Abstract
Background& Aims
The accuracy of creatinine-based estimated GFR (eGFR) in assessing the prevalence of chronic kidney disease (CKD) and associated mortality after liver transplantation (LTx) is unknown. Using measured GFR (mGFR) by iothalamate clearance, we determined the prevalence of the entire spectrum of renal dysfunction and the impact of CKD on mortality after LTx.
Methods
A database that prospectively tracks all LTx recipients at this academic transplant program from 1985 to 2012 was queried to identify all adult primary LTx recipients. Our post-LTx protocol incorporates GFR measurement by iothalamate clearance at regular intervals. A multistate model was used to assess the prevalence of CKD, kidney transplant and death after LTx. Time-dependent Cox regression analysis was performed to evaluate the impact of mGFR and eGFR changes on survival.
Results
A total of 1,211 transplant recipients were included. At the time of LTx, the median age was 54 years, 60% were male and 86% were Caucasian. At 25 years after LTx, 54% of patients died, 9% underwent kidney transplantation, whereas 7%, 21% and 18% had mGFR >60, 59–30 and <30 ml/min/1.73m2 respectively. The risk of death increased when mGFR decreased below 30 ml/min/1.73m2: HR= 2.67 (95% CI=1. 80–3.96 ) for GFR=29-15 ml/min/1.73m2 and HR= 5.47(95% CI=3.10–9.65 ) for GFR<15 ml/min/1.73m2. Compared to mGFR, eGFR underestimated mortality risk in LTx recipients with an eGFR of 30–90 ml/min/1.73m2.
Conclusion
An overwhelming majority of LTx recipients develop CKD. The risk of death increases exponentially when GFR<30 ml/min/1.73m2. Creatinine-based eGFR underestimates the mortality risk in a large proportion of patients.
Keywords: renal failure, iothalamate clearance, prevalence, outcomes
INTRODUCTION
Advances in immunosuppression and perioperative management have revolutionized liver transplantation (LTx) outcomes in the past 3 decades [1]. Improved long-term survival has led in turn to increased prevalence of ‘late’ complications after LTx such as chronic kidney disease (CKD). CKD has become one of the leading causes of morbidity and death after LTx [2]. Although calcineurin inhibitor-toxicity is typically considered a major contributor, other risk factors for CKD include perioperative acute kidney injury, diabetes mellitus, hypertension and chronic hepatitis C infection [3, 4, 5].
The reported prevalence of CKD after LTx ranges between 10% and 45% [6–17]. The wide range is largely attributed to the different criteria used to define CKD and different follow-up lengths, which make the existing literature difficult to compare. Moreover, renal function was assessed using creatinine-based equations, which is influenced by factors other than renal function, namely muscle mass [18] and tend to overestimate GFR in patients after liver transplantation [19]. Studies using measured GFR (mGFR) to assess the prevalence and mortality impact of CKD in these patients are limited [20]. Most of the studies describe the prevalence and mortality risk associated with advanced stages of kidney failure, which affect a relatively small proportion of LTx patients. These studies underestimate the true burden of CKD, because they do not assess the long-term outcomes in liver recipients who do not reach these end stages [21].
In this work, we analyze the prevalence of CKD by its severity and assess its impact on patient survival. We take advantage of the data resources available to this program from protocolized measurement of GFR by iothalamate clearance in liver recipients. Using mGFR, we aimed to determine i) the prevalence of the entire spectrum of renal dysfunction after LTx and ii) the impact of CKD on patient survival after LTx.
METHODS
Patients
Data on all adults undergoing primary LTx at Mayo Clinic, Rochester, MN between 1985 and 2012 were extracted from a prospective database tracking all LTx recipients. We excluded patients who underwent simultaneous multiple organ transplant and those who underwent a repeated LTx. The study was approved by our institutional review board.
Data
All laboratory data, including mGFR, were extracted from the LTx database and the institutional laboratory file. Outcome of follow-up, including patient survival and kidney transplantation (KTx), was also extracted from the LTx database, supplemented by the institutional registration file to determine the vital status of patients who may have lost to follow-up.
GFR was measured by iothalamate clearance [22, 23]. In addition, estimated GFR (eGFR) was calculated using serum creatinine values and the Modified Diet in Renal Disease (MDRD-4) equation [24]. Measured or eGFR were capped at 150 ml/min/1.73m2, as some results were implausibly high (e.g., > 200 ml/min/1.73m2). Serum creatinine results from 1985–2006 were re-calibrated by subtracting 0.14 mg/dL from the original value, in concordance with the serum creatinine assay standardization in October 2006. All serum creatinine data were used except those within 30 days prior to death, since those results may be reflective of the multi-organ failure leading to the patient’s death, rather than representing CKD predictive of death.
Standard definitions of chronic kidney disease stages were used, as per the Kidney Disease Improving Global Outcomes (KDIGO 2012) guidelines. CKD stage 3a was defined by GFR of 45–59 ml/min/1.73m2; CKD stage 3b by GFR of 30–44 ml/min/1.73m2, CKD stage 4 by GFR of 15–29 ml/min/1.73m2, and CKD stage 5 by GFR < 15 ml/min/1.73m2. Given the variability of serum creatinine, the mean of the 2 lowest creatinine values over the prior 6 months was used for these definitions when eGFR was analyzed.
Analytical approach
In describing the prevalence of post-LTx CKD, a multistate model was utilized. A multistate model is an extension of a competing risk model, in which patients can go through different intermediate states before reaching the final state. The model allowed patients to move back and forth between CKD stages until reaching the final state of either KTx or death, transition out of which was not allowed. For example if a patient received KTx and then subsequently died, the patient remained in the KTx state. This method appreciates the dynamic transitions between CKD stages, as well as the occurrence of KTx and death over time.
Renal function at LTx, at 4 months, and yearly after LTx was assessed separately by eGFR and, when available, by mGFR. Given the goal of the analysis being long term effect of CKD, we excluded deaths that occurred in the immediate (i.e., <4 months) post-LTx period. Similarly, in light of reversible events that may affect renal function acutely in the immediate post-operative period, data in the first 4 months post-LTx were ignored.
Because of the long study period, we divided the data into two eras – pre-MELD (1985–2001) and MELD (2002–2012). Initial analyses were performed for the entire study period and then each era was considered separately.
In the second part of the analysis, we determined the age and sex-adjusted impact of post-LTx renal function on survival. The time-dependent Cox regression analysis was used to estimate the effect of a GFR result obtained at any time during the follow-up (separately for mGFR and eGFR). Smoothing splines were used to graphically assess the relation between GFR and mortality and to identify any thresholds in GFR at which the relation changes. To evaluate the extent to which eGFR may incorrectly assess mortality risk, we constructed a reclassification table, which compared the proportion of patients in each eGFR category who actually belonged in a different CKD stage when assessed by mGFR. Using Cox regression models, we determined the age and sex adjusted risk of death among subjects who were reclassified to a higher or lower GFR range, in comparison to the subjects who were not reclassified [25]. All statistical analyses were conducted using R software.
RESULTS
Patients
A total of 1,266 patients met the eligibility criteria. Of these, 1,211 (96%) were alive at 4 months after LTx and were included in the analysis. The median age at LTx was 54 (range 19–73) years; the majority of the recipients were male and white (Table 1). The median follow-up time for the overall study period was 6 (range 0–26.4) years, expectedly longer for the pre-MELD era (11 years) than for the MELD era (3 years). GFR was measured at least once at LTx, at 4 months or later post-LTx in 95 % of patients (mean of 5 measurements per patient). Over the entire study period, a total of 6,246 iothalamate measurements and 34,353 creatinine values for eGFR assessment were available. Approximately 54% of patients died in the study period. The causes of death included malignancy (25%), graft failure (14%), infections (13%), cardiovascular events (7%), renal failure (5%), other (18%) and were unknown in 18% of cases .
Table 1.
Patient characteristics at transplantation.
| Parameter | Patients N=1211 |
|---|---|
| Age, y (median, IQR) | 54 (46–60) |
| Male (%) | 724 (60) |
| Race (%) | |
| White | 1046 (86) |
| Black | 22 (2) |
| Asian | 36 (3) |
| Other | 107 (9) |
| Liver disease etiology | |
| Viral | 28% |
| Alcoholic | 14% |
| Cholestatic | 26% |
| Other | 32% |
| Bilirubin, mg/dL(median, IQR) | 4.5 (2.4–8.8) |
| Creatinine, mg/dL (median, IQR) | 1.0 (0.8–1.3) |
| INR (median, IQR) | 1.4 (1.2–1.7) |
| Sodium, mmol/L (median, IQR) | 136 (132–139) |
| MELD (median, IQR) | 17 (12–22) |
| Patients (%) by eGFR ranges | |
| ≥ 60 ml/min/1.73 m2 | 878 (72.9) |
| 59-30 ml/min/1.73 m2 | 240 (19.9) |
| 29-15 ml/min/1.73 m2 | 60 (5.0) |
| <15 ml/min/1.73 m2 | 26 (2.2) |
Prevalence of chronic kidney disease after liver transplantation
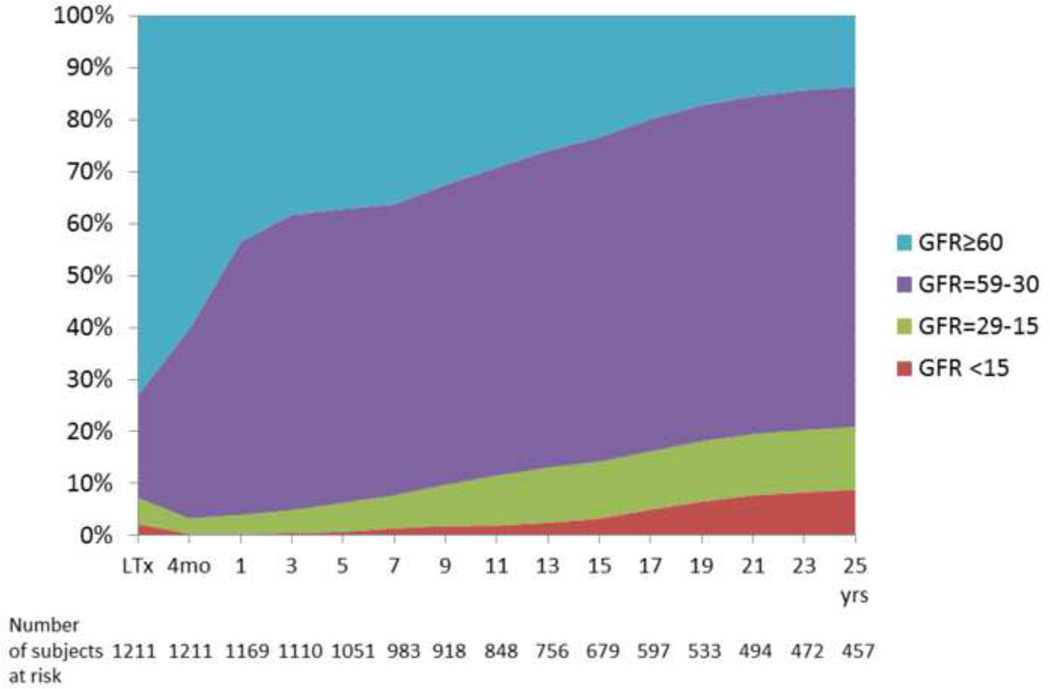
Based on estimated GFR data, the median (IQR) GFR decreased from 84 ( 58–109 ) ml/min/1.73m2 at the time of LTx to 66 (52–86) ml/min/1.73m2 post-LTx at 4 months post-transplant. A significant increase in the prevalence of CKD was noted as early as 4 months post –LTx, when 40% of subjects already had CKD stage 3 or worse (GFR below 60 ml/min/1.73m2) (Figure 1A and Supplementary Table). In an attempt to determine risk factors for early CKD, we reviewed a random sample of 30 subjects with normal renal function at LTx who developed CKD (GFR < 60ml/min/1.73m2) 4 months afterwards. Exhaustive review of all perioperative and subsequent medical events in those patients revealed that postoperative acute kidney injury was present in only 8 patients (27%). No other risk factors for renal dysfunction, such as cause of liver disease leading to LTx or presence of comorbidities (e.g., diabetes and pre-existing or de novo hypertension) appeared to result in a precipitous decrease in renal function. Instead, in the majority of cases, the renal dysfunction was gradually progressive, with subtle increase in serum creatinine (by 0.3 mg/ml/1.73m2) starting after a median of 20 days postoperatively .
Fig. 1.
Posttransplant renal function in patients who remain alive and without kidney transplant. (A) Estimated GFR by MDRD 4 (B) Measured GFR by iothalamate clearance.
Table 2 shows the prevalence of kidney disease, assessed by mGFR. The proportion of patients without CKD (i.e., GFR>60) decreased progressively over time to 41 % at 1 year, 38% at 5 years, 28% at 10 years and 7% at 25 years, in part due to patient death as well as increasing prevalence of CKD. Throughout the follow-up, CKD stage 3 (GFR=30–59 ml/min/1.73m2) was the most predominant state; of all transplant recipients, 48% had CKD stage 3 at 1 year, 41% at 10 years and 21% at 25 years. The proportion of patients who developed severe CKD (stage 4 or worse: GFR<30 ml/min/1.73m2 or KTx) at 1, 10 and 25 years after LTx was 8%, 11% and 18%, respectively. At 25 years after LTx, the proportion of liver graft recipients ever undergoing KTx was 9%, while 3.3% of patients received definitive renal replacement therapy without kidney transplantation . When compared to mGFR, MDRD equation tended to underestimate the fraction of patients with normal GFR and severe CKD (stage 4 or worse), and overestimate moderate CKD (GFR=30–59 ml/min/1.73m2) (Supplementary Table).
Table 2.
Distribution of measured GFR (ml/min/1.73m2), kidney transplant and death after liver transplantation.
| Time post LTx |
% subjects with GFR>60 |
% subjects with GFR= 59-30 |
% subjects with GFR= 29-15 |
% subjects with GFR<15 |
% subjects with kidney transplant |
Death |
|---|---|---|---|---|---|---|
| 1 year | 41 | 48 | 7 | 0.4 | 0.2 | 3 |
| 5 years | 38 | 41 | 7 | 1 | 1 | 12 |
| 10 years | 28 | 37 | 7 | 1 | 3 | 24 |
| 15 years | 17 | 30 | 8 | 2 | 5 | 39 |
| 20 years | 9 | 23 | 7 | 2 | 8 | 50 |
| 25 years | 7 | 21 | 7 | 2 | 9 | 54 |
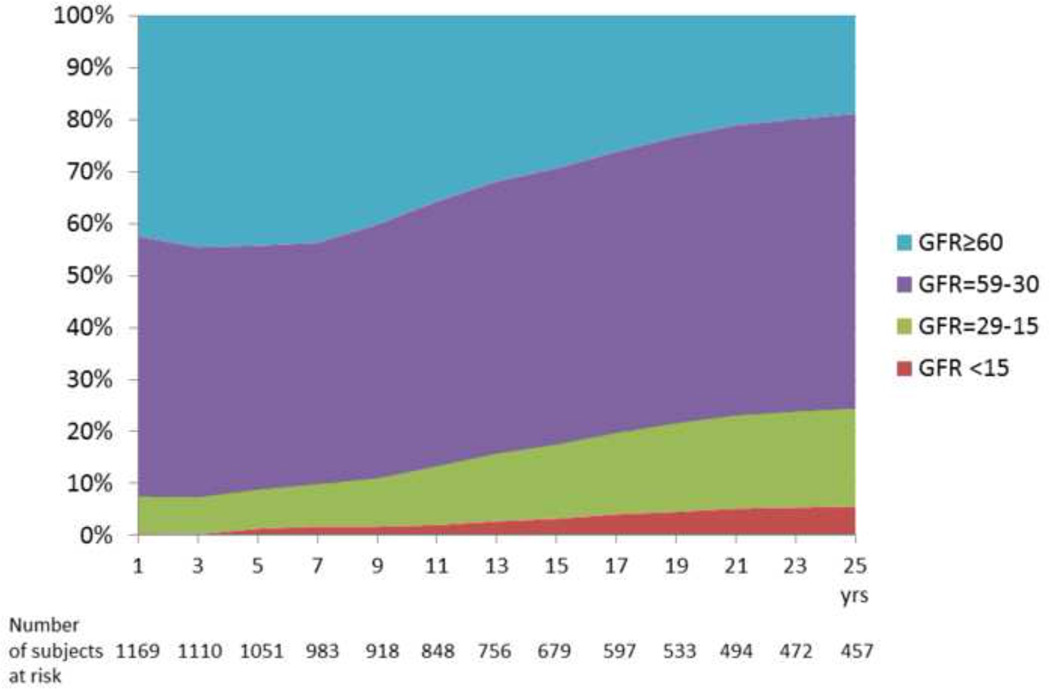
Figure 1B displays the data reported in Table 2 while subtracting deaths and KTx, thus describing the proportion (point prevalence) of each measured GFR range . At 25 years after LTx, of the 457 patients who were alive and KTx-free, only 19 % maintained a mGFR above 60 ml/min/1.73m2, while 57% had GFR of 59-30 ml/min/m2 (stage 3 CKD), 19% had GFR of 29-15 ml/min/1.73m2 (stage 4 CKD) and 5 % had GFR<15 ml/min/1.73m2 (stage 5 CKD).
When the data from the 2 eras were compared for the first 9 years after LTx, the distribution of the GFR ranges was similar (Supplementary Figure). Compared to the pre-MELD era, patients in the MELD era were older (median age 55 vs 50, p<0.0001) and more predominantly male (64% vs 53%, p=0.0001). There were no statistically significant differences in the levels of creatinine, bilirubin, INR and sodium between the 2 eras. The proportion of patients with GFR above 60 ml/min/1.73m2 at the time of LTx was similar between the 2 ears (72.9% vs 73.0%, p=0.09).The proportion of patients who developed severe CKD (including KTx recipients) at 9 years after LTx was 9.6 % and 6.2 % in the pre-MELD and MELD eras, respectively.
Impact of post-transplant renal function on mortality
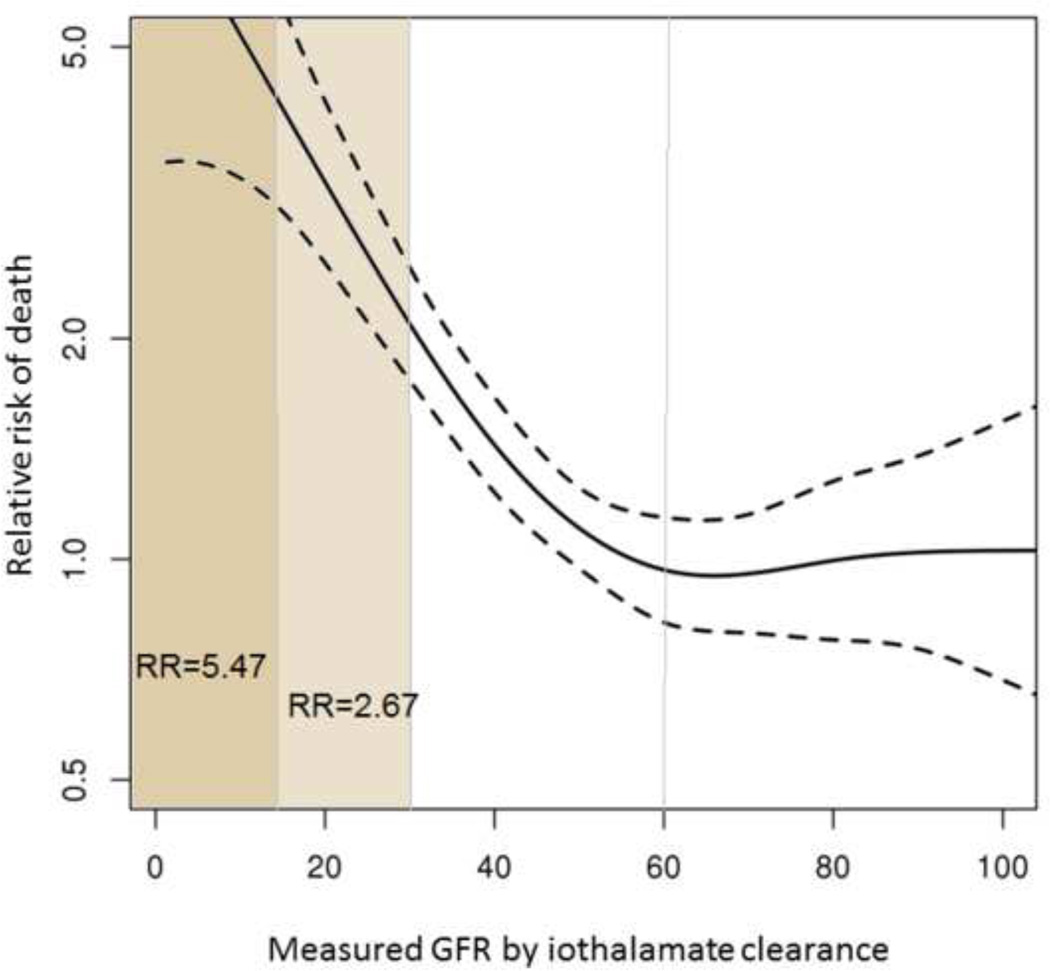
Figure 2 shows the association between mGFR and mortality analyzed in a time-dependent fashion. As GFR decreases, the risk of death increases exponentially. The shape of the curve suggests that renal function starts to affect survival when mGFR decreases below 60 ml/min/1.73m2. Referenced to subjects whose GFR is above 60 ml/min/1.73m2, the age and gender adjusted hazard ratio (HR) for mortality was 2.67 (95% CI 1.80–3.96) for GFR of 29-15 ml/min/1.73m2 and 5.47 (95% CI 3.10–9.65) for GFR less than 15 ml/min/1.73m2. In other words, if GFR is measured, for example, at 29 ml/min/1.73m at any time during the follow-up, the patient has 2.67 times the risk of death compared to all recipients alive and followed for the same amount of time. Moderate CKD (GFR 59-30 ml/min/1.73m2) was associated with an adjusted HR of 1.19 (95% CI 0.87–1.64) , which was not statistically significant (p=0.27).
Fig. 2. Impact of measured GFR on mortality.
The risk of death increases significantly when measured GFR decreased <30 ml/min/1.73m2. Dashed lines represent the 95% confidence interval.
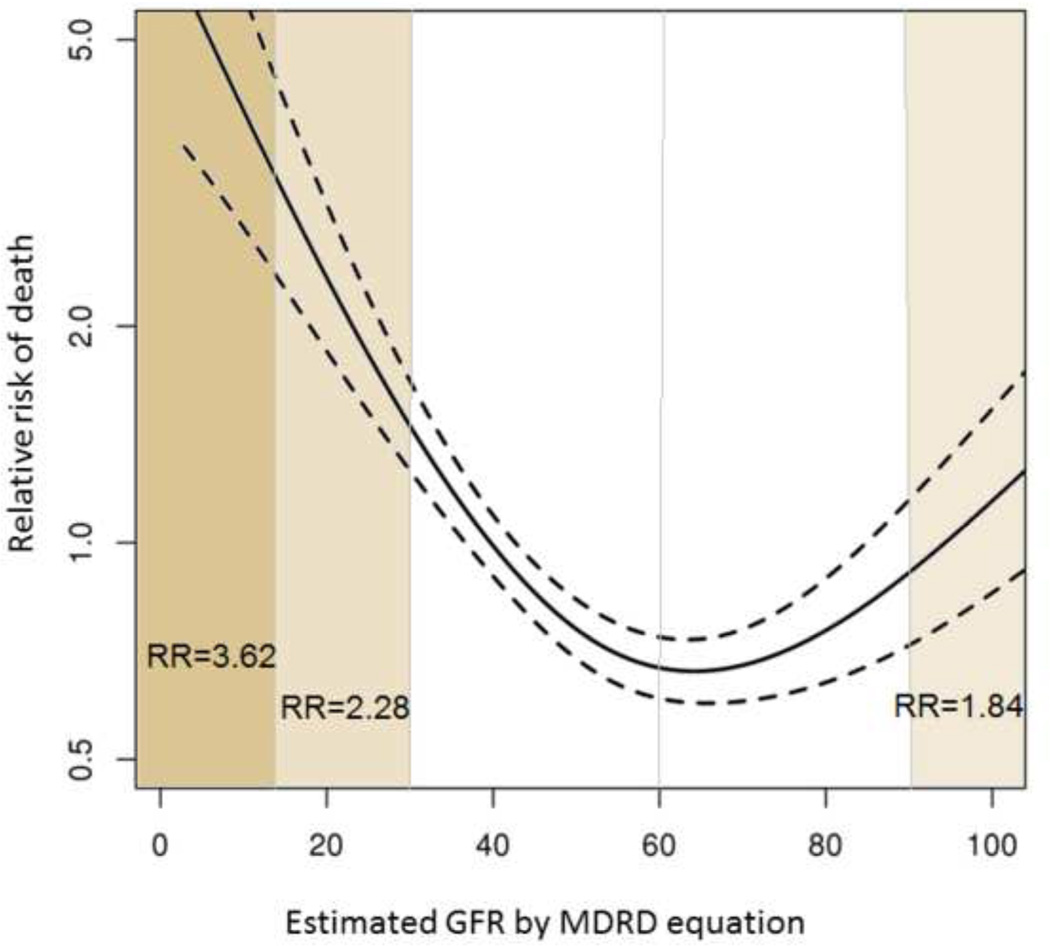
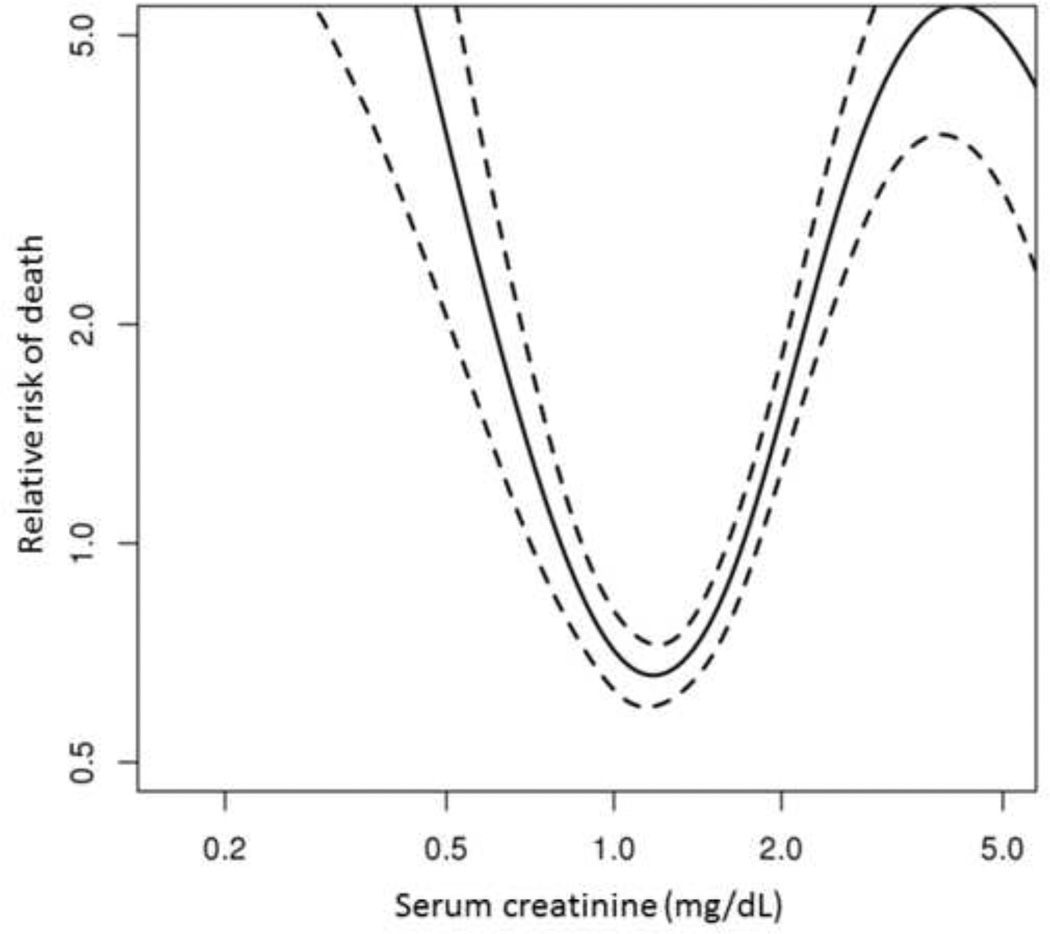
To evaluate to what extent eGFR may be a surrogate indicator of survival, a similar time-dependent analysis was performed using estimated GFR. As seen in Figure 3, the effect on survival is overall comparable to that with mGFR. As expected, a decline in eGFR is associated with an increase in mortality risk: age and gender adjusted HRs of 2.28 (95% CI 1.52–3.43) and 3.62 (95% CI 1.97–6.66) for GFR of 29-15 ml/min/1.73 m2 and below 15 ml/min/1.73 m2, respectively. The shape of the curve suggests that eGFR is less sensitive in detecting increased mortality – reduced survival is not apparent until eGFR decreases below 45 ml/min/1 .73m2. Notably, unlike mGFR, the relationship is bidirectional, in that increased mortality is also seen with normal to high eGFR. The effect is statistically significant: the adjusted hazard ratio at eGFR greater than 90 ml/min/1.73 m2 (using GFR of 89-60 ml/min/1.73 m2 as reference) was 1.84 (95% CI 1.15–2.95) . This spurious trend is further examined in Figure 4, which describes the association with mortality and serum creatinine. It is clear that the relation is U-shaped: the risk of death increases in both directions as serum creatinine increases or decreases away from around 1.0 mg/dL (adjusted HR= 1.89 for each ± unit ln (creatinine), 95% CI 1.39–2.58).
Fig. 3. Impact of estimated GFR on mortality.
The risk of death increases exponentially when estimated GFR decreased below 30 ml/min/1.73m2. GFR>90 ml/min/1.73m2 is also associated with an increased risk of death. Dashed lines represent the 95% confidence interval.
Fig. 4. Impact of serum creatinine on mortality.
The risk of death is increased both at high and low creatinine levels. Dashed lines represent the 95% confidence interval.
The discrepant effect of eGFR in comparison to mGFR is further evaluated in Table 3, which summarizes the risk of death associated with the reclassification of eGFR category by mGFR. Overall, a high proportion of participants were reclassified to a different GFR category. Notably, reclassification occurred most often for eGFR of 45–59 ml/min/1.73m2, where more than half (53.6%) of subjects had mGFR in a different category. Reclassification occurred not as ubiquitously, yet still quite frequently, for eGFR<30 ml/min/1.73m2 (31.5%). Reclassification to a lower GFR range by mGFR (i.e., overestimation of GFR by eGFR) was associated with a significantly increased risk of death across all categories. For example, of the patients with eGFR between 60–89 ml/min/1.73m2, 24% were reclassified to a lower GFR and had a relative increase in risk of death of 2.37. Reclassification to a higher GFR range by mGFR tended to decrease the risk of death, but the association was not statistically significant.
Table 3.
Risk of death associated with reclassification of estimated GFR by measured GFR.
| eGFR ml/min/1.73m2 |
Number of observations |
Reclassification to higher GFR by mGFR |
No reclassification | Reclassification to lower GFR by mGFR |
|||
|---|---|---|---|---|---|---|---|
| % of observations |
HR (95% CI) | % of observations |
HR (95% CI) |
% of observations | HR (95% CI) | ||
| ≥ 90 | 352 | N/A | N/A | 57.7% | Reference | 42.3% | 2.25 (0.98–5.21) |
| 60–89 | 1591 | 15.6% | 0.76 (0.35–1.66) |
60.2% | Reference | 24.3% | 2.37 (1.52–3.68) |
| 45–59 | 1805 | 29.3% | 0.89 (0.54–1.45) |
46.4% | Reference | 24.3% | 1.70 (1.05–2.74) |
| 30–44 | 1698 | 28.2% | 0.55 (0.28–1.08) |
547% | Reference | 17.1% | 2.80 (1.83–4.29) |
| <30 | 463 | 31.5% | 1.39 (0.60–3. 20 ) |
68.5% | Reference | N/A | N/A |
HR was adjusted for age and sex
DISCUSSION
As of 2014, CKD remains a prevalent complication after liver transplantation and carries a substantial mortality risk. In this study, we describe the full spectrum of renal dysfunction, assessed by measured GFR, after LTx in a large sample of subjects followed up to 25 years. An overwhelming majority of liver recipients develop moderate to severe CKD within the first year post-LTx, while only a small fraction of patients maintain normal renal function long-term. Of all patients undergoing LTx, 18% had severe CKD (defined by GFR<30 ml/min/1.73m2 or KTx) at 25 years. The point prevalence of severe CKD among liver transplant recipients who were alive and without a kidney transplant at 25 years post-LT (mean age 67 years) is strikingly higher than the prevalence of severe CKD in the general population of 60–69 years of age (25% versus 0.4%) [26]. Similarly, only 18% of LTx recipients who survive 25 years have normal renal function, in contrast to 39% of subjects within the same age range from the general population .
Renal dysfunction is a well-recognized complication of LTx. In a large SRTR analysis, severe CKD (defined as eGFR <30ml/min/1.73m2 or listing for KTx) occurred in 26% of liver graft recipients 10 years after LTx [6]. In other studies, the cumulative incidence of post-LTx CKD ranged between 18% [7] and 39% [11] more than 10 years after LTx. In the only other study based on mGFR data, the cumulative incidence of renal failure (GFR <40 ml/min/1.73m2) at 5 years [20] was 27%, although the study was limited by its small sample size.
In our study, the prevalence of severe CKD assessed by mGFR was lower than that described in most of the existing literature. It is generally accepted that creatinine based eGFR underestimates the degree of renal dysfunction in patients with liver disease. In our study, the prevalence of severe CKD as estimated by MDRD was indeed lower than that assessed by mGFR. One potential explanation as to the lower frequency of CKD in our data than in other studies may be the different methodology used to obtain the results, i.e. multistate model versus cumulative incidence. Details describing the methods used in other studies are not available, which makes further comparison difficult.
Another possible explanation for the difference is informative censoring in other studies, where patients with advanced CKD may be more likely to remain in follow-up, whereas our follow-up protocol encourages even healthier patients to return to the Transplant Clinic for follow-up many years after their transplantation. Another theoretical possibility is that a number of ‘renal sparing’ efforts instituted in our patients may have led to better preservation of renal function. However, as the effectiveness of these measures to preserve renal function is limited, the extent to which this may have contributed to the difference is uncertain.
Consistent with previous studies [27] [28], the decline in GFR post-LTx in this analysis is bi-phasic. The first phase, seen in the first 4 months, represents a steep decline in GFR, as shown by the slope in Figure 1A during the first year following LTx. Although the majority of patients (72%) had a GFR>60 ml/min/1.73m2 at the time of LTx, almost half of the patients alive at 1 year already had moderate CKD. Postoperative kidney injury was identified in only a minority of subjects. In the majority of cases the decrease of renal function within the first 4 months post-LTx was attributed to the nephrotoxicity of the immunosuppressive agents. This underscores the importance of prevention and timely reversal of perioperative kidney injury, as well as diligent monitoring of renal function early post-LTx. The ensuing second phase is more gradual yet persistently progressive for years to come. The majority (50–60%) of our liver recipients who survive without a KTx have stage 3 CKD (GFR= 30–59 ml/min/1.73m2). Although in our study the risk of death was not significantly increased, moderate CKD and its associated comorbidities cause substantial health burden both in patient suffering and healthcare resource expenditure.
Since the introduction of MELD-based liver graft allocation in 2002 [29], the proportion of patients with renal dysfunction undergoing LTx has increased [30]. This trend has translated to a higher prevalence of CKD or ESRD post-LTx in the MELD era [31] [8] [27]. Although the proportion of our patients with GFR >60ml/min/1.73m2 at the time of LTx was lower in the MELD era, the prevalence of severe CKD and KTx after LTx did not increase between the pre-MELD and the MELD eras (Supplementary Figure). The apparent dissociation between pre- and post-LTx renal dysfunction may be a result of judicious application of simultaneous liver-kidney transplantation in patients at high risk of progression to ESRD following LTx alone. In addition, as was alluded to earlier, various renal sparing protocols were put in place in the more recent era, including the use of IL-2 antibody induction to delay and minimize the use of calcineurin inhibitors in the immediate post-LTx period and switch to sirolimus in the maintenance phase. Close follow-up of renal function including measurement of GFR at regular intervals, and protocolized, multidisciplinary management of comorbidity such as diabetes and hypertension may have also contributed.
The other unique feature of this analysis is time-dependent modeling of the impact of renal dysfunction on patient survival. Most prior studies addressing this question utilized time-fixed methods, correlating renal dysfunction at a certain initial time-point, e.g., at or shortly after LTx with subsequent survival [32] [33] [34]. Since time-fixed analyses fail to take into account CKD developing after the initial assessment, the results are not applicable to patients being seen in follow-up and they tend to underestimate the true impact of renal dysfunction on mortality. In contrast, time-dependent analysis makes use of all the available GFR data and the risk is assessed with each GFR data point during the follow-up, an approach that is more reflective of our clinical practice.
The time-dependent analysis suggests that the risk of mortality starts to increase as early as at mGFR of 60 ml/min/1.73m2. In our study, CKD stage 3 increased the mortality risk by 16% when assessed by iothalamate clearance, although the increased risk did not reach statistical significance. Clearly, further decrease in GFR to lower than 30 ml/min/1.73m2 is associated with worse prognosis. Compared to subjects with GFR of 60 ml/min/1.73m2 and above, GFR of 29-15 ml/min/1.73m2 conveys a mortality risk 2.7 times higher and values below 15 ml/min/1.73m2 increase the risk of death more than fivefold (HR= 5.5).
Another notable limitation in the existing literature reporting mortality impact of CKD after LTx is the use of serum creatinine or creatinine-based eGFR to study this association. In keeping with previous findings in patients with CKD [35], our study shows limited reliability of creatinine-based GFR estimation in predicting mortality. Indeed, eGFR underestimated the mortality risk in a significant proportion of patients with mild-moderate renal dysfunction (24% of patients with CKD stage 2, 24% of patients with CKD stage 3a and 17% of patients with CKD stage 3b) (Table 3). On the other hand, as illustrated by the U-shaped curves in Figures 3 and 4, a high-normal eGFR (> 90 ml/min/1.73m2) is associated with an increased mortality risk. This paradoxical finding, previously reported in the nephrology literature [36], can be attributed to the fact that low creatinine (thus high GFR) is a non-specific indicator of poor general health in patients with malnutrition, sarcopenia or low body mass index. Thus, a normal renal function, as estimated by MDRD may give a false sense of safety in these subjects. Inaccurate GFR estimation by MDRD at low serum creatinine levels could be another potential explanation for this finding, given that almost half of the patients with high-normal GFR were reclassified to lower GFR by iothalamate clearance (Table 3). However, this is unlikely, since the vast majority of these patients were reclassified to a GFR of 60–89 ml/min/1.73m2, a range where the mortality risk is not significantly increased based on renal function alone.
The strength of this study is the use of measured GFR, presented separately from eGFR starting at 1 year post-LTx. GFR at LTx and at 4 months was estimated, since it is not feasible to schedule an iothalamate clearance test on the day of transplantation. Although our protocol specifies GFR to be measured annually, real life events not uncommonly hindered complete adherence, due to events such as other urgent medical issues taking precedence, scheduling conflict, or patient refusal. Of the 1,211 patients, 95% had at least one iothalamate clearance test, with a mean of 5 measurements per patient. Despite this limitation, our results are based on a large number of GFR measurements (n=6,246).
In summary, based on assessment of the full spectrum of renal dysfunction as measured by iothalamate clearance in a large number of patients followed up to 25 years, CKD is a common complication of LTx. Notably, the vast majority of liver graft recipients end up with GFR<60 ml/min/1.73m2, i.e., stage 3 CKD, within the first year after LTx, a threshold level at which long term morbidity and mortality begin to rise. In contrast, eGFR underestimates the renal dysfunction and the risk of death in a significant proportion of patients, particularly in those with GFR between 90 and 30 ml/min/1.73m2. As the current therapeutic options to reverse CKD in our patients are limited, these data highlight the need for an emphasis on preventive and expectant management based on early recognition of renal dysfunction, which may require actual measurement of GFR. As less nephrotoxic immunosuppressive regimens evolve, paying attention to renal morbidity may become even more important for practitioners in the future in order to prevent long-term complications and reduce mortality, thereby achieving further optimization of the outcome of liver transplantation.
Supplementary Material
Acknowledgments
Financial support: National Institute of Diabetes and Digestive and Kidney Diseases DK-34238 and DK-92336 (WRK).
Glossary
- LTx
liver transplantation
- eGFR
estimated glomerular filtration rate
- mGFR
measured glomerular filtration rate
- CKD
chronic kidney disease
- MDRD
modified diet in renal disease
- KTx
kidney transplantation
- MELD
model for end-stage liver disease
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors of this manuscript have no conflicts of interest to disclose as described by Journal of Hepatology.
REFERENCES
- 1.Jain A, Singhal A, Fontes P, Mazariegos G, DeVera ME, Cacciarelli T, et al. One thousand consecutive primary liver transplants under tacrolimus immunosuppression: a 17- to 20-year longitudinal follow-up. Transplantation. 2011;91:1025–1030. doi: 10.1097/TP.0b013e3182129215. [DOI] [PubMed] [Google Scholar]
- 2.Watt KD, Pedersen RA, Kremers WK, Heimbach JK, Charlton MR. Evolution of causes and risk factors for mortality post-liver transplant: results of the NIDDK long-term follow-up study. Am J Transplant. 2010;10:1420–1427. doi: 10.1111/j.1600-6143.2010.03126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim JY, Akalin E, Dikman S, Gagliardi R, Schiano T, Bromberg J, et al. The variable pathology of kidney disease after liver transplantation. Transplantation. 2010;89:215–221. doi: 10.1097/TP.0b013e3181c353e5. [DOI] [PubMed] [Google Scholar]
- 4.McGuire BM, Julian BA, Bynon JS, Jr, Cook WJ, King SJ, Curtis JJ, et al. Brief communication: Glomerulonephritis in patients with hepatitis C cirrhosis undergoing liver transplantation. Annals of internal medicine. 2006;144:735–741. doi: 10.7326/0003-4819-144-10-200605160-00007. [DOI] [PubMed] [Google Scholar]
- 5.Pillebout E, Nochy D, Hill G, Conti F, Antoine C, Calmus Y, et al. Renal histopathological lesions after orthotopic liver transplantation (OLT) Am J Transplant. 2005;5:1120–1129. doi: 10.1111/j.1600-6143.2005.00852.x. [DOI] [PubMed] [Google Scholar]
- 6.Ojo AO, Held PJ, Port FK, Wolfe RA, Leichtman AB, Young EW, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003;349:931–940. doi: 10.1056/NEJMoa021744. [DOI] [PubMed] [Google Scholar]
- 7.Gonwa TA, Mai ML, Melton LB, Hays SR, Goldstein RM, Levy MF, et al. End-stage renal disease (ESRD) after orthotopic liver transplantation (OLTX) using calcineurin-based immunotherapy: risk of development and treatment. Transplantation. 2001;72:1934–1939. doi: 10.1097/00007890-200112270-00012. [DOI] [PubMed] [Google Scholar]
- 8.Sharma P, Schaubel DE, Guidinger MK, Goodrich NP, Ojo AO, Merion RM. Impact of MELD-based allocation on end-stage renal disease after liver transplantation. Am J Transplant. 2011;11:2372–2378. doi: 10.1111/j.1600-6143.2011.03703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burra P, Senzolo M, Masier A, Prestele H, Jones R, Samuel D, et al. Factors influencing renal function after liver transplantation. Results from the MOST, an international observational study. Digestive and liver disease. 2009;41:350–356. doi: 10.1016/j.dld.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 10.Sharma P, Welch K, Eikstadt R, Marrero JA, Fontana RJ, Lok AS. Renal outcomes after liver transplantation in the model for end-stage liver disease era. Liver transplantation. 2009;15:1142–1148. doi: 10.1002/lt.21821. [DOI] [PubMed] [Google Scholar]
- 11.Moreno JM, Cuervas-Mons V, Rubio E, Pons F, Herreros de TA, Turrion VS, et al. Chronic renal dysfunction after liver transplantation in adult patients: prevalence, risk factors, and impact on mortality. Transplantation proceedings. 2003;35:1907–1908. doi: 10.1016/s0041-1345(03)00642-0. [DOI] [PubMed] [Google Scholar]
- 12.Giusto M, Berenguer M, Merkel C, Aguilera V, Rubin A, Ginanni Corradini S, et al. Chronic Kidney Disease After Liver Transplantation: Pretransplantation Risk Factors and Predictors During Follow-Up. Transplantation. 2013;95:1148–1153. doi: 10.1097/TP.0b013e3182884890. [DOI] [PubMed] [Google Scholar]
- 13.LaMattina JC, Mezrich JD, Fernandez LA, D'Alessandro AM, Djamali A, Musat AI, et al. Native kidney function following liver transplantation using calcineurin inhibitors: single-center analysis with 20 years of follow-up. Clinical transplantation. 2013;27:193–202. doi: 10.1111/ctr.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fisher NC, Nightingale PG, Gunson BK, Lipkin GW, Neuberger JM. Chronic renal failure following liver transplantation: a retrospective analysis. Transplantation. 1998;66:59–66. doi: 10.1097/00007890-199807150-00010. [DOI] [PubMed] [Google Scholar]
- 15.Gayowski T, Singh N, Keyes L, Wannstedt CF, Wagener MM, Vargas H, et al. Late-onset renal failure after liver transplantation: role of posttransplant alcohol use. Transplantation. 2000;69:383–388. doi: 10.1097/00007890-200002150-00013. [DOI] [PubMed] [Google Scholar]
- 16.Schmitz V, Laudi S, Moeckel F, Puhl G, Stockmann M, Tran ZV, et al. Chronic renal dysfunction following liver transplantation. Clinical transplantation. 2008;22:333–340. doi: 10.1111/j.1399-0012.2008.00806.x. [DOI] [PubMed] [Google Scholar]
- 17.Lynn M, Abreo K, Zibari G, McDonald J. End-stage renal disease in liver transplants. Clinical transplantation. 2001;15(Suppl 6):66–69. doi: 10.1034/j.1399-0012.2001.00013.x. [DOI] [PubMed] [Google Scholar]
- 18.Rule AD, Bailey KR, Schwartz GL, Khosla S, Lieske JC, Melton LJ., 3rd For estimating creatinine clearance measuring muscle mass gives better results than those based on demographics. Kidney international. 2009;75:1071–1078. doi: 10.1038/ki.2008.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonwa TA, Jennings L, Mai ML, Stark PC, Levey AS, Klintmalm GB. Estimation of glomerular filtration rates before and after orthotopic liver transplantation: evaluation of current equations. Liver transplantation. 2004;10:301–309. doi: 10.1002/lt.20017. [DOI] [PubMed] [Google Scholar]
- 20.Cohen AJ, Stegall MD, Rosen CB, Wiesner RH, Leung N, Kremers WK, et al. Chronic renal dysfunction late after liver transplantation. Liver transplantation. 2002;8:916–921. doi: 10.1053/jlts.2002.35668. [DOI] [PubMed] [Google Scholar]
- 21.O'Riordan A, Wong V, McCormick PA, Hegarty JE, Watson AJ. Chronic kidney disease post-liver transplantation. Nephrology, dialysis, transplantation. 2006;21:2630–2636. doi: 10.1093/ndt/gfl247. [DOI] [PubMed] [Google Scholar]
- 22.Wilson DM, Bergert JH, Larson TS, Liedtke RR. GFR determined by nonradiolabeled iothalamate using capillary electrophoresis. Am J Kidney Dis. 1997;30:646–652. doi: 10.1016/s0272-6386(97)90488-1. [DOI] [PubMed] [Google Scholar]
- 23.Rule AD, Gussak HM, Pond GR, Bergstralh EJ, Stegall MD, Cosio FG, et al. Measured and estimated GFR in healthy potential kidney donors.[erratum appears in Am J Kidney Dis. 2004 Dec;44(6):1126] American Journal of Kidney Diseases. 2004;43:112–119. doi: 10.1053/j.ajkd.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 24.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Annals of internal medicine. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 25.Shlipak MG, Matsushita K, Arnlov J, Inker LA, Katz R, Polkinghorne KR, et al. Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med. 2013;369:932–943. doi: 10.1056/NEJMoa1214234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003;41:1–12. doi: 10.1053/ajkd.2003.50007. [DOI] [PubMed] [Google Scholar]
- 27.Israni AK, Xiong H, Liu J, Salkowski N, Trotter JF, Snyder JJ, et al. Predicting End-Stage Renal Disease After Liver Transplant. Am J Transplant. 2013 doi: 10.1111/ajt.12257. [DOI] [PubMed] [Google Scholar]
- 28.Sanchez EQ, Melton LB, Chinnakotla S, Randall HB, McKenna GJ, Ruiz R, et al. Predicting renal failure after liver transplantation from measured glomerular filtration rate: review of up to 15 years of follow-up. Transplantation. 2010;89:232–235. doi: 10.1097/TP.0b013e3181c42ff9. [DOI] [PubMed] [Google Scholar]
- 29.Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–470. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 30.Gonwa TA, McBride MA, Anderson K, Mai ML, Wadei H, Ahsan N. Continued influence of preoperative renal function on outcome of orthotopic liver transplant (OLTX) in the US: where will MELD lead us? Am J Transplant. 2006;6:2651–2659. doi: 10.1111/j.1600-6143.2006.01526.x. [DOI] [PubMed] [Google Scholar]
- 31.Thuluvath PJ, Guidinger MK, Fung JJ, Johnson LB, Rayhill SC, Pelletier SJ. Liver transplantation in the United States, 1999–2008. Am J Transplant. 2010;10:1003–1019. doi: 10.1111/j.1600-6143.2010.03037.x. [DOI] [PubMed] [Google Scholar]
- 32.Pawarode A, Fine DM, Thuluvath PJ. Independent risk factors and natural history of renal dysfunction in liver transplant recipients. Liver transplantation. 2003;9:741–747. doi: 10.1053/jlts.2003.50113. [DOI] [PubMed] [Google Scholar]
- 33.Nair S, Verma S, Thuluvath PJ. Pretransplant renal function predicts survival in patients undergoing orthotopic liver transplantation. Hepatology. 2002;35:1179–1185. doi: 10.1053/jhep.2002.33160. [DOI] [PubMed] [Google Scholar]
- 34.Al Riyami D, Alam A, Badovinac K, Ivis F, Trpeski L, Cantarovich M. Decreased survival in liver transplant patients requiring chronic dialysis: a Canadian experience. Transplantation. 2008;85:1277–1280. doi: 10.1097/TP.0b013e31816c4e6b. [DOI] [PubMed] [Google Scholar]
- 35.Astor BC, Levey AS, Stevens LA, Van Lente F, Selvin E, Coresh J. Method of glomerular filtration rate estimation affects prediction of mortality risk. Journal of the American Society of Nephrology : JASN. 2009;20:2214–2222. doi: 10.1681/ASN.2008090980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tonelli M, Klarenbach SW, Lloyd AM, James MT, Bello AK, Manns BJ, et al. Higher estimated glomerular filtration rates may be associated with increased risk of adverse outcomes, especially with concomitant proteinuria. Kidney Int. 2011;80:1306–1314. doi: 10.1038/ki.2011.280. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.