Abstract
The p21 activated kinases (Paks) are well known effector proteins for the Rho GTPases Cdc42 and Rac. The Paks contain 6 members, which fall into 2 families of proteins. The first family consists of Paks 1, 2, and 3, and the second consists of Paks 4, 5, and 6. While some of the Paks are ubiquitously expressed, others have more restrictive tissue specificity. All of them are found in the nervous system. Studies using cell culture, transgenic mice, and knockout mice, have revealed important roles for the Paks in cytoskeletal organization and in many aspects of cell growth and development. This review discusses the basic structures of the Paks, and their roles in cell growth, development, and in cancer.
Keywords: neurobiology, oncogenesis, p21-activated kinases, pak, protein kinases, substrates
Introduction to the Pak Kinases
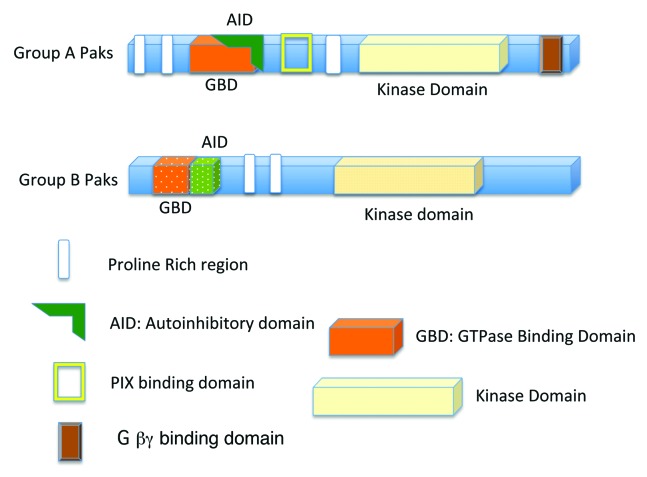
The p21 activated kinase (PAK) family of serine/threonine kinases are effector proteins for the Rho GTPases Cdc42 and Rac. They bind to Cdc42 and Rac through a GTPase Binding Domain (GBD), also sometimes known as a Cdc42 Rac Interactive Binding (CRIB) domain. The Paks fall into 2 categories, group A and group B, also referred to as group I and group II, based on their sequences and functions (see Fig. 1).
Figure 1. Basic structures of the group A Paks (Paks 1, 2, and 3), and group B Paks (Paks 4, 5, and 6). Group A Paks have an Autoinhibitory Domain (AID) overlapping the GBD (GTPase Binding Domain), while the Group B Paks have a related sequence adjacent to the GBD.
Group A includes mammalian Pak1, Pak2, and Pak3.1-3 Homologs can also be found in other organisms including C. elegans, Xenopus, and Drosophila. In fact, the Paks are considered to be related to the yeast protein Ste20, which is also a serine/threonine kinase with a GBD domain. Each of the group A Paks has an N-terminal regulatory domain and a carboxyl terminal kinase domain. Within the regulatory domain is the GBD, which binds to activated Rac or Cdc42. The group A Paks also have several other conserved motifs, as illustrated in Figure 1. Binding to Cdc42 or Rac stimulates the Paks’ kinase activities by relieving an intramolecular interaction between the kinase domain and an autoinhibitory domain (AID), as discussed below. Paks 1, 2, and 3 share a high level of sequence homology, but they all have different tissue specific expression patterns. Pak2 is found in all tissues, whereas Paks 1 and 3 have more restricted expression patterns. Pak1 is found in several tissues including mammary gland, muscle, and spleen,4,5 and all of the group A Paks are highly expressed in the nervous system.5
The group B Paks, like the group A Paks, also have N-terminal GBD and carboxyl terminal kinase domains, and they have a sequence that is related to the AID, but they do not contain the other conserved domains found in the group A Paks (see Fig. 1). Furthermore, the GBD and kinase domains of the group B Paks have only approximately 50% identity with those of Paks 1, 2, and 3, and the regulatory domains outside of the GBD are completely different from the group A Paks. PAK4 is the founding member of the group B Paks.6 PAK4 binds most strongly to Cdc42, and less efficiently to Rac. PAK4 and the other group B Paks share some substrates in common with the group A Paks, but also have some of their own unique substrates.7,8 PAK4 is often considered to be ubiquitous because it can be found in all tissues. In many adult tissues, however, PAK4 levels are low, while in embryogenesis its levels are quite high. Pak5 and Pak6, in contrast, have more of a tissue restricted expression pattern and they are especially high in the adult brain.9-12 Pak6 is also found in testes and prostate, and it has an important role in androgen receptor signaling,11,13,14 indicating that in addition to functioning as Rho GTPase targets, the group B Paks also have Rho GTPase independent functions.
Pak Structure and Activation
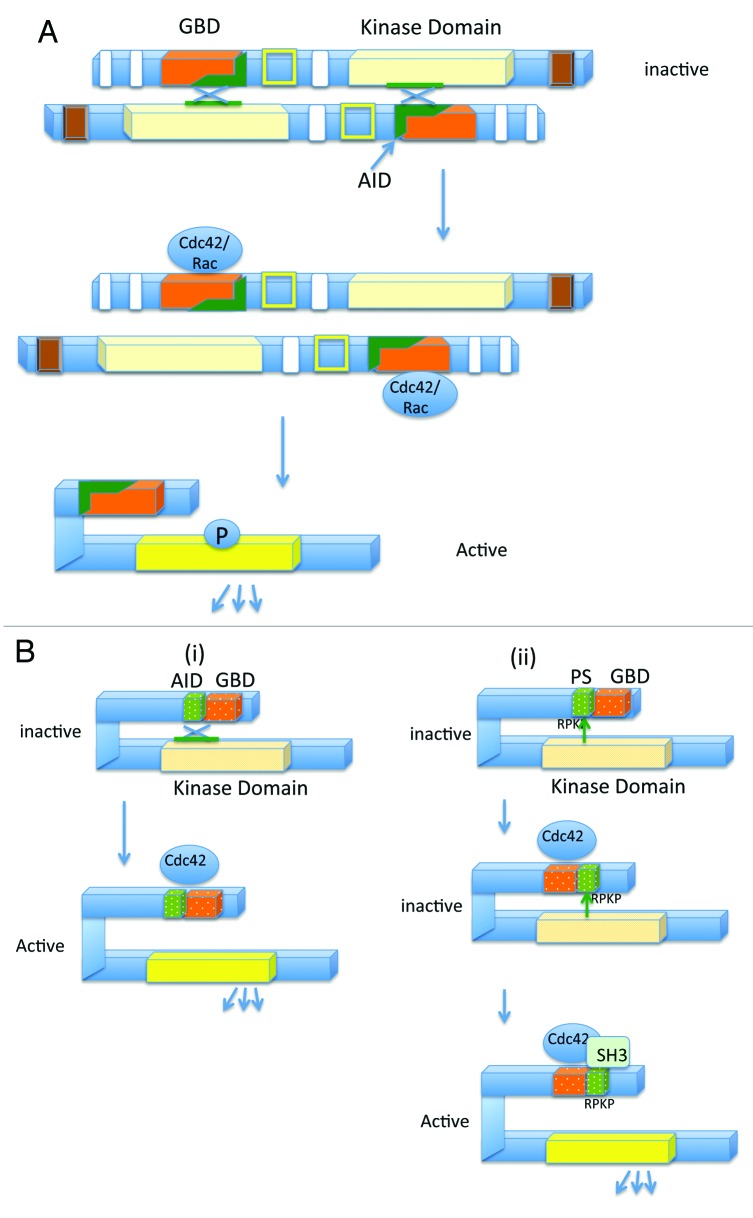
The group A and group B Paks are regulated by different mechanisms,15,16 and the current models for the regulation of the 2 groups of Paks are shown in Figure 2. The group A Paks are notable for having an autoinhibitory domain (AID), which overlaps the GBD, and plays an important role in the control of Pak activation.17,18 Group A Paks function as dimers, where the AID binds in trans to the Pak catalytic domain on the dimerizing Pak. This interaction prevents autophosphorylation at the activation loop (A-loop), and subsequent activation of Pak’s kinase activity. Activation occurs when Pak binds to Cdc42 or Rac, along with lipids. Cdc42/Rac binding to the Pak GBD disrupts the interaction between the AID and the dimerizing Pak. This in turn leads to a conformational change and causes the Pak to become a monomer, which subsequently becomes autophosphorylated on the A-loop and several other sites, and activated16,17,19-22 (see Fig. 2).
Figure 2. Models representing the activation mechanism of the group A and group B Paks. (A) Activation of the group A Paks: Inactivated group A Paks form dimers, where the AID of one Pak binds the kinase domain of the dimerizing Pak, and inactivates it. Binding to Cdc42 or Rac can relieve this inhibition, resulting in autophosphorylation and kinase activation. (B) Two different models for regulation of the group B Paks: In the first model (i), the AID binds to the kinase domain of the monomeric Pak, in trans, resulting in an inactive conformation. Binding of Cdc42 relieves the inhibition and leads to Pak activation. Unlike the group A Paks, the group B Paks are constitutively phosphorylated, but the kinase takes on an active conformation upon Cdc42 binding. In the second model (ii), the autoinhibitory pseudosubstrate (PS) containing the sequence RPKP is recognized by the kinase domain. This interaction inhibits Pak kinase activity. Cdc42 binding relocalizes Pak within the cell, and proteins containing SH3 domains, such as Src, subsequently activate Pak by competing with the Pak kinase domain, for interacting with the pseudosubstrate domain (modified from refs. 15, 16, and 24)
The group B Paks were at first not thought to have autoinhibitory domains, but later work revealed AID-like domains in Paks 4, 5, and 6.15,23,24 Nevertheless, group B Paks are activated by completely different mechanisms than the group A Paks, and they function as monomers rather than as dimers.15 In fact, the group B Paks are usually found to be constitutively autophosphorylated at the A-loop, even in quiescent cells.15 Instead of regulating A-loop autophosphorylation, the group B Pak AID is thought to allosterically modify the constitutively phosphorylated kinase so that it becomes active.15 Exactly how the AID like domains work in the group B Paks is controversial, but one prevailing model is that the AID binds to the Pak catalytic domain in cis, which keeps the kinase in an inactive conformation, even though it is constitutively phosphorylated. When GTP bound Cdc42 binds the GBD, this interaction is disrupted, leading to a conformational change which results in Pak activation15 (see Fig. 2). Recently, a second model for group B Pak activation has also been proposed for Pak4,24 though Pak5 and Pak6 could operate by a similar mechanism. The model involves an autoinhibitory pseudosubstrate (PS), which like the AID, is adjacent to the GBD. The PS is a proline rich region, and thus has the potential to interact with proteins that contain SH3 domains. According to the model, the PS is recognized by the Pak kinase domain, leading to an interaction between the 2 domains. This interaction is disrupted when the PS domain binds to proteins that contain SH3 domains, resulting in relief of autoinhibition. In fact, Pak4 was shown to be activated by the Src SH3 domain, thus supporting this model. This model relies on a 2-step activation process. First, Cdc42 or Rac bind to the Pak4 GBD. This would affect the localization of Pak, presumably bringing it to cellular regions containing other activating proteins and substrates. Upon relocalization, the second signal would come into play. This second signal involves binding by SH3 domain containing proteins, which results in relief of autoinhibition and activation of the kinase (see Fig. 2). Interestingly, upon a screen for mutations, mutations in the autoinhibitory PS region were found in both Pak5 and Pak6 in human cancer cells, including lung cancer and melanoma,24 suggesting that disruption of this regulatory mechanism may be associated with cancer.
While the Pak kinases are known for their regulation by Rho GTPases, a number of other signaling proteins can also play key roles in Pak activation. Pak1 can in fact even lie upstream to Rac, by binding to the Pak interacting exchange factor (PIX), which is a guanine nucleotide exchange factor (gef) for Rac.18,25,26 Binding of Pak1 to Pix causes Pak1 to localize to focal complexes.25 In PC12 cells, this interaction causes an increase in neurite outgrowth.26 PIX also interacts with the G protein coupled receptor kinase interacting target (GIT1), and Pak1 is found in a trimeric complex with PIX/GIT1. Both PIX and GIT1 have key roles in targeting Pak1 to focal complexes.27 GIT1 activates PAK in this complex, and while PIX appears to play an important role in this activation process, activation can occur independently of Cdc42/Rac binding.27 In addition to its role in focal complexes, the PAK/PIX/GIT complex also has a role at the centrosome.28 As cells near M phase, Pak1 is recruited to the centrosomes where it interacts with GIT1/PIX complex. Similar to what is seen at focal complexes, recruiting Pak1 to the centrosome activates Pak, and this is independent of Cdc42 or Rac, but is dependent on centrosome integrity.28
Group B Paks can also be regulated by complex mechanisms. In MDCK epithelial cells, PAK4 is activated by Hepatocyte Growth Factor (HGF). HGF activation of PAK4 is dependent on PI3 Kinase. Once cells are stimulated with HGF, PAK4 localizes to the cell periphery. In turn, PAK4 modulates cell adhesion and the organization of the cytoskeleton.29 PAK4 also binds to the cytoplasmic domain of the keratinocyte growth factor (KGF) receptor in a transformed kidney cell line, and it has important roles in cell survival pathways downstream to KGF.30 Finally, Pak6 plays a role in androgen receptor signaling,11,13,14 in a pathway that is independent of the Rho GTPases.
Pak Kinases and Cytoskeletal Organization
The Paks are effectors of Cdc42 and Rac, which are key cytoskeletal regulatory proteins. Likewise, major functions of the Paks include their roles in regulating the cytoskeleton, consequently affecting cell shape, motility, and adhesion. The Paks control the cytoskeleton primarily through the regulation of polymerized actin structures, particularly the formation of filopodia and lamellipodia, but they can also act upon microtubule organization. The major role for the group B Paks in cytoskeletal organization are their roles in the formation of filopodia in response to Cdc42.6 Activated Pak5 was shown to lead to cytoskeletal changes that are associated with neuronal structure. These include induction of filopodia and the formation of neurite like extensions in neuroblastoma cells.9 Not surprisingly, many Pak substrates are known for their roles in cytoskeletal organization. Group A Paks, for example, phosphorylate a serine residue on the regulatory myosin light chain (MLC)31-34 in neural cells. Myosins are important cytoskeletal regulatory proteins that interact with actin. They fall into a family of proteins found in muscle cells, smooth muscle cells, and non-muscle cells. Phosphorylation of MLC by Pak stabilizes polymerized actin, and in neuronal cells it contributes to the regulation of dendritic spine formation.35 Group A Paks also phosphorylate the regulatory MLC of myosin VI, a nonconventional myosin involved in membrane trafficking and cell migration36.
In other types of cells Pak can have the opposite effect and lead to decreased MLC phosphorylation, and this is associated with stress fiber dissolution. Pak proteins lead to formation of polymerized actin structures such as lamellipodia and filopodia, but they also lead to the dissolution of stress fibers.8 Stress fibers are polymerized actin structures that exert tension on the cell and that are directly linked to focal adhesions. Dissolution of stress fibers thus also leads to loss of focal adhesions.8 In the case of Pak1 and Pak2, this has been shown to be mediated by phosphorylation of Myosin Light Chain Kinase (MLCK) in fibroblasts, epithelial cells, and endothelial cells.37,38 Phosphorylation of MLCK decreases MLCK’s kinase activity, leading to decreased MLC phosphorylation and subsequent stress fiber dissolution.37 MLCK is not a direct substrate for PAK4, and yet PAK4 also causes stress fiber dissolution.8 Instead, PAK4 phosphorylates GefH1, which in turn inhibits Rho activation. Since Rho leads to stress fiber formation, its inhibition leads to a decrease in stress fibers.39 Pak1 also phosphorylates GefH1 although on different sites.33,40 This phosphorylation affects the crosstalk between actin and microtubule networks39
Another substrate for both group A and group B Paks is LIM Kinase 1 (LIMK1).41 When it is phosphorylated, LIMK phosphorylates the actin depolymerization protein cofilin, thereby inhibiting actin depolymerization.42,43 The phosphorylation of LIMK1 by Pak kinases therefore represents another mechanism by which the Paks promote the formation or stability of polymerized actin structures such as filopodia and lamellipodia. In addition to direct phosphorylation of LIMK1, PAK4 also activates LIMK1 indirectly, via inhibition of SlingShot Phosphatase (SSH1), a LIM Kinase phosphatase.44 LIMK1 phosphorylation by PAK4 is also regulated by DGCR6L. DGCR6L binds to PAK4, and this interaction enhances LIMK phosphorylation, leading to increased migration of gastric cells.45
Filamin A (FLNa) is another cytoskeletal regulatory protein that is targeted by the Paks.46 FLNa is an actin binding protein which connects actin filaments to the cell membrane. FLNa is phosphorylated by Pak1, which in turn controls actin stability. Interestingly, FLNa can also bind to the Pak1 GBD and stimulate PAK1 kinase activity, indicating the presence of a positive feedback loop.33,46 Another mechanism by which Paks control actin-cytoskeletal organization is via its actions on the ARP 2/3 complex. The ARP 2/3 complex controls actin nucleation and branching. Phosphorylation of the p41-ARC subunit of ARP 2/3 by Pak1 stimulates the assembly of the complex at the cellular cortex of migrating cells. This plays an important role in constitutive and growth-factor induced cell motility.47
In addition to their affects on the actin cytoskeleton, the Paks can also affect microtubules. One outcome of this is the regulation of mitotic spindles. Pak1 affects mitotic spindle function and organization by interacting with Tubulin Cofactor B (TCoB), a cofactor in the assembly of α/β-tubulin. It phosphorylates TCoB on Ser65 and Ser128 and co-localizes with TCoB on newly polymerized microtubules and centrosomes.47 Phosphorylation of TCoB by Pak1 is essential for microtubule polymerization. Coordinate dysregulation of Pak1 and TCoB can promote multiple spindle formation, as seen in human breast tumors. Pak1 thus plays an important role in maintaining microtubule dynamics, which is crucial for mitotic spindle function. Another mechanism by which Pak1 regulates mitotic spindle function during mitosis is by phosphorylating centrosome-located Polo-like kinase1 and Aurora-A kinase, 2 important mitotic regulators The Git-Pix complex activates Pak1 by recruiting it to the centrosome. Activated Pak1 then phosphorylates Plk1 and Aurora A kinase. Pak1 phosphorylates Plk1 at Ser49 following which both PlK1 and Aurora A Kinase co-localize on spindle poles, the central spindle, and the midbody. This is important for establishing a functional bipolar spindle.48 Activated Pak1 activates Aurora-A by phosphorylating 2 sites, Thr288 and Ser342.28 Enhanced Aurora-A activity can result in abnormal mitotic spindle organization and anchorage-independent growth in human breast epithelial cells.49 Paks also play important roles in cell motility by regulating leading edge microtubule dynamics. Microtubules in the protruding edge of migrating cells exhibit decreased catastrophic frequency and increased net growth. Pak1 has been shown to phosphorylate stathmin at Ser16 in vitro in Hep-2 cells. This leads to downregulation of stathmin, which is a protein that normally inhibits tubulin polymerization. Consequently, stathmin phosphorylation enhances cell motility.50 Xenopus and mammalian PAK4 were shown to phosphorylate the GTPase Ran, and PAK4 mediated phosphorylation of Ran regulates the assembly of Ran-dependent complexes on the mitotic spindle, pointing to a role for this complex in mitosis.51
The group A and group B Paks share some substrates in common, but also have some non-overlapping substrates. Several of the group B Pak substrates are linked to cell adhesion. Pak5 binds to p120-catenin in vitro, resulting in p120 phosphorylation. A constitutively active PAK4 mutant also leads to phosphorylation of p120.52 p120 catenin has important roles in controlling cell shape and adhesion. It also has a role in anchorage independent cell growth,53 an important hallmark of cancer. PAK4 phosphorylates the cytoplasmic tail of β-5 integrin, which subsequently has important implications in cell adhesion and migration.54 Phosphorylation of p120 and β-5 integrin by the group A Paks has not yet been reported. Group A Paks are also implicated in cell adhesion, however, and one important mediator is GIT1. GIT1, a Pak1 substrate, is a GAP protein for the Arf GTPase. PAK1 phosphorylates GIT1, and this increases GIT1 binding to paxillin, a focal adhesion adaptor protein. This entire pathway is important for regulating focal adhesion turnover.28,55
Biological Roles of the Pak Kinases
The Paks roles in cytoskeletal organization, along with their expression in the nervous system, has led many researchers to study their roles in neurons. Cytoskeletal organization and cell shape changes are important in neuronal cells, especially during development when neurons extend axons and dendrites and connections are made with target cells.56-58 During growth cone guidance and elongation, for example, filopodia and lamellipodia move toward attractive cues and away from repulsive cues, and neurite extension occurs when these structures are stabilized.37,41 Because the formation of filopodia and lamellipodia is so important for neurite development, there has been considerable interest in the roles for the Rho GTPases and Paks in neurons. Rho GTPases are expressed in the nervous system, and they have been shown to have important roles in the regulation of neuronal development and neurite outgrowth in several systems,57-65 as well as in the formation of dendritic spines.35
As targets of the Rho GTPases, Paks also have important roles in regulating neuronal development and morphology. The 2 PAK related proteins that have been isolated from Drosophila both have roles in neuronal development. Drosophila PAK (DPAK) (56), which falls into group A, is expressed at relatively high levels in axons and growth cones of the developing visual system, and it has been shown to be necessary for photoreceptor axon guidance.66 Mushroom Body Tiny (MBT) is a Drosophila Pak that falls into group B.67 Mutations in the mbt gene result in a decreased number of Kenyon cells in the Drosophila mushroom body, a structure of Drosophila brain, indicating that it is important for the regulation of neuronal survival or proliferation.67
In humans, Pak3 is so far the most clinically relevant Pak with respect to the nervous system. This is because mutations in PAK3 have been linked to nonsyndromatic X-linked mental retardation (MRX).68 A subset of individuals with this disorder (referred to as (MRX30) have point mutations in Pak3, resulting in a truncated protein. The mutation truncates the kinase domain, but Pak3 is still capable of binding Cdc42 and Rac. A mutated Pak3 can result in aberrant or absent neuronal connections, leading to decreased neuronal connectivity, as seen in MRX30 patients. Furthermore, Pak3 kinase activity is required for activation of JNK1 and p38 MAP kinase pathways, and these pathways play important roles in transcriptional activation in post-synaptic neurons.69 Other Paks and Pak targets are also important in the nervous system. A defect in the PAK target LIMK1, for example, is linked to the neurological disorder Williams Syndrome, and prevents neurons from making their proper connections.70 Consistent with this, overexpression of the LIMK target cofilin promotes neurite outgrowth in cell lines.71 Expression of PAK1 triggers neurite outgrowth in PC12 cells,61 and an activated PAK5 promotes filopodia formation and neurite outgrowth in N1E-115 neuroblastoma cells.
One interesting function of the Pak proteins is their role in dendritic spine morphology.. Dendritic spines are long, thin, actin-rich protrusions that are the main sites of excitatory synaptic input for most of neurons. Formation and maintenance of synaptic plasticity is mediated by Rac, and this is important for cognitive functions such as learning and memory. Locally regulated activation of Rac by synaptic targeting of the GIT1-PIX complex plays an important role in synapse formation.28,35 Once activated, Rac binds to Pak1 and Pak3. Pak1 and Pak3 activates the downstream effector MLC by phosphorylating it on Ser19. Active MLC localizes to dendritic spines where it stabilizes the actin network which leads to increased actomyosin contractility, which is essential for formation of dendritic spines and excitatory synapses. Mislocalization of GIT1-PIX complex away from the synapses can cause mislocalized activation of Rac. This can lead to formation of multiple dendritic protrusions, a common spine morphology in patients with mental retardation. Thus, the GIT1-Pak Pix complex plays a key role in spine morphogenesis. Alterations in this complex are associated with decreased neuronal connectivity and cognitive defects such as those seen in nonsyndromic mental retardation.28,35
All of the Paks are expressed in the nervous system, and mouse knockouts have given important clues as to their functions. Pak1 knockouts have abnormalities in neuronal function. Specifically, synaptic plasticity is altered in such a way that the knockout mice are deficient in long-term depression (LTD), indicating that Pak1 is important for synapse function. Dendritic spines of the Pak1 knockout mice are also abnormal, having low levels of polymerized actin compared with those of control mice.
Pak3 knockout mice appear normal,72 including the brain and nervous system. However late phase LTP (L-LTP) is reduced in Pak3 knockout mice, and this is associated with a decrease in phosphorylation of the transcription factor CREB. CREB is required for L-LTP and memory formation, and interestingly, the knockout mice also have deficits in memory retention. Pak1 knockout mice show many similarities to Pak3 knockout mice. Pak1/Pak3 double knockout mice have learning and memory deficits, and display hyperactive behavior. They also exhibit less complex neuronal morphology including reduced dendrite length and reduction in the amount of dendritic tips, indicating that these Paks are important for branch formation.
PAK4 has been conditionally deleted in the nervous system.73 These conditional knockout mice are born normally, but display growth retardation and die prematurely. The brains show loss of neuroepithelial adherens junctions, and a dramatic decrease in proliferation of cortical and striatal neuronal progenitor cells. In vitro analyses also revealed deficits in proliferation and self-renewal of neural progenitor cells. The knockout mice only live until approximately 4 wk after birth, and after this point severe hydrocephalus is evident. These studies indicate that PAK4 plays important roles in the development of the brain, especially in the control of neural progenitor cell proliferation.
Unlike PAK4, which is ubiquitously expressed during development, Pak5 and Pak6 have more restrictive tissue specific expression patterns, and are particularly high in the nervous system. Interestingly, Pak5 and Pak6 knockout mice appear phenotypically normal and have normal life spans. When Pak5/Pak6 double knockouts were observed in depth, however, noticeable abnormalities were detectable. Specifically, the double knockouts have lower activity levels compared with the wild-type mice, and they display less aggressive behavior. When learning tests were performed, the mice also displayed significant deficits in both learning and memory.74 Pak5 and Pak6 levels are high in the cortex, hippocampus, and striatum, and interestingly these are structures with highly important roles in cognitive functions. While the Pak5/Pak6 knockout brains appear phenotypically normal as assessed by histology, differences are seen when neurons are cultured in vitro. Specifically, cultured neurons from the knockouts have abnormally small growth cones and a reduction in neurite outgrowth.
It is interesting that there are such big differences between the phenotypes of the PAK4 knockouts and the Pak5/Pak6 knockout mice. These differences are most likely due to the different expression patterns of these proteins. Specifically, while PAK4 levels are highest in embryogenesis, Pak5 and Pak6 levels are usually higher after birth, particularly in the brain. This can explain why PAK4 appears to function mostly in early neuronal differentiation, while Pak5 and Pak6 have roles later in neuronal development or maintenance.
Developmental Functions of Pak Kinases as Assessed by Studying Knockout Mice
The biological functions of the Pak kinases have been studied in part by developing the knockout mice described above. Both Pak2 and PAK4 are embryonic lethal when they are knocked out, indicating an essential role for these proteins in embryogenesis. The embryos have abnormalities in multiple organs, including the heart and brain.75,76 Pak1 knockout mice are viable and appear normal. They do, however, have subtle abnormalities in the immune system.72 Wild-type bone marrow derived mast cells (BMMCs), when antigen sensitized and challenged with IgE, trigger rapid release of histamine containing granules. In contrast, BMMCs derived from Pak1 knockout mice failed to be stimulated with IgE. They were also incompetent in F-actin disassembly following allergen stimulation, resulting in defective mast cell degranulation.77 The exact mechanism is unknown, but likely involves lowered activation of ERK, JNK and p38 pathways in Pak1 knockout mice, which play important roles in mast cell function. Another abnormality in Pak1 knockout mice is a deficit in glucose homeostasis. This includes inefficient insulin secretion, and abnormal glucose clearance. This is reminiscent of the biological role for Pak1 in humans, where Pak1 levels are reduced in type 2 diabetic islets.78 Pak1 is thus directly implicated in the control of glucose metabolism.
Both groups of Paks have significant roles in the heart. When Pak1 is conditionally deleted in the heart, the mice appear normal. However, when Pak1 was conditionally deleted in cardiomyocytes and mice were subjected to pressure overload, significant increases were observed in heart weight/tibia length ratio and cross-sectional cardiomyocyte area. This is due to attenuated JNK pathway stimulation in the knockout mice, which is usually induced in wild-type mice when subjected to pressure overload. Thus, Pak1 acts as an anti-hypertrophic agent, revealing a link between Pak1 and ventricular hypertrophy, and indicating that Pak1 knockouts are more susceptible to heart failure when the heart is subjected to pressure overload.72,79 Deletion of PAK4 leads to embryonic lethality, but among the different abnormalities in the PAK4 knockout embryos, is a dramatic abnormality in heart development.76 These include thinning of the myocardial walls of the bulbus cordis and the ventricle, and severe dilation and distortion of the sinus venosus region.76 PAK4’s role in cytoskeletal organization is in many ways consistent with a role in cardiac development. Heart muscle cells, like all muscle cells, have a complex and unique cytoskeletal organization. The major cytoskeletal component of the heart cell is the sarcomere. The sarcomere is a complex of actin and myosin and a network of associated proteins, and it is absolutely required for proper heart cell structure and for contractility. Conditional deletion of PAK4 in the heart leads to severely disorganized sarcomeric structures. The abnormalities include a dramatic reduction in the level of F-actin, while the normally repetitive pattern of α-actinin appears less organized. Disruption of sarcomeric structure in the PAK4 knockout cardiomyocytes also affects beating (contractility) of the cells. In PAK4 knockout cardiomyocytes and embryos, the levels of LIMK1 and phospho-LIMK1 are reduced. Likewise, the levels of phospho-cofilin, the LIMK substrate, are also reduced.80 These studies indicate that PAK4 has an important role in cardiomyocyte development, with a specific role in the sarcomere. Interestingly, Pak1 is also associated with the sarcomere. In fact, Pak1 has been shown to localize to the sarcomeric Z disc,81 though a precise role for Pak proteins in sarcomeric structure has not been defined.
PAK4 null embryos have a marked reduction in blood vessels, throughout the embryo and extraembryonic tissue.82 Although some early vessels form, there is almost a complete lack of branching. Blood vessel development and branching require complex cytoskeletal changes. The role for PAK4 in cytoskeletal organization could help explain its role in blood vessel formation, since formation of vessels requires precise control of the cytoskeleton.
Paks and Cancer
As described above, the Pak kinases and Rho GTPases have important roles in normal development. When they are improperly expressed, however, they are frequently associated with cancer. This is consistent with their roles in controlling cell survival, proliferation, cytoskeletal organization, and migration.74,83-89 Neither the group A nor the group B Paks are frequently mutated in human cancer. Instead, overexpression and gene amplification of the Pak kinases, however, are commonly seen in cancer. Pak1 and PAK4 are the Paks that are most strongly associated with cancer. Both Pak1 and PAK4 genes are found on chromosomal regions that are frequently amplified in cancer.90 While gene amplification is one way that Pak proteins become overproduced in cancer, there are also other mechanisms leading to Pak overexpression. Pak1, for example, has been shown to be overexpressed via a mechanism that involves microRNA downregulation.90,91
Paks and cell growth control
Among the group B Paks, PAK4 is involved in many cellular activities that are associated with the control of cell growth, which are important for the oncogenic process. For example, expression of PAK4 is associated with increased cell survival and prevention of apoptosis,74,84 Conversely, cells lacking PAK4 have an increased susceptibility toward apoptosis.85 This is important, because increased survival is an important part of tumor formation and growth. PAK4 promotes cell survival by different mechanisms in different situations. When serum is removed from cells, PAK4 can protect the cells from apoptosis by phosphorylating the pro-apoptotic protein Bad.84 When cells are exposed to ligands that bind to death domain receptors, however, such as TNF and Fas receptors, PAK4 protects cells by a different mechanism. In this case, PAK4 inhibits recruitment of caspase-8 to the DISC complex that forms on the cytoplasmic side of the receptor.74,84 This leads to inhibition of the caspase cascade, and the entire process is independent of the kinase activity of PAK4.74,84 The mechanisms of PAK4 induced survival in this case involve activation of cell survival pathways, leading to NFKB and ERK activation.85 Pak5 and Pak1 also protect cells from apoptosis,92,93 via a pathway involving Raf and Bad. Both Pak1 and Pak5 phosphorylate Ser338 on Raf, stimulating translocation of Raf1 to the mitochondria. Phosphorylated Raf-1 forms a complex with the Bcl-2 proto-oncogene. This complex leads to phosphorylation of the pro-apoptotic protein Bad at Ser112, which prevents its binding to Bcl-2. This prevents the release of pro-apoptotic factors from the mitochondria, thereby inhibiting apoptosis.92,94 Another mechanism by which Pak1 can prevent cells from undergoing apoptosis is by stimulation of transcription factor NFkappaB, which can promote cell survival, proliferation and angiogenesis,95,96 and which inhibits the pro-apoptotic factor FKHR.97 Pak5 overexpression also inhibits camptothecin induced apoptosis in colorectal cancer cells, and this is mediated by inhibition of caspase-8.93
Like activated Cdc42,98-100 activated PAK4 promotes anchorage independent growth in immortalized fibroblasts,8,39 an important hallmark of oncogenic transformation. In fact, activated PAK4 is as efficient as oncogenic Ras, a very strong oncogene, in promoting foci in soft agar.8 Consistent with this effect, dominant negative PAK4 partially inhibits focus formation in response to oncogenic Dbl in fibroblasts,8 and in some cells it also inhibits transformation by oncogenic Ras.12
Metastasis, which is one of the most challenging aspects of cancer treatment, is tightly linked with cell migration and invasiveness. There is evidence that PAK4 has a direct role in this process. When activated PAK4 is overexpressed in pancreatic ductal cells, it leads to increased migration and increased invasiveness in in vitro assays. In contrast, blocking PAK4 reduces invasiveness in a pancreatic tumor cell line.101 PAK4 overexpression was also shown to promote invasion and migration, as well as proliferation, of choriocarcinoma cells. Here again, inhibition of PAK4 has the opposite effect and blocks migration and proliferation.102 Reducing PAK4 levels in prostate cancer cells also decreases cell migration and leads to reduced cell-adhesion turnover rates, indicating that PAK4 has a role in prostate cancer cell migration and adhesion.
Paks are overexpressed in human cancers
PAK4 is associated with a number of different types of cancer.12,103-106 Occasionally, point mutations have been found in PAK4, as is the case in some colorectal cancers,107 but most often overexpression of wild-type PAK4 is sufficient for oncogenesis. The mechanism of PAK4 overexpression in cancer is an important area of investigation. In some cases, PAK4 gene amplification is linked to cancer. The PAK4 gene is located on a chromosomal region (19q13.2) that is often found to be amplified in cancer. For example, the PAK4 gene was shown to be amplified in a series of pancreatic cancer samples, including pancreas ductal adenocarcinomas (PDACs),101,108,109 and squamous cell carcinomas,110 The 19q13.2 region is also often highly overexpressed in aggressive breast cancers with basal-like features.111
PAK4 mRNA levels were found to be elevated in almost all members of a panel of 60 tumor cell lines, which represent a range of different types of cancers.12 In contrast, PAK4 levels are low in most normal tissues. PAK4 is overexpressed in a subset of gastric tumors, and overexpression of PAK4 is associated with poor survival rates in patients with these types of tumors.105 Liver cancer is also linked to high PAK4 expression. In human hepatocellular cancer carcinomas (HCC),105 PAK4 is often found to be overexpressed and activated, and this correlates with overexpression of the CDK5 Kinase Associated Protein CDK5RAP3. CDK5RAP3 in turn, is linked with tumor aggressiveness and invasiveness.112 Further evidence for the role for PAK4 in liver cancer comes from studying microRNAs.113 The microRNA miR-199a/b-3p is highly expressed in liver, but consistently decreased in HCC. This microRNA, which has an antitumor effect in cells, inhibits PAK4 expression, as well as downstream ERK activation.113 This evidence strongly links PAK4 to liver cancer.
Ovarian cancer is also frequently associated with high PAK4 levels, as well as an increase in phosphorylated PAK4. High PAK4 levels in these types of tumors are highly linked with metastasis, poor survival, and reduced chemosensitivity.106 Reduction of PAK4 in ovarian cancer cells reduces cell migration, invasion, and proliferation, and abrogates a number of signaling pathways associated with cell proliferation. It also blocks the ability of the cells to form tumors in mice. In contrast, overexpression of PAK4 in ovarian cancer cells increases cell migration and invasion.106
Pak5 overexpression has been seen in some colorectal cancers, and Pak5 plays a role in invasiveness of colorectal cancer cells.114 Pak6 protein levels are elevated in some prostate and breast cancer cell lines,115 and Pak6 mRNA levels are also overproduced in some cancer cells.12 Pak6 levels are elevated in prostate tumors that relapsed after androgen deprivation therapy, and it plays a role in motility and stress responses of tumor cells.115 Inhibition of Pak6, combined with irradiation, decreases survival of prostate cancer cells.116 This indicates that Pak6 is linked to radiosensitivity in prostate cancer cells. The role for Pak6 in prostate cancer is complex though, because it was also identified as a gene that is sometimes hypermethylated in prostate cancer, which is more often associated with suppression of tumorigenesis.117 The exact role for Pak6 in prostate cancer thus remains to be fully clarified.
Pak1 is overexpressed in a number of different types of tumors, including those of the breast, kidney, colon.47,118 Although in some cases Pak1 kinase activity is high in tumors, it is almost always found in its wild-type form, without known activating mutations. High Pak1 levels were seen in invasive prostate cancer cells, compared with the non-invasive ones. Pak6 levels are also high in prostate cancer cells, but unlike Pak1, it is not correlated with the metastatic potential of the cells. Pak1 was shown to play a predominant role in microinvasion of the cells, and is necessary for prostate tumor growth and micrometastasis. Pak1 stimulates invasiveness of prostate cancer cells via its actions on the cytoskeletal network that enhance the directional migration of these cells. It also stimulates prostate tumor growth through enhanced expression of various tumor-promoting factors such as MMP9 and reduced expression of TGFβ, a factor that inhibits tumor growth.119
Pak1 DNA copy number, mRNA and protein expression are upregulated in human melanoma. Dysregulated Pak1 expression, however, had a negative correlation with BRAF mutation. While BRaf mutation is associated with a subset of melanoma, Pak1 upregulation is associated primarily with melanomas that lack the BRAF oncogenic mutation. This is significant, because wild-type BRaf melanoma has no effective targeted therapy. These observations raise the possibility that targeted Pak1 inhibition could serve as a pharmacologically effective treatment for wild-type BRAF melanomas.120 PAK4 and Pak5 have been implicated in colon cancer cell transformation, but it is Pak1 that is most commonly associated with colon cancer. Pak1 mediates cell proliferation, migration and survival of colon cancer cells by regulating the Wnt, Erk and Akt Pathways.121
Pak kinases and breast cancer
Pak1 is frequently overexpressed in breast cancer, and expression levels of endogenous Pak1 correlate with invasiveness and increased survival.47 PAK4 is also frequently overexpressed in breast cancer.12,103,104 Like Pak1, PAK4 is often found in its wild-type form in tumors.101,108
Transgenic mice have been generated that express a constitutively active Pak1 mutant (Pak1T423E) in the mammary gland. These mice develop mammary tumors, but at low penetrance and with a long latency period, suggesting that other genetic events are required in the transformation process.122 The breast cancer cell line (MDA-MB-631) forms tumors when injected into the flanks of severe combined immune deficiency (SCID) mice. Dominant negative Pak1 (Pak1K299R) and a Pak1 inhibitor (the Pak1 inhibitory domain: PID) lead to a significant reduction in the sizes of tumors formed by these cells. These results indicate that Pak1 kinase activity is required for tumor formation in this breast cancer cell line.123 The MCF10A progression cell line series is useful as an in vitro model of breast cancer. This panel of cells consists of MCF10A, neoT, ATI, and DCIS cells, which are all derived from MCF10A cells. MCF10A represent normal breast epithelium,124 and the other cells are models for increasing levels of oncogenic transformation.125-127 When grown in 3D culture, the cell lines in the series develop into increasingly disorganized acinar structures. PAK4 levels increase in the more malignant versions of the cells.128 Likewise, Pak1 expression and phosphorylation increase in the more malignant versions of the cells,129 Dominant negative Pak1 can partially reverse the abnormal morphologies of the malignant cells, and it also inhibits proliferation and migration of all cells in the series. Overexpression of exogenous wild-type or activated Pak1, however, has no effects on cell proliferation, invasion, or acinar structure.129 Overexpression of activated Pak1 in MCF7 breast cancer cells, however, leads to abnormal centrosome number and abnormal spindle organization. This leads to aneuploidy, which can result in loss of tumor suppressor genes and accumulation of oncogenes.47
Her2/neu/ErbB2 is a growth factor receptor that is frequently overexpressed in breast cancer, and recent evidence points to a role for Pak1 in ErbB2 positive breast cancer.123 Activation of ErbB2 in MCF10A cells led to a decrease in apoptosis and an increase in proliferation in 3D cultures, and this corresponded with increased Pak1 kinase activity.123 Expression of a constitutively active mutant of Pak1 (Pak1 L107F) has similar effects, and inhibition of Pak1 kinase activity blocks the effects of ErbB2. Pak1 is activated in breast cancer cells that are estrogen receptor (ER) negative and that overexpress oncogenic Erb-B2.123 When Pak1 activity is blocked, ErbB2 induced transformation of MCF10A is abrogated. Blocking Pak1 also inhibits the ability of ErbB2 positive breast cancer cell lines to form tumors in mice. Finally, an activated Pak1 mutant can bypass the requirement for ErbB2 activity in transformation.123
The mouse mammary epithelial cell line iMMEC has been used as a model to study the role for PAK4 in breast cancer.130 iMMECs can be grown in 3D culture conditions where they grow into spherical acini that resemble the acinar structures that form in normal non-cancerous breast epithelia.131 As is the case in normal mammary epithelium, wild-type iMMECs have nearly undetectable levels of PAK4. When wild-type iMMECs are stably transfected with wild-type PAK4, however, the cells grow into acini that have features usually associated with oncogenesis. The acini become abnormally large, and their lumens never become completely empty. They have a larger layer of epithelial cells surrounding the lumens, and the cells within the structures have increased levels of cell proliferation and decreased levels of apoptosis,130 Many of these abnormalities are reminiscent of changes that occur during pre-cancerous conditions and early tumorigenesis. Some of the changes, such as the partial filling of the luminal space with cells, are reminiscent of atypical hyperplasia and DCIS.132 When iMMECs transfected with wild-type PAK4 are implanted into the mammary fat pads of mice, the mice develop mammary tumors at a high frequency,130 indicating that wild-type PAK4 can be a driving force in oncogenic transformation of mammary cells. Oncogenes such as ErbB2 and oncogenic Ras also cause oncogenic transformation of iMMECs.131,133 Interestingly, these oncogenes also lead to high levels of PAK4. These studies all point to an important role for PAK4 in the signaling pathways leading to mammary tumorigenesis.130
Paks and Neurofibromatosis
Neurofibromatosis types 1 and 2 (NF1 and NF2) are caused by loss of function of the NF1 or NF2 tumor suppressor genes. The disease is associated with tumors that form in the nervous system, particularly in Schwann cells. Interestingly, loss of these genes are associated with improper activation of Pak1, and Pak1 appears to be important for growth of NF tumors.90,134 The NF1 gene product, neurofibromin, is a GAP protein. Its loss leads to activation of the Ras pathway. Ras normally leads to activation of several different pathways, including the PI3 Kinase pathway. Since PI3 Kinase can also lead to Cdc42 and Rac activation and ultimately to Pak activation, Pak is a good candidate for a target operating downstream to NF1 inactivation. In fact, dominant negative Pak is strong inhibitor of Ras transformation in Schwann cells and peripheral nerve sheet tumor cells from an NF1 patient.90,134 NF2 functions differently from NF1. The NF2 gene product is Merlin, which is a tumor suppressor that is associated with the cytoskeleton. Merlin is highly expressed in Schwann cells and cells of the nervous system. It has a growth suppressive role and mediates contact dependent growth inhibition. Pak1 phosphorylates Merlin, and this phosphorylation inhibits its growth suppressive activity. Pak2 and Pak6 can also phosphorylate Merlin.135,136 There is also an inhibitory feedback mechanism from Merlin to Pak. Merlin can bind to Pak and prevent its activation, but once Merlin gets phosphorylated it undergoes a conformational change and disrupts its interaction with Pak1, which then becomes activated. Consequently, inactivation of Merlin corresponds to dysregulated Pak1 activity and cytoskeletal changes associated with cancer. Overall Pak1 is considered an important target for both NF1 and NF2, particularly because of these roles in Schwann cells.135,136 Similarly, both Pak2 and Pak6 have also been shown to phosphorylate Merlin.135,136
Conclusions and Future Directions
The Pak kinases are effector proteins for the Rho GTPases Cdc42 and Rac. They have a wide range of cellular functions, including the control of cytoskeletal organization, cell growth, and cell survival. In animal models they have been shown to have important roles in development, including development of the heart, and the growth and development of the nervous system. The Paks are implicated in human disorders, and as discussed in this review, mutations in of Pak3 are associated with mental retardation. Several Pak family members, especially Pak1 and Pak4, are frequently found to be overexpressed in human cancer, and have been implicated in oncogenic transformation in cells and in mice. Because of this strong link between Pak proteins and cancer, there has been considerable interest in developing Pak inhibitors. Development of Pak inhibitors has challenges, because the different Paks share strong sequence similarity, and because of similarities between the kinase domains of many different protein kinases. Several different inhibitors, however, have been generated.137 IPA-3 (p21-activated kinase inhibitor 3), for example, is an allosteric inhibitor of Pak1, and is specific for the group A Paks.137,138 Peptide inhibitors have also been generated, including the PAK1 AID,139 and TAT-PAK18,140,141 the latter of which blocks the growth of ovarian cancer cell lines. Pak1 shRNA inhibits cell proliferation,142 suggesting that shRNA may be another strategy for blocking the Paks. FL172143 and OS-2144 are examples of kinase inhibitors with some specificity toward the group A Paks. Pfizer has developed the inhibitor, PF-3758309, which was designed specifically to block the Pak4 kinase activity, but which turned out to be broadly inhibitory toward both group A and group B Paks, and also other kinases, including AMPK (AMP-dependent kinase) and RSK (ribosomal S6 kinase).145,146 In vitro results for this inhibitor were initially encouraging, because when it was tested on a panel of 92 tumor cell lines, half of them exhibited IC50 (inhibitory concentration for 10% maximal effects) values of less than 10 nM. The compound also inhibits tumor growth in mouse xenograft models, and it reduces proliferation and inhibits apoptosis in HCT116 colon tumor cells.146 Results from clinical trials on human patients, however, have been hampered by a lack of detectable tumor responses, as well as undesirable PK characteristics of the drug, and adverse side effects.147,148 A second Pak4 inhibitor, LCH-779944 has also been developed.149 LCH-779944 inhibits Pak4 activity more modestly than PF-3758309, and also has some inhibitory activity toward Paks 1, 5, and 6. EGFR phosphorylation and c-Src phosphorylation were also abrogated in cells treated with the inhibitor. LCH-779944 reduces proliferation and invasion of gastric cancer cells in vitro, as well as filopodia formation and cell elongation, but it has not been tested in tumors in animals or clinically in cancer patients. Genentech has recently generated a highly specific group B Pak small molecule inhibitor called compound 17,150 which leads to decreased migration and invasiveness in 2 breast cancer cell lines. These types of inhibitors are promising for the future treatment of disease, but important challenges lie ahead. These include development of the most specific inhibitors possible, and development of a better understanding of the signaling pathways linked to the Pak proteins, so that these pathways can be most effectively blocked or modified for the treatment of human disease.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Daniels RH, Bokoch GM. p21-activated protein kinase: a crucial component of morphological signaling? Trends Biochem Sci. 1999;24:350–5. doi: 10.1016/S0968-0004(99)01442-5. [DOI] [PubMed] [Google Scholar]
- 2.Knaus UG, Bokoch GM. The p21Rac/Cdc42-activated kinases (PAKs) Int J Biochem Cell Biol. 1998;30:857–62. doi: 10.1016/S1357-2725(98)00059-4. [DOI] [PubMed] [Google Scholar]
- 3.Sells MA, Chernoff J. Emerging from the Pak: the p21-activated protein kinase family. Trends Cell Biol. 1997;7:162–7. doi: 10.1016/S0962-8924(97)01003-9. [DOI] [PubMed] [Google Scholar]
- 4.Manser E, Leung T, Salihuddin H, Zhao ZS, Lim L. A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature. 1994;367:40–6. doi: 10.1038/367040a0. [DOI] [PubMed] [Google Scholar]
- 5.Whale A, Hashim FN, Fram S, Jones GE, Wells CM. Signalling to cancer cell invasion through PAK family kinases. Front Biosci (Landmark Ed) 2011;16:849–64. doi: 10.2741/3724. [DOI] [PubMed] [Google Scholar]
- 6.Abo A, Qu J, Cammarano MS, Dan C, Fritsch A, Baud V, Belisle B, Minden A. PAK4, a novel effector for Cdc42Hs, is implicated in the reorganization of the actin cytoskeleton and in the formation of filopodia. EMBO J. 1998;17:6527–40. doi: 10.1093/emboj/17.22.6527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dan C, Kelly A, Bernard O, Minden A. Cytoskeletal changes regulated by the PAK4 serine/threonine kinase are mediated by LIM kinase 1 and cofilin. J Biol Chem. 2001;276:32115–21. doi: 10.1074/jbc.M100871200. [DOI] [PubMed] [Google Scholar]
- 8.Qu J, Cammarano MS, Shi Q, Ha KC, de Lanerolle P, Minden A. Activated PAK4 regulates cell adhesion and anchorage-independent growth. Mol Cell Biol. 2001;21:3523–33. doi: 10.1128/MCB.21.10.3523-3533.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dan C, Nath N, Liberto M, Minden A. PAK5, a new brain-specific kinase, promotes neurite outgrowth in N1E-115 cells. Mol Cell Biol. 2002;22:567–77. doi: 10.1128/MCB.22.2.567-577.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pandey A, Dan I, Kristiansen TZ, Watanabe NM, Voldby J, Kajikawa E, Khosravi-Far R, Blagoev B, Mann M. Cloning and characterization of PAK5, a novel member of mammalian p21-activated kinase-II subfamily that is predominantly expressed in brain. Oncogene. 2002;21:3939–48. doi: 10.1038/sj.onc.1205478. [DOI] [PubMed] [Google Scholar]
- 11.Yang F, Li X, Sharma M, Zarnegar M, Lim B, Sun Z. Androgen receptor specifically interacts with a novel p21-activated kinase, PAK6. J Biol Chem. 2001;276:15345–53. doi: 10.1074/jbc.M010311200. [DOI] [PubMed] [Google Scholar]
- 12.Callow MG, Clairvoyant F, Zhu S, Schryver B, Whyte DB, Bischoff JR, Jallal B, Smeal T. Requirement for PAK4 in the anchorage-independent growth of human cancer cell lines. J Biol Chem. 2002;277:550–8. doi: 10.1074/jbc.M105732200. [DOI] [PubMed] [Google Scholar]
- 13.Lee SR, Ramos SM, Ko A, Masiello D, Swanson KD, Lu ML, Balk SP. AR and ER interaction with a p21-activated kinase (PAK6) Mol Endocrinol. 2002;16:85–99. doi: 10.1210/mend.16.1.0753. [DOI] [PubMed] [Google Scholar]
- 14.Schrantz N, da Silva Correia J, Fowler B, Ge Q, Sun Z, Bokoch GM. Mechanism of p21-activated kinase 6-mediated inhibition of androgen receptor signaling. J Biol Chem. 2004;279:1922–31. doi: 10.1074/jbc.M311145200. [DOI] [PubMed] [Google Scholar]
- 15.Baskaran Y, Ng YW, Selamat W, Ling FT, Manser E. Group I and II mammalian PAKs have different modes of activation by Cdc42. EMBO Rep. 2012;13:653–9. doi: 10.1038/embor.2012.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Radu M, Semenova G, Kosoff R, Chernoff J. PAK signalling during the development and progression of cancer. Nat Rev Cancer. 2014;14:13–25. doi: 10.1038/nrc3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pirruccello M, Sondermann H, Pelton JG, Pellicena P, Hoelz A, Chernoff J, Wemmer DE, Kuriyan J. A dimeric kinase assembly underlying autophosphorylation in the p21 activated kinases. J Mol Biol. 2006;361:312–26. doi: 10.1016/j.jmb.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 18.Zhao ZS, Manser E. PAK family kinases: Physiological roles and regulation. Cell Logist. 2012;2:59–68. doi: 10.4161/cl.21912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lei M, Lu W, Meng W, Parrini MC, Eck MJ, Mayer BJ, Harrison SC. Structure of PAK1 in an autoinhibited conformation reveals a multistage activation switch. Cell. 2000;102:387–97. doi: 10.1016/S0092-8674(00)00043-X. [DOI] [PubMed] [Google Scholar]
- 20.Buchwald G, Hostinova E, Rudolph MG, Kraemer A, Sickmann A, Meyer HE, Scheffzek K, Wittinghofer A. Conformational switch and role of phosphorylation in PAK activation. Mol Cell Biol. 2001;21:5179–89. doi: 10.1128/MCB.21.15.5179-5189.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bokoch GM. Biology of the p21-activated kinases. Annu Rev Biochem. 2003;72:743–81. doi: 10.1146/annurev.biochem.72.121801.161742. [DOI] [PubMed] [Google Scholar]
- 22.Parrini MC, Lei M, Harrison SC, Mayer BJ. Pak1 kinase homodimers are autoinhibited in trans and dissociated upon activation by Cdc42 and Rac1. Mol Cell. 2002;9:73–83. doi: 10.1016/S1097-2765(01)00428-2. [DOI] [PubMed] [Google Scholar]
- 23.Ching YP, Leong VY, Wong CM, Kung HF. Identification of an autoinhibitory domain of p21-activated protein kinase 5. J Biol Chem. 2003;278:33621–4. doi: 10.1074/jbc.C300234200. [DOI] [PubMed] [Google Scholar]
- 24.Ha BH, Davis MJ, Chen C, Lou HJ, Gao J, Zhang R, Krauthammer M, Halaban R, Schlessinger J, Turk BE, et al. Type II p21-activated kinases (PAKs) are regulated by an autoinhibitory pseudosubstrate. Proc Natl Acad Sci U S A. 2012;109:16107–12. doi: 10.1073/pnas.1214447109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manser E, Loo TH, Koh CG, Zhao ZS, Chen XQ, Tan L, Tan I, Leung T, Lim L. PAK kinases are directly coupled to the PIX family of nucleotide exchange factors. Mol Cell. 1998;1:183–92. doi: 10.1016/S1097-2765(00)80019-2. [DOI] [PubMed] [Google Scholar]
- 26.Obermeier A, Ahmed S, Manser E, Yen SC, Hall C, Lim L. PAK promotes morphological changes by acting upstream of Rac. EMBO J. 1998;17:4328–39. doi: 10.1093/emboj/17.15.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loo T-H, Ng Y-W, Lim L, Manser E. GIT1 activates p21-activated kinase through a mechanism independent of p21 binding. Mol Cell Biol. 2004;24:3849–59. doi: 10.1128/MCB.24.9.3849-3859.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao ZS, Lim JP, Ng YW, Lim L, Manser E. The GIT-associated kinase PAK targets to the centrosome and regulates Aurora-A. Mol Cell. 2005;20:237–49. doi: 10.1016/j.molcel.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 29.Wells CM, Abo A, Ridley AJ. PAK4 is activated via PI3K in HGF-stimulated epithelial cells. J Cell Sci. 2002;115:3947–56. doi: 10.1242/jcs.00080. [DOI] [PubMed] [Google Scholar]
- 30.Lu Y, Pan ZZ, Devaux Y, Ray P. p21-activated protein kinase 4 (PAK4) interacts with the keratinocyte growth factor receptor and participates in keratinocyte growth factor-mediated inhibition of oxidant-induced cell death. J Biol Chem. 2003;278:10374–80. doi: 10.1074/jbc.M205875200. [DOI] [PubMed] [Google Scholar]
- 31.Ramos E, Wysolmerski RB, Masaracchia RA. Myosin phosphorylation by human cdc42-dependent S6/H4 kinase/gammaPAK from placenta and lymphoid cells. Recept Signal Transduct. 1997;7:99–110. [PubMed] [Google Scholar]
- 32.Van Eyk JE, Arrell DK, Foster DB, Strauss JD, Heinonen TY, Furmaniak-Kazmierczak E, Côté GP, Mak AS. Different molecular mechanisms for Rho family GTPase-dependent, Ca2+-independent contraction of smooth muscle. J Biol Chem. 1998;273:23433–9. doi: 10.1074/jbc.273.36.23433. [DOI] [PubMed] [Google Scholar]
- 33.Szczepanowska J. Involvement of Rac/Cdc42/PAK pathway in cytoskeletal rearrangements. Acta Biochim Pol. 2009;56:225–34. [PubMed] [Google Scholar]
- 34.Chew TL, Masaracchia RA, Goeckeler ZM, Wysolmerski RB. Phosphorylation of non-muscle myosin II regulatory light chain by p21-activated kinase (gamma-PAK) J Muscle Res Cell Motil. 1998;19:839–54. doi: 10.1023/A:1005417926585. [DOI] [PubMed] [Google Scholar]
- 35.Zhang H, Webb DJ, Asmussen H, Niu S, Horwitz AF. A GIT1/PIX/Rac/PAK signaling module regulates spine morphogenesis and synapse formation through MLC. J Neurosci. 2005;25:3379–88. doi: 10.1523/JNEUROSCI.3553-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buss F, Kendrick-Jones J, Lionne C, Knight AE, Côté GP, Paul Luzio J. The localization of myosin VI at the golgi complex and leading edge of fibroblasts and its phosphorylation and recruitment into membrane ruffles of A431 cells after growth factor stimulation. J Cell Biol. 1998;143:1535–45. doi: 10.1083/jcb.143.6.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanders LC, Matsumura F, Bokoch GM, de Lanerolle P. Inhibition of myosin light chain kinase by p21-activated kinase. Science. 1999;283:2083–5. doi: 10.1126/science.283.5410.2083. [DOI] [PubMed] [Google Scholar]
- 38.Goeckeler ZM, Masaracchia RA, Zeng Q, Chew TL, Gallagher P, Wysolmerski RB. Phosphorylation of myosin light chain kinase by p21-activated kinase PAK2. J Biol Chem. 2000;275:18366–74. doi: 10.1074/jbc.M001339200. [DOI] [PubMed] [Google Scholar]
- 39.Callow MG, Zozulya S, Gishizky ML, Jallal B, Smeal T. PAK4 mediates morphological changes through the regulation of GEF-H1. J Cell Sci. 2005;118:1861–72. doi: 10.1242/jcs.02313. [DOI] [PubMed] [Google Scholar]
- 40.Zenke FT, Krendel M, DerMardirossian C, King CC, Bohl BP, Bokoch GM. p21-activated kinase 1 phosphorylates and regulates 14-3-3 binding to GEF-H1, a microtubule-localized Rho exchange factor. J Biol Chem. 2004;279:18392–400. doi: 10.1074/jbc.M400084200. [DOI] [PubMed] [Google Scholar]
- 41.Edwards DC, Sanders LC, Bokoch GM, Gill GN. Activation of LIM-kinase by Pak1 couples Rac/Cdc42 GTPase signalling to actin cytoskeletal dynamics. Nat Cell Biol. 1999;1:253–9. doi: 10.1038/12963. [DOI] [PubMed] [Google Scholar]
- 42.Arber S, Barbayannis FA, Hanser H, Schneider C, Stanyon CA, Bernard O, Caroni P. Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature. 1998;393:805–9. doi: 10.1038/31729. [DOI] [PubMed] [Google Scholar]
- 43.Yang N, Higuchi O, Ohashi K, Nagata K, Wada A, Kangawa K, Nishida E, Mizuno K. Cofilin phosphorylation by LIM-kinase 1 and its role in Rac-mediated actin reorganization. Nature. 1998;393:809–12. doi: 10.1038/31735. [DOI] [PubMed] [Google Scholar]
- 44.Soosairajah J, Maiti S, Wiggan O, Sarmiere P, Moussi N, Sarcevic B, Sampath R, Bamburg JR, Bernard O. Interplay between components of a novel LIM kinase-slingshot phosphatase complex regulates cofilin. EMBO J. 2005;24:473–86. doi: 10.1038/sj.emboj.7600543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li X, Ke Q, Li Y, Liu F, Zhu G, Li F. DGCR6L, a novel PAK4 interaction protein, regulates PAK4-mediated migration of human gastric cancer cell via LIMK1. Int J Biochem Cell Biol. 2010;42:70–9. doi: 10.1016/j.biocel.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 46.Vadlamudi RK, Li F, Adam L, Nguyen D, Ohta Y, Stossel TP, Kumar R. Filamin is essential in actin cytoskeletal assembly mediated by p21-activated kinase 1. Nat Cell Biol. 2002;4:681–90. doi: 10.1038/ncb838. [DOI] [PubMed] [Google Scholar]
- 47.Dummler B, Ohshiro K, Kumar R, Field J. Pak protein kinases and their role in cancer. Cancer Metastasis Rev. 2009;28:51–63. doi: 10.1007/s10555-008-9168-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maroto B, Ye MB, von Lohneysen K, Schnelzer A, Knaus UG. P21-activated kinase is required for mitotic progression and regulates Plk1. Oncogene. 2008;27:4900–8. doi: 10.1038/onc.2008.131. [DOI] [PubMed] [Google Scholar]
- 49.Vadlamudi RK, Adam L, Wang RA, Mandal M, Nguyen D, Sahin A, Chernoff J, Hung MC, Kumar R. Regulatable expression of p21-activated kinase-1 promotes anchorage-independent growth and abnormal organization of mitotic spindles in human epithelial breast cancer cells. J Biol Chem. 2000;275:36238–44. doi: 10.1074/jbc.M002138200. [DOI] [PubMed] [Google Scholar]
- 50.Daub H, Gevaert K, Vandekerckhove J, Sobel A, Hall A. Rac/Cdc42 and p65PAK regulate the microtubule-destabilizing protein stathmin through phosphorylation at serine 16. J Biol Chem. 2001;276:1677–80. doi: 10.1074/jbc.C000635200. [DOI] [PubMed] [Google Scholar]
- 51.Bompard G, Rabeharivelo G, Frank M, Cau J, Delsert C, Morin N. Subgroup II PAK-mediated phosphorylation regulates Ran activity during mitosis. J Cell Biol. 2010;190:807–22. doi: 10.1083/jcb.200912056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wong LE, Reynolds AB, Dissanayaka NT, Minden A. p120-catenin is a binding partner and substrate for Group B Pak kinases. J Cell Biochem. 2010;110:1244–54. doi: 10.1002/jcb.22639. [DOI] [PubMed] [Google Scholar]
- 53.Dohn MR, Brown MV, Reynolds AB. An essential role for p120-catenin in Src- and Rac1-mediated anchorage-independent cell growth. J Cell Biol. 2009;184:437–50. doi: 10.1083/jcb.200807096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li Z, Zhang H, Lundin L, Thullberg M, Liu Y, Wang Y, Claesson-Welsh L, Strömblad S. p21-activated kinase 4 phosphorylation of integrin beta5 Ser-759 and Ser-762 regulates cell migration. J Biol Chem. 2010;285:23699–710. doi: 10.1074/jbc.M110.123497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hoefen RJ, Berk BC. The multifunctional GIT family of proteins. J Cell Sci. 2006;119:1469–75. doi: 10.1242/jcs.02925. [DOI] [PubMed] [Google Scholar]
- 56.Bentley D, O’Connor TP. Cytoskeletal events in growth cone steering. Curr Opin Neurobiol. 1994;4:43–8. doi: 10.1016/0959-4388(94)90030-2. [DOI] [PubMed] [Google Scholar]
- 57.Mueller BK. Growth cone guidance: first steps towards a deeper understanding. Annu Rev Neurosci. 1999;22:351–88. doi: 10.1146/annurev.neuro.22.1.351. [DOI] [PubMed] [Google Scholar]
- 58.Suter DM, Forscher P. An emerging link between cytoskeletal dynamics and cell adhesion molecules in growth cone guidance. Curr Opin Neurobiol. 1998;8:106–16. doi: 10.1016/S0959-4388(98)80014-7. [DOI] [PubMed] [Google Scholar]
- 59.Brown MD, Cornejo BJ, Kuhn TB, Bamburg JR. Cdc42 stimulates neurite outgrowth and formation of growth cone filopodia and lamellipodia. J Neurobiol. 2000;43:352–64. doi: 10.1002/1097-4695(20000615)43:4<352::AID-NEU4>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 60.Luo L, Liao YJ, Jan LY, Jan YN. Distinct morphogenetic functions of similar small GTPases: Drosophila Drac1 is involved in axonal outgrowth and myoblast fusion. Genes Dev. 1994;8:1787–802. doi: 10.1101/gad.8.15.1787. [DOI] [PubMed] [Google Scholar]
- 61.Daniels RH, Hall PS, Bokoch GM. Membrane targeting of p21-activated kinase 1 (PAK1) induces neurite outgrowth from PC12 cells. EMBO J. 1998;17:754–64. doi: 10.1093/emboj/17.3.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kaufmann N, Wills ZP, Van Vactor D. Drosophila Rac1 controls motor axon guidance. Development. 1998;125:453–61. doi: 10.1242/dev.125.3.453. [DOI] [PubMed] [Google Scholar]
- 63.Lamoureux P, Altun-Gultekin ZF, Lin C, Wagner JA, Heidemann SR. Rac is required for growth cone function but not neurite assembly. J Cell Sci. 1997;110:635–41. doi: 10.1242/jcs.110.5.635. [DOI] [PubMed] [Google Scholar]
- 64.Steven R, Kubiseski TJ, Zheng H, Kulkarni S, Mancillas J, Ruiz Morales A, Hogue CW, Pawson T, Culotti J. UNC-73 activates the Rac GTPase and is required for cell and growth cone migrations in C. elegans. Cell. 1998;92:785–95. doi: 10.1016/S0092-8674(00)81406-3. [DOI] [PubMed] [Google Scholar]
- 65.Zipkin ID, Kindt RM, Kenyon CJ. Role of a new Rho family member in cell migration and axon guidance in C. elegans. Cell. 1997;90:883–94. doi: 10.1016/S0092-8674(00)80353-0. [DOI] [PubMed] [Google Scholar]
- 66.Harden N, Lee J, Loh HY, Ong YM, Tan I, Leung T, Manser E, Lim L. A Drosophila homolog of the Rac- and Cdc42-activated serine/threonine kinase PAK is a potential focal adhesion and focal complex protein that colocalizes with dynamic actin structures. Mol Cell Biol. 1996;16:1896–908. doi: 10.1128/mcb.16.5.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Melzig J, Rein KH, Schäfer U, Pfister H, Jäckle H, Heisenberg M, Raabe T. A protein related to p21-activated kinase (PAK) that is involved in neurogenesis in the Drosophila adult central nervous system. Curr Biol. 1998;8:1223–6. doi: 10.1016/S0960-9822(07)00514-3. [DOI] [PubMed] [Google Scholar]
- 68.Allen KM, Gleeson JG, Bagrodia S, Partington MW, MacMillan JC, Cerione RA, Mulley JC, Walsh CA. PAK3 mutation in nonsyndromic X-linked mental retardation. Nat Genet. 1998;20:25–30. doi: 10.1038/1675. [DOI] [PubMed] [Google Scholar]
- 69.Bagrodia S, Dérijard B, Davis RJ, Cerione RA. Cdc42 and PAK-mediated signaling leads to Jun kinase and p38 mitogen-activated protein kinase activation. J Biol Chem. 1995;270:27995–8. doi: 10.1074/jbc.270.47.27995. [DOI] [PubMed] [Google Scholar]
- 70.Stanyon CA, Bernard O. LIM-kinase1. Int J Biochem Cell Biol. 1999;31:389–94. doi: 10.1016/S1357-2725(98)00116-2. [DOI] [PubMed] [Google Scholar]
- 71.Meberg PJ, Bamburg JR. Increase in neurite outgrowth mediated by overexpression of actin depolymerizing factor. J Neurosci. 2000;20:2459–69. doi: 10.1523/JNEUROSCI.20-07-02459.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kelly ML, Chernoff J. Mouse models of PAK function. Cell Logist. 2012;2:84–8. doi: 10.4161/cl.21381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tian Y, Lei L, Minden A. A key role for Pak4 in proliferation and differentiation of neural progenitor cells. Dev Biol. 2011;353:206–16. doi: 10.1016/j.ydbio.2011.02.026. [DOI] [PubMed] [Google Scholar]
- 74.Gnesutta N, Minden A. Death receptor-induced activation of initiator caspase 8 is antagonized by serine/threonine kinase PAK4. Mol Cell Biol. 2003;23:7838–48. doi: 10.1128/MCB.23.21.7838-7848.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Arias-Romero LE, Chernoff J. A tale of two Paks. Biol Cell. 2008;100:97–108. doi: 10.1042/BC20070109. [DOI] [PubMed] [Google Scholar]
- 76.Qu J, Li X, Novitch BG, Zheng Y, Kohn M, Xie JM, Kozinn S, Bronson R, Beg AA, Minden A. PAK4 kinase is essential for embryonic viability and for proper neuronal development. Mol Cell Biol. 2003;23:7122–33. doi: 10.1128/MCB.23.20.7122-7133.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Allen JD, Jaffer ZM, Park SJ, Burgin S, Hofmann C, Sells MA, Chen S, Derr-Yellin E, Michels EG, McDaniel A, et al. p21-activated kinase regulates mast cell degranulation via effects on calcium mobilization and cytoskeletal dynamics. Blood. 2009;113:2695–705. doi: 10.1182/blood-2008-06-160861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang Z, Oh E, Clapp DW, Chernoff J, Thurmond DC. Inhibition or ablation of p21-activated kinase (PAK1) disrupts glucose homeostatic mechanisms in vivo. J Biol Chem. 2011;286:41359–67. doi: 10.1074/jbc.M111.291500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu W, Zi M, Naumann R, Ulm S, Jin J, Taglieri DM, Prehar S, Gui J, Tsui H, Xiao RP, et al. Pak1 as a novel therapeutic target for antihypertrophic treatment in the heart. Circulation. 2011;124:2702–15. doi: 10.1161/CIRCULATIONAHA.111.048785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nekrasova T, Minden A. Role for p21-activated kinase PAK4 in development of the mammalian heart. Transgenic Res. 2012;21:797–811. doi: 10.1007/s11248-011-9578-7. [DOI] [PubMed] [Google Scholar]
- 81.Ke Y, Wang L, Pyle WG, de Tombe PP, Solaro RJ. Intracellular localization and functional effects of P21-activated kinase-1 (Pak1) in cardiac myocytes. Circ Res. 2004;94:194–200. doi: 10.1161/01.RES.0000111522.02730.56. [DOI] [PubMed] [Google Scholar]
- 82.Tian Y, Lei L, Cammarano M, Nekrasova T, Minden A. Essential role for the Pak4 protein kinase in extraembryonic tissue development and vessel formation. Mech Dev. 2009;126:710–20. doi: 10.1016/j.mod.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 83.Eswaran J, Soundararajan M, Knapp S. Targeting group II PAKs in cancer and metastasis. Cancer Metastasis Rev. 2009;28:209–17. doi: 10.1007/s10555-008-9181-4. [DOI] [PubMed] [Google Scholar]
- 84.Gnesutta N, Qu J, Minden A. The serine/threonine kinase PAK4 prevents caspase activation and protects cells from apoptosis. J Biol Chem. 2001;276:14414–9. doi: 10.1074/jbc.M011046200. [DOI] [PubMed] [Google Scholar]
- 85.Li X, Minden A. PAK4 functions in tumor necrosis factor (TNF) alpha-induced survival pathways by facilitating TRADD binding to the TNF receptor. J Biol Chem. 2005;280:41192–200. doi: 10.1074/jbc.M506884200. [DOI] [PubMed] [Google Scholar]
- 86.Paliouras GN, Naujokas MA, Park M. Pak4, a novel Gab1 binding partner, modulates cell migration and invasion by the Met receptor. Mol Cell Biol. 2009;29:3018–32. doi: 10.1128/MCB.01286-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ahmed T, Shea K, Masters JR, Jones GE, Wells CM. A PAK4-LIMK1 pathway drives prostate cancer cell migration downstream of HGF. Cell Signal. 2008;20:1320–8. doi: 10.1016/j.cellsig.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 88.Gringel A, Walz D, Rosenberger G, Minden A, Kutsche K, Kopp P, Linder S. PAK4 and alphaPIX determine podosome size and number in macrophages through localized actin regulation. J Cell Physiol. 2006;209:568–79. doi: 10.1002/jcp.20777. [DOI] [PubMed] [Google Scholar]
- 89.Bao W, Thullberg M, Zhang H, Onischenko A, Strömblad S. Cell attachment to the extracellular matrix induces proteasomal degradation of p21(CIP1) via Cdc42/Rac1 signaling. Mol Cell Biol. 2002;22:4587–97. doi: 10.1128/MCB.22.13.4587-4597.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ye DZ, Field J. PAK signaling in cancer. Cell Logist. 2012;2:105–16. doi: 10.4161/cl.21882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Reddy SD, Ohshiro K, Rayala SK, Kumar R. MicroRNA-7, a homeobox D10 target, inhibits p21-activated kinase 1 and regulates its functions. Cancer Res. 2008;68:8195–200. doi: 10.1158/0008-5472.CAN-08-2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cotteret S, Jaffer ZM, Beeser A, Chernoff J. p21-Activated kinase 5 (Pak5) localizes to mitochondria and inhibits apoptosis by phosphorylating BAD. Mol Cell Biol. 2003;23:5526–39. doi: 10.1128/MCB.23.16.5526-5539.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang X, Gong W, Qing H, Geng Y, Wang X, Zhang Y, Peng L, Zhang H, Jiang B. p21-activated kinase 5 inhibits camptothecin-induced apoptosis in colorectal carcinoma cells. Tumour Biol. 2010;31:575–82. doi: 10.1007/s13277-010-0071-3. [DOI] [PubMed] [Google Scholar]
- 94.Jin S, Zhuo Y, Guo W, Field J. p21-activated Kinase 1 (Pak1)-dependent phosphorylation of Raf-1 regulates its mitochondrial localization, phosphorylation of BAD, and Bcl-2 association. J Biol Chem. 2005;280:24698–705. doi: 10.1074/jbc.M413374200. [DOI] [PubMed] [Google Scholar]
- 95.Frost JA, Swantek JL, Stippec S, Yin MJ, Gaynor R, Cobb MH. Stimulation of NFkappa B activity by multiple signaling pathways requires PAK1. J Biol Chem. 2000;275:19693–9. doi: 10.1074/jbc.M909860199. [DOI] [PubMed] [Google Scholar]
- 96.Friedland JC, Lakins JN, Kazanietz MG, Chernoff J, Boettiger D, Weaver VM. alpha6beta4 integrin activates Rac-dependent p21-activated kinase 1 to drive NF-kappaB-dependent resistance to apoptosis in 3D mammary acini. J Cell Sci. 2007;120:3700–12. doi: 10.1242/jcs.03484. [DOI] [PubMed] [Google Scholar]
- 97.Mazumdar A, Kumar R. Estrogen regulation of Pak1 and FKHR pathways in breast cancer cells. FEBS Lett. 2003;535:6–10. doi: 10.1016/S0014-5793(02)03846-2. [DOI] [PubMed] [Google Scholar]
- 98.Lin R, Bagrodia S, Cerione R, Manor D. A novel Cdc42Hs mutant induces cellular transformation. Curr Biol. 1997;7:794–7. doi: 10.1016/S0960-9822(06)00338-1. [DOI] [PubMed] [Google Scholar]
- 99.Lin R, Cerione RA, Manor D. Specific contributions of the small GTPases Rho, Rac, and Cdc42 to Dbl transformation. J Biol Chem. 1999;274:23633–41. doi: 10.1074/jbc.274.33.23633. [DOI] [PubMed] [Google Scholar]
- 100.Qiu RG, Abo A, McCormick F, Symons M. Cdc42 regulates anchorage-independent growth and is necessary for Ras transformation. Mol Cell Biol. 1997;17:3449–58. doi: 10.1128/mcb.17.6.3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kimmelman AC, Hezel AF, Aguirre AJ, Zheng H, Paik JH, Ying H, Chu GC, Zhang JX, Sahin E, Yeo G, et al. Genomic alterations link Rho family of GTPases to the highly invasive phenotype of pancreas cancer. Proc Natl Acad Sci U S A. 2008;105:19372–7. doi: 10.1073/pnas.0809966105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang HJ, Siu MK, Yeung MC, Jiang LL, Mak VC, Ngan HY, Wong OG, Zhang HQ, Cheung AN. Overexpressed PAK4 promotes proliferation, migration and invasion of choriocarcinoma. Carcinogenesis. 2011;32:765–71. doi: 10.1093/carcin/bgr033. [DOI] [PubMed] [Google Scholar]
- 103.Kim JH, Kim HN, Lee KT, Lee JK, Choi SH, Paik SW, Rhee JC, Lowe AW. Gene expression profiles in gallbladder cancer: the close genetic similarity seen for early and advanced gallbladder cancers may explain the poor prognosis. Tumour Biol. 2008;29:41–9. doi: 10.1159/000132570. [DOI] [PubMed] [Google Scholar]
- 104.Liu Y, Xiao H, Tian Y, Nekrasova T, Hao X, Lee HJ, Suh N, Yang CS, Minden A. The pak4 protein kinase plays a key role in cell survival and tumorigenesis in athymic mice. Mol Cancer Res. 2008;6:1215–24. doi: 10.1158/1541-7786.MCR-08-0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ahn HK, Jang J, Lee J, Se Hoon P, Park JO, Park YS, Lim HY, Kim KM, Kang WK. P21-activated kinase 4 overexpression in metastatic gastric cancer patients. Transl Oncol. 2011;4:345–9. doi: 10.1593/tlo.11145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Siu MKY, Chan HY, Kong DS, Wong ESY, Wong OGW, Ngan HYS, Tam KF, Zhang H, Li Z, Chan QKY, et al. p21-activated kinase 4 regulates ovarian cancer cell proliferation, migration, and invasion and contributes to poor prognosis in patients. Proc Natl Acad Sci U S A. 2010;107:18622–7. doi: 10.1073/pnas.0907481107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Parsons DW, Wang TL, Samuels Y, Bardelli A, Cummins JM, DeLong L, Silliman N, Ptak J, Szabo S, Willson JK, et al. Colorectal cancer: mutations in a signalling pathway. Nature. 2005;436:792. doi: 10.1038/436792a. [DOI] [PubMed] [Google Scholar]
- 108.Chen S, Auletta T, Dovirak O, Hutter C, Kuntz K, El-ftesi S, Kendall J, Han H, Von Hoff DD, Ashfaq R, et al. Copy number alterations in pancreatic cancer identify recurrent PAK4 amplification. Cancer Biol Ther. 2008;7:1793–802. doi: 10.4161/cbt.7.11.6840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mahlamäki EH, Kauraniemi P, Monni O, Wolf M, Hautaniemi S, Kallioniemi A. High-resolution genomic and expression profiling reveals 105 putative amplification target genes in pancreatic cancer. Neoplasia. 2004;6:432–9. doi: 10.1593/neo.04130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Begum A, Imoto I, Kozaki K, Tsuda H, Suzuki E, Amagasa T, Inazawa J. Identification of PAK4 as a putative target gene for amplification within 19q13.12-q13.2 in oral squamous-cell carcinoma. Cancer Sci. 2009;100:1908–16. doi: 10.1111/j.1349-7006.2009.01252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yu W, Kanaan Y, Bae YK, Gabrielson E. Chromosomal changes in aggressive breast cancers with basal-like features. Cancer Genet Cytogenet. 2009;193:29–37. doi: 10.1016/j.cancergencyto.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mak GW, Chan MM, Leong VY, Lee JM, Yau TO, Ng IO, Ching YP. Overexpression of a novel activator of PAK4, the CDK5 kinase-associated protein CDK5RAP3, promotes hepatocellular carcinoma metastasis. Cancer Res. 2011;71:2949–58. doi: 10.1158/0008-5472.CAN-10-4046. [DOI] [PubMed] [Google Scholar]
- 113.Hou J, Lin L, Zhou W, Wang Z, Ding G, Dong Q, Qin L, Wu X, Zheng Y, Yang Y, et al. Identification of miRNomes in human liver and hepatocellular carcinoma reveals miR-199a/b-3p as therapeutic target for hepatocellular carcinoma. Cancer Cell. 2011;19:232–43. doi: 10.1016/j.ccr.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 114.Gong W, An Z, Wang Y, Pan X, Fang W, Jiang B, Zhang H. P21-activated kinase 5 is overexpressed during colorectal cancer progression and regulates colorectal carcinoma cell adhesion and migration. Int J Cancer. 2009;125:548–55. doi: 10.1002/ijc.24428. [DOI] [PubMed] [Google Scholar]
- 115.Kaur R, Yuan X, Lu ML, Balk SP. Increased PAK6 expression in prostate cancer and identification of PAK6 associated proteins. Prostate. 2008;68:1510–6. doi: 10.1002/pros.20787. [DOI] [PubMed] [Google Scholar]
- 116.Zhang M, Siedow M, Saia G, Chakravarti A. Inhibition of p21-activated kinase 6 (PAK6) increases radiosensitivity of prostate cancer cells. Prostate. 2010;70:807–16. doi: 10.1002/pros.21114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang Y, Yu Q, Cho AH, Rondeau G, Welsh J, Adamson E, Mercola D, McClelland M. Survey of differentially methylated promoters in prostate cancer cell lines. Neoplasia. 2005;7:748–60. doi: 10.1593/neo.05289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kumar R, Gururaj AE, Barnes CJ. p21-activated kinases in cancer. Nat Rev Cancer. 2006;6:459–71. doi: 10.1038/nrc1892. [DOI] [PubMed] [Google Scholar]
- 119.Goc A, Al-Azayzih A, Abdalla M, Al-Husein B, Kavuri S, Lee J, Moses K, Somanath PR. P21 activated kinase-1 (Pak1) promotes prostate tumor growth and microinvasion via inhibition of transforming growth factor β expression and enhanced matrix metalloproteinase 9 secretion. J Biol Chem. 2013;288:3025–35. doi: 10.1074/jbc.M112.424770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ong CC, Jubb AM, Jakubiak D, Zhou W, Rudolph J, Haverty PM, Kowanetz M, Yan Y, Tremayne J, Lisle R, et al. P21-activated kinase 1 (PAK1) as a therapeutic target in BRAF wild-type melanoma. J Natl Cancer Inst. 2013;105:606–7. doi: 10.1093/jnci/djt054. [DOI] [PubMed] [Google Scholar]
- 121.Ye DZ, Field J. PAK signaling in cancer. Cell Logist. 2012;2:105–16. doi: 10.4161/cl.21882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang RA, Zhang H, Balasenthil S, Medina D, Kumar R. PAK1 hyperactivation is sufficient for mammary gland tumor formation. Oncogene. 2006;25:2931–6. doi: 10.1038/sj.onc.1209309. [DOI] [PubMed] [Google Scholar]
- 123.Arias-Romero LE, Villamar-Cruz O, Pacheco A, Kosoff R, Huang M, Muthuswamy SK, Chernoff J. A Rac-Pak signaling pathway is essential for ErbB2-mediated transformation of human breast epithelial cancer cells. Oncogene. 2010;29:5839–49. doi: 10.1038/onc.2010.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Soule HD, Maloney TM, Wolman SR, Peterson WD, Jr., Brenz R, McGrath CM, Russo J, Pauley RJ, Jones RF, Brooks SC. Isolation and characterization of a spontaneously immortalized human breast epithelial cell line, MCF-10. Cancer Res. 1990;50:6075–86. [PubMed] [Google Scholar]
- 125.Miller FR, Santner SJ, Tait L, Dawson PJ. MCF10DCIS.com xenograft model of human comedo ductal carcinoma in situ. J Natl Cancer Inst. 2000;92:1185–6. doi: 10.1093/jnci/92.14.1185A. [DOI] [PubMed] [Google Scholar]
- 126.Basolo F, Elliott J, Tait L, Chen XQ, Maloney T, Russo IH, Pauley R, Momiki S, Caamano J, Klein-Szanto AJ, et al. Transformation of human breast epithelial cells by c-Ha-ras oncogene. Mol Carcinog. 1991;4:25–35. doi: 10.1002/mc.2940040106. [DOI] [PubMed] [Google Scholar]
- 127.Dawson PJ, Wolman SR, Tait L, Heppner GH, Miller FR. MCF10AT: a model for the evolution of cancer from proliferative breast disease. Am J Pathol. 1996;148:313–9. [PMC free article] [PubMed] [Google Scholar]
- 128.So JY, Lee HJ, Kramata P, Minden A, Suh N. Lee., H.J., Kramata, P, Minden, A., Suh, N., Differential expression of key signaling proteins in MCF10 cell lines, a human breast cancer progression model. Mol Cell Pharmacol. 2012;4:31–40. [PMC free article] [PubMed] [Google Scholar]
- 129.Li Q, Mullins SR, Sloane BF, Mattingly RR. p21-Activated kinase 1 coordinates aberrant cell survival and pericellular proteolysis in a three-dimensional culture model for premalignant progression of human breast cancer. Neoplasia. 2008;10:314–29. doi: 10.1593/neo.07970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Liu Y, Chen N, Cui X, Zheng X, Deng L, Price S, Karantza V, Minden A. The protein kinase Pak4 disrupts mammary acinar architecture and promotes mammary tumorigenesis. Oncogene. 2010;29:5883–94. doi: 10.1038/onc.2010.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Karantza-Wadsworth V, White E. A mouse mammary epithelial cell model to identify molecular mechanisms regulating breast cancer progression. Methods Enzymol. 2008;446:61–76. doi: 10.1016/S0076-6879(08)01604-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Debnath J, Mills KR, Collins NL, Reginato MJ, Muthuswamy SK, Brugge JS. The role of apoptosis in creating and maintaining luminal space within normal and oncogene-expressing mammary acini. Cell. 2002;111:29–40. doi: 10.1016/S0092-8674(02)01001-2. [DOI] [PubMed] [Google Scholar]
- 133.Karantza-Wadsworth V, Patel S, Kravchuk O, Chen G, Mathew R, Jin S, White E. Autophagy mitigates metabolic stress and genome damage in mammary tumorigenesis. Genes Dev. 2007;21:1621–35. doi: 10.1101/gad.1565707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tang Y, Marwaha S, Rutkowski JL, Tennekoon GI, Phillips PC, Field J. A role for Pak protein kinases in Schwann cell transformation. Proc Natl Acad Sci U S A. 1998;95:5139–44. doi: 10.1073/pnas.95.9.5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Xiao GH, Beeser A, Chernoff J, Testa JR. p21-activated kinase links Rac/Cdc42 signaling to merlin. J Biol Chem. 2002;277:883–6. doi: 10.1074/jbc.C100553200. [DOI] [PubMed] [Google Scholar]
- 136.Kissil JL, Johnson KC, Eckman MS, Jacks T. Merlin phosphorylation by p21-activated kinase 2 and effects of phosphorylation on merlin localization. J Biol Chem. 2002;277:10394–9. doi: 10.1074/jbc.M200083200. [DOI] [PubMed] [Google Scholar]
- 137.Coleman N, Kissil J. Recent advances in the development of p21-activated kinase inhibitors. Cell Logist. 2012;2:132–5. doi: 10.4161/cl.21667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Deacon SW, Beeser A, Fukui JA, Rennefahrt UEE, Myers C, Chernoff J, Peterson JR. An isoform-selective, small-molecule inhibitor targets the autoregulatory mechanism of p21-activated kinase. Chem Biol. 2008;15:322–31. doi: 10.1016/j.chembiol.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhao Z-S, Manser E, Chen XQ, Chong C, Leung T, Lim L. A conserved negative regulatory region in alphaPAK: inhibition of PAK kinases reveals their morphological roles downstream of Cdc42 and Rac1. Mol Cell Biol. 1998;18:2153–63. doi: 10.1128/mcb.18.4.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hashimoto H, Sudo T, Maruta H, Nishimura R. The direct PAK1 inhibitor, TAT-PAK18, blocks preferentially the growth of human ovarian cancer cell lines in which PAK1 is abnormally activated by autophosphorylation at Thr 423. Drug Discov Ther. 2010;4:1–4. [PubMed] [Google Scholar]
- 141.Maruta H, He H, Tikoo A, Nur-e-Kamal M. Cytoskeletal tumor suppressors that block oncogenic RAS signaling. Ann N Y Acad Sci. 1999;886:48–57. doi: 10.1111/j.1749-6632.1999.tb09399.x. [DOI] [PubMed] [Google Scholar]
- 142.Yi C, Wilker EW, Yaffe MB, Stemmer-Rachamimov A, Kissil JL. Validation of the p21-activated kinases as targets for inhibition in neurofibromatosis type 2. Cancer Res. 2008;68:7932–7. doi: 10.1158/0008-5472.CAN-08-0866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Maksimoska J, Feng L, Harms K, Yi C, Kissil J, Marmorstein R, Meggers E. Targeting large kinase active site with rigid, bulky octahedral ruthenium complexes. J Am Chem Soc. 2008;130:15764–5. doi: 10.1021/ja805555a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Feng L, Geisselbrecht Y, Blanck S, Wilbuer A, Atilla-Gokcumen GE, Filippakopoulos P, Kräling K, Celik MA, Harms K, Maksimoska J, et al. Structurally sophisticated octahedral metal complexes as highly selective protein kinase inhibitors. J Am Chem Soc. 2011;133:5976–86. doi: 10.1021/ja1112996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhao ZS, Manser E. Do PAKs make good drug targets? F1000 Biol Rep. 2010;2:70–3. doi: 10.3410/B2-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Murray BW, Guo C, Piraino J, Westwick JK, Zhang C, Lamerdin J, Dagostino E, Knighton D, Loi CM, Zager M, et al. Small-molecule p21-activated kinase inhibitor PF-3758309 is a potent inhibitor of oncogenic signaling and tumor growth. Proc Natl Acad Sci U S A. 2010;107:9446–51. doi: 10.1073/pnas.0911863107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rosen LS, Blumenkopf TA, Breazna A, Darang S, Gallo JD, Goldman J, Want D, Mileshkin L, Eckhardt SG. Phase 1, dose-escalateion, safety, pharmacokinetic and pharmacodynamic study of single agent PF-03758309, an oral PAK inhibitor, in patients with advanced solid tumors. AACR-NCI-EORTC International Conference: Molecular Targets and Cancer Therapeutics, 2011. 10(11): p. ABSTRACT A177. [Google Scholar]
- 148.Crawford JJ, Hoeflich KP, Rudolph J. p21-Activated kinase inhibitors: a patent review. Expert Opin Ther Pat. 2012;22:293–310. doi: 10.1517/13543776.2012.668758. [DOI] [PubMed] [Google Scholar]
- 149.Zhang J, Wang J, Guo Q, Wang Y, Zhou Y, Peng H, Cheng M, Zhao D, Li F. LCH-7749944, a novel and potent p21-activated kinase 4 inhibitor, suppresses proliferation and invasion in human gastric cancer cells. Cancer Lett. 2012;317:24–32. doi: 10.1016/j.canlet.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 150.Staben ST, Feng J, Lyle K, Belvin M, Boggs J, Burch JD, Chua C, Cui H, DiPasquale AG, Friedman LS, et al. Back pocket flexibility provides group II PAK selectivity for type 1-1/2 kinase inhibitors. J Med Chem. 2014 doi: 10.1021/jm401768t. In press. [DOI] [PubMed] [Google Scholar]