Abstract
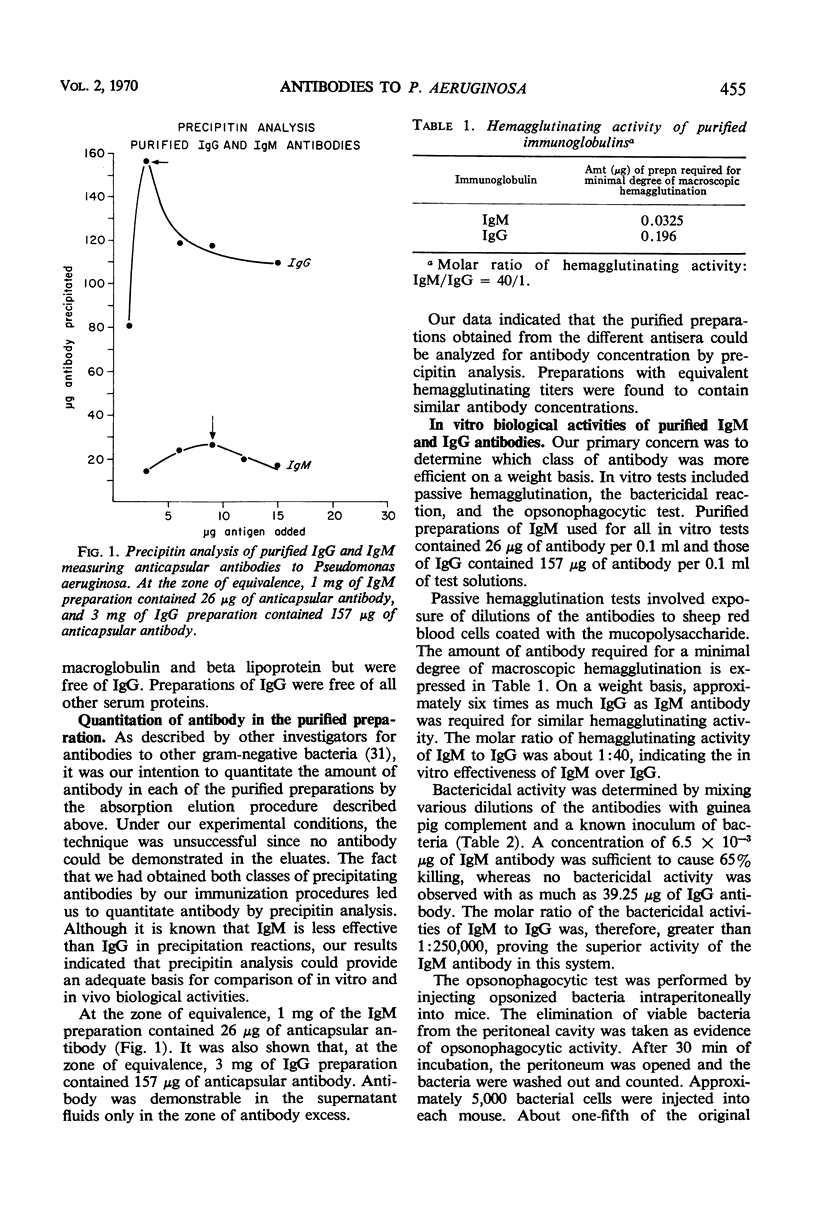
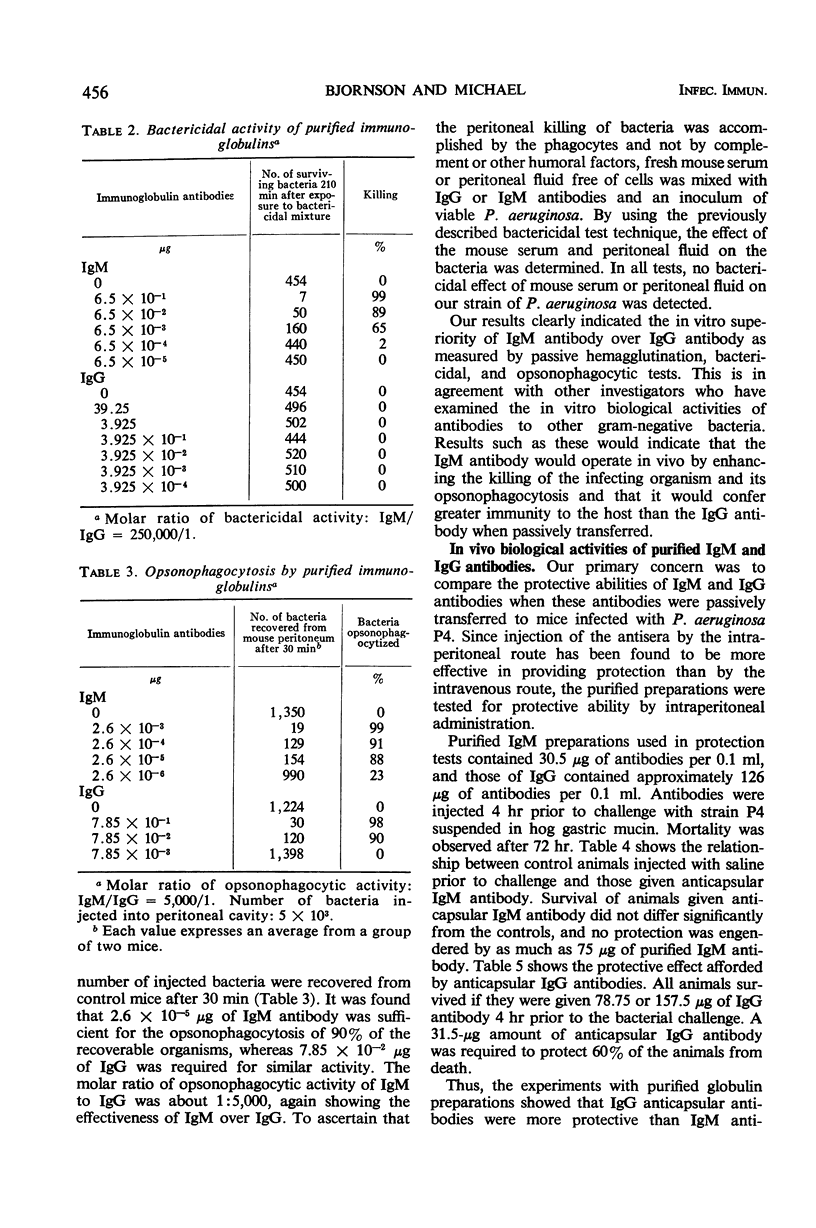
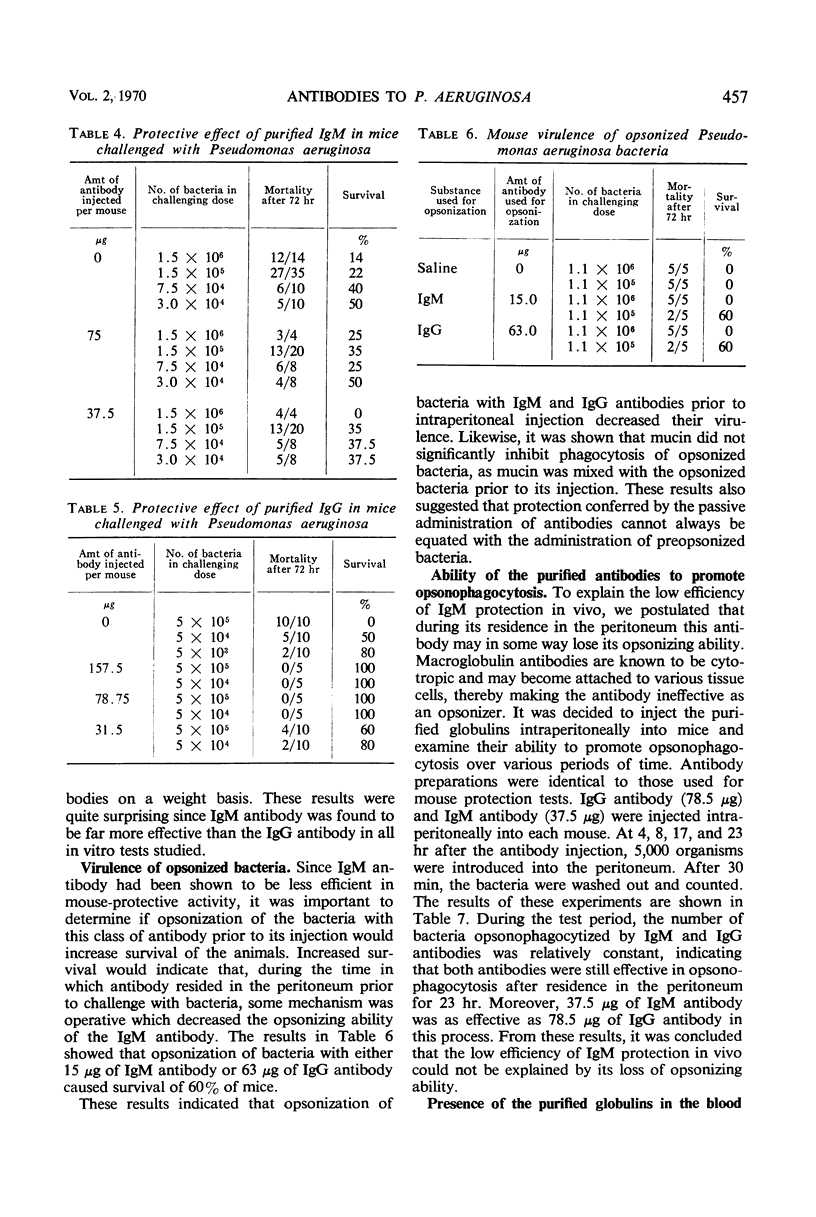
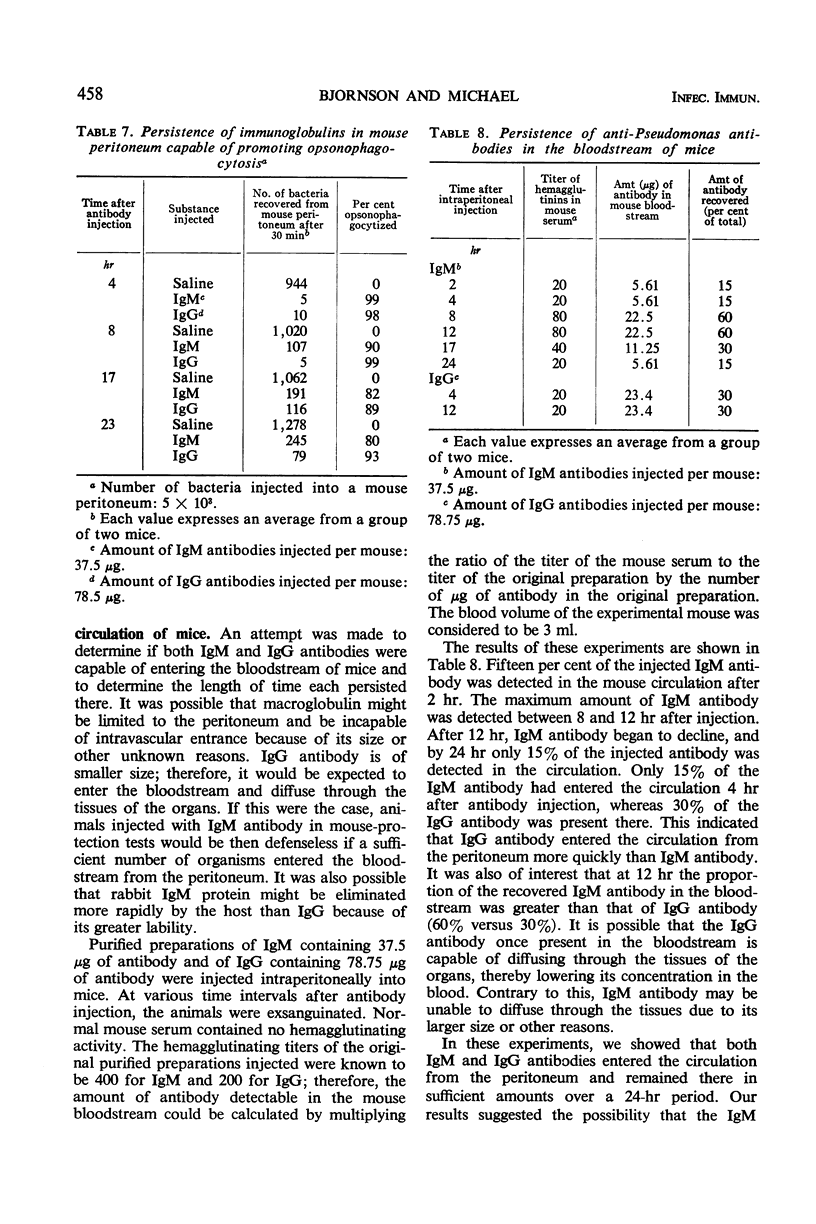
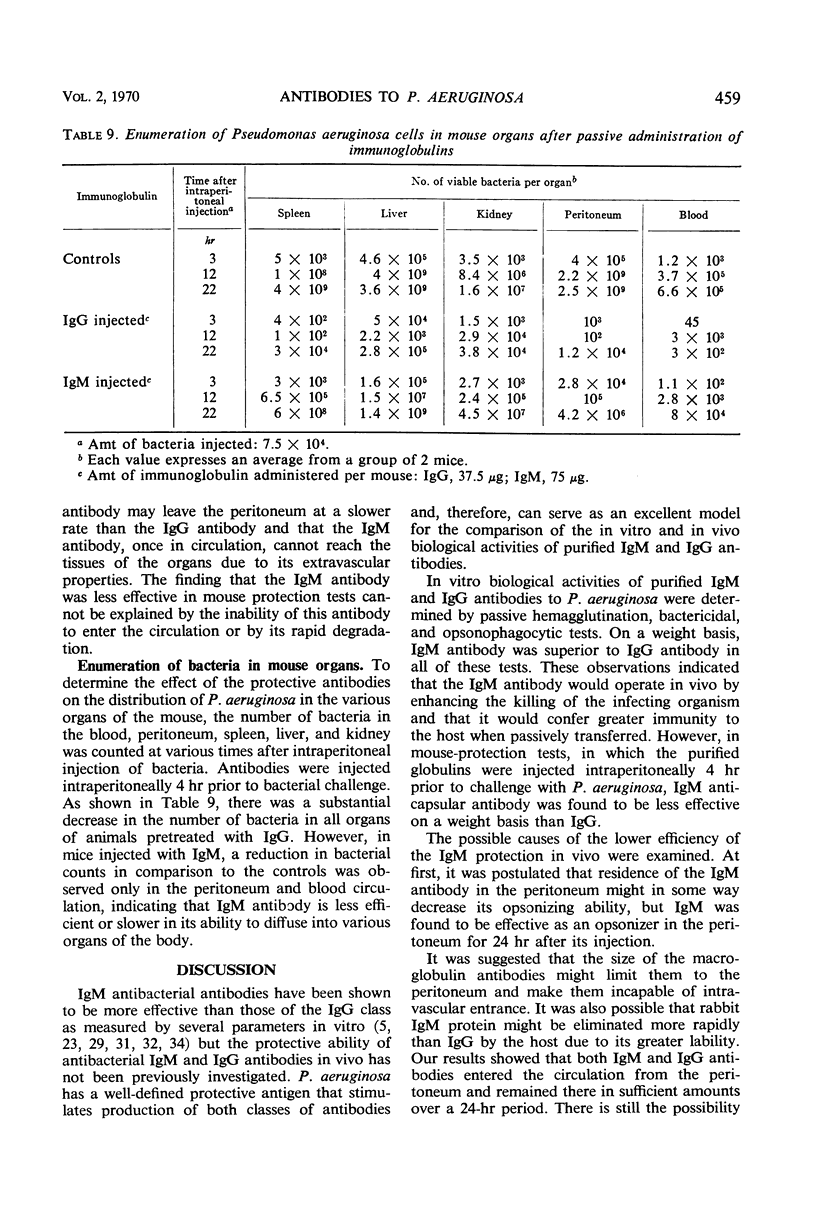
Immunoglobulin (Ig)M and IgG antibodies, prepared in the rabbit against the protective antigen of Pseudomonas aeruginosa P4, were compared as to their biological activities in vitro and in vivo. In vitro biological activities of these antibodies were determined by passive hemagglutination, bactericidal, and opsonophagocytic tests. Increased effectiveness of IgM over IgG on a molar basis was demonstrated in all of these tests. However, in mouse protection tests, in which the purified globulins were injected intraperitoneally 4 hr prior to challenge with P. aeruginosa suspended in hog gastric mucin, IgM anticapsular antibody was found to be less effective than IgG antibody. The exact mechanism whereby IgG antibody exerts more protective ability than IgM antibody is still unknown. We present evidence to suggest that the difference in activity between the two classes of antibody is due to the ability of the IgG antibody to enter the bloodstream more rapidly than the IgM antibody and also to the ability of IgG to diffuse rapidly through the tissues of the organs.
Full text
PDF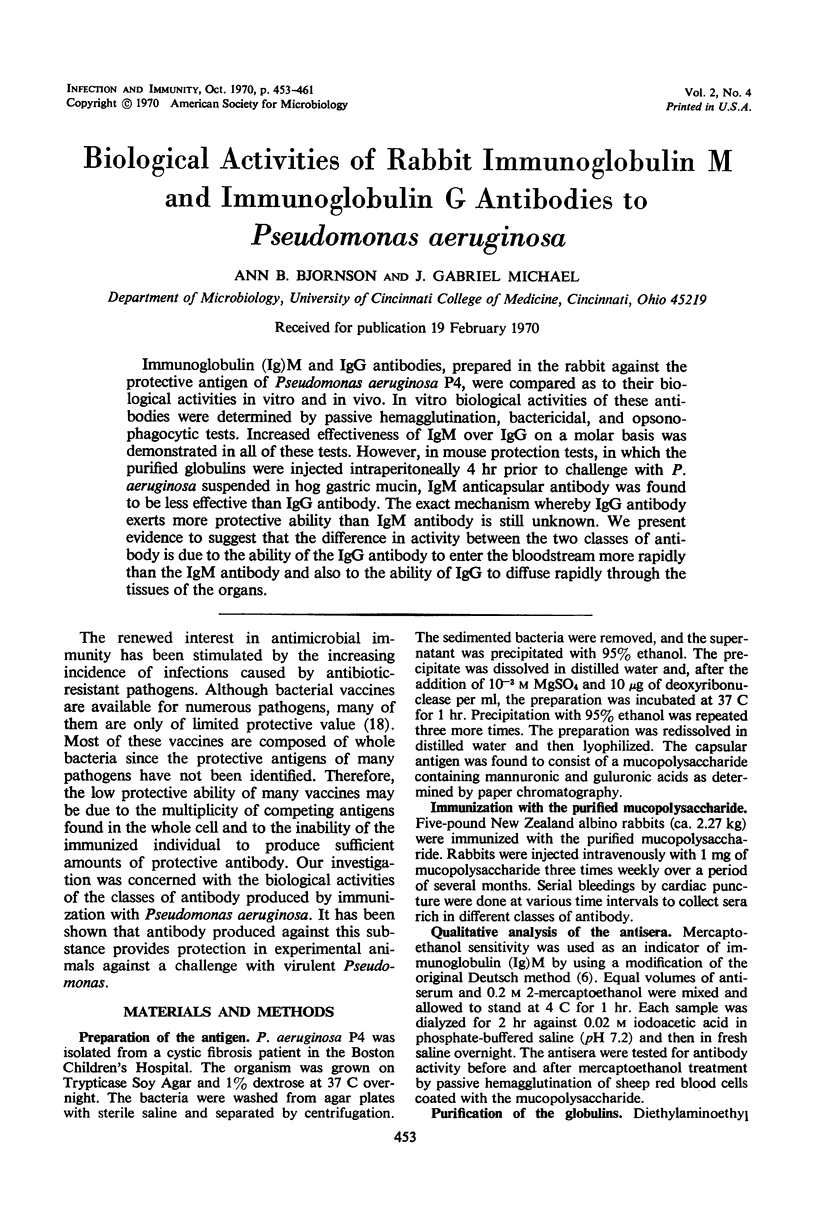



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander J. W., Brown W., Walker H., Mason A. D., Jr, Moncrief J. A. Studies on the isolation of an infection-protective antigen from Psudomonas aeruginosa. Surg Gynecol Obstet. 1966 Nov;123(5):965–977. [PubMed] [Google Scholar]
- Alms T. H., Bass J. A. Immunization against Pseudomonas aeruginosa. I. Induction of protection by an alcohol-precipitated fraction from the slime layer. J Infect Dis. 1967 Jun;117(3):249–256. doi: 10.1093/infdis/117.3.249. [DOI] [PubMed] [Google Scholar]
- Alms T. H., Bass J. A. Immunization against Pseudomonas aeruginosa. II. Purification and characterization of the protective factor from the alcohol-precipitated fraction. J Infect Dis. 1967 Jun;117(3):257–264. doi: 10.1093/infdis/117.3.257. [DOI] [PubMed] [Google Scholar]
- Altemeier W. A., 3rd, Bellanti J. A., Buescher E. L. The IgM response of children to Salmonella typhosa vaccine. I. Measurements of anti-typhoid concentrations and their proportions of total serum IgM globulins. J Immunol. 1969 Nov;103(5):917–923. [PubMed] [Google Scholar]
- DEUTSCH H. F., MORTON J. I. Dissociation of human serum macroglobulins. Science. 1957 Mar 29;125(3248):600–601. doi: 10.1126/science.125.3248.600. [DOI] [PubMed] [Google Scholar]
- Del Guercio P., Tolone G., Andrade F. B., Biozzi G., Binaghi R. A. Opsonic, cytophilic and agglutinating activity of guinea-pig gamma-2 and gamma-M anti-Salmonella antibodies. Immunology. 1969 Mar;16(3):361–371. [PMC free article] [PubMed] [Google Scholar]
- Dolby J. M., Dolby D. E. The antibody activities of 19S and 7S fractions from rabbit antisera to Bordetella pertussis. Immunology. 1969 Jun;16(6):737–747. [PMC free article] [PubMed] [Google Scholar]
- FAHEY J. L., McCOY P. F., GOULIAN M. Chromatography of serum proteins in normal and pathologic sera: the distribution of protein-bound carbohydrate and cholesterol, siderophilin, thyroxin-binding protein, B12-binding protein, alkaline and acid phosphatases, radio-iodinated albumin and myeloma proteins. J Clin Invest. 1958 Feb;37(2):272–284. doi: 10.1172/JCI103606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FEINGOLD D. S., OSKI F. PSEUDOMONAS INFECTION. TREATMENT WITH IMMUNE PLASMA. Arch Intern Med. 1965 Sep;116:326–328. doi: 10.1001/archinte.1965.03870030006002. [DOI] [PubMed] [Google Scholar]
- FELLER I., KAMEI I. PSEUDOMONAS VACCINE AND HYPERIMMUNE PLASMA IN THE TREATMENT OF BURNED PATIENTS. Surg Forum. 1964;15:46–47. [PubMed] [Google Scholar]
- FISHER M. W., MANNING M. C. Studies on the immunotherapy of bacterial infections. I. The comparative effectiveness of human gamma-globulin against various bacterial species in mice. J Immunol. 1958 Jul;81(1):29–31. [PubMed] [Google Scholar]
- FLODIN P., KILLANDER J. Fractionation of human-serum proteins by gel filtration. Biochim Biophys Acta. 1962 Oct 8;63:402–410. doi: 10.1016/0006-3002(62)90104-x. [DOI] [PubMed] [Google Scholar]
- FOX J. E., LOWBURY E. J. Immunity to Pseudomonas pyocyanea in man. J Pathol Bacteriol. 1953 Apr;65(2):519–531. doi: 10.1002/path.1700650224. [DOI] [PubMed] [Google Scholar]
- Fisher M. W., Devlin H. B., Gnabasik F. J. New immunotype schema for Pseudomonas aeruginosa based on protective antigens. J Bacteriol. 1969 May;98(2):835–836. doi: 10.1128/jb.98.2.835-836.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAINES S., LANDY M. Prevalence of antibody to Pseudomonas in normal human sera. J Bacteriol. 1955 Jun;69(6):628–633. doi: 10.1128/jb.69.6.628-633.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlings-Petersen B. T., Pondman K. W. The relationship between complement, the heterogeneity of antibody and phagocytosis. Bibl Haematol. 1965;23:829–833. doi: 10.1159/000384374. [DOI] [PubMed] [Google Scholar]
- Gotschlich E. C., Goldschneider I., Artenstein M. S. Human immunity to the meningococcus. IV. Immunogenicity of group A and group C meningococcal polysaccharides in human volunteers. J Exp Med. 1969 Jun 1;129(6):1367–1384. doi: 10.1084/jem.129.6.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. J., Jackson D. M., Lowbury E. J. Antiserum and antibiotic in the prophylaxis of burns against Pseudomonas aeruginosa. Br J Plast Surg. 1966 Jan;19(1):43–57. doi: 10.1016/s0007-1226(66)80007-3. [DOI] [PubMed] [Google Scholar]
- Keefer C. S., Spink W. W. STUDIES OF GONOCOCCAL INFECTION. IV. THE EFFECT OF MUCIN ON THE BACTERIOLYTIC POWER OF WHOLE BLOOD AND IMMUNE SERUM. J Clin Invest. 1938 Jan;17(1):23–30. doi: 10.1172/JCI100924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU P. V., ABE Y., BATES J. L. The roles of various fractions of Pseudomonas aeruginosa in its pathogenesis. J Infect Dis. 1961 Mar-Apr;108:218–228. doi: 10.1093/infdis/108.2.218. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MICHAEL J. G., ROSEN F. S. ASSOCIATION OF "NATURAL" ANTIBODIES TO GRAM-NEGATIVE BACTERIA WITH THE GAMMA-1-MACROGLOBULINS. J Exp Med. 1963 Oct 1;118:619–626. doi: 10.1084/jem.118.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MICHAEL J. G., WHITBY J. L., LANDY M. Studies on natural antibodies to gram-negative bacteria. J Exp Med. 1962 Jan 1;115:131–146. doi: 10.1084/jem.115.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLICAN R. C., RUST J. D. Efficacy of rabbit pseudomonas antiserum in experimental Pseudomonas aeruginosa infection. J Infect Dis. 1960 Nov-Dec;107:389–394. doi: 10.1093/infdis/107.3.389. [DOI] [PubMed] [Google Scholar]
- MILLICAN R. C., RUST J., ROSENTHAL S. M. Gamma globulin factors protective against infections from Pseudomonas and other organisms. Science. 1957 Sep 13;126(3272):509–511. doi: 10.1126/science.126.3272.509-a. [DOI] [PubMed] [Google Scholar]
- NETER E., BERTRAM L. F., ARBESMAN C. E. Demonstration of Escherichia coli 055 and 0111 antigens by means of hemagglutination test. Proc Soc Exp Biol Med. 1952 Feb;79(2):255–257. doi: 10.3181/00379727-79-19343. [DOI] [PubMed] [Google Scholar]
- Olitzki L. MUCIN AS A RESISTANCE-LOWERING SUBSTANCE. Bacteriol Rev. 1948 Jun;12(2):149–172. doi: 10.1128/br.12.2.149-172.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike R. M., Schulze M. L., Chandler C. H. Agglutinating and precipitating capacity of rabbit anti-Salmonella typhosa gamma G and gamma M-antibodies during prolonged immunization. J Bacteriol. 1966 Oct;92(4):880–886. doi: 10.1128/jb.92.4.880-886.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBBINS J. B., KENNY K., SUTER E. THE ISOLATION AND BIOLOGICAL ACTIVITIES OF RABBIT GAMMA M- AND GAMMA G-ANTI-SALMONELLA TYPHIMURIUM ANTIBODIES. J Exp Med. 1965 Aug 1;122:385–402. doi: 10.1084/jem.122.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley D., Turner K. J. Number of molecules of antibody required to promote phagocytosis of one bacterium. Nature. 1966 Apr 30;210(5035):496–498. doi: 10.1038/210496a0. [DOI] [PubMed] [Google Scholar]
- SCHEIDEGGER J. J. Une micro-méthode de l'immuno-electrophorèse. Int Arch Allergy Appl Immunol. 1955;7(2):103–110. [PubMed] [Google Scholar]
- Smith J. W., Barnett J. A., May F. P., Sanford J. P. Comparison of the opsonic activity of gamma-G- and gamma-M-anti-Proteus globulins. J Immunol. 1967 Feb;98(2):336–343. [PubMed] [Google Scholar]



