnosis, a rate that increases up to 80% in patients with advanced disease [7]. The origin of cancer-related anemia is often multifactorial. It is a consequence of dysfunction of iron metabolism, inadequate production of erythropoietin (Epo) and inadequate response of the bone marrow to endogenous Epo, suppressed hematopoiesis through bone marrow infiltration, increased destruction of red blood cells, reduced number of erythroid progenitor cells in the bone marrow and production of inflammatory cytokines [8]–[10]. As the clinical symptoms of anemia start slowly, hemoglobin (Hb) level represents the most important predictor in guiding anemia evaluation and treatment. According to the World Health Organization (WHO), the cut-off levels for anemia were defined as Hb levels <13 g/dl in males and Hb levels <12 g/dl in females [11]. Recent studies indicate that a low Hb level is an unfavorable prognostic factor in diverse cancer types, including non-small cell lung cancer (NSCLC), ovarian carcinoma and pancreatic cancer [12]–[14]. To the best of our knowledge, only one similar sized study in surgical treated STS patients reported about a poor prognostic value of Hb levels [15]. Therefore, the present study was conducted to externally validate the prognostic significance of pre-operative Hb levels on cancer-specific survival (CSS) and overall survival (OS) in a large cohort of STS patients who underwent curative surgery.
Materials and Methods
Subjects
Three hundred and sixty seven patients with histologically confirmed STS who have been operated between March 1998 and August 2013 at the Department of Orthopaedic Surgery, Medical University of Graz, were retrospectively analyzed. Patients without available laboratory parameters or histological proven diagnosis were not included in this study. All patients were of Caucasians ancestry. The date of last follow-up documentation was the 1st July, 2014. All patients were included in the follow-up program of the Department of Orthopaedic Surgery and the Division of Clinical Oncology, Medical University of Graz, providing follow-up examinations in regular intervals (3 month intervals in years 1–3, 6 months intervals in years 4–5, and 12 month intervals in years 6–15 after diagnosis). The laboratory data, including pre-operative Hb, was obtained by pre-operative determination one to three days before surgery was performed. Follow-up investigations included clinical check-up and radiological analyses (computed tomography alternating with X-ray of the chest, local magnetic resonance imaging, and abdominal ultrasound). Clinico-pathological data including histopathological diagnosis and tumor grade were retrospectively obtained from the patients history. For the present study, all histological specimens were centrally reviewed by an independent experienced soft tissue pathologist (B. LA). All sarcomas were diagnosed according to the current WHO classification of soft tissue and bone tumors [1]. Tumors were graded according to the French Federation of Cancer Centres Sarcoma Group (FNCLCC) grading system if possible or tumor grade was defined by tumor entity [16]. Cases formerly classified as malignant fibrous histiocytomas have been re-classified according to the current diagnostic criteria [1], [17]. This study has been approved by the Institutional Review Board (IRB) of the Medical University of Graz. Written informed consent was obtained from all participants.
Statistical analysis
The primary endpoint of this study was CSS, which was calculated from the date of diagnosis to the date of cancer-related death. Secondary endpoint included OS which was defined as time between diagnosis and death of any cause. For anemia, the pre-published and most commonly used cut-off value of Hb level <13 g/dl in males and Hb level <12 g/dl in females was applied [12], [15]. The association between the Hb level and clinico-pathological parameters was evaluated by non-parametric tests (Mann-Whitney U and chi square test). Patients’ clinical endpoints were calculated using the Kaplan-Meier method and compared by the log rank test. Backward stepwise multivariate Cox proportion analysis was performed to determine the influence of age, gender, tumor size, grade and depth and Hb levels on CSS and OS. Hazard ratios (HRs) estimated from the Cox analysis were reported as relative risks with corresponding 95% confidence intervals (CIs). All statistical analyses were performed using the Statistical Package for Social Sciences version 20.0 (SPSS Inc., Chicago, IL, USA) or R program. A two-sided p<0.05 was considered statistically significant.
Results
One hundred and eighty three male patients and 184 female patients with STS were included in the study. The median follow-up time was 37 months (interquartile range 14 to 88 months). Forty seven patients were lost to follow-up. The 367 patients were histologically classified as follows: 101 myxofibrosarcomas, 94 liposarcomas, 43 leiomyosarcomas, 29 synovial sarcoma, 13 malignant peripheral nerve sheath tumours (MPNSTs) and 87 other histological subtypes. The primary tumour site was localized at the upper extremities (n = 86), lower extremities (n = 219), thorax/trunk (n = 52), retroperitoneal/intraabdominal (n = 6) and head/neck (n = 4). The tumor depth was defined superficial in 120 patients and deep in 247 patients. All STS patients underwent surgery, and 43 (11.7%) were administered adjuvant chemotherapy. Two hundred twenty-five (61.3%) of the 367 sarcoma patients received adjuvant radiation therapy for the primary tumour site. The resection margins were determined as wide in 331 and marginal in 36 STS patients. Of the 367 STS patients, 70 (19.1%) developed metastatic disease, of which 62 (16.9%) died due to their advanced disease state. Thirty three patients (9%) presented with local recurrence. Overall 136 (37.1%) patients died of any cause by their most recent follow-up.
We validated the pre-published values of Hb level <13 g/dl in males and Hb level <12 g/dl in females as the cut-offs for the continuous Hb. Consequently, we separated the STS patients into two groups according to low Hb (<13 g/dl in males and <12 g/dl in females) levels or high Hb (≥13 g/dl in males and ≥12 g/dl in females) levels and tested the association between pre-operative Hb levels and other clinico-pathological factors. We found a statistically significant association between a low Hb level and older age, higher tumor grade, deep tumor location and larger tumor size (p<0.05), whereas no correlation with gender, tumor site and histologic subtype could be demonstrated (Table 1).
Table 1. The relation between clinico-pathological parameters and pre-operative Hb levels of patients with soft tissue sarcoma (n = 367).
| Characteristics | Hb female <12 g/dlor male <13 g/dl | Hb female ≥12 g/dlor male ≥13 g/dl | p-value |
| Age at diagnosis (yrs.) | |||
| <60 | 10 | 88 | 0.009 |
| ≥60 | 60 | 209 | |
| Gender | |||
| Female | 34 | 150 | 0.771 |
| Male | 36 | 147 | |
| Tumor depth | |||
| Superficial | 12 | 108 | 0.002 |
| Deep | 58 | 189 | |
| Tumor grade | |||
| G1+G2 | 10 | 130 | <0.001 |
| G3 | 60 | 167 | |
| Tumor size | |||
| <5 cm | 7 | 86 | <0.001 |
| 5–10 cm | 27 | 124 | |
| >10 cm | 36 | 87 | |
| Tumor site | |||
| Upper extremity | 14 | 72 | 0.729 |
| Lower extremity | 46 | 173 | |
| Thoracic/trunk | 9 | 43 | |
| Retroabdominal/intraabdominal | 1 | 5 | |
| Head/neck | 0 | 4 | |
| Histologic subtype | |||
| Liposarcoma | 12 | 82 | 0.178 |
| Myxofibrosarcoma | 23 | 78 | |
| Leiomyosarcoma | 10 | 33 | |
| Synovial sarcoma | 2 | 27 | |
| MPNST | 2 | 11 | |
| Other | 21 | 66 |
MPNST, malignant peripheral nerve sheath tumor.
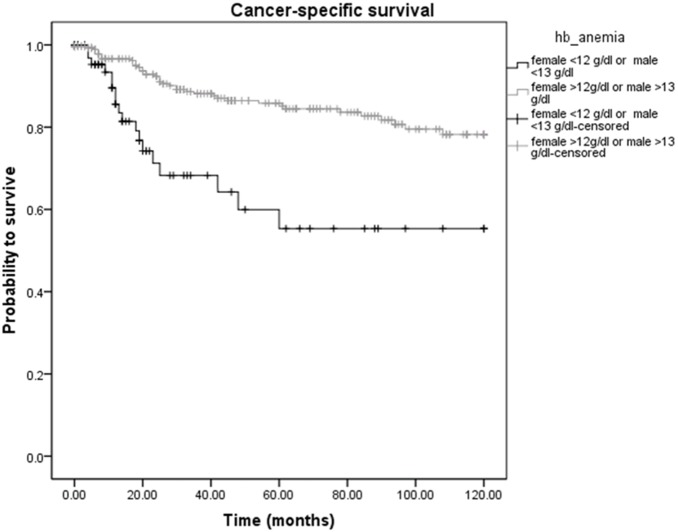
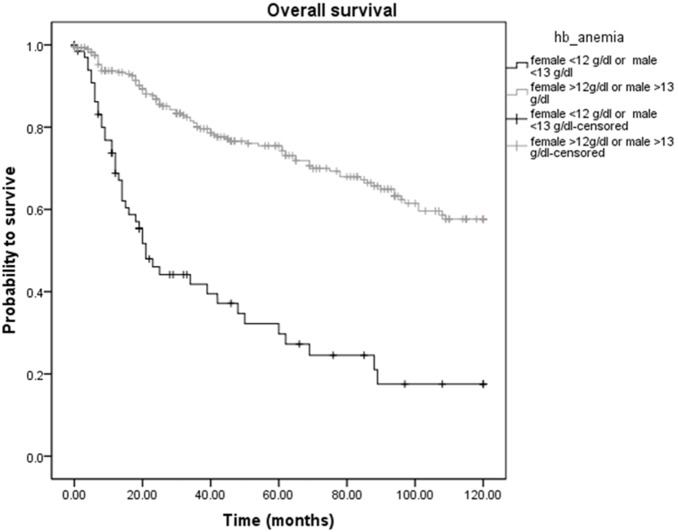
Analyzing the Hb levels into more detail, among the 367 STS patients, cancer-related death was diagnosed in 18 of 70 (25.7%) patients with low Hb levels and in 40 of 297 (13.5%) patients with high Hb levels (p<0.05). Overall deaths occurred in 44 (62.9%) patients with low Hb levels and in 82 (27.6%) patients with high Hb levels, respectively (p<0.05). Figures 1 and 2 show the Kaplan-Meier curves for CSS and OS and reveal that a low Hb level is a significant factor for decreased CSS and OS in STS patients (p<0.001 for CSS and OS, log-rank test).
Figure 1. Kaplan-Meier curve for cancer-specific survival regarding high (≥13 g/dl in males and ≥12 g/dl in females) versus low (<13 g/dl in males and <12 g/dl in females) hemoglobin levels (p<0.001).
Figure 2. Kaplan-Meier curve for overall survival regarding high (≥13 g/dl in males and ≥12 g/dl in females) versus low (<13 g/dl in males and <12 g/dl in females) hemoglobin levels (p<0.001).
To investigate whether serum Hb levels and other clinical-pathological factors are associated with clinical outcome of STS patients, univariate and multivariate Cox proportional models for CSS and OS were calculated. Univariate analysis identified a high tumor grade (G1+G2 versus G3, p = 0.002) and a low Hb level (<13 g/dl in males and <12 g/dl in females versus ≥13 g/dl in males and ≥12 g/dl in females, p<0.001) as poor prognostic factors for CSS in our study cohort (Table 2). For OS, we found a significant association between an older age (<60 versus ≥60, p = 0.002), a high tumor grade (G1+G2 versus G3, p<0.001) and a low Hb level (<13 g/dl in males and <12 g/dl in females versus ≥13 g/dl in males and ≥12 g/dl in females, p<0.001) and poor clinical outcome (Table 3). In the multivariate analysis that included age, gender, tumor grade, tumor depth, tumor size and the Hb level, we determined tumor grade and Hb level as independent prognostic factors for CSS (HR = 2.34, 95% CI = 1.28–4.30, p = 0.006; HR = 0.46, 95% CI = 0.25–0.85, p = 0.012; Table 2) and age, tumor grade and Hb level for OS (HR = 1.77, 95% CI = 1.10–2.85, p = 0.019; HR = 2.55, 95% CI = 1.67–3.89, p<0.001; HR = 0.34, 95% CI = 0.23–0.51, p<0.001; Table 3).
Table 2. Univariate and multivariate Cox proportional analysis regarding cancer-specific survival.
| Parameter | Univariate analysis | Multivariate analysis | ||
| HR (95% Cl) | p-value | HR (95% Cl) | p-value | |
| Age at operation (yrs.) | ||||
| <60 | 1 (referent) | 1 (referent) | ||
| ≥60 | 1.98 (1.01–3.90) | 0.048 | 1.75 (0.87–3.48) | 0.115 |
| Gender | ||||
| Female | 1 (referent) | 1 (referent) | ||
| Male | 0.92 (0.56–1.52) | 0.757 | 0.78 (0.46–1.30) | 0.335 |
| Tumor depth | ||||
| Superficial | 1 (referent) | 1 (referent) | ||
| Deep | 1.36 (0.76–2.43) | 0.301 | 1.22 (0.65–2.31) | 0.538 |
| Tumor grade | ||||
| G1+G2 | 1 (referent) | 1 (referent) | ||
| G3 | 2.45 (1.38–4.35) | 0.002 | 2.34 (1.28–4.30) | 0.006 |
| Tumor size | ||||
| <5 cm | 1 (referent) | 1 (referent) | ||
| 5–10 cm | 1.34 (0.96–1.87) | 0.091 | 1.26 (0.86–1.84) | 0.238 |
| ≥10 cm | ||||
| Hb level | ||||
| <13 g/dl in males and | ||||
| >12 g/dl in females | 1 (referent) | 1 (referent) | ||
| ≥13 g/dl in males and | 0.33 (0.19–0.57) | <0.001 | 0.46 (0.25–0.85) | 0.012 |
| ≥12 g/dl in females | ||||
Table 3. Univariate and multivariate Cox proportional analysis regarding overall survival.
| Parameter | Univariate analysis | Multivariate analysis | ||
| HR (95% Cl) | p-value | HR (95% Cl) | p-value | |
| Age at operation (yrs.) | ||||
| <60 | 1 (referent) | 1 (referent) | ||
| ≥60 | 2.08 (1.30–3.30) | 0.002 | 1.77 (1.10–2.85) | 0.019 |
| Gender | ||||
| Female | 1 (referent) | 1 (referent) | ||
| Male | 0.94 (0.67–1.32) | 0.718 | 0.83 (0.58–1.17) | 0.281 |
| Tumor depth | ||||
| Superficial | 1 (referent) | 1 (referent) | ||
| Deep | 1.09 (0.75–1.59) | 0.648 | 0.99 (0.65–1.50) | 0.956 |
| Tumor grade | ||||
| G1+G2 | 1 (referent) | 1 (referent) | ||
| G3 | 2.92 (1.95–4.35) | <0.001 | 2.55 (1.67–3.89) | <0.001 |
| Tumor size | ||||
| <5 cm | 1 (referent) | 1 (referent) | ||
| 5–10 cm | 1.21 (0.96–1.51) | 0.103 | 1.14 (0.88–1.47) | 0.320 |
| ≥10 cm | ||||
| Hb level | ||||
| <13 g/dl in males and | ||||
| >12 g/dl in females | 1 (referent) | 1 (referent) | ||
| ≥13 g/dl in males and | 0.26 (0.18–0.37) | <0.001 | 0.34 (0.23–0.51) | <0.001 |
| ≥12 g/dl in females | ||||
Overall, in liposarcoma patients the incidence of anaemia was 12.8% (12 out of 94 patients) compared to 19% in the whole STS cohort. We analysed the incidence of anemia in these patients and found that the frequency of anaemia was significantly higher in patients with grade 2 (15%) or 3 (30.4%) liposarcomas compared to grade 1 (3.8%) liposarcoma patients (p = 0.006).
Discussion
In the present study, we found an independently significant association between low pre-operative Hb levels and poor clinical outcome in STS patients.
Pre-treatment anemia, indicated by low Hb levels, was reported to negatively influence clinical outcome in various types of cancer. Aoe et al reported a reduced median survival time (MST) in a large cohort of 611 NSCLC patients with anemia, defined as a Hb level <13 g/dl in males and <12 g/dl in females, at first presentation [12]. In pancreatic cancer, pre-operative Hb levels <12 g/dl were significantly associated with poor survival [13]. Another study, that included 206 patients with ovarian carcinoma, showed a decreased OS in patients with low Hb levels [14]. In STS, Nakamura et al demonstrated in 376 patients that low Hb levels (Hb levels <13 g/dl in men and <12 g/dl in women) correlated with established poor prognostic factors, including larger tumor size, higher tumor grade and older age. Furthermore, STS patients with anemia showed an independent association with reduced disease-specific survival (DSS) and event-free survival (EFS) [15]. In line with these findings, in the present study, low Hb levels were found predominantly in STS patients presenting with well-known factors associated with worse prognosis, such as high tumor grade, deep tumor location, large tumor size and older age. Similarly, we demonstrated that low Hb levels were significantly associated with decreased CSS in uni- and multivariate analysis. Additionally, an independent association between low Hb level and OS was observed in our study. These results might be explained by the fact that, on the one hand, in patients with malignancies, anemia might result from the extent of cancer-burden, and on the other hand may also be caused by co-morbidities that result in decreased survival, such as coagulation disorders, hemolysis, renal insufficiency, bleeding, or nutritional deficiencies [18]–[20]. Interestingly, in a subgroup analysis, Nakamura et al observed a higher incidence of anemia in patients with malignant fibrous histiocytomas (MFHs) and a low incidence of anemia in liposarcoma patients and attributed this to the fact that most of the liposarcoma patients presented with grade 1 liposarcomas. They also analyzed the relationship between anaemia, survival, and event separately for patients with malignant fibrous histiocytomas (MFHs) and liposarcomas and found that a low Hb level was a significant adverse prognostic factor for EFS in MFHs and liposarcomas. Moreover, patients with MFHs and anemia showed a significantly poorer DSS than those without anemia, whereas in liposarcomas, the rates of DSS did not significantly differ between those with and without anaemia [15]. To clarify these findings, in the present study, we analyzed the incidence of anemia in liposarcoma patients separately with respect to the grade and found that the frequency of anemia was higher in patients with grade 2 or 3 liposarcomas compared to grade 1 liposarcomas.
Based on recent data from experimental and clinical studies, there is increasing evidence that low Hb levels are associated with a poor tumor oxygenation and that up to 50–60% of locally advanced solid tumors may exhibit hypoxic tissue areas [21]. Hypoxia in solid tumors has been associated with malignant progression in terms of recurrence, loco-regional spread and distant metastasis, mediated by proteomic and genomic changes activating angiogenesis, anaerobic metabolism and other processes that enable tumor cells to survive or escape their oxygen-deficient environment, thus promoting the selection and expansion of more aggressive tumor clones [22]. Multivariate analyses have shown that hypoxia is a powerful prognostic factor in various cancer types, including STS. Brizel et al reported a significantly lower 18-month actuarial disease-free survival (DFS) rate in STS patients with tumor median oxygen pressure (pO2) values of <10 mmHg compared to patients with median pO2>10 mmHg (35% versus 70%, p = 0.01). Additionally, they demonstrated that the median pO2 for metastasized tumors was lower than for non-metastasized tumors [23]. In another study that investigated the relationship between tumor oxygenation and cell proliferation in STS, a significant association between the median pO2 and tumor cell potential doubling time was found, reporting the fastest proliferating tumor cells in the poorest oxygenated tumors [24]. Thus, we hypothesize that one reason for the poor clinical outcome in STS patients with low Hb levels in the present study might be due to tumor hypoxia. On the other hand, some cytokines, such as interleukin-6 (IL-6), have been demonstrated to induce anemia [25], [26]. It has been shown that IL-6 induces the liver to produce hepcidin. Hepcidin decreases iron absorption from the bowel and the gut and blocks iron utilization in the bone marrow, so that it is not available for erythropoiesis, resulting in cancer-related anemia [25]. Rutkowski et al reported that increased serum levels of IL-6 were found in the majority of STS patients included in their study [27].
In conclusion, the findings in our study suggest that low pre-operative Hb levels are significantly associated with decreased CSS and OS in STS patients after curative surgery. In context with the previously published study by Nakamura et al., we independently confirmed (or externally validated) their findings [15]. As both studies used a middle European cohort of Caucasian ancestry, evidence is supported that hemoglobin levels in STS patients are a general prognostic factor in other central European countries or Caucasians in the North American hemisphere. We identified anemia as an additional prognostic factor that might help to allow a more accurate strategy for stratifying patients with STS for risk of tumor-related and unrelated death. Markers like the haemoglobin can provide improvements in individual therapy modalities and follow-up schedules [4], [5]. In STS patients, administration of adjuvant chemotherapy is controversially discussed. Consequently, easily accessible, cheap and robust prognostic factors, such as Hb levels, might be helpful to allow treatment options to be tailored to the individual risk situation. With accurate prediction, patients at low risk for disease recurrence can be spared the toxicity of further treatment, whereas patients at high risk might be considered as candidates for additional adjuvant systemic therapy, novel experimental or more aggressive treatment approaches or more stringent follow-up schedules. As with all retrospective studies, our cohort is not without limitations. There have been several different surgeons involved over the years in the treatment of patients, and some other factors could also led to a selection bias, especially as we only included patients that were physically appropriate for a surgical procedure. In addition, we had no information about the influence of blood transfusions or administration of erythropoiesis stimulating agents on prognosis. Although the present investigations are limited due to their retrospective study design and the mixture of various histologic subtypes of STS, our data indicates that Hb levels, which are frequently measured in the routinely tested complete blood count panel, may represent a potentially important variable that can be included with other clinico-pathological parameters and laboratory findings to create new cancer prognosis assessment models.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper, tables and figures.
Funding Statement
The authors have no support or funding to report.
References
- 1.Fletcher CDM, Bridge JA, Hogendoorn P, Mertens F (2013) WHO Classification of Tumours of Soft Tissue and Bone. In: WHO Classification of Tumours. 4th edn, Vol. 5. Lyon, France: IARC Press.
- 2. Cormier JN, Pollock RE (2004) Soft tissue sarcomas. CA Cancer J Clin 54: 94–109. [DOI] [PubMed] [Google Scholar]
- 3. Kattan MW, Leung DH, Brennan MF (2002) Postoperative nomogram for 12-year sarcoma-specific death. J Clin Oncol 20: 791–796. [DOI] [PubMed] [Google Scholar]
- 4. Szkandera J, Gerger A, Liegl-Atzwanger B, Absenger G, Stotz M, et al. (2014) The lymphocyte/monocyte ratio predicts poor clinical outcome and improves the predictive accuracy in patients with soft tissue sarcomas. Br J Cancer 110: 435–440. [DOI] [PubMed] [Google Scholar]
- 5. Szkandera J, Gerger A, Liegl-Atzwanger B, Absenger G, Stotz M, et al. (2013) Validation of the prognostic relevance of plasma C-reactive protein levels in soft-tissue sarcoma patients. Br J Cancer 109: 2316–2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Szkandera J, Absenger G, Liegl-Atzwanger B, Pichler M, Stotz M, et al. (2013) Elevated preoperative neutrophil/lymphocyte ratio is associated with poor prognosis in soft-tissue sarcoma patients. Br J Cancer 108: 1677–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Knight K, Wade S, Balducci L (2004) Prevalence and outcomes of anemia in cancer: a systematic review of the literature. Am J Med 116 Suppl 7A11S–26S. [DOI] [PubMed] [Google Scholar]
- 8. De Rienzo DP, Saleem A (1990) Anemia of chronic disease: a review of pathogenesis. Tex Med 86: 80–83. [PubMed] [Google Scholar]
- 9. Miller CB, Jones RJ, Piantadosi S, Abeloff MD, Spivak JL (1990) Decreased erythropoietin response in patients with the anemia of cancer. N Engl J Med 322: 1689–1692. [DOI] [PubMed] [Google Scholar]
- 10. Ludwig H, Fritz E (1998) Anemia in cancer patients. Semin Oncol 25: 2–6. [PubMed] [Google Scholar]
- 11.de Benoist B, McLean E, Egli I, Cogswell M (2008) Worldwide prevalence of anaemia 1993–2005: WHO global database on anaemia. Geneva, Switzerland: WHO Press. 4.
- 12. Aoe K, Hiraki A, Maeda T, Katayama H, Fujiwara K, et al. (2005) Serum hemoglobin level determined at the first presentation is a poor prognostic indicator in patients with lung cancer. Intern Med 44: 800–804. [DOI] [PubMed] [Google Scholar]
- 13. Ruiz-Tovar J, Martín-Pérez E, Fernández-Contreras ME, Reguero-Callejas ME, Gamallo-Amat C (2011) Identification of prognostic factors in pancreatic cancer. Cir Cir 79: 313–322. [PubMed] [Google Scholar]
- 14. Obermair A, Handisurya A, Kaider A, Sevelda P, Kölbl H, et al. (1998) The relationship of pretreatment serum hemoglobin level to the survival of epithelial ovarian carcinoma patients: a prospective review. Cancer 83: 726–731. [PubMed] [Google Scholar]
- 15. Nakamura T, Grimer R, Gaston C, Carter S, Tillman R, et al. (2013) The relationship between pretreatment anaemia and survival in patients with adult soft tissue sarcoma. J Orthop Sci 18: 987–993. [DOI] [PubMed] [Google Scholar]
- 16. Coindre JM (2006) Grading of soft tissue sarcomas: review and update. Arch Pathol Lab Med 130: 1448–1453. [DOI] [PubMed] [Google Scholar]
- 17. Liegl-Atzwanger B, Hofmann G, Leithner A, Beham A (2009) Undifferentiated high-grade pleomorphic sarcoma (UHPS): Diagnostic criteria, differential diagnosis, and treatment. An attempt to erasure the misnomer MFH. Eur Surg 41: 143–149. [Google Scholar]
- 18. Steensma DP (2008) Is anemia of cancer different from chemotherapy-induced anemia? J Clin Oncol 26: 1022–1024. [DOI] [PubMed] [Google Scholar]
- 19. Dicato M, Plawny L, Diederich M (2010) Anemia in cancer. Ann Oncol 21 Suppl 7167–172. [DOI] [PubMed] [Google Scholar]
- 20. Adamson JW (2008) The anemia of inflammation/malignancy: mechanisms and management. Hematology Am Soc Hematol Educ Program 159–165. [DOI] [PubMed] [Google Scholar]
- 21. Vaupel P, Mayer A, Höckel M (2006) Impact of hemoglobin levels on tumor oxygenation: the higher, the better? Strahlenther Onkol 182: 63–71. [DOI] [PubMed] [Google Scholar]
- 22. Vaupel P (2004) The role of hypoxia-induced factors in tumor progression. Oncologist 9 Suppl 5: 10–17. [DOI] [PubMed] [Google Scholar]
- 23. Brizel DM, Scully SP, Harrelson JM, Layfield LJ, Bean JM, et al. (1996) Tumor oxygenation predicts for the likelihood of distant metastases in human soft tissue sarcoma. Cancer Res 56: 941–943. [PubMed] [Google Scholar]
- 24. Nordsmark M, Høyer M, Keller J, Nielsen OS, Jensen OM, et al. (1996) The relationship between tumor oxygenation and cell proliferation in human soft tissue sarcomas. Int J Radiat Oncol Biol Phys 35: 701–708. [DOI] [PubMed] [Google Scholar]
- 25. Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, et al. (2004) IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest 113: 1271–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nieken J, Mulder NH, Buter J, Vellenga E, Limburg PC, et al. (1995) Recombinant human interleukin-6 induces a rapid and reversible anemia in cancer patients. Blood 86: 900–905. [PubMed] [Google Scholar]
- 27. Rutkowski P, Kaminska J, Kowalska M, Ruka W, Steffen J (2002) Cytokine serum levels in soft tissue sarcoma patients: correlations with clinico-pathological features and prognosis. Int J Cancer 100: 463–471. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper, tables and figures.