Abstract
Background
In 2008, the United States Preventive Services Task Force recommended against prostate specific antigen (PSA) testing for cancer screening in men age 75+.
Purpose
To assess PSA screening by primary care physicians (PCPs) before and after recommendations.
Methods
In 2013, this retrospective cohort study analyzed PCPs in Texas with 20+ male patients aged 75+ in both 2007 and 2010, with Parts A and B Medicare. The main outcome was percent of PCP’s male patients 75+ who received PSA testing ordered by the PCP in 2007 and 2010, with no recent symptoms suggestive of prostate cancer.
Results
In both 2007 and 2010, 1,083 PCPs cared for at least 20 men aged 75 or older. The rate of PSA screening ordered by PCPs was 33.2% in 2007 and 30.6% in 2010. In multilevel analyses controlling for patient characteristics, the variation in PSA screening attributable to the PCP (intraclass correlation coefficient) increased from 23% in 2007 to 26% in 2010, p<0.001. Men with PCPs older than age 60 had 9% lower odds (95% CI, 1–17%) in 2010 compared to 2007 of receiving a PSA test, vs. a 4% increase (95% CI, 4% decrease to 12% increase) in men with PCPs aged 50 or younger. Patients with Board Certified PCPs had a 12% lower odds (95% CI, 8% to 16%) from 2007 to 2010, vs. 2% increase (95% CI 11% decrease to 18% increase) in men with PCPs without board certification.
Conclusions
The USPSTF recommendation did not increase consensus among PCPs regarding PSA screening of older men.
Introduction
The introduction of routine prostate specific antigen (PSA) testing was associated with increases in the number of men diagnosed with and treated for prostate cancer in the U.S. [1] For example, for every 100,000 men aged 66–74 receiving PSA testing in the US in 2007, an additional 4,894 men underwent prostate cancer biopsy, and 1,597 were treated [2].
Prostate cancer is especially problematic in older men, who may benefit little from its diagnosis and treatment. Because of this, in 2008 the US Preventive Services Task Force (USPSTF) specifically recommended against PSA screening in men aged 75 and older. [3] Other organizations, including the American Cancer Society and the American Urological Association, also recommend against routine PSA testing in men over 70 [4] or in men with life expectancy less than 10–15 years. [5], [6] Nevertheless, high rates of PSA testing continue, with relatively modest decreases in PSA testing rates in men over age 75. [7]–[12] One complicating issues is distrust among physicians and patients of guidelines that recommend less care [13], [14].
Physician recommendation is a major driver of testing, along with patient knowledge and preferences. [15], [16] A previous study used Medicare data to describe substantial variation in PSA screening rates among different primary care physicians (PCPs). [10] Variation among providers is thought to reflect lack of evidence, and variation should decrease as underlying evidence grows and provider consensus increases. [17], [18] This report compares PSA screening rates by Texas PCPs for their male patients aged 75 years and older one year before and two years after the 2008 USPSTF recommendation against testing in men aged 75 and older. The hypothesis was that overall PSA testing rates would drop from 2007 to 2010, similar to other studies,[7], [11], [12] and that variations in PSA testing rates would also decrease among PCPs.
Methods
The overall approach was to identify PCPs who cared for at least 20 men aged 75 and older in both 2007 and 2010, and examine the percent of each PCP’s patients who underwent PSA testing in those years. Given the variation among PCPs,[10] 20 patients are sufficient to provide testing estimates with a reliability of >0.85 [19].
Ethics Statement
The UTMB institutional review board approved this retrospective study. Patient and provider information were de-identified in the Medicare claims data. Informed consent was not obtained because patient and provider information were de-identified in the Medicare claims data.
Identification of Patients and Primary Care Physicians
Using 100% Texas Medicare claims data for 2004–10, two cohorts of men were identified. The first included men aged 75 years or older as of 1/1/2007; residing in Texas in 2007; with no claims related to prostate cancer in 2004–06 (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] diagnosis codes: 185, V104.6, 222.2, 233.4, 236.5; ICD-9-CM procedure codes: 60.21, 60.29, 60.3–60.6; and Current Procedure Terminology [CPT] codes: 55801, 55810, 55812, 55815, 55821, 55842, 55845); and with continuous Medicare Parts A and B without health maintenance organization (HMO) coverage during 2004–07. [20], [21] The second cohort, selected using the same criteria, included men aged 75 years or older as of 1/1/2010. Information on each man’s demographics, Medicare coverage and HMO enrollment was obtained from the Medicare enrollment files. Men were then selected from both cohorts with an identifiable PCP. A man’s PCP was identified by the method of Shah et al. [22] as the physician who saw that man on two or more occasions in an outpatient setting for evaluation and management (CPT codes 99201–99205 and 99211–99215) in 2007 or 2010 and had a Health Care Financing Administration (HCFA) specialty code in family medicine, general practice, internal medicine or geriatrics. Physicians were identified by the National Provider Identifier (NPI). If a man had more than one identified physician, the one who provided the most evaluation and management services was assigned as his PCP. In the case of ties, the most recently visited physician was assigned as the PCP. The sample was then restricted to men with an identifiable PCP who had at least 20 such patients in both 2007 and 2010. The final study cohorts contained 37,264 men in 2007 and 45,692 men in 2010, cared for by 1,083 PCPs.
Patient Characteristics
Men were categorized by age (75–79, 80–84 and 85+ years). Comorbidity was assessed by the Elixhauser comorbidity measure based on Medicare Carrier files (claims for physician services), Outpatient Statistical Analysis Files (claims for hospital outpatient services) and Medicare Provider Analysis and Review files (claims for inpatient stays) in 2006 or 2009. The number of comorbidities was categorized as none, 1, 2, 3 or≥4. [20], [21] Race/ethnicity, obtained from the Medicare Part D denominator file, was categorized as White, Black, Hispanic/Latino, or Other/Unknown. Medicaid eligibility (yes or no) was used as a proxy for poverty and was measured by state buy-in fields in the Medicare enrollment file. The patient’s county of residence was categorized into urban, non-urban and rural, according to definitions developed by the US Department of Agriculture. [23] The percentage of high school graduates in the patient’s zip code area was obtained from the US Census data.
PCP characteristics
PCP age and gender in 2007 and board certification in 2007 and 2010 were obtained from the American Medical Association (AMA) Masterfile, which were linked with Medicare claims via the provider NPI. A PCP’s specialty in 2007 was obtained from the HCFA specialty field in the carrier files and was categorized as Family Medicine (including family medicine and general practice) or Internal Medicine (including general internal medicine and geriatrics).
PSA Testing
Each man in the 2007 and 2010 cohorts was assessed for claims for any PSA testing (Carrier files with CPT codes of 84153 and Healthcare Common Procedural Coding Systems Code of G0103) in 2007 and 2010, respectively. Both PSA testing ordered by any physician and that ordered by a man’s own PCP were identified, but for most analyses the outcome was PSA testing ordered by the PCP. Men were excluded who had any diagnoses in the three months prior to PSA test that suggested symptoms associated with prostate cancer (e.g., hematuria, weight loss, urinary obstruction), because such diagnoses or symptoms suggest that the PSA was obtained as a diagnostic test and not a screening test [8], [9].
Statistical Analysis
In 2013, descriptive analysis was used to summarize the patient and PCP characteristics and PSA testing rates in 2007 and 2010 stratified by these characteristics. Multilevel logistic regression modeling was done separately for the 2007 and 2010 cohorts to (1) evaluate variation among PCPs using intra-class correlation (ICC) statistics; (2) evaluate associations between PCP characteristics and PSA testing, adjusting for patient characteristics; (3) estimate PSA testing rates for each PCP, adjusting for patient characteristics and within-PCP clustering; and (4) identify PCPs with a significantly lower or higher than average PSA testing rate. The 1,083 PCPs were then ranked based on their adjusted PSA testing rates, from lowest to highest, for both 2007 and 2010. The ICCs for PCPs from the 2007 and 2010 multilevel models were compared using Levine’s test for equal variance. Finally, a model was constructed including both the 2007 and 2010 cohorts, and tested for interactions between year (2007 or 2010) and PCP characteristics. SAS version 9.2 (SAS Institute, Cary, NC) was used for all statistical analyses.
Results
In all, 1,083 PCPs had >20 men aged 75+ in their patient panels in both 2007 (total men = 37,264) and 2010 (total men = 45,692). Table 1 presents the percent of patients who underwent PSA screening in 2007 and 2010, stratified by characteristics of patients and their PCPs, and also the odds ratios for receiving PSA screening adjusted for all characteristics in a multivariable multilevel model. The 2007 rate of PSA screening ordered by the patient’s PCP (33.2%) decreased to 30.6% in 2010 (p<0.001). The rates of all PSA screening by any physician were 45.2% in 2007 and 42.4% in 2010 (p<0.001). In both 2007 and 2010, the odds of PSA testing declined for patients with increasing age or greater comorbidities. Patient race/ethnicity and socioeconomic status had little effect. PCP characteristics independently associated with higher odds of PSA testing included a greater number of men aged 75 years or older in their patient panels, and Internal Medicine specialty.
Table 1. Patient and primary care physician characteristics and their associations with prostate specific antigen (PSA) screening.
| Patient Characteristics* | Number of patients (% receiving PSA screening ordered by PCP) | OR (95% CI) | ||
| 2007 | 2010 | 2007 | 2010 | |
| Overall | 37,264 (33.2) | 45,692 (30.6) | − | − |
| Age (years) | ||||
| 75–79 | 17,487 (39.1) | 15,728 (37.8) | 1.00 | 1.00 |
| 80–84 | 11,682 (31.8) | 15,654 (31.6) | 0.76 (0.72, 0.80) | 0.75 (0.71, 0.79) |
| 85+ | 8,095 (22.6) | 14,310 (21.6) | 0.42 (0.40, 0.45) | 0.42 (0.39, 0.44) |
| Race/Ethnicity | ||||
| White | 31,348 (34.0) | 38,037 (31.0) | 1.00 | 1.00 |
| Black | 905 (30.5) | 1,165 (30.6) | 0.96 (0.81, 1.14) | 1.01 (0.87, 1.17) |
| Hispanic | 4,603 (28.4) | 5,922 (28.1) | 0.99 (0.89, 1.11) | 1.08 (0.97, 1.19) |
| Other | 382 (34.0) | 548 (29.9) | 1.10 (0.84, 1.45) | 0.83 (0.65, 1.06) |
| Numbers of comorbidities | ||||
| 0 | 5,591 (35.2) | 5,936 (33.9) | 1.00 | 1.00 |
| 1 | 11,323 (37.2) | 12,758 (34.8) | 1.11 (1.03, 1.20) | 1.07 (0.99, 1.15) |
| 2 | 9,191 (33.5) | 11,611 (31.5) | 0.92 (0.85, 1.00) | 0.90 (0.83, 0.98) |
| 3 | 5,297 (31.6) | 6,973 (27.8) | 0.83 (0.76, 0.91) | 0.74 (0.68, 0.81) |
| 4+ | 5,462 (23.9) | 8,414 (22.9) | 0.56 (0.51, 0.62) | 0.57 (0.52, 0.62) |
| Medicaid eligibility | ||||
| No | 33,719 (33.9) | 41,477 (31.0) | 1.00 | 1.00 |
| Yes | 3,545 (27.0) | 4,215 (27.1) | 0.84 (0.75, 0.94) | 0.93 (0.84, 1.04) |
| Urban/Rural | ||||
| Metro | 28,882 (33.8) | 35,104 (30.8) | 1.00 | 1.00 |
| Non-Metro | 7,657 (31.3) | 9,743 (29.7) | 0.96 (0.88, 1.06) | 1.07 (0.98, 1.17) |
| Rural | 702 (30.5) | 839 (30.3) | 1.08 (0.87, 1.33) | 1.13 (0.93, 1.39) |
| Percent high school graduates in the zip code area | ||||
| <75% | 8,786 (30.8) | 10,275 (28.4) | 1.00 | 1.00 |
| 75–83% | 8,642 (23.2) | 10,757 (28.4) | 0.96 (0.88, 1.04) | 0.96 (0.88, 1.04) |
| 84–90% | 9,332 (25.0) | 11,426 (30.9) | 1.05 (0.96, 1.14) | 1.03 (0.95, 1.12) |
| >90% | 9,404 (25.2) | 11,888 (34.1) | 1.10 (1.01, 1.20) | 1.06 (0.98, 1.15) |
| PCP Characteristics | Number of PCPs (% of their patients receiving PSA ordered by PCP) | OR (95% CI) | ||
| 2007 | 2010 | 2007 | 2010 | |
| Overall | 1,083 (33.2%) | 1083 (30.6%) | − | − |
| Age (years) | ||||
| < = 50 | 432 (31.0%) | (29.6%) | 1.00 | 1.00 |
| 50–60 | 438 (33.8%) | (30.4%) | 1.03 (0.89, 1.19) | 0.95 (0.81, 1.11) |
| >60 | 202 (36.5%) | (32.2%) | 1.15 (0.95, 1.39) | 1.03 (0.84, 1.27) |
| Gender | ||||
| Female | 45 (26.3%) | 45 (23.8%) | 1.00 | 1.00 |
| Male | 1,027 (33.5%) | 1,027 (30.6%) | 1.31 (0.93, 1.84) | 1.30 (0.89, 1.89) |
| Number of Male Patients 75+ in 2007/2010 in their patient panel | ||||
| 20–25 | 345 (29.3%) | 139 (24.7%) | 1.00 | 1.00 |
| 26–35 | 365 (31.7%) | 344 (28.8%) | 1.10 (0.93, 1.30) | 1.29 (1.01, 1.65) |
| 36–50 | 243 (35.4%) | 336 (35.4%) | 1.35 (1.12, 1.62) | 1.47 (1.15, 1.88) |
| >50 | 130 (36.2%) | 264 (31.4%) | 1.30 (1.04, 1.63) | 1.36 (1.05, 1.76) |
| Specialty | ||||
| Family Medicine | 442 (31.2%) | 442 (28.9%) | 1.00 | 1.00 |
| Internal Medicine | 641 (34.4%) | 641 (31.6%) | 1.13 (0.98, 1.30) | 1.16 (1.00, 1.35) |
| Board Certified in 2007/2010 | ||||
| Yes | 828 (33.6%) | 790 (30.5%) | 1.00 | 1.00 |
| No | 70 (29.6%) | 108 (29.0%) | 0.92 (0.70, 1.22) | 0.94 (0.74, 1.20) |
*There are missing data for patient race/ethnicity (n = 26 in 2007 and n = 20 in 2010) urban/rural (n = 23 in 2007 and n = 6 in 2010) and education (n = 1100 in 2007 and n = 1344 in 2010); PCP characteristics are missing data on age (n = 11 in 2007 and 2010) and board certification (n = 188) in 2007 and 2010).
The models shown in Table 1 were then used to estimate PSA testing rates for each PCP in 2007 and 2010, adjusted for patient characteristics. Figure 1 presents cumulative distributions of adjusted PSA testing rates for each of the 1,083 PCPs in 2007 and 2010. PCPs varied substantially in testing rates in both years. PCPs with rates significantly higher or lower than the mean rate are indicated by bold lines. In 2007, 258 (24%) PCPs had rates significantly greater than the mean, with an average rate of 57.5%, while 172 (16%) PCPs had significantly lower rates, with an average rate of 9.7%. For 2010, the number of PCPs significantly higher and lower than the mean increased (p<0.001), with 302 (28%) PCPs with significantly higher rates (average rate 55.0%) and 231 (21%) with significantly lower rates (average rate 8.5%).
Figure 1. Cumulative distribution of 1,083 Texas primary care physicians (PCPs) by the adjusted percentage of their male patients age 75 and older who underwent prostate specific antigen (PSA) testing ordered by the PCP in 2007 (top panel) and 2010 (bottom panel).
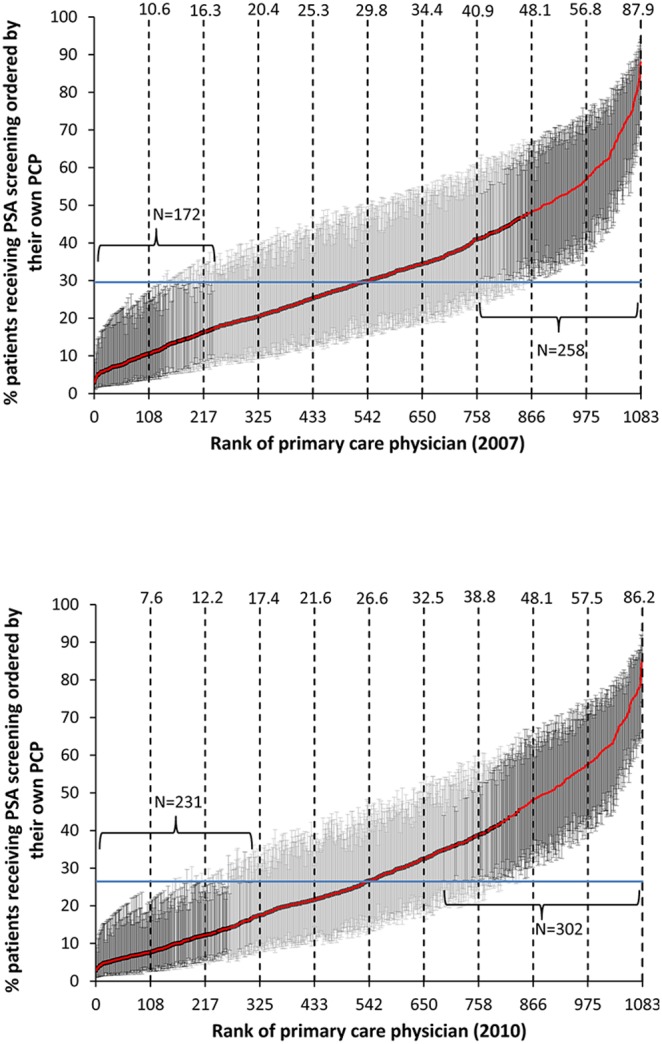
Only PCPs with at least 20 male patients 75+ in their panels in both years are included. The vertical lines denote the 95% confidence intervals of the estimates, derived from the multilevel models presented in Table 1. Dark lines indicate PCPs whose PSA testing rate was significantly different from the mean rate for all PCPs.
Also shown in Figure 1 are the rates by decile of PCP rank. For example, PCPs in the lowest decile in 2007 had rates <10.6%, with an average rate for those PCPs of 7.7%. In 2010 the lowest decile was <7.6%, with an average rate of 6.0%. In contrast, the cut points and average rates for the top decile of PCPs actually increased slightly between 2007 and 2010.
The multilevel models presented in Table 1 also show the ICC at the PCP level for each year. In 2007 the ICC was 0.23; in 2010, it increased to 0.26 (p<0.001). This is consistent with the cumulative distributions shown in Figure 2, showing greater dispersion in PSA testing rates of PCPs in 2010 versus 2007. In both years, specific patient characteristics (age, comorbidity, education, etc.) explained less than 4% of the variance in receipt of PSA screening.
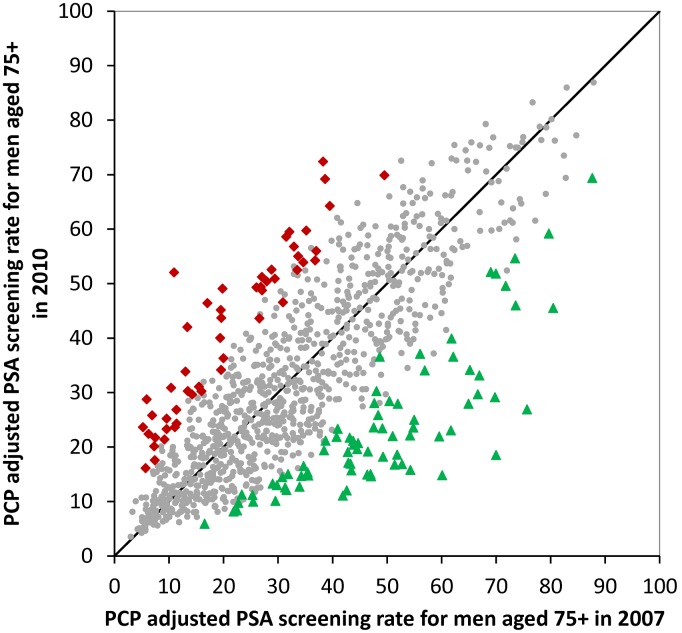
Figure 2. Scatterplot of the adjusted prostate specific antigen (PSA) screening rates in men 75+ for 1,083 PCPs in 2007 vs. 2010.
The 51 PCPs with significantly higher rates in 2010 are indicated with red, while the 77 PCPs with significantly lower rates are in green. The results were generated from a multilevel model adjusting for patient characteristics and including both the 2007 and 2010 data in the same model.
There was good stability in the PCP ranking between 2007 and 2010, with a Pearson correlation co-efficient of 0.63. Of the 216 PCPs in the top quintile of screening rates in 2007, 74.1% were in the first (49.1%) or second (25.0%) quintile in 2010. Similarly, of the 258 PCPs in Figure 1 with PSA screening rates significantly higher than the mean rate in 2007, 178 (69.0%) had significantly higher rates in 2010. Figure 2 graphs the adjusted PSA screening rates for each of the 1,083 PCPs in 2007 vs. 2010. It also shows the PCPs whose screening rates significantly increased (n = 51, indicated in red) or decreased (n = 77, indicated in green).
To determine if specific PCP characteristics were associated with changes in PSA screening rates by PCPs from 2007 to 2010, a multilevel multivariable model was constructed combining both years of data and including year (2010 vs. 2007) as a variable. After controlling for patient and PCP characteristics, the odds of receiving a PSA test from one’s PCP decreased 9% between 2007 and 2010 (OR = 0.91, 95% CI = 0.87; 0.96). This model showed significant interactions between year (2010 vs. 2007) and PCP age, board certification, and size of the PCP panel. As shown in Table 2, patients of PCPs older than age 60 experienced a 9% lower odds of receiving a PSA test in 2010 compared to 2007, versus a 4% increase for patients whose PCP was younger than age 50. Patients of PCPs with board certification experienced a 12% decrease in odds of PSA testing versus a 2% increase for patients of PCPs without board certification. Patients with PCPs with a high volume of older men in their panels also experienced a drop in odds of undergoing PSA screening.
Table 2. PCP characteristics associated with change in PSA screening rates from 2007 to 2010.
| PCP Characteristics | OR (95% CI) of receivingPSA screening, 2010 vs. 2007 |
| Age (years) | |
| < = 50 | 1.04 (0.96, 1.12) |
| 51–60 | 0.97 (0.90, 1.04) |
| >60 | 0.91 (0.83, 0.99) |
| Number of Patients 75+ | |
| 20–25 | 1.03 (0.95, 1.12) |
| 26–35 | 0.97 (0.90, 1.05) |
| 36–60 | 1.00 (0.92, 1.08) |
| >50 | 0.88 (0.80, 0.96) |
| Board Certified | |
| Yes | 0.88 (0.84, 0.92) |
| No | 1.02 (0.89, 1.18) |
PCP: primary care physician; PSA: prostate specific antigen.
Discussion
In the 1970’s, Wennberg and colleagues described geographic variations in the receipt of surgical procedures. [17] The variation was much higher for operations with less clear-cut indications (such as tonsillectomy) than for those with clear indications (such as appendectomy). Wennberg et al. termed this phenomenon “preference sensitive care.” More recently, these investigators have expanded this concept to medical testing. [18], [24] Using clinical vignettes of patient scenarios, they found little variation in diagnostic and treatment decisions when the supporting evidence was clear cut, but considerable variation in situations with poor evidence. [18] With PSA testing, variation among PCPs actually increased after publication of the USPSTF recommendations, suggesting that consensus statements and guidelines did not reduce uncertainty.
The variation in PSA testing among PCPs is striking. PCPs in the lowest decile of testing differed nine- to ten-fold in PSA rates vs. those in the top decile. No other behaviors appear to have this high level of variation among PCPs. [19], [25]–[29] For example, previous reports show ICCs at the provider level of 0.10 and 0.09 for receipt of mammography[25] and colorectal cancer screening,[26] respectively, compared to the ICC of 0.26 for PSA screening in 2010.
PSA testing rates by PCPs overall decreased, from 33.2% in 2007 to 30.6% in 2010. However, as shown in Figure 1, PSA testing rates among physicians in the upper decile of PSA testing actually increased. The overall decrease in rates was driven by increases in the number of PCPs with lower rates. The cut points for the bottom seven deciles of PCPs in 2010 are all lower than in 2007. The result is a significant increase in variability among physicians, and an increase in the amount of overall variability in PSA testing attributable to the PCP.
The decline in PSA testing rates was greater in board certified and older PCPs. Several studies have found board certification associated with better adherence to guidelines,[30], [31] but those same studies tend to find that younger, more recently trained physicians are more adherent to guidelines. Patients of older PCPs (vs. younger PCPs) had higher odds of testing in 2007, but this association disappeared by 2010.
Investigators using interviews of PCPs have also documented considerable variation in PSA screening behavior. [7] Two groups surveyed PCPs after the release of the 2011 draft USPSTF recommendations against PSA screening in men of any age. [32], [33] PCPs varied considerably in whether they agreed with the recommendations and whether their PSA screening behavior would change as a result. Patient attitudes and preferences also clearly contribute to overtesting. For example, Schwartz et al.,[34] in a national telephone survey in 2001–02, found that 73% of males disagreed that they would ever stop getting PSA screening; 77% said they would try to continue the test even if their physician recommended against it. In a qualitative study, Torke et al. [35] found that older adults view cancer screening as a moral obligation.
Another contributing factor to the variation among providers in PSA screening may be the lack of complete consensus in the recommendations on PSA screening provided by various professional organizations. In general, organizations representing primary care and/or preventive medicine have recommended against PSA screening, while oncology and urology organizations have a broader spectrum of views. The US Preventive Services Task Force and primary care organizations such as the American College of Physicians and the American Academy of Family Practice recommend against PSA screening at any age. [3], [36], [37] The American Cancer Society and the American Urological Association recommend a patient-centered individualized approach to decisions, but discourage testing in older men. [4], [5] The Large Urology Group Practice Association and some other urology groups are more favorable to PSA screening, though they still discourage screening in men with less than 10 years life expectancy. [38], [39] All of the organizations generate press releases and have public websites to disseminate their diverse and conflicting recommendation, which presumably contributes to the lack of clarity among PCPs and their older male patients.
However, even given these differences in recommendations, there may be more consensus than is realized about PSA screening in older men. Even the most pro-screening groups do not recommend it for men with less than 10 years life expectancy. Using a validated algorithm to predict life expectancy using a man’s age and degree of comorbidity, [40] it is not possible to define a cohort of men aged 80 or older with a life expectancy of greater than 10 years. Only 12% of 80 year old men survive for 10 years, and it is not possible to prospectively identify them. Nevertheless, we found >30% such men received PSA screening in 2007 and 2010.
Previous reports have documented overuse of PSA screening in older men. Walter and colleagues [9] studied PSA testing in 2003 of men aged 70 and older cared for at US Veteran’s Affairs (VA) facilities, and found little influence of health status on testing rates. For example, with men aged 85 years and older, 34% in the best health and 36% in the worst health had PSA tests. Bynum et al. [8] analyzed 2003 Medicare data and found an overall PSA screening rate of 17.2% for men aged 80 years and older, with variation in testing rates across regions from 2% to 38%. The current data, from 2007 and 2010, suggest no improvement in these patterns. Ross et al. [11] compared PSA testing rates for men 75 and older in the months immediately before and after change in USPSTF recommendations, using the 5% Medicare non cancer sample from the Surveillance Epidemiology and End Results (SEER) Tumor Registry, representing 28% of the US population. They noted a 2% absolute decrease in testing rates. Howard et al. [12] compared testing rates on approximately 2,400 men aged 75 and older in 2006–07 to 2200 in 2009–10 in the Medicare Current Beneficiary Survey and found a 5.3% absolute decrease in testing rates between 2006 and 2010. In contrast, self-reported PSA screening rates did not change between 2005 and 2010 in men aged 75 and older in the national Health Interview Survey. [41] Other reports have found little[42] or no[43] change in PSA testing rates in response to the publication of clinical trials. The overall picture, then, is of modest effects of published evidence and consensus recommendations on PSA screening, with substantial and increasing variation in screening behavior among PCPs. Part of the variation in PCP testing behavior likely reflects variations in the attitudes of their patient panels about such testing. Perhaps the increasing variations among PCPs reflect differences in willingness or ability to confront the issue with patients who equate screening with good medical care.
A major implication of these findings relates to targeting interventions to discourage PSA testing, particularly of men with limited life expectancy. The very high variability among PCPs suggests that PCPs would be an excellent target for intervention efforts. For example, overtesting rates have been suggested as quality measures of PCPs. [44], [45] Other approaches could include eliminating reimbursement for screening PSA tests in, for example, men aged 80 and older. Medicare recently decided to continue reimbursement for PSA screening with no age limitation [46].
The study has limitations. First, while we excluded patients with a history of prostate cancer, and those with recent symptoms suggestive of prostate cancer, this method undoubtedly did not eliminate all cases where the PSA testing was in response to symptoms, and not true screening. However, it is not plausible that the prevalence of such symptoms would vary greatly among men cared for by different PCPs. Second, our sample was restricted to men in fee-for-service Medicare. Evidence suggests that screening rates in HMOs and in the VA system are lower. [9], [47] Third, the study was limited to Texas. PSA screening rates are somewhat higher in southern states than in other regions. [8] Finally, an ongoing concern about physician profiling is reliability, which reflects an estimate of how much of measured variability is due to real differences in behavior. [19], [48] For example, Adams et al. [48] showed that most measures of physician-level resource utilization for specific episodes of care had reliabilities of less than 0.70, indicating poor reliability. Because of the high variation among PCPs in PSA testing, these estimates of PSA testing rates had a reliability of >0.85 for PCPs with 20 or more patients in their panel. All PCPs assessed had at least 20 male patients aged 75+ in their panels.
In conclusion, the continued high levels of PSA testing in older men, and the high variation among PCPs in rates of PSA testing, suggest that interventions at the PCP level may be useful.
Data Availability
The authors confirm that, for approved reasons, some access restrictions apply to the data underlying the findings. Data on Medicare beneficiaries is available at the Centers for Medicare and Medicaid Services; detail are at (https://www.cms.gov/Research-Statistics-Data-and-Systems/CMS-Information-Technology/AccesstoDataApplication/index.html). Data on providers is taken from the American Medical Association (AMA) Masterfile, available from the AMA Division of Survey and Data Resources. Information on accessing this data is available at (http://www.ama-assn.org/ama/pub/about-ama/physician-data-resources/ama-database-licensing.page?).
Funding Statement
The study was funded by The Comparative Effectiveness Research on Cancer in Texas (CERCIT) grant RP101207 from the Cancer Prevention and Research Institute of Texas; grant K05CA134923 from the National Institutes of Health; grant R24HS022134 from the Agency for Healthcare Research and Quality; and The University of Texas Medical Branch Clinical and Translational Science Award UL1TR00007. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Welch HG, Albertsen PC (2009) Prostate cancer diagnosis and treatment after the introduction of prostate-specific antigen screening: 1986–2005. J Natl Cancer Instit 101: 1325–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Howrey BT, Kuo YF, Lin YL, Goodwin JS (2013) The impact of PSA screening on prostate cancer mortality and overdiagnosis of prostate cancer in the United States. J Gerontol A Bio Sci Med Sci 68: 56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. U.S. Preventives Services Task Force (2008) Screening for prostate cancer: U.S. Preventives Services Task Force recommendation statement. Ann Intern Med 149: 185–191. [DOI] [PubMed] [Google Scholar]
- 4. Carter HB, Albertsen PC, Barry MJ, Etzioni R, Freedland SJ, et al. (2013) Early detection of prostate cancer: AUA Guideline. J Urol 190: 419–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Smith RA, Cokkinides V, Eyre HJ (2006) American Cancer Society guidelines for the early detection of cancer, 2006. CA Cancer J Clin 56: 11–25. [DOI] [PubMed] [Google Scholar]
- 6. American Urological Association (AUA) (2000) Prostate-specific antigen (PSA) best practice policy. Oncology (Williston Park) 14: 267–272. [PubMed] [Google Scholar]
- 7. Guerra CE, Gimotty PA, Shea JA, Pagán JA, Schwartz JS, et al. (2008) Effect of guidelines on primary care physician use of PSA screening: results from the Community Tracking Study Physician Survey. Med Decis Making 28: 681–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bynum J, Song Y, Fisher E (2010) Variation in prostate-specific antigen screening in men aged 80 and older in fee-for-service Medicare. J Am Geriatr Soc 58: 674–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Walter LC, Bertenthal D, Lindquist K, Konety BR (2006) PSA screening among elderly men with limited life expectancies. JAMA 296: 2336–2342. [DOI] [PubMed] [Google Scholar]
- 10. Jaramillo E, Tan A, Yang L, Kuo YF, Goodwin JS (2013) Variation among primary care physicians in prostate specific antigen testing of older men. JAMA 310: 1622–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ross JS, Wang R, Long JB, Gross CP, Ma X (2012) Impact of the 2008 US Preventive Services Task Force recommendations to discontinue prostate cancer screening among male Medicare beneficiaries. Arch Intern Med 172: 1601–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Howard DH, Tangka FK, Guy GP, Ekweume DU, Lipscomb J (2013) Prostate cancer screening in men ages 75 and older fell by 8 percentage points after task for recommendation. Health Affairs 32: 596–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Steinbrook R (2007) Guidance for guidelines. N Engl J Med 356: 331–333. [DOI] [PubMed] [Google Scholar]
- 14. Gerber AS, Patashnik EM, Doherty D, Dowling C (2010) A national survey reveals public skepticism about research-based treatment guidelines. Health Aff (Millwood) 10: 1882–1884. [DOI] [PubMed] [Google Scholar]
- 15. Hawley ST, Earp JA, O'Malley M, Ricketts TC (2000) The role of physician recommendation in women's mammography use: is it a 2-stage process? Med Care 38: 392–403. [DOI] [PubMed] [Google Scholar]
- 16. Nichols C, Holt CL, Shipp M, Eloubeidi M, Fouad MN, et al. (2009) Physician knowledge, perceptions of barriers, and patient colorectal cancer screening practices. Am J Med Qual 24: 116–122. [DOI] [PubMed] [Google Scholar]
- 17.Wennberg JE (2010) Tracking medicine: A researcher's quest to understand health care. New York: Oxford University Press. 344 p.
- 18. Sirovich B, Gallagher PM, Wennberg DE, Fisher ES (2008) Discretionary decision making by primary care physicians and the cost of U.S. health care. Health Aff (Millwood) 27: 813–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hofer TP, Hayward RA, Greenfield S, Wagner EH, Kaplan SH, et al. (1999) The unreliability of individual physician “report cards” for assessing the costs and quality of care of a chronic disease. JAMA 281: 2098–2105. [DOI] [PubMed] [Google Scholar]
- 20. Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, et al. (2005) Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 43: 1130–1139. [DOI] [PubMed] [Google Scholar]
- 21. Elixhauser A, Steiner C, Harris DR, Coffey RM (1998) Comorbidity measures for use with administrative data. Med Care 36: 8–27. [DOI] [PubMed] [Google Scholar]
- 22. Shah BR, Hux JE, Laupacis A, Zinman B, Cauch-Dudek K, et al. (2007) Administrative data algorithms can describe ambulatory physician utilization. Health Serv Res 42: 1783–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.US Department of Agriculture Economic Research Service (2013) Rural-Urban Continuum Codes. USDA. http://www.ers.usda.gov/data-products/rural-urban-continuum-codes/documentation.aspx. Accessed 20 June 2014.
- 24. Song Y, Skinner J, Bynum J, Sutherland J, Wennberg JE, et al. (2010) Regional variations in diagnostic practices. N Eng J Med 363: 45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tan A, Kuo YF, Elting LS, Goodwin JS (2013) Refining physician quality indicators for screening mammography in older women: Distinguishing appropriate use from overuse. J Am Geriatr Soc 61: 380–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Singal AK, Lin YL, Kuo YF, Riall T, Goodwin JS (2013) Primary care physicians and disparities in colorectal cancer screening in the elderly. Health Serv Res 48: 95–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cowen ME, Strawderman RL (2002) Quantifying the physician contribution to managed care pharmacy expenses: a random effects approach. Med Care 40: 650–661. [DOI] [PubMed] [Google Scholar]
- 28. Beaulieu MD, Blais R, Jacques A, Battista RN, Lebeau R, et al. (2001) Are patients suffering from stable angina receiving optimal medical treatment? QJM 94: 301–308. [DOI] [PubMed] [Google Scholar]
- 29. Sixma HJ, Spreeuwenberg PM, van der Pasch MA (1998) Patient satisfaction with the general practitioner: a two-level analysis. Med Care 36: 212–229. [DOI] [PubMed] [Google Scholar]
- 30. Chen J, Rathore SS, Wang Y, Radford MJ, Krumholz HM (2006) Physician board certification and the care and outcomes of elderly patients with acute myocardial infarction. J Gen Intern Med 21: 238–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Norcini J, Lipner R, Kimball H (2001) The certification status of generalist physicians and the mortality of their patients after acute myocardial infarction. Acad Med 76: S21–S23. [DOI] [PubMed] [Google Scholar]
- 32. Tasian GE, Cooperberg MR, Potter MD, Cowan JE, Greene KL, et al. (2012) PSA screening: determinants of primary-care physician practice patterns. Prostate Cancer Prostatic Dis 15: 189–194. [DOI] [PubMed] [Google Scholar]
- 33. Pollack CE, Noronha G, Green GE, Bhavsar NA, Carter HB (2012) Primary care provider’s response to the US Preventive Services Task Force draft recommendations on screening for prostate cancer. Arch Intern Med 172: 668–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schwartz LM, Woloshin S, Fowler FJ Jr, Welch HG (2004) Enthusiasm for cancer screening in the United States. JAMA 291: 71–78. [DOI] [PubMed] [Google Scholar]
- 35. Torke AM, Schwartz PH, Holtz LR, Montz K, Sachs GA (2013) Older adults and forgoing cancer screening: “I think it would be strange.”. JAMA Intern Med 173: 526–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.AAFP, USPSTF Issue Final Recommendation Against Routine PSA-based Screening for Prostate Cancer. http://www.aafp.org/news/health-of-the-public/20120522psascreenrec.html. Accessed 7/16/2014.
- 37. Moyer VA (2012) U.S. Preventive Services Task Force (2012) Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 157: 120–134. [DOI] [PubMed] [Google Scholar]
- 38. Murphy DG, Ahlering T, Catalona WJ, Crowe H, Crow J, et al. (2014) The Melbourne Concensus Statement on the early detection of prostate cancer. BJUI International 113: 186–188. [DOI] [PubMed] [Google Scholar]
- 39.LUGPA Position Statement: PSA Screening. http://lugpa.org/latest-news/lugpa-position-statement-psa-screening/, accessed 7/16/14.
- 40. Tan A, Kuo Y, Goodwin JS (2013) Predicting life expectancy for community dwelling older adults using Medicare claims data. Am J Epidemiol 178: 974–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pasad SM, Drazer MW, Dezheng H, Hu JC, Eggener SE (2012) 2008 US Preventive Task Force recommendations and prostate cancer screening rates. JAMA 307: 1692–1694. [DOI] [PubMed] [Google Scholar]
- 42. Zeliadt SB, Hoffman RM, Etzioni R, Gore JL, Kessler LG, et al. (2011) Influence of publication of US and European prostate cancer screening trials on PSA testing practices. J Natl Cancer Inst 103: 520–523. [DOI] [PubMed] [Google Scholar]
- 43. Goodwin JS, Tan A, Jaramillo E, Kuo YF (2013) Prostate-specific antigen testing in men aged 40–64 years: impact of publication of clinical trials. J Natl Cancer Inst 105: 743–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Katz MH (2012) Overuse of health care: where are the data? Arch Intern Med 172: 178 doi:10.1001/archinternmed.2011.1253 [DOI] [PubMed] [Google Scholar]
- 45. Lee SJ, Walter LC (2011) Quality indicators for older adults: preventing unintended harms. JAMA 306: 1481–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Medicare Learning Network (2012) Reminder – Medicare provides coverage of prostate cancer screening for eligible Medicare beneficiaries. MLN Matters Number: SE0709. Centers for Medicare and Medicaid Services. http://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MN/MLNMattersArticles/downloads/SE0709.pdf. Accessed 20 June 2014.
- 47. Wallner L, Frencher S, Hsu JW, Loo R, Huang J, et al. (2012) Prostate cancer screening trends in a large, integrated health care system. Perm J 16: 4–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Adams JL, Mehrotra A, Thomas JW, McGlynn EA (2010) Physician cost profiling ― reliability and risk of misclassification. N Engl J Med 362: 1014–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that, for approved reasons, some access restrictions apply to the data underlying the findings. Data on Medicare beneficiaries is available at the Centers for Medicare and Medicaid Services; detail are at (https://www.cms.gov/Research-Statistics-Data-and-Systems/CMS-Information-Technology/AccesstoDataApplication/index.html). Data on providers is taken from the American Medical Association (AMA) Masterfile, available from the AMA Division of Survey and Data Resources. Information on accessing this data is available at (http://www.ama-assn.org/ama/pub/about-ama/physician-data-resources/ama-database-licensing.page?).