Abstract
Rho-GTPases belong to the Ras superfamily and are crucial signal transducing proteins downstream of many receptors. In general, the Rho-GTPases function as molecular switches, cycling between inactive (GDP-bound) and active (GTP-bound) states. The activated GTP bound Rho-GTPases interact with a broad spectrum of effectors to regulate a plethora of biological pathways including cytoskeletal dynamics, motility, cytokinesis, cell growth, apoptosis, transcriptional activity and nuclear signaling. Recently, gene targeting in mice allowed the selective inactivation of different Rho-GTPases and has advanced our understanding of the physiological role of these proteins, particularly in the immune system. Particularly, these proteins are key signaling molecules in T lymphocytes, which are generated in the thymus and are major players in the immune system. The scope of this review is to discuss recent data obtained in Rho-GTPases deficient mice by focusing on the role-played by Rho-GTPases in T-lymphocyte development, migration, activation and differentiation.
Keywords: Rho-GTPases, T-cell development, T-cell migration, T-cell differentiation
Introduction
The Ras superfamily of small GTP-binding proteins is divided into several subgroups, including the Rho-GTPase (guanosine triphosphatases) family of proteins, which play key roles in signal transduction and regulation of gene expression in almost all cell types, including immune cells. Most members of the mammalian Rho-GTPases cycle between an inactive GDP-bound state and an active GTP-bound state.1 This transition is regulated by guanine nucleotide exchange factors (GEFs) and GTPase activating proteins (GAPs). GEFs activate Rho-GTPases by promoting the exchange of GDP for GTP.2 GAPs inhibit Rho-GTPases by stimulating their intrinsic GTP hydrolysis activity.3 In addition, guanine nucleotide dissociation inhibitors (GDIs) can bind the GDP-bound Rho-GTPases in the cytoplasm, thereby modifying their normal intracellular localization.4 However, it should be noted that other members of the family (such as RhoH, RhoU, and RhoV) are predominantly GTP-bound and not regulated by GEFs and GAPs.5,6 To date, mammalian Rho-GTPases comprise a family of 23 Rho-GTPases, which are regulated by 79 GEFs, 65 GAPs and 3 GDIs.7-9 This large numbers of GEFs and GAPs relative to Rho-GTPases remain unexplained. Upon GTP binding, GTPases undergo conformational changes that give them the selective ability to bind effector proteins and to elicit specific biochemical functions. Rho-GTPases control cytoskeletal dynamics, as well as numerous signaling pathways, due to their capacity to bind and to activate a large number of downstream effector molecules, which include serine/threonine kinases, lipid kinases and adaptor proteins. Rho-GTPases have been implicated in the control of a wide range of biological processes such as proliferation, gene expression, migration, and apoptosis. Most of the current knowledge on Rho-GTPases cellular functions was obtained from studies using dominant negative or constitutively active mutants. However, these methods seem to have several limitations related to specificity, dosage and clonal variation.10 Recently, gene targeting of individual Rho-GTPases in mice has revealed that they actually regulate many basic functions of T lymphocytes such as activation, cell division, migration and adhesion.11-13 In this review, we will discuss the recent finding obtained in these animal models to highlight the role of Rho-GTPases in the thymic development of T cells, in T cell migration in lymphoid organs or inflammatory tissues and in T cell activation and differentiation.
Rho-GTPases and Thymic Development of T Cells
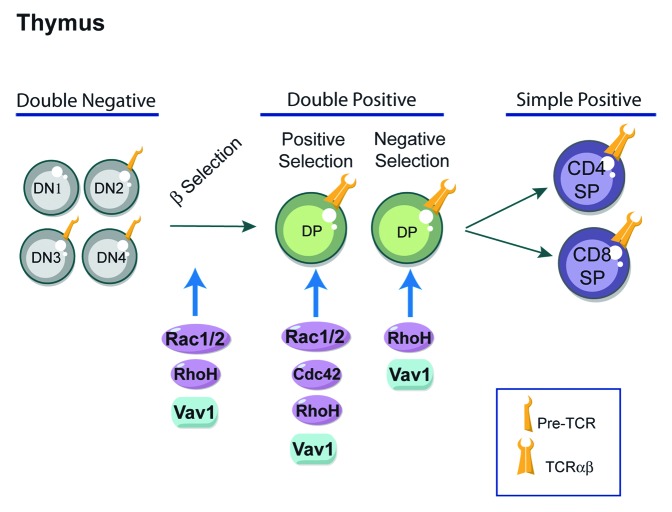
The development and maturation of T lymphocytes in the thymus is an essential process for the formation of the peripheral immune system.14 A key stage in T-cell development in the thymus is the selection of cells that have successfully rearranged their T-cell receptor (TCR) β locus (Fig. 1). This occurs in T-cell precursors, which do not express the CD4 and CD8 co-receptors and are thus called double negative thymocytes (DN).15 The different DN stages of thymic differentiation can be traced by the sequential pattern of expression of CD44 and CD25 molecules. CD44+CD25- (DN1) are the first progenitors, followed by CD44+CD25+ (DN2) stage during which TCRβ rearrangements begin. The selection of TCRβ occurs in CD44-CD25+ (DN3) cells. Cells that successfully rearrange their TCRβ locus express a functional receptor complex known as the pre-TCR, composed of a TCRβ chain, the p-Tα subunit and the signaling subunits of the CD3 antigen.15,16 When the pre-TCR is expressed at the cell surface, it promotes cell survival and entry into the cell cycle.17 At this stage, cells downregulate CD25 and transit to the DN4 pre-T-cell subset. DN4 T cells proliferate and differentiate into CD4+CD8+ double positive cells (DP), undergo TCRα rearrangements and express a mature αβ TCR complex. When a TCR has the correct ability to interact with a self-peptide loaded major histocompatibility complex (MHC) molecule, signaling from the TCR on DP cells results in positive or negative selection depending on its binding avidity to self peptide-MHC complexes.18-20 DP cells with a MHC class I-restricted TCR differentiate into CD4-CD8+ single positive (CD8+ SP) cells, whereas MHC class II-restricted cells are directed into CD4+CD8- single positive (CD4+ SP) cells. If T cells express no TCR or a TCR with negligible affinity for self-peptides, they are eliminated via a cell death pathway called death by neglect. Cell death is also induced when the TCR interacts too strongly with self peptide-MHC complexes. This process of activation-induced apoptosis is referred as “negative selection” and plays a key role in central tolerance, immune homeostasis and prevention of autoimmunity. The mature CD4+ or CD8+ T cells migrate to peripheral lymphoid organs (e.g., spleen and lymph nodes) to mediate immune responses. There has been considerable interest in the signal transduction molecules that mediate the transition of thymocytes beyond the pre-T cell stages of thymocyte differentiation. In this context, crucial responses are mediated by Rho-GTPases that influence different stages of thymic development21-24 (Fig. 1).
Figure 1. Rho-GTPases and thymic development of T cells. Differentiation of T cells occurs within the thymus. Most T cells develop along 3 steps: CD4- CD8- double negative (DN), CD4+ CD8+ double positive (DP) and mature CD4+ or CD8+ single positive (SP) stages. Key regulatory signals are mediated through both the pre-TCR and TCR complexes. Signals from the pre-TCR allow cells to differentiate into DP cells and proliferate (β-selection). DP T cells rearrange their TCR and undergo further maturation process that leads to positive selection or nedative selection depenting to TCR binding avidity. DP cells with a MHC class I-restricted TCR differentiate into CD8+ SP T cells, whereas MHC class II-restricted cells are directed into CD4+ SP T cells. The Rho-GTPases shown to influence thymic selection using Rho-GTPases-deficient mice are indicated in the figure and discussed in more detail in the text.
The implication of Rho-GTPases in thymocyte development was first suggested in mice lacking Vav1,25 a GEF that preferentially catalyzes the guanine nucleotide exchange on Rac1 but also on RhoA, RhoG and Cdc42 (Cell-division cycle 42) Rho-GTPases.26,27 Studies of Vav1-deficient mice have shown that the development of T cells is partially blocked at the pre-TCR checkpoint in the thymus and is strongly blocked in both positive and negative selection of T cells.28-31 Recently, the generation of mice that express a mutant Vav1 protein that lacks GEF activity but retains GEF-independent functions, clearly demonstrated that this effect on thymic selection is dependent on Vav1 GEF function.32 Rac-GTPases of the Rho family comprises Rac1, Rac2, Rac3 and RhoG.1,33-35 RhoG, Rac2 and Rac3 knockout mice do not show major developmental abnormalities, whereas conventional Rac1 deficiency causes early embryonic lethality.36 This experimental limitation could be circumvented by the use of conditional knockout mice that exhibit T-cell lineage-specific deletion of Rac1.37,38 The roles of Rac1 and Rac2 in T cell development have been investigated using mice deficient in either or both GTPases. Whereas T-cell development or positive/negative selection were not overtly perturbed in mice lacking either Rac1 or Rac2, the deletion of Rac1 in combination with Rac2 had a dramatic effect on thymocyte development.37,38 Mice lacking both GTPases had a marked block in the transition of thymocytes from the DN to DP compartments, resulting in very few DP, CD4SP, and CD8SP thymocytes thus reflecting a key role of Rac1 and Rac2 for efficient β-selection. Furthermore, Rac1 and Rac2 are required for positive selection of DP thymocytes.37,38 Thus, these studies revealed that Rac1 and Rac2 share redundant but essential roles in multiple stages of T cell development.37,38
Cdc42 loss-of-function causes death in utero.39 Conditional deletion of Cdc42 in the T-cell lineage blocks thymopoiesis at the DP stage, resulting in a significant increase of DP thymocytes and a reduction in mature CD4+ and CD8+ T cells.40,41 Indeed, positive selection of CD4+CD8+ DP thymocytes is defective in Cdc42−/− mice41 and CD4+ and CD8+ SP thymocytes from these mice show increased cell apoptosis in response to anti-CD3/CD28 stimulation, defective proliferation and aberrant TCR signaling.41 Thus, a combined effect of Cdc42 on positive selection, survival, proliferation and TCR signaling contributes to its regulation of thymic T-cell development. Besides, mice deficient in RhoC or RhoG did not show any alterations in thymic T-cell development,35,42 indicating that these Rho-GTPases are dispensable for T-lymphocyte development. Concerning RhoB deficient mice, no immune phenotype was described so far.43 The RhoA deficiency results in early death of mouse embryos and thus has been uninformative with regards its potential role in lymphocyte development and function.10 The generation of T-cell specific conditional RhoA knockout could be a great tool to analyze the importance of this Rho-GTPase in T-cell functions.
RhoH is highly expressed in mouse and human T cells.44,45 Although RhoH belongs to the Rho family of small GTPases, it lacks GTPase activity and functions instead as an adaptor for ZAP70, Lck, and Csk in T cells. Recent mouse genetic studies have shown that RhoH is crucial for thymocyte maturation at the pre-TCR stage and plays an important role in positive and negative thymic selection.46-48 Indeed, loss of RhoH leads to striking defects at 2 important T-cell development transition points, DN3 to DN4 and DP to SP, resulting in abnormally increased DN subpopulation and reduced SP subpopulations.46,47 In line with these defects, the proliferation of RhoH-null DN3 and DN4 cells is decreased, whereas apoptosis of DN4, DP, CD4SP, and CD8SP cells is significantly increased.46,47 It has been suggested that RhoH is important for pre-TCR and TCR signaling because it allows the efficient interaction of ZAP70 with the LAT signalosome, thus regulating thymocyte development. Besides, RhoH has also been shown to maintain the tyrosine kinase Lck in its inactive state and this might explain the impact of RhoH on the DN stage development, since Lck is one of the key regulators at this stage.49
Rho-GTPases and T-Cell Migration
Mature CD4 and CD8 T cells exit the thymus and circulate continuously through the bloodstream, secondary lymphoid tissues and lymphatic vessels. This trafficking is fundamental to increase the probability for T cells to encounter their specific antigen and is critical for effective immune surveillance.50 In peripheral lymphoid organs, the presentation of peptides by antigen presenting cells (APCs) to T cells induces their activation and differentiation into effector T cells. The fully activated T cells then returns into the bloodstream where they transmigrate into the target tissue and initiate pathogen clearance. The cytoarchitecture of T cells differs dramatically on whether the cell is circulating within the bloodstream, migrating through tissues or interacting with APCs.51 Blood T cells enter lymph nodes through a specialized vasculature called high endothelial venules (HEVs). In the bloodstream, the interaction of T lymphocytes with HEVs via L-selectin (CD62L) and the peripheral lymph node addressin (PNAd) on endothelial cells causes T cells to slow down and roll on the endothelium. Subsequently, binding of either the CCL19 or CCL21 chemokines present on endothelial cells to their receptor CCR7 expressed on T cells results in activation of leukocyte function–associated antigen 1 (LFA-1, αLβ2) and α4β1 (VLA-4) integrins. These, in turn, bind to their ligands (ICAM-1 and ICAM-2 for LFA-1) and (VCAM-1 for VLA-4) on endothelial cells, leading to the arrest of the lymphocyte on the endothelium and, ultimately, its transmigration across the endothelial wall into of the lymph node. Thus, T-cell migration across the endothelium is a complex process that follows a sequence of extensively regulated events, including rolling, integrin activation through chemokine signaling, firm adhesion and diapedesis.52 These events are controlled by the engagement of distinct classes of surface receptors, which include integrins and chemokine receptors. Rho-GTPases are important components of the signaling cascades mediated by these classes of receptors and regulate a variety of molecules involved in cytoskeletal rearrangements underlying leukocyte migration.33,53
In order to migrate, T cells undergo a dramatic re-organization of membrane domains and of the cytoskeleton, to acquire a polarized morphology with an actin-rich lamellipodium at the leading edge and a uropod at the trailing edge.54-56 Rho-GTPase signaling is compartmentalized in these distinct regions, with Rac1 and Cdc42 acting at the leading edge and RhoA proteins at the rear.57 Although lymphocyte polarity has not been analyzed in Rho-GTPases deficient mice, there is evidence that RhoA, Rac1 and Cdc42 influence T-cell polarity.12,54,58 In response to chemokine stimulation, Rho-GTPases are required to mediate lymphocyte adhesion. Indeed, CXCL12 activates Rac1 and promotes T-lymphocyte adhesion by converting VLA-4 integrins to a high avidity state, resulting in the prompt arrest of T cells on the endothelium, while inhibition of Rac1 impairs CXCL12-mediated adhesion.59 T cells from Rac2-deficient mice are partially defective in chemotaxis in response to CCL19, CCL21, and CXCL12 and in homing to lymph nodes.60 The partial nature of this phenotype suggests that there might be redundancy between Rac2 and other Rho-GTPases in transducing signals from chemokine receptors. Indeed, Rac1- and Rac2-deficient T cells are severely compromised in their ability to undergo either chemotaxis or chemokinesis in response to CCR7 or CXCR4 stimulation and home very inefficiently to lymph nodes and spleen.61 RhoA is required to transduce chemokine receptor signals to the activation of LFA-1 and therefore supports chemokine-regulated homing of circulating lymphocytes to secondary lymphoid organs.62 In contrast, RhoH is a negative regulator of chemokine induced LFA-1 activation.63,64
Rho-GTPases at the Immunological Synapse
In lymph nodes, only T lymphocytes that have encountered their specific antigens are retained, implying that T cell migration is selectively inhibited by interaction with antigen-bearing APC. TCR engagement causes cytoskeleton reorganization resulting in T-cell polarization toward APCs, enhanced T-cell/APC interactions, and formation of the immunological synapse (IS).65 Productive interactions between T and APC trigger full-blown T-cell activation, which, in conjunction with various cytokines, drives clonal expansion and differentiation into effector T cells. The variations in structure, duration and composition of the IS have a strong impact on the outcome of T-cell activation and the functional features of effector cells, leading to either activation or tolerance. The assembly of T-cell signaling molecules is intimately related to the reorganization of the actin/myosin and microtubule cytoskeleton and involves activation of Rho-GTPases. After the formation of the IS, the Rac1-dependent actin-rich lamellipodium observed in migrating cells is retained and forms a dynamic structure in close contact with the APC surface. In contrast, the uropod disappears likely resulting from an arrest in RhoA activity at the back whereas active RhoA is maintained at the IS.66 Active Cdc42 is also locally triggered at the IS66 and Cdc42-deficient T cells display an impaired TCR clustering and actin polymerization at the T-cell/APC interface.41,67 Rho-GTPases regulate the localization and activity of the ezrin/radixin/moesin (ERM) family of proteins. ERM proteins bind to a variety of membrane receptors and to phosphoinositides through their N-terminal FERM domain, and to actin filaments via their C-terminus, thereby acting as linkers between the actin cytoskeleton and membrane receptors. Inactivation of ERM proteins by dephosphorylation leads to a transient increase in cell deformability and enables closer contacts between the T cell and the APC.68 The activation of Rac leads to ERM inactivation68 while the activation of RhoA elicits an opposite effect. In addition, analysis of Rac1- and Rac2-deficient T cells show that these proteins play a redundant role in the regulation of ERM activity.61 The clustering of lipid rafts at the IS also depends on Rac.69 Thus, Rho-GTPases orchestrate biochemical or cytoskeletal pathways leading to T-cell polarization toward APCs and therefore play a key role in T-cell activation. Compromising these pathways results in perturbation of T-cell response to stimulation and impacts on effector T-cell differentiation.
Rho-GTPases and T Cell Receptor Signaling
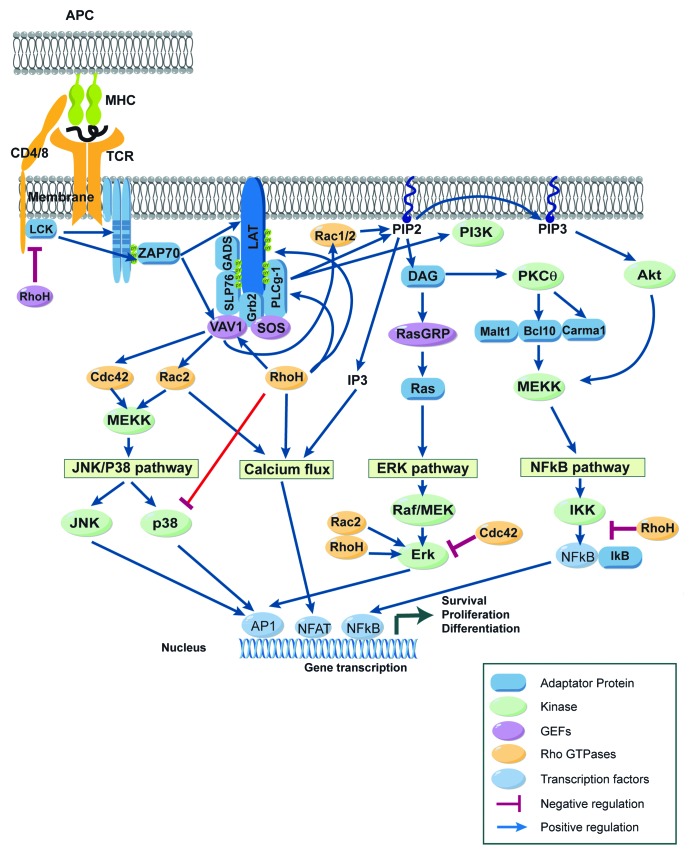
The TCR complex is composed of the variable α/β subunits that recognize peptide/MHC complexes and of the invariant signal transduction subunits of the CD3 antigen. When a T cell encounters its cognate antigen presented by APC, the TCR is engaged and triggers a complex cascade of biochemical events, leading to cytoskeletal reorganization and transcriptional activation of multiple genes, ultimately culminating by T-cell activation and proliferation (Fig. 2). Engagement of TCRs triggers the phosphorylation of the immunoreceptor tyrosine-based activation motifs (ITAMs) of four components of the TCR/CD3 complex, i.e., CD3γ, CD3δ, CD3ε, and TCR-ζ,70,71 mediated by the Src family tyrosine kinase Lck. When phosphorylated, ITAMs recruit the SH2 domain containing tyrosine kinase ZAP70. Once recruited, ZAP70 is activated by Lck and phosphorylates the transmembrane adaptor protein LAT on multiple tyrosine residues. The phosphorylation of LAT allows recruitment of a whole range of signaling molecules, including Grb2, GADS, PLCγ1, SLP76, Cbl, and Vav1. Then, distinct signaling pathways are initiated including (1) the Mitogen-Activated Protein Kinase (MAPK) pathway, including extracellular signal–regulated kinase (ERK), c-Jun NH2-terminal kinase (JNK) and p38, (2) the Ca2+/Calcineurin/NFAT pathway, and (3) the NF-κB pathway. Ultimately, these signaling pathways induce the nuclear translocation of effector molecules or transcription factors like AP-1, NFAT, and NF-κB, which control the gene expression program in T cells (Fig. 2).
Figure 2. Rho-GTPases regulate signaling pathways induced after TCR engagement. Schematic view of the signaling events induced in mature T cells after the recognition of an antigen presenting cell (APC) expressing peptide-MHC complexes. Binding of the T-cell receptor (TCR) to its ligand leads to a complex cascade of biochemical events that initiate distinct signaling pathways including (1) the Mitogen Activated Protein Kinase (MAPK) pathway including extracellular signal–regulated kinase (ERK), c-Jun NH2-terminal kinase (JNK) and p38, (2) the Ca/calcineurin/NFAT pathway, and (3) the NF-κB pathway. Eventually, these signaling pathways result in the activation of transcription factors like AP-1, NFAT, and NF-κB, which control the gene expression program characteristic of activated T cells. Rho-GTPases regulate positively (blue arrows) or negatively (red lines) these TCR signaling pathways. For more details, see text.
After TCR engagement, RhoA, Cdc42 and Rac1 are always localized at the IS, suggesting that they are key elements for TCR signaling.66 Indeed, Rac1 and Rac2 are activated upon TCR engagement72 and TCR-induced activation of Rac proteins, in turn, has been implicated in the regulation of MAPKs,73 PI3K,74 and calcium responses74 and thus in the control of the transcriptional activity of AP-1, NFAT, and NF-κB. Consistent with a key role for Rac proteins in TCR signaling, T cells from Rac2−/− mice exhibit reduced ERK1/2 and p38 activation and decreased calcium mobilization.75 However, TCR activation in Rac1 and Rac2 deficient T cells leads to normal levels of p38, Erk and ZAP70 activation, but reduced Akt activation.38 T cells from Cdc42 deficient mice exhibit enhanced TCR-induced proliferation associated with increased ERK1/2 MAP kinase suggesting that Cdc42 suppresses ERK.40 The enhanced activation of ERK in Cdc42−/− T cells is contradictory with observations made in other cell types, suggesting that Cdc42 is a positive regulator of ERK activity.76 RhoH acts as a regulator for ZAP70, Lck, and Csk in T cells.46,49 RhoH is crucial for the tyrosine phosphorylation of LAT, PLCγ1, and Vav1 and for the activation of Erk and calcium influx signaling.46,47 RhoH also binds and stabilizes Lck in its inactivated state through the recruitment of Csk.49 RhoH has also been proposed to function as a negative regulator of other Rho-GTPases, particularly Rac proteins45,77 (Fig. 2).
Rho-GTPases and T Cell Differentiation
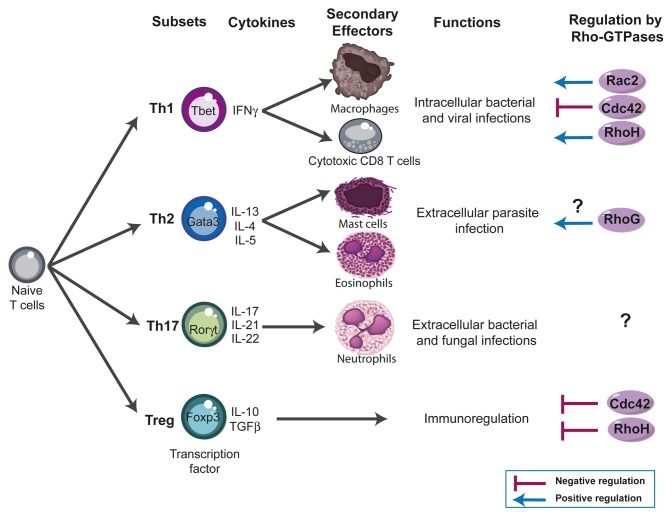
After productive TCR engagement, effector CD4 (Th) and CD8 (Tc) T cells differentiate into functionally different subsets based on their specific patterns of cytokine secretion.78-82 Type 1 T cells (Th1 and Tc1) secrete primarily interleukin-2 (IL-2) and interferon gamma (IFN-γ), express the transcription factor T-bet and protect against intracellular pathogens (Fig. 3). In contrast, type 2 T cells (Th2 and Tc2) secrete IL-4, IL-5, and IL-13, express the transcription factor GATA3 and protect against extracellular parasites. Type 17 T cells (Th17 and Tc17) are characterized by their secretion of IL-17, IL-21, and IL-22, express the retinoic acid receptor-related orphan nuclear receptor (ROR)-γ transcription factor and may play an important role in host defense against extracellular bacterial and fungal infections. While these T-cell subsets have specific effector functions in clearing infections, their dysregulation causes immunopathology. For instance, allergic diseases such as asthma are thought to arise through excessive type 2 responses, whereas many autoimmune diseases involve excessive type 1 and type 17 responses. Regulatory T cells (Treg) are characterized by the expression of the transcription factor Foxp3,83,84 and are key players in the prevention of autoimmune responses and the termination of immune responses85,86 (Fig. 3). Although the current knowledge on the role of Rho-GTPases in T-cell differentiation is at its early stage, in particular for CD8 T cells, there is evidence that these signaling molecules could play an important role. For example, Vav1 deficiency is associated with impaired IL-4 production and enhanced Th1-cell development.87 Rac2 has been shown to activate Th1-specific signaling, is preferentially expressed in the Th1 subset and is required for the production and release of IFN-γ.88 However, another study showed only a minor skewing of the T-cell response toward the Th1 phenotype in Rac2-deficient mice challenged with Leishmania major.60 In contrast, Cdc42-deficient mice show an enhanced differentiation to Th1 and CD8 effector cells (but not Th2) and exhibit exacerbated liver damage in an induced model of autoimmune disease.41 Cdc42 has also been shown to play a role in IFN-γ exocytosis at the IS.89 Thus, while essential for TCR clustering and actin polarization in the course of mature IS formation, Cdc42 plays restrictive role in T-cell differentiation and autoimmunity.41 RhoG deficiency has no impact on Th1/Th2 differentiation, except for a slight decrease in IL-5 production from Th2-differentiated RhoG-deficient cells.35 Concerning RhoH, it is more expressed in Th1 cells compared with the Th2 subset, suggesting a role for RhoH in the functional differentiation of T cells.45 Regarding Treg, we have shown recently that a constitutively active form of Vav1 favors thymic development of Foxp3 regulatory T cells.90 Moreover, Cdc42−/− mice show an increased frequency of Foxp3+ regulatory T cells.41 Similarly, RhoH deficient mice also exhibit increased frequency of Treg cells.48 However, the absolute number of Treg is reduced, indicating that RhoH deficiency impacts differently on Treg and conventional T-cell development.
Figure 3. Rho-GTPases influence - lymphocyte differentiation. Four major subsets of CD4 T cells have been defined; T helper (Th) 1, Th2, Th17, and regulatory T cells (Treg). These subsets exert their immune functions through secretion of distinct patterns of cytokines and activation of different effector cells: Th1 mediate clearance of intracellular pathogens by producing IFN-γ and by activating macrophages and cytotoxic effector CD8 T cells. Th2 cells are involved in the elimination of parasitic organisms by secreting IL-4, IL-5, and IL-13 and by activating basophils and eosinophils. Th17 cells are involved in the clearance of intracellular pathogens by producing IL-17 and activating neutrophils. Similar subsets have been also described within CD8 T cells compartment (not shown). Foxp3 Treg play a crucial role in downregulating immune responses by suppressing several actors of the immune responses. The Rho-GTPases shown to influence - cell differentiation using deficient mice are indicated in the figure and discussed in more detail in the text.
Conclusions and Future Directions
It is clear that the analysis of the in vivo function of Rho-GTPases family has only just begun and much more remains to be discovered concerning their implication in immune system homeostasis. So far, knockouts of only 9 of the 23 Rho-GTPase family members have been described. These knockout mice revealed novel information about the important role of Rho-GTPases in T-cell signaling through antigen receptors, chemokine receptors and integrins. Those Rho-GTPases are involved in key processes for the physiology of T lymphocytes, such as development, activation, differentiation and migration. The level of regulation applied on Rho-GTPases is outstanding by its complexity. Challenges ahead will be to investigate more deeply the degree of regulation and crosstalk among different Rho-GTPases, in order to better understand the contribution of each member of the family to a given specific signal delivered after receptors engagement. In this regard, crossbreeding of various Rho-GTPases and Rho-GTPase regulators knockout mice will probably provide further insight into their molecular mechanisms. Given the new knowledge provided by detailed analysis of the Rho-GTPase knockouts mice so far, we can expect a plethora of information on Rho-GTPases from these mouse models in a near future.
Although the impact of Rho-GTPases deficiency on thymic selection is well documented, their role in peripheral T cells in the context of normal or pathological immune responses has not been clarified yet. This is mainly related to the lymphopenia induced by the defect in thymic selection that has been observed in the majority of Rho-GTPases deficient mice. This lymphopenia results in the proliferation and differentiation of naïve T cells into memory-like cells, in the absence of overt antigenic stimulation. This may influence the orientation of immune responses by affecting the differentiation of T-cell subsets.91,92 Therefore, the generation of conditional knockout mouse lines, in which Rho-GTPase is expressed in the thymus (before positive selection) and deleted exclusively in mature peripheral T cells, would be of great interest to analyze the role of these signaling molecules in peripheral T-cell activation and differentiation. This will help to define the role and potential therapeutic value of specific Rho-GTPase-mediated signaling pathways in human diseases such as cancer, autoimmunity and allergy.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We would like to thank Daniel Dunia, Loic Dupré, and Christophe Pedros for their critical comments on the manuscript. We thank Emmanuel Dejean for graphic design (emmanueldejean@gmail.com). The authors are grateful for funding support from Association Française contre les Myopathies, Agence Nationale de la Recherche (ANR-08-GENO-041-01), Association de Recherche sur la Sclérose En Plaques, Fight-MG (FP7-Health-2009-242210) and région Midi Pyrénées.
References
- 1.Van Aelst L, D’Souza-Schorey C. Rho GTPases and signaling networks. Genes Dev. 1997;11:2295–322. doi: 10.1101/gad.11.18.2295. [DOI] [PubMed] [Google Scholar]
- 2.Zheng Y. Dbl family guanine nucleotide exchange factors. Trends Biochem Sci. 2001;26:724–32. doi: 10.1016/S0968-0004(01)01973-9. [DOI] [PubMed] [Google Scholar]
- 3.Moon SY, Zheng Y. Rho GTPase-activating proteins in cell regulation. Trends Cell Biol. 2003;13:13–22. doi: 10.1016/S0962-8924(02)00004-1. [DOI] [PubMed] [Google Scholar]
- 4.Olofsson B. Rho guanine dissociation inhibitors: pivotal molecules in cellular signalling. Cell Signal. 1999;11:545–54. doi: 10.1016/S0898-6568(98)00063-1. [DOI] [PubMed] [Google Scholar]
- 5.Wennerberg K, Der CJ. Rho-family GTPases: it’s not only Rac and Rho (and I like it) J Cell Sci. 2004;117:1301–12. doi: 10.1242/jcs.01118. [DOI] [PubMed] [Google Scholar]
- 6.Aspenström P, Ruusala A, Pacholsky D. Taking Rho GTPases to the next level: the cellular functions of atypical Rho GTPases. Exp Cell Res. 2007;313:3673–9. doi: 10.1016/j.yexcr.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 7.Rossman KL, Der CJ, Sondek J. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol. 2005;6:167–80. doi: 10.1038/nrm1587. [DOI] [PubMed] [Google Scholar]
- 8.Tcherkezian J, Lamarche-Vane N. Current knowledge of the large RhoGAP family of proteins. Biol Cell. 2007;99:67–86. doi: 10.1042/BC20060086. [DOI] [PubMed] [Google Scholar]
- 9.DerMardirossian C, Bokoch GM. GDIs: central regulatory molecules in Rho GTPase activation. Trends Cell Biol. 2005;15:356–63. doi: 10.1016/j.tcb.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Wang L, Zheng Y. Cell type-specific functions of Rho GTPases revealed by gene targeting in mice. Trends Cell Biol. 2007;17:58–64. doi: 10.1016/j.tcb.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 11.Heasman SJ, Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol. 2008;9:690–701. doi: 10.1038/nrm2476. [DOI] [PubMed] [Google Scholar]
- 12.Tybulewicz VL, Henderson RB. Rho family GTPases and their regulators in lymphocytes. Nat Rev Immunol. 2009;9:630–44. doi: 10.1038/nri2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rougerie P, Delon J. Rho GTPases: masters of T lymphocyte migration and activation. Immunol Lett. 2012;142:1–13. doi: 10.1016/j.imlet.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Ciofani M, Zúñiga-Pflücker JC. The thymus as an inductive site for T lymphopoiesis. Annu Rev Cell Dev Biol. 2007;23:463–93. doi: 10.1146/annurev.cellbio.23.090506.123547. [DOI] [PubMed] [Google Scholar]
- 15.von Boehmer H, Aifantis I, Feinberg J, Lechner O, Saint-Ruf C, Walter U, Buer J, Azogui O. Pleiotropic changes controlled by the pre-T-cell receptor. Curr Opin Immunol. 1999;11:135–42. doi: 10.1016/S0952-7915(99)80024-7. [DOI] [PubMed] [Google Scholar]
- 16.Fehling HJ, von Boehmer H. Early alpha beta T cell development in the thymus of normal and genetically altered mice. Curr Opin Immunol. 1997;9:263–75. doi: 10.1016/S0952-7915(97)80146-X. [DOI] [PubMed] [Google Scholar]
- 17.Aifantis I, Mandal M, Sawai K, Ferrando A, Vilimas T. Regulation of T-cell progenitor survival and cell-cycle entry by the pre-T-cell receptor. Immunol Rev. 2006;209:159–69. doi: 10.1111/j.0105-2896.2006.00343.x. [DOI] [PubMed] [Google Scholar]
- 18.Jameson SC, Bevan MJ. T-cell selection. Curr Opin Immunol. 1998;10:214–9. doi: 10.1016/S0952-7915(98)80251-3. [DOI] [PubMed] [Google Scholar]
- 19.Sebzda E, Mariathasan S, Ohteki T, Jones R, Bachmann MF, Ohashi PS. Selection of the T cell repertoire. Annu Rev Immunol. 1999;17:829–74. doi: 10.1146/annurev.immunol.17.1.829. [DOI] [PubMed] [Google Scholar]
- 20.Germain RN. T-cell development and the CD4-CD8 lineage decision. Nat Rev Immunol. 2002;2:309–22. doi: 10.1038/nri798. [DOI] [PubMed] [Google Scholar]
- 21.Na S, Li B, Grewal IS, Enslen H, Davis RJ, Hanke JH, Flavell RA. Expression of activated CDC42 induces T cell apoptosis in thymus and peripheral lymph organs via different pathways. Oncogene. 1999;18:7966–74. doi: 10.1038/sj.onc.1203122. [DOI] [PubMed] [Google Scholar]
- 22.Galandrini R, Henning SW, Cantrell DA. Different functions of the GTPase Rho in prothymocytes and late pre-T cells. Immunity. 1997;7:163–74. doi: 10.1016/S1074-7613(00)80519-1. [DOI] [PubMed] [Google Scholar]
- 23.Henning SW, Galandrini R, Hall A, Cantrell DA. The GTPase Rho has a critical regulatory role in thymus development. EMBO J. 1997;16:2397–407. doi: 10.1093/emboj/16.9.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gomez M, Tybulewicz V, Cantrell DA. Control of pre-T cell proliferation and differentiation by the GTPase Rac-I. Nat Immunol. 2000;1:348–52. doi: 10.1038/79808. [DOI] [PubMed] [Google Scholar]
- 25.Crespo P, Schuebel KE, Ostrom AA, Gutkind JS, Bustelo XR. Phosphotyrosine-dependent activation of Rac-1 GDP/GTP exchange by the vav proto-oncogene product. Nature. 1997;385:169–72. doi: 10.1038/385169a0. [DOI] [PubMed] [Google Scholar]
- 26.Bustelo XR. Regulatory and signaling properties of the Vav family. Mol Cell Biol. 2000;20:1461–77. doi: 10.1128/MCB.20.5.1461-1477.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rapley J, Tybulewicz VL, Rittinger K. Crucial structural role for the PH and C1 domains of the Vav1 exchange factor. EMBO Rep. 2008;9:655–61. doi: 10.1038/embor.2008.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fischer KD, Zmuldzinas A, Gardner S, Barbacid M, Bernstein A, Guidos C. Defective T-cell receptor signalling and positive selection of Vav-deficient CD4+ CD8+ thymocytes. Nature. 1995;374:474–7. doi: 10.1038/374474a0. [DOI] [PubMed] [Google Scholar]
- 29.Tarakhovsky A, Turner M, Schaal S, Mee PJ, Duddy LP, Rajewsky K, Tybulewicz VL. Defective antigen receptor-mediated proliferation of B and T cells in the absence of Vav. Nature. 1995;374:467–70. doi: 10.1038/374467a0. [DOI] [PubMed] [Google Scholar]
- 30.Turner M, Mee PJ, Walters AE, Quinn ME, Mellor AL, Zamoyska R, Tybulewicz VL. A requirement for the Rho-family GTP exchange factor Vav in positive and negative selection of thymocytes. Immunity. 1997;7:451–60. doi: 10.1016/S1074-7613(00)80367-2. [DOI] [PubMed] [Google Scholar]
- 31.Zhang R, Alt FW, Davidson L, Orkin SH, Swat W. Defective signalling through the T- and B-cell antigen receptors in lymphoid cells lacking the vav proto-oncogene. Nature. 1995;374:470–3. doi: 10.1038/374470a0. [DOI] [PubMed] [Google Scholar]
- 32.Saveliev A, Vanes L, Ksionda O, Rapley J, Smerdon SJ, Rittinger K, Tybulewicz VL. Function of the nucleotide exchange activity of vav1 in T cell development and activation. Sci Signal. 2009;2:ra83. doi: 10.1126/scisignal.2000420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–35. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 34.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–14. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 35.Vigorito E, Bell S, Hebeis BJ, Reynolds H, McAdam S, Emson PC, McKenzie A, Turner M. Immunological function in mice lacking the Rac-related GTPase RhoG. Mol Cell Biol. 2004;24:719–29. doi: 10.1128/MCB.24.2.719-729.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sugihara K, Nakatsuji N, Nakamura K, Nakao K, Hashimoto R, Otani H, Sakagami H, Kondo H, Nozawa S, Aiba A, et al. Rac1 is required for the formation of three germ layers during gastrulation. Oncogene. 1998;17:3427–33. doi: 10.1038/sj.onc.1202595. [DOI] [PubMed] [Google Scholar]
- 37.Dumont C, Corsoni-Tadrzak A, Ruf S, de Boer J, Williams A, Turner M, Kioussis D, Tybulewicz VL. Rac GTPases play critical roles in early T-cell development. Blood. 2009;113:3990–8. doi: 10.1182/blood-2008-09-181180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guo F, Cancelas JA, Hildeman D, Williams DA, Zheng Y. Rac GTPase isoforms Rac1 and Rac2 play a redundant and crucial role in T-cell development. Blood. 2008;112:1767–75. doi: 10.1182/blood-2008-01-132068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen F, Ma L, Parrini MC, Mao X, Lopez M, Wu C, Marks PW, Davidson L, Kwiatkowski DJ, Kirchhausen T, et al. Cdc42 is required for PIP(2)-induced actin polymerization and early development but not for cell viability. Curr Biol. 2000;10:758–65. doi: 10.1016/S0960-9822(00)00571-6. [DOI] [PubMed] [Google Scholar]
- 40.Guo F, Hildeman D, Tripathi P, Velu CS, Grimes HL, Zheng Y. Coordination of IL-7 receptor and T-cell receptor signaling by cell-division cycle 42 in T-cell homeostasis. Proc Natl Acad Sci U S A. 2010;107:18505–10. doi: 10.1073/pnas.1010249107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guo F, Zhang S, Tripathi P, Mattner J, Phelan J, Sproles A, Mo J, Wills-Karp M, Grimes HL, Hildeman D, et al. Distinct roles of Cdc42 in thymopoiesis and effector and memory T cell differentiation. PLoS One. 2011;6:e18002. doi: 10.1371/journal.pone.0018002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hakem A, Sanchez-Sweatman O, You-Ten A, Duncan G, Wakeham A, Khokha R, Mak TW. RhoC is dispensable for embryogenesis and tumor initiation but essential for metastasis. Genes Dev. 2005;19:1974–9. doi: 10.1101/gad.1310805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu AX, Rane N, Liu JP, Prendergast GC. RhoB is dispensable for mouse development, but it modifies susceptibility to tumor formation as well as cell adhesion and growth factor signaling in transformed cells. Mol Cell Biol. 2001;21:6906–12. doi: 10.1128/MCB.21.20.6906-6912.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gu Y, Jasti AC, Jansen M, Siefring JE. RhoH, a hematopoietic-specific Rho GTPase, regulates proliferation, survival, migration, and engraftment of hematopoietic progenitor cells. Blood. 2005;105:1467–75. doi: 10.1182/blood-2004-04-1604. [DOI] [PubMed] [Google Scholar]
- 45.Li X, Bu X, Lu B, Avraham H, Flavell RA, Lim B. The hematopoiesis-specific GTP-binding protein RhoH is GTPase deficient and modulates activities of other Rho GTPases by an inhibitory function. Mol Cell Biol. 2002;22:1158–71. doi: 10.1128/MCB.22.4.1158-1171.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dorn T, Kuhn U, Bungartz G, Stiller S, Bauer M, Ellwart J, Peters T, Scharffetter-Kochanek K, Semmrich M, Laschinger M, et al. RhoH is important for positive thymocyte selection and T-cell receptor signaling. Blood. 2007;109:2346–55. doi: 10.1182/blood-2006-04-019034. [DOI] [PubMed] [Google Scholar]
- 47.Gu Y, Chae HD, Siefring JE, Jasti AC, Hildeman DA, Williams DA, Rho H. RhoH GTPase recruits and activates Zap70 required for T cell receptor signaling and thymocyte development. Nat Immunol. 2006;7:1182–90. doi: 10.1038/ni1396. [DOI] [PubMed] [Google Scholar]
- 48.Oda H, Tamehiro N, Patrick MS, Hayakawa K, Suzuki H. Differential requirement for RhoH in development of TCRαβ CD8αα IELs and other types of T cells. Immunol Lett. 2013;151:1–9. doi: 10.1016/j.imlet.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 49.Wang H, Zeng X, Fan Z, Lim B. RhoH modulates pre-TCR and TCR signalling by regulating LCK. Cell Signal. 2011;23:249–58. doi: 10.1016/j.cellsig.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 50.Bromley SK, Mempel TR, Luster AD. Orchestrating the orchestrators: chemokines in control of T cell traffic. Nat Immunol. 2008;9:970–80. doi: 10.1038/ni.f.213. [DOI] [PubMed] [Google Scholar]
- 51.Lafouresse F, Vasconcelos Z, Cotta-de-Almeida V, Dupré L. Actin cytoskeleton control of the comings and goings of T lymphocytes. Tissue Antigens. 2013;82:301–11. doi: 10.1111/tan.12193. [DOI] [PubMed] [Google Scholar]
- 52.Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–6. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 53.Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–69. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- 54.Sánchez-Madrid F, del Pozo MA. Leukocyte polarization in cell migration and immune interactions. EMBO J. 1999;18:501–11. doi: 10.1093/emboj/18.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ratner S, Sherrod WS, Lichlyter D. Microtubule retraction into the uropod and its role in T cell polarization and motility. J Immunol. 1997;159:1063–7. [PubMed] [Google Scholar]
- 56.Dustin ML. Visualization of cell-cell interaction contacts-synapses and kinapses. Adv Exp Med Biol. 2008;640:164–82. doi: 10.1007/978-0-387-09789-3_13. [DOI] [PubMed] [Google Scholar]
- 57.Krummel MF, Macara I. Maintenance and modulation of T cell polarity. Nat Immunol. 2006;7:1143–9. doi: 10.1038/ni1404. [DOI] [PubMed] [Google Scholar]
- 58.Stowers L, Yelon D, Berg LJ, Chant J. Regulation of the polarization of T cells toward antigen-presenting cells by Ras-related GTPase CDC42. Proc Natl Acad Sci U S A. 1995;92:5027–31. doi: 10.1073/pnas.92.11.5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.García-Bernal D, Wright N, Sotillo-Mallo E, Nombela-Arrieta C, Stein JV, Bustelo XR, Teixidó J. Vav1 and Rac control chemokine-promoted T lymphocyte adhesion mediated by the integrin alpha4beta1. Mol Biol Cell. 2005;16:3223–35. doi: 10.1091/mbc.E04-12-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Croker BA, Handman E, Hayball JD, Baldwin TM, Voigt V, Cluse LA, Yang FC, Williams DA, Roberts AW. Rac2-deficient mice display perturbed T-cell distribution and chemotaxis, but only minor abnormalities in T(H)1 responses. Immunol Cell Biol. 2002;80:231–40. doi: 10.1046/j.1440-1711.2002.01077.x. [DOI] [PubMed] [Google Scholar]
- 61.Faroudi M, Hons M, Zachacz A, Dumont C, Lyck R, Stein JV, Tybulewicz VL. Critical roles for Rac GTPases in T-cell migration to and within lymph nodes. Blood. 2010;116:5536–47. doi: 10.1182/blood-2010-08-299438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Giagulli C, Scarpini E, Ottoboni L, Narumiya S, Butcher EC, Constantin G, Laudanna C. RhoA and zeta PKC control distinct modalities of LFA-1 activation by chemokines: critical role of LFA-1 affinity triggering in lymphocyte in vivo homing. Immunity. 2004;20:25–35. doi: 10.1016/S1074-7613(03)00350-9. [DOI] [PubMed] [Google Scholar]
- 63.Cherry LK, Li X, Schwab P, Lim B, Klickstein LB. RhoH is required to maintain the integrin LFA-1 in a nonadhesive state on lymphocytes. Nat Immunol. 2004;5:961–7. doi: 10.1038/ni1103. [DOI] [PubMed] [Google Scholar]
- 64.Baker CM, Comrie WA, Hyun YM, Chung HL, Fedorchuk CA, Lim K, Brakebusch C, McGrath JL, Waugh RE, Meier-Schellersheim M, et al. Opposing roles for RhoH GTPase during T-cell migration and activation. Proc Natl Acad Sci U S A. 2012;109:10474–9. doi: 10.1073/pnas.1114214109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221–7. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- 66.Singleton KL, Roybal KT, Sun Y, Fu G, Gascoigne NR, van Oers NS, Wülfing C. Spatiotemporal patterning during T cell activation is highly diverse. Sci Signal. 2009;2:ra15. doi: 10.1126/scisignal.2000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tskvitaria-Fuller I, Seth A, Mistry N, Gu H, Rosen MK, Wülfing C. Specific patterns of Cdc42 activity are related to distinct elements of T cell polarization. J Immunol. 2006;177:1708–20. doi: 10.4049/jimmunol.177.3.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Faure S, Salazar-Fontana LI, Semichon M, Tybulewicz VL, Bismuth G, Trautmann A, Germain RN, Delon J. ERM proteins regulate cytoskeleton relaxation promoting T cell-APC conjugation. Nat Immunol. 2004;5:272–9. doi: 10.1038/ni1039. [DOI] [PubMed] [Google Scholar]
- 69.Villalba M, Bi K, Rodriguez F, Tanaka Y, Schoenberger S, Altman A. Vav1/Rac-dependent actin cytoskeleton reorganization is required for lipid raft clustering in T cells. J Cell Biol. 2001;155:331–8. doi: 10.1083/jcb.200107080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Iwashima M. Kinetic perspectives of T cell antigen receptor signaling. A two-tier model for T cell full activation. Immunol Rev. 2003;191:196–210. doi: 10.1034/j.1600-065X.2003.00024.x. [DOI] [PubMed] [Google Scholar]
- 71.Guy CS, Vignali DA. Organization of proximal signal initiation at the TCR:CD3 complex. Immunol Rev. 2009;232:7–21. doi: 10.1111/j.1600-065X.2009.00843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sanui T, Inayoshi A, Noda M, Iwata E, Oike M, Sasazuki T, Fukui Y. DOCK2 is essential for antigen-induced translocation of TCR and lipid rafts, but not PKC-theta and LFA-1, in T cells. Immunity. 2003;19:119–29. doi: 10.1016/S1074-7613(03)00169-9. [DOI] [PubMed] [Google Scholar]
- 73.Jacinto E, Werlen G, Karin M. Cooperation between Syk and Rac1 leads to synergistic JNK activation in T lymphocytes. Immunity. 1998;8:31–41. doi: 10.1016/S1074-7613(00)80456-2. [DOI] [PubMed] [Google Scholar]
- 74.Arrieumerlou C, Randriamampita C, Bismuth G, Trautmann A. Rac is involved in early TCR signaling. J Immunol. 2000;165:3182–9. doi: 10.4049/jimmunol.165.6.3182. [DOI] [PubMed] [Google Scholar]
- 75.Yu H, Leitenberg D, Li B, Flavell RA. Deficiency of small GTPase Rac2 affects T cell activation. J Exp Med. 2001;194:915–26. doi: 10.1084/jem.194.7.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chuang TH, Hahn KM, Lee JD, Danley DE, Bokoch GM. The small GTPase Cdc42 initiates an apoptotic signaling pathway in Jurkat T lymphocytes. Mol Biol Cell. 1997;8:1687–98. doi: 10.1091/mbc.8.9.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chae HD, Lee KE, Williams DA, Gu Y. Cross-talk between RhoH and Rac1 in regulation of actin cytoskeleton and chemotaxis of hematopoietic progenitor cells. Blood. 2008;111:2597–605. doi: 10.1182/blood-2007-06-093237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Constant S, Pfeiffer C, Woodard A, Pasqualini T, Bottomly K. Extent of T cell receptor ligation can determine the functional differentiation of naive CD4+ T cells. J Exp Med. 1995;182:1591–6. doi: 10.1084/jem.182.5.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Constant SL, Bottomly K. Induction of Th1 and Th2 CD4+ T cell responses: the alternative approaches. Annu Rev Immunol. 1997;15:297–322. doi: 10.1146/annurev.immunol.15.1.297. [DOI] [PubMed] [Google Scholar]
- 80.Nakayama T, Yamashita M. The TCR-mediated signaling pathways that control the direction of helper T cell differentiation. Semin Immunol. 2010;22:303–9. doi: 10.1016/j.smim.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 81.Sad S, Marcotte R, Mosmann TR. Cytokine-induced differentiation of precursor mouse CD8+ T cells into cytotoxic CD8+ T cells secreting Th1 or Th2 cytokines. Immunity. 1995;2:271–9. doi: 10.1016/1074-7613(95)90051-9. [DOI] [PubMed] [Google Scholar]
- 82.Saxena A, Martin-Blondel G, Mars LT, Liblau RS. Role of CD8 T cell subsets in the pathogenesis of multiple sclerosis. FEBS Lett. 2011;585:3758–63. doi: 10.1016/j.febslet.2011.08.047. [DOI] [PubMed] [Google Scholar]
- 83.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 84.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–41. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 85.Wing K, Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat Immunol. 2010;11:7–13. doi: 10.1038/ni.1818. [DOI] [PubMed] [Google Scholar]
- 86.Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10:490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- 87.Tanaka Y, So T, Lebedeva S, Croft M, Altman A. Impaired IL-4 and c-Maf expression and enhanced Th1-cell development in Vav1-deficient mice. Blood. 2005;106:1286–95. doi: 10.1182/blood-2004-10-4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li B, Yu H, Zheng W, Voll R, Na S, Roberts AW, Williams DA, Davis RJ, Ghosh S, Flavell RA. Role of the guanosine triphosphatase Rac2 in T helper 1 cell differentiation. Science. 2000;288:2219–22. doi: 10.1126/science.288.5474.2219. [DOI] [PubMed] [Google Scholar]
- 89.Chemin K, Bohineust A, Dogniaux S, Tourret M, Guégan S, Miro F, Hivroz C. Cytokine secretion by CD4+ T cells at the immunological synapse requires Cdc42-dependent local actin remodeling but not microtubule organizing center polarity. J Immunol. 2012;189:2159–68. doi: 10.4049/jimmunol.1200156. [DOI] [PubMed] [Google Scholar]
- 90.Colacios C, Casemayou A, Dejean AS, Gaits-Iacovoni F, Pedros C, Bernard I, Lagrange D, Deckert M, Lamouroux L, Jagodic M, et al. The p.Arg63Trp polymorphism controls Vav1 functions and Foxp3 regulatory T cell development. J Exp Med. 2011;208:2183–91. doi: 10.1084/jem.20102191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.King C, Ilic A, Koelsch K, Sarvetnick N. Homeostatic expansion of T cells during immune insufficiency generates autoimmunity. Cell. 2004;117:265–77. doi: 10.1016/S0092-8674(04)00335-6. [DOI] [PubMed] [Google Scholar]
- 92.Le Campion A, Gagnerault MC, Auffray C, Bécourt C, Poitrasson-Rivière M, Lallemand E, Bienvenu B, Martin B, Lepault F, Lucas B. Lymphopenia-induced spontaneous T-cell proliferation as a cofactor for autoimmune disease development. Blood. 2009;114:1784–93. doi: 10.1182/blood-2008-12-192120. [DOI] [PubMed] [Google Scholar]