Abstract
Rho GTPases regulate a diverse range of cellular functions primarily through their ability to modulate microtubule dynamics and the actin-myosin cytoskeleton. Both of these cytoskeletal structures are crucial for a mitotic cell division. Specifically, their assembly and disassembly is tightly regulated in a temporal manner to ensure that each mitotic stage occurs in the correct sequential order and not prematurely until the previous stage is completed. Thus, it is not surprising that the Rho GTPases, RhoA, and Cdc42, have reported roles in several stages of mitosis: cell cortex stiffening during cell rounding, mitotic spindle formation, and bi-orient attachment of the spindle microtubules to the kinetochore and during cytokinesis play multiple roles in establishing the division plane, assembly, and activation of the contractile ring, membrane ingression, and abscission. Here, I review the molecular mechanisms regulating the spatial and temporal activation of RhoA and Cdc42 during mitosis, and how this is critical for mitotic progression and completion.
Keywords: RhoA, Cdc42, metaphase, cytokinesis, spindle assembly, actin, cytoskeleton, microtubules, cleavage furrow, abscission
Mitosis results in the generation of two independent daughter cells. It is the final phase of the cell cycle and consists of six distinct sequential stages—prophase, prometaphase, metaphase, anaphase, telophase, and cytokinesis (Fig. 1). Mitotic progression is highly regulated and involves dynamic modulation of cell shape and morphology primarily through remodelling of the actin and microtubule cytoskeletons. Rho GTPases are key regulators of both processes in a diverse spectrum of cellular functions due to their ability to elicit a cellular response through activation of a large suite of effector proteins.
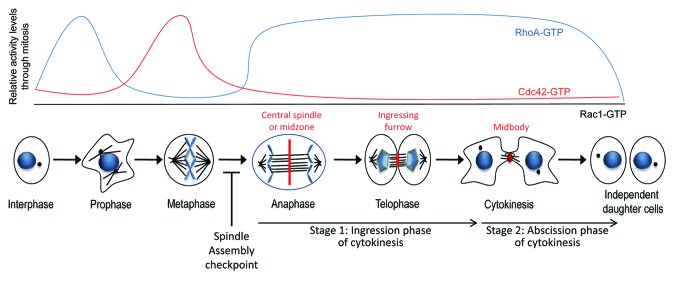
Figure 1. Phases of mitosis and the relative activity levels of Rho GTPases throughout mitosis. A profile of the relative activity levels (upper panel) of RhoA-GTP (blue), Cdc42-GTP (red), and Rac1-GTP (black) corresponding with the phases of mitosis (lower panel) is shown.
The Rho GTPase family of proteins is approximately 20 kDa and there are >22 members in humans. The best characterized can be subdivided into three major groups based on sequence: Rho, Rac and Cdc42.1 Rho GTPases cycle between an active GTP-bound and an inactive GDP-bound state. This cycle is tightly regulated, primarily by two classes of regulatory molecules: (1) GTPase-activating proteins (GAPs), which enhance the relatively slow intrinsic GTPase activity of Rho proteins leading to its inactivation; and (2) guanine nucleotide-exchange factors (GEFs), which catalyze the exchange of GDP for GTP in vivo promoting Rho activity.1 A third set of regulatory proteins have emerged and are the guanine nucleotide-dissociation inhibitors (GDIs), which sequester Rho GTPases in the cytosol in a GDP-bound state.2
The role of Rho GTPases in animal cell division, once thought to be limited to cytokinesis, has now been shown to extend to all mitotic stages. Recent work indicates that they may function during mitotic onset for cell rounding as well as during metaphase for spindle orientation and chromosome congression. Assessment of HeLa mitotic extracts for GTP-bound RhoA, Cdc42, and Rac1 as a measure of their activity revealed that GTP-Cdc42 peaks in metaphase (chromosome alignment), whereas GTP-RhoA increases in anaphase reaching maximal levels during telophase (cytokinesis).3 GTP-Rac1 levels do not alter during mitosis. Consistent with this idea, Cdc42 appears to be the primary driver of Rho signaling during metaphase4 and several RhoA-mediated signaling pathways are essential for cytokinesis.5 In contrast, active Rac1 does not have any known roles in mitosis but rather its inactivation appears to be required. Analysis of Rho signaling pathways in mitosis by RNAi-mediated depletion has identified several Rho GEFs (such as Ect2, GEF-H1, and MyoGEF) as well as many GAPs (such as MgcRacGAP, p190RhoGAP, and MP-GAP), as key regulators of mitotic progression as depletion of any one of these proteins results in multinucleated cells. The expression level of Rho GEFs (Ect2, GEF-H1) and GAPs (MgcRacGAP, p190RhoGAP) involved in mitosis, peak at the G2/M boundary.6-8 Only one RhoGDI has been associated with mitosis to date.9 However, given their importance in regulating Rho function, future investigation is likely to identify other RhoGDIs with mitotic roles.
This review focuses on the role and regulation of Rho GTPases during mitosis, specifically the molecular mechanisms that regulate their temporal localization and activation throughout mitosis to ensure that mitotic events take place in a sequential manner to achieve two independent daughter cells. In this review, I summarize the literature surrounding the roles of RhoA, Cdc42, and Rac1 as well as their regulators, GAPs and GEFs, during mitosis, specifically focusing on their role in mammalian cells.
Prophase: Mitotic Onset
At the onset of mitosis, the cell undergoes profound changes in its architecture to detach from the substrate and become round in shape (Fig. 1). This is important for subsequent mitotic events such as spindle assembly and positioning as well as chromosome capture. Cell rounding involves extensive rearrangement of the actin cytoskeleton, de-adhesion, and an increase in cortical rigidity.10,11 Recent time-lapse microscopy analysis of HeLa cells reveals that cell rounding is one of the earliest mitotic events in prophase occurring prior to centrosome separation and visible chromatin condensation followed by nuclear envelope breakdown.12 Upon mitotic entry, RhoA activity is elevated12,13 and active RhoA concentrates to the cell cortex.12,14 Here, it is the primary driver of actin remodeling resulting in both mitotic cell rounding and cortical stiffening. It is not involved in loss of adhesion. Instead this involves inactivation of the small GTPase Rap1.10 RhoA mediates cell rounding and cortical stiffening through activation of its downstream effectors, Rho kinase (ROCK) and subsequent phosphorylation of myosin II12,13 in an analogous pathway for contractile ring assembly and function during the ingression phase of cytokinesis.
A rise in Cdk1 activity occurs concomitant with cell rounding,15 indicating that Cdk1/cyclin B1-mediated phosphorylation may regulate timing of this process. Indeed, upon mitotic onset Cdk1/cyclin B1 mediates the phosphorylation of the RhoA regulators, the GEF Ect2,12,16,17 and the GAP p190RhoGAP.13 The resulting outcome is a global increase in RhoA activity.13 Ect2 (also known as Pebble in Drosophila) is an essential RhoA-GEF.16,18-22 It localizes to the nucleus of interphase cells and upon mitotic entry translocates from the nucleus to the cytoplasm prior to nuclear envelope breakdown.12,20,23 This coincides with phosphorylation at T341 and T412 by Cdk1/cyclin B1. Specifically, T341 phosphorylation causes Ect2 to fold into an inactive conformation through an intramolecular interaction between the N-terminal BRCT domains and the catalytic DH-PH region.16 This autoinhibition is relieved upon binding of the phosphorylated form of MgcRacGAP to the BRCT domains. Presumably this would occur at the cell cortex as both MgcRacGAP and Ect2 can bind phospholipids and this interaction is essential for Ect2 activity.24,25 Thus, what is the role of Ect2 phosphorylation? It may facilitate the binding of proteins to prepare Ect2 for activation. Cdk1-mediated phosphorylation of Ect2 at T412 during G2/M creates a phosphospecific binding site for Plk1. Cells expressing the Ect2-T412A mutant exhibit reduced accumulation of GTP-RhoA and impaired induction of cortical hyperactivity.17 Spatial regulation of Ect2 also seems to be important for correct temporal activation of RhoA and cell rounding. Mislocalization of Ect2 to the cytoplasm leads to premature cell rounding as evident by cells that express a nuclear localization signal Ect2 mutant that renders it in the cytoplasm.12 Nuclear export of Ect2 coincides with an increase in Cdk1 activity and nuclear import of cyclin B1. Thus it is possible that Ect2 is phosphorylated in the nucleus and this drives its nuclear export. Phosphorylation would therefore maintain Ect2 in an inactive conformation at this point during mitosis to prevent premature activation of RhoA, i.e., prior to nuclear envelope breakdown. Only once Ect2 reached the cell cortex would the autoinhibition be relieved to activate RhoA.
Pro-Metaphase and/or Metaphase: Spindle Formation and Chromosome Alignment
Following cell rounding and nuclear envelope breakdown, the mitotic spindle is assembled to assist in alignment of chromosomes along the center of the cell (metaphase plate) and subsequent equal segregation of sister chromatids into daughter cells (Fig. 1).26,27 The mitotic spindle is a bipolar structure composed of microtubules and associated motor proteins. Microtubules initially emanate from the spindle poles and/or centrosomes toward the cell equator and attach to the kinetochore of a chromosome with their plus ends. This population of microtubules is termed kinetochore- or K-fibers. This attachment allows rapid movement of the bound chromosome such that the sister kinetochore attaches to a microtubule growing from the opposing centrosome. The result is a correctly bi-orientated chromosome at the metaphase plate with a stable microtubule-kinetochore attachment. Once all chromosomes have achieved this, the cell is considered to be in metaphase and chromosome segregation during anaphase can proceed. The mitotic spindle consists of two other populations of microtubules: (1) microtubules that do not attach to kinetochores, but also emanate toward the cell center and are called central spindle or non-kinetochore microtubules and (2) microtubules that emanate from the centrosomes circumferentially and anchor at the cell cortex are called astral microtubules, which function to maintain correct spindle orientation. Premature or aberrant chromosome segregation results in aneuploidy and is avoided by activity of the spindle assembly checkpoint (SAC). This signaling pathway consists of a number of protein complexes that monitor mitotic spindle assembly, delaying anaphase onset until all chromosomes have formed stable bipolar attachments to the spindle via kinetochores.28
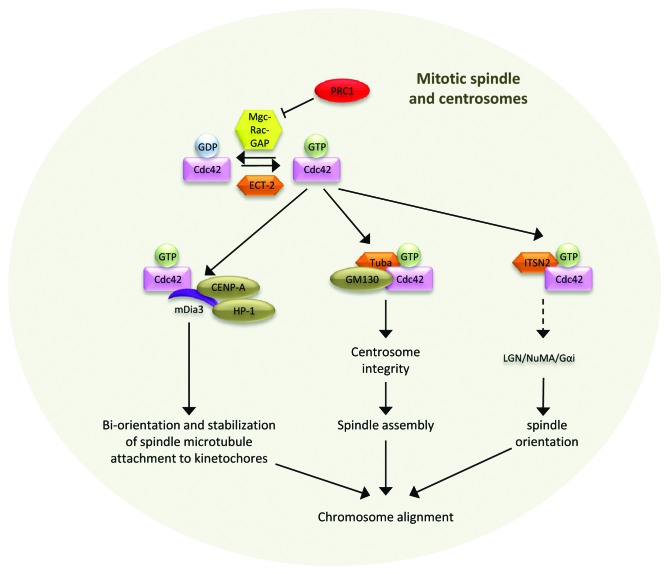
The primary Rho GTPase that functions during metaphase is Cdc42 with several diverse roles that contribute to spindle orientation in polarized epithelial cells as well as centrosome integrity for spindle formation and bi-orient chromosome attachment to spindle microtubules (Fig. 2). All three roles contribute to chromosome alignment at the metaphase plate.
Figure 2. Cdc42-mediated signaling at the mitotic spindle and centrosomes during prometaphase and/or metaphase. In contrast to other mitotic stages, the primary Rho GTPase driving prometaphase and/or metaphase transition is Cdc42, which locates to the mitotic spindle and centrosomes. Here, it is activated by several GEFs (Ect-2, Tuba, and ITSN-2). Cdc42 is also a target of MgcRacGAP. However, its activity it inhibited by binding to PRC1, thus favoring Cdc42 activation. Depending on the localized pool of Cdc42-GTP, it interacts with mDia3/CENP-A/HP-1 (mitotic spindle), GM130/Tuba (Golgi), or ITSN-2 (centrosomes) to regulate a specific metaphase function. As such Cdc42 has roles in (1) bi-orientation and stabilization of spindle microtubule attachment to kinetochores, (2) spindle assembly, and (3) spindle orientation. All three roles contribute to chromosome alignment at the metaphase plate.
Role for Cdc42 in spindle orientation
In contrast to the localization and function of RhoA, Cdc42 localizes to the spindle and centrosomes during metaphase where it contributes to spindle orientation and formation for chromosome alignment.29 Cdc42 is a master regulator of cell polarity, and thus its role during metaphase appears to be particularly important for formation of epithelial organs, where it functions in the monolayer of polarized cells that enclose the central lumen.30 Orientation of the mitotic spindle is paramount in these cells to ensure division occurs in the correct plane. As such, the metaphase function of Cdc42 has been linked to regulation of the formation of the central lumen in a 3D Madin-Darby canine kidney model as well as in a Caco-2 cell model of mammalian intestinal epithelial morphogenesis.29,31 At the centrosome, Cdc42 co-localizes with the Cdc42 specific GEF, intersectin 2 (ITSN2). Spindle positioning during asymmetric divisions is believed to be governed by the interaction between astral microtubules and the cell cortex. This involves the motor protein dynein, which is cortically anchored by a conserved protein complex consisting of LGN, nuclear mitotic apparatus (NuMA) and Gαi.32 Cells depleted of either Cdc42 or ITSN2 display incorrect spindle orientation.29 Silencing of ITSN2 also results in a loss of centrosomal Cdc42 with a corresponding dispersal throughout the cytoplasm. Disruption of LGN causes a similar lumen formation defective phenotype to depletion of ITSN2 and Cdc42.29 Thus, it is proposed that the role of ITSN2/Cdc42 in spindle orientation is mediated through an LGN-dependent pathway. To date, this ITSN2/Cdc42 metaphase role has only been demonstrated in polarized epithelial cells and thus may not contribute to spindle orientation in other cell types.
Role for Cdc42 in maintenance of centrosome integrity
The Golgi is in close physical proximity to the centrosome in mammalian cells. This association is poorly understood; however, it is becoming evident that the Golgi contributes to organization of the structure and function of the centrosome.33 Depletion of the Golgi proteins, GM130 and GRASP65, results in fragmented mitotic centrosomes and multipolar spindles.34,35 The mechanism of action of these Golgi proteins remains unknown. Co-immunoprecipitation assays revealed that GM130 forms a complex with the Cdc42 GEF, Tuba, as well as Cdc42 and that formation of this complex is required for Tuba-mediated activation of Cdc42 at the Golgi and/or centrosome region of mitotic U2OS and HeLa cells.36 Blocking either Tuba or Cdc42 activity phenocopy GM130 depletion of aberrant, non-functional centrosomes. Expression of constitutively active Cdc42 can overcome the requirement for GM130 in centrosome regulation, indicating that Cdc42 functions downstream of GM130. Thus, Cdc42 functions during metaphase to maintain centrosome integrity and unlike its role in spindle orientation this role does not appear to be exclusive to polarized epithelial cells. The mechanism(s) of how Cdc42 maintains centrosome integrity will be revealed by identifying its target effectors.
Role for Cdc42 in regulating bi-orient attachment of spindle microtubules to kinetochores
Evidence for a Rho GTPase in bi-orient attachment of spindle microtubules to kinetochores during metaphase was first demonstrated in cells treated with Clostridium difficile toxin B, which inactivates all Rho subfamily members.4 Staining for CENP-A, a kinetochore marker, and MAD2, a mitotic checkpoint protein that only localizes to unattached kinetochores, revealed that these cells are impaired in their ability to align their chromosomes at the metaphase plate, and have a loss of microtubule attachment to these chromosomes. The resulting outcome is an accumulation of chromosomes near each pole that are either mono-oriented or without microtubule attachment. These cells either fail to progress through mitosis, most likely resulting in cell death or premature exit to interphase without a distinct anaphase to produce aberrant tetraploid nuclei. Cdc42 was identified as being the responsible Rho GTPase as cells overexpressing the dominant negative form of Cdc42-N17 phenocopied toxin B treatment.4 In contrast, overexpression of RhoA-N19 and Rac1-N17 results in a cytokinesis failure phenotype and no mitotic defects, respectively. Consistent with a role for Cdc42 during metaphase, only active Cdc42 locates along the mitotic spindle, in addition to its localization at the spindle poles.4,37 The downstream effector of Cdc42 metaphase function is likely mDia3, a member of the formin-homology (FH) family of proteins that induces actin polymerization and promotes the alignment and stabilization of microtubules.4,38 mDia3 binds active Cdc42-GTP and forms a complex with CENP-A at the kinetochores of condensed chromosomes juxtaposed with p150Glued, a component of the dynactin–dynein complex that anchors MTs to the kinetochore.39 mDia3 kinetochore localization is disrupted in toxin B-treated cells. Thus, it is proposed that the Cdc42/mDia3-signaling pathway is not involved in the initial attachment of microtubules to kinetochores but plays an important role in subsequent, stable bi-orientated microtubule attachment for proper chromosome alignment and segregation. Another potential Cdc42 effector during metaphase is p21-activated kinase 1 (Pak1), as it also localizes to the mitotic spindle and its loss causes monopolar and multipolar spindles, and misaligned chromosomes.40,41 The role of Cdc42 in regards to Pak1 activation during metaphase is still yet to be determined. These findings suggest a direct involvement of Cdc42 at the kinetochore. However, it is also possible that its role is indirect by stabilizing the core histone H3 variant CENP-A, which lies at the centromere and is responsible for recruiting kinetochore-binding proteins. Modulation of Cdc42 activity and depletion of Cdc42 causes a significant reduction in centrosome-localized CENP-A.42 This would result in impaired chromosome attachment and subsequent segregation. In an analogous manner to Cdc42 depletion, depletion of Rac1 also results in a decrease in CENP-A localization at the centromere.42 However, in contrast to Cdc42,4,37 Rac1 does not locate to the mitotic spindle or the centromere nor does its depletion cause chromosome attachment errors.42 Therefore, a role for Rac1 in CENP-A regulation during metaphase is not likely nor is it the primary Rho GTPase that functions during metaphase.
Although the evidence for a role for Cdc42 during metaphase is limited, it is becoming evident that it is the primary Rho GTPase that functions during this mitotic stage with several roles that all contribute to stabilization of the mitotic spindle for chromosome alignment. Thus, the metaphase roles of Cdc42 are most likely not mutually exclusive. For example, given centrosome integrity is required for spindle assembly and subsequent attachment to the kinetochores of chromosomes for alignment at the metaphase plate, it is possible that the roles for Cdc42 in these two processes are linked.
Cytokinesis: Final Separation to Generate Two Independent Cells
Cytokinesis is the final stage of cell division that generates two separate daughter cells. Cytokinesis in animal cells can be subdivided broadly into two steps: (1) membrane ingression and (2) membrane abscission (Fig. 1).43 These steps are highly ordered processes that begin immediately following chromosome segregation and need to occur in a sequential manner for cytokinesis to be executed correctly. Membrane ingression involves two phases. First, the site of division is determined by recruiting RhoA. Second, the membrane ingresses, which requires the assembly and activity of the actin-myosin II contractile ring.43 Membrane abscission also involves two phases. This first phase involves completion of ingression such that the membrane is fully constricted by the end of telophase, whereby the anti-parallel microtubules forming the mitotic spindle and associated proteins are compressed to form an intracellular bridge between nascent daughter cells. At the center of the bridge is the midbody, consisting of several proteins,44 such as γ-tubulin,45 centriolin,46 and the exocyst complex.47 They assemble into a single ring structure, called the midbody ring (MR).47 The MR is not contractile and is unable to account for abscission on its own. It appears to act as a recruitment scaffold for the abscission machinery. The final phase is the physical separation of the cytoplasmic contents into two independent cells. Vesicle trafficking is a major hallmark of this stage of cytokinesis, with vesicle fusion occurring adjacent to the MR and is thought to be the site of abscission. After abscission the MR may be inherited by one of the daughter cells.47 Cytokinesis failure can occur at any one of the steps in this process due to an array of errors such as protein mislocalization, deregulated protein phosphorylation, and erroneous signaling. Depending on the stage of cytokinesis that the error occurs, membrane ingression may not be initiated, or if partially completed will regress or if the midbody has formed then the nascent daughter cells may remain connected due to persistent bridge formation. Nevertheless, the resultant outcome is the generation of a binucleated (or aneuploid cell), which can lead to genomic instability and thus contribute to the initiation and/or progression of tumorigenesis.
The role of several Rho signaling components in cytokinesis is specifically exemplified in the differentiation of megakaryocytes where cytokinesis failure is necessary for these hematopoietic cells to give rise to blood platelets. In this case, this process of polyploid formation is termed endomitosis. Downregulation of the Rho/ROCK pathway48 as well as repression of Ect2 and GEF-H1 transcrption49 results in a block in myosin II activation at the ingressing furrow. The result aborts ring contraction to allow endomitosis. Overexpression of either GEF forces megakaryocytes to complete cytokinesis, generating 2N cells.
Membrane ingression
Position of division site
Immediately after chromosome segregation, cells must ensure that the spindle poles are on opposing sides of the division plane so that the segregated chromosomes are equally distributed into each of the two newly forming cells. In animal cells, the division plane is determined at the metaphase to anaphase transition and its position is dictated in part by three separate pools of microtubules that collectively form the mitotic spindle:50,51 (1) polar astral microtubules (emanating from the spindle pole to sites away from the ingression site) most likely aid in positioning of the ingression site by inhibiting cortical contraction52-54 by spatially regulating myosin recruitment),55,56 (2) equatorial astral microtubules (emanating from the spindle poles toward the ingression site) are thought to contribute to stabilization of the equatorial cortical region,57 as well as provide positive signals that stimulate formation and contraction for membrane ingression),58 and (3) central spindle (a set of anti-parallel microtubule bundles between the spindle poles) deliver positive signals to the midzone region, which are especially important for later cytokinetic stages. The roles of each microtubule population are partly redundant but collectively they ensure that a robust division site is established.59,60
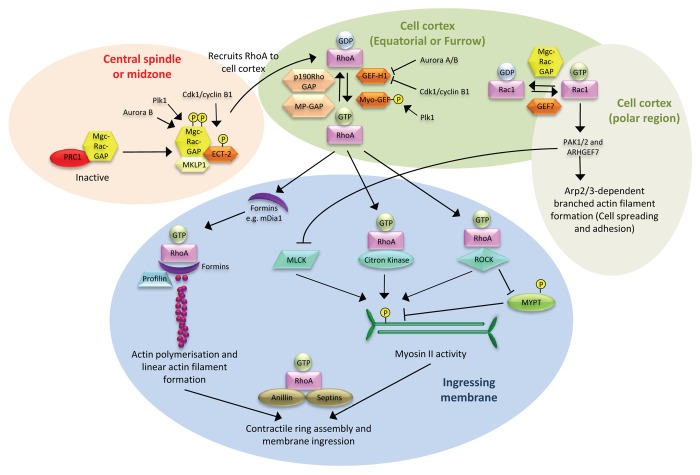
RhoA is essential for defining the site to initiate formation of the ingressing furrow in animals cells (Fig. 3).19,61-64 Its activation is restricted spatially to a narrow zone at the equatorial cell cortex upon anaphase onset.22,65-68 Disruption of this restricted spatial regulation of RhoA that results in a wider zone of activation can lead to failure of the initiation of membrane ingression.22 The narrow zone of RhoA activation is achieved by RhoA activators whose localization is restricted to the central spindle.
Figure 3. Spatial regulation of the signaling pathways mediated by Rho GTPases during the ingression phase of cytokinesis. Upon anaphase onset, the site of the division plane is established. This involves in-part the interaction between the centralspindlin complex and Ect-2, which is facilitated by phosphorylation, and occurs at the central spindle and/or midzone. RhoA is subsequently recruited to the equator of the cell cortex where its activation is tightly regulated. Its activity is confined to a narrow zone (defining the division plane) by several Rho GEFs (GEF-H1, Myo-GEF) and GAPs (p190RhoGAP, MP-GAP). At the same time, cell cortex localized Rac1 is maintained in an inactive state by MgcRacGAP to inhibit the formation of Arp2/3-mediated branched actin filaments. However, this pathway may be active at the polar regions to allow for cell elongation. At the equatorial cell cortex, RhoA activates the effectors, mDia1 for linear actin filament polymerization, as well as the kinases, MLCK, ROCK, and citron kinase, to activate myosin II. The outcome is assembly and activation of the actin-myosin II contractile ring to mediate membrane ingression. Anillin and septin proteins maintain all furrow components within this narrow zone for efficient and complete membrane ingression.
Regulation of RhoA by GEFs
Activation of Ect2 upon mitotic entry16,20,21 could activate RhoA globally and thus additional mechanisms contribute to equatorial activation of RhoA. The spatial regulation of RhoA GEFs and GAPs is thought to underpin the mechanisms of correct spatial activation of RhoA. During metaphase and anaphase, Ect2 localizes to the mitotic spindle and associates with the centralspindlin complex, which is a heterotetrameric complex consisting of kinesin-like MKLP1 and the GAP MgcRacGAP (also known as Cyk4).19,22,69,70 The central spindlin complex binds to the plus-ends of the central spindle non-kinetochore microtubules where it is required for microtubule bundling.69,70 Here, MgcRacGAP recruits and binds Ect2 in a phosphorylation-dependent manner. Thus, Ect2 is stabilized in an active conformation to allow interact with RhoA.22 Depletion of MKLP122 or disruption of the central spindle71,72 results in mis-localization of MgcRacGAP and Ect2 from the central spindle and subsequent broadening of the RhoA activation zone. These cells do not accumulate the necessary components for ingression in a spatially narrow region on the cell cortex and thus this first phase of cytokinesis fails.22 The T341E phospho-mimetic form of Ect2 is only weakly catalytically active whereas the T341A mutant is fully active and binds MgcRacGAP.16 Therefore, Cdk1-mediated phosphorylation of Ect2 likely maintains Ect2 in an inactive conformation to prevent binding to MgcRacGAP until it is positioned correctly to activate RhoA. Identification of the phosphatase responsible for dephosphorylating T341 will be important to identify and likely will locate to the central spindle and/or cell cortex as phospholipid binding is essential for Ect2 activity.24,25 MgcRacGAP also binds phospholipids.73 The interaction site of MgcRacGAP on the plasma membrane could demark the site of RhoA activation and initiation of cytokinesis.
Other GEFs with a reported role in the initiation of cytokinesis are the microtubule-regulated exchange factor, GEF-H1, and the myosin-interacting GEF, MyoGEF. Both can catalyze RhoA activation and locate to the central spindle. GEF-H1 is responsible for catalyzing RhoA activation in response to Ect2-dependent localization and initiation of cytokinesis. GEF-H1 localizes to the spindle, particularly at the tips of cortical microtubules and perturbation of its function induces asymmetric membrane ingression.74 Like Ect2, GEF-H1 activity is also regulated by phosphorylation whereby Aurora A/B and Cdk1/Cyclin B mediated phosphorylation of GEF-H1 results in inhibition of its catalytic activity. Thus, RhoA activation at the equatorial cell cortex is likely to coincide with downregulation of Cdk1 activity upon the onset of anaphase and/or chromosome segregation. This would ensure that initiation of membrane ingression does not occur prematurely. Consistent with this idea, dephosphorylation of GEF-H1 occurs just prior to the initiation of cytokinesis, accompanied by GEF-H1-dependent GTP loading on RhoA.74 On the other hand, MyoGEF spindle and cell cortex localization is not dependent on Ect2, but instead it is required for Ect2 localization at the central spindle.75 MyoGEF spindle localization is dependent on the centrosome/spindle pole-associated protein (CSPP) and Plk1, as depletion of either protein by RNAi interferes with the localization of MyoGEF at the spindle pole and central spindle.75,76 This dependence on Plk1 is most likely due to phosphorylation as Plk1 mediates MyoGEF phosphorylation at T574. In contrast to the role of phosphorylation in regulating GEF-H1 function,74 this modification stimulates MyoGEF activity toward RhoA.76 RNAi-mediated depletion of CSPP or MyoGEF causes defects in mitosis and cytokinesis, such as metaphase arrest and regression of the invaginating membrane resulting in formation of multinucleated cells.75,77 It also causes mis-localization of activated RhoA and the phosphorylated (active) form of nonmuscle myosin II.75 Thus, the temporal regulation required to initiate cytokinesis is tightly regulated by three GEFs (Ect2, GEF-H1, and MyoGEF). Of these, Ect2 appears to be the major GEF responsible as RNAi-mediated depletion of Ect2 has the most penetrant cytokinesis failure phenotype. Nevertheless, these GEFs function in converging pathways to ensure spatially correct activation of RhoA to establish the site of membrane ingression and this is temporally coordinated with chromosomal segregation.
Regulation of RhoA by GAPs
The presence of a GAP (MgcRacGAP) and a GEF (Ect2) in the same complex at the central spindle that contribute to RhoA localized activation is puzzling. Specifically, the role of MgcRacGAP is unclear. It was suggested to mediate RhoA inactivation78,79 and thus thought that constant RhoA cycling between active and inactive states is required for the proper localization and function of RhoA.79 However, the primary role of MgcRacGAP appears to be indirect via recruiting and contributing to Ect2 activation, which in turn is required for RhoA cortical localization.19,22,69,70 MgcRacGAP is a Plk1 substrate, and phosphorylation is required to bind and recruit Ect2.80,81 Microtubules, as well as PRC1 facilitate Plk1 phosphorylation of MgcRacGAP.81 PRC1 binding to MgcRacGAP has also been shown to decrease its GAP activity and prevent Aurora B-mediated phosphorylation.37 Overexpression of an Aurora B phospho-mimetic form of MgcRacGAP results in abnormal spindle morphology and therefore PRC1 binding prevents premature activation of MgcRacGAP or at least prevents its activation at the spindle midzone.37 This raises the question of what are the targets of the GAP function of MgcRacGAP. Evidence suggests that it is Rac1 (discussed below).82-84 This raises the question of what is the identity of the GAPs that regulate RhoA during cytokinesis? p190RhoGAP co-localizes with RhoA at the cell cortex of anaphase cells. Overexpression of p190RhoGAP results in abnormal positioning of the ingression site and this correlates with mis-localization of active RhoA, leading to failed or abnormal ingression and multinucleation. Its levels decrease during late mitosis by a ubiquitin-mediated mechanism,8 consistent with the hypothesis that high RhoA-GTP levels are required for completion of cytokinesis. A systematic and thorough screen of 67 potential RhoGAPs in humans has recently been performed identifying M phase GAP (MP-GAP) as the most likely major GAP regulator of RhoA during mitosis.85 RhoA is excessively activated in cells depleted of MP-GAP correlating with formation of large cortical protrusions and cytokinesis failure. In these cells, RhoA accumulates around the entire cell cortex but only when centrosomal asters are simultaneously disrupted.85 Thus, p190RhoGAP and MP-GAP are the two primary RhoGAPs to function during the initiation of cytokinesis as regulators of RhoA activity. However, these molecules do not function alone and RhoA accumulation, site selection for ingression and subsequent ring contraction are also regulated by other factors such as astral microtubules
In summary, the interplay and collaborative effort of several GAPs (MgcRacGAP, p190RhoGAP, MP-GAP) and GEFs (Ect2, GEF-H1, and MyoGEF) are responsible for modulating RhoA activity at the correct equatorial cell cortex zone, and are therefore critical for the spatial organization of the division site and subsequent membrane ingression.
Regulation of actin and myosin contractile ring and cleavage furrow formation
Membrane ingression and invagination of the cell membrane is driven by a contractile ring, composed of parallel filaments of actin and non-muscle myosin II.86 Myosin comprises two heavy chains, two essential light chains, and two regulatory myosin light chains (MLC). Phosphorylation of MLC on T18 and S19 drives assembly of the actin-myosin II contractile ring87 and activity of the myosin II motor,88,89 respectively. The major phosphorylation site is S19, which allows myosin II to interact with actin to assemble an actin-myosin II complex and initiate contraction.90
A second cytoskeletal regulator of cytokinesis is the septin family of GTP-binding proteins that form filamentous complexes.91 Of the 12 septin genes in mammals, Sept2, Sept4, Sept7, and Sept9 localize to the cell cortex, contractile ring, and midbody of mitotic cells.92-97 Cytokinesis is disrupted by micro-injection of anti-Sept2 or anti-Sept9 antibodies and by transfection of siRNAs against Sept2, Sept7, or Sept9.92,94,95 Despite mechanistic differences in cytokinesis among distant organisms, septins are required for a robust cytokinesis in D. melanogaster,98 C. elegans,99 and mammals.92 Septin filaments associate with actin filaments at the site of cytokinesis initation, where they co-operate to achieve membrane ingression.95,100 These two filament systems are thought to be linked indirectly by the actin-binding protein anillin as it can bind phosphorylated myosin II, actin filaments and septins.91,93,101-103 In yeast, septin filaments are thought to provide a diffusion barrier that restricts membrane proteins in the ingressing zone.104,105 For example, to retain active RhoA within the narrow zone required for initiation of and subsequent membrane ingression. However, this role for septins is unclear in mammalian cells. Instead, the primary role of septins appears to be in regulating anillin levels during the final stages of ingression.102,103
RhoA is essential for establishing organization within the ingressing zone as well as stimulating ingression by not only promoting actin and myosin II assembly and function but by also interacting with the scaffold proteins anillin and septins. RhoA stimulates actin polymerization through the activation of formins. Formins nucleate the formation of unbranched long, straight actin filaments in response to RhoA.106 One key mechanism preventing premature actin polymerization is the autoinhibition of formins through intramolecular binding of a Diaphanous autoinhibitory domain (DAD) to a conserved N-terminal regulatory element.107 This is alleviated upon binding of active RhoA,108-110 and thus actin polymerization is restricted to the ingressing membrane region. The mammalian formin mDia1 (a RhoA effector) binds selectively to the GTP-bound form of RhoA, as well as to profilin, thereby inducing actin polymerization.110 Inhibition of mDia1 by antibody injection causes cytokinesis failure.111 Downstream of RhoA-mDia1, in addition to its role in actin polymerization, mDia1 has been implicated in regulating Src activation, which appears to play a role in cytokinesis completion;112 however this is most likely during the abscission stage.
RhoA is a key regulator of myosin activity via several pathways. Myosin ATPase activity is not required for myosin II localization to the ingressing membrane113-115 and instead its recruitment is dependent on activated RhoA.19,116,117 Of the three kinases, myosin regulatory light chain kinase (MLCK), ROCK, and Citron kinase, that contribute to myosin activation by phosphorylating T18 and S19 on MRLC, the latter two are activated by RhoA.118 Both ROCK and Citron kinase localize to the ingression site and this is thought to be dependent on RhoA. However, this dependency relationship between RhoA and Citron kinase is under debate. Nevertheless, they both activate myosin by phosphorylating MRLC at S19119 and T18 and S19,120 respectively. Overexpression of kinase-active mutants causes abnormal contractions resulting in multinucleated cells.118 ROCK also cross-links straight anti-parallel actin filaments.121 MLC phosphorylation is regulated in part by myosin phosphatase (MYPT1), which downregulates myosin II contractility by dephosphorylating S19 on MLC. MYPT1 consists of a myosin binding subunit (MBS), a catalytic subunit (delta isoform of PP1) and an additional small subunit. ROCK, but not Citron kinase,120 also acts to maintain active myosin by phosphorylating T853 on the MBS subunit of MYPT1. Thereby inhibiting the phosphatase activity of MYPT1 by causing dissociation of the catalytic subunit of PP1δ.122,123
As for its role in activating RhoA for initiation of cytokinesis, MyoGEF continues to function as a RhoA activator at the ingression site via interaction with non-muscle myosin II to advance membrane ingression.77 Citron kinase was thought to be a second RhoA regulator during ingression. However, two recent manuscripts provide evidence to indicate that Citron kinase is not a canonical RhoA effector for ingression124,125 as interaction of the D. melanogaster Citron kinase ortholog Sticky with RhoA is not dependent on RhoA status. Instead their findings indicate that Sticky is a regulator of RhoA during the later stages of cytokinesis when transitioning to midbody formation. RhoA fails to form a compact ring in late cytokinesis after Sticky depletion or overexpression of a Sticky kinase-dead mutant.124,125 This indicates that RhoA midbody localization during late stages of cytokinesis is dependent on Sticky, specifically its kinase activity. Similar findings have since been shown in mammalian cells during the latter stages of ingression,126 suggesting that Citron kinase is important for maintaining active RhoA at the midbody during its formation. Thus, the primary Rho effector regulating myosin activity for membrane ingression appears to be ROCK while Citron kinase is likely to maintain active RhoA during the final stage of ingression converging with midbody formation.
Both activated RhoA and Citron kinase (Sticky) interact with anillin at the cleavage furrow and are required for its localization at this region.125,127-130 Anillin contains conserved N-terminal actin and myosin binding domains and is required to maintain active myosin in the equatorial plane during cytokinesis, suggesting it links these filament systems for coordinated action to achieve contraction. RhoA interacts with the conserved C-terminal domain in anillin that shares homology with the RhoA binding protein Rhotekin. This C-terminal region is essential for its function and localization128 and thus anillin is thought to act as a scaffold protein to link RhoA with the ring components actin and myosin to maintain efficient contraction. Although furrows can form and initiate ingression in the absence of anillin, furrows cannot form in anillin-depleted cells in which the central spindle is also disrupted, revealing that anillin can also act at an early stage of cytokinesis.128 In line with this idea, the central spindlin component MgcRacGAP (RacGAP50C) is directly involved in targeting anillin to the cleavage furrow in Drosophila, but not in mammalian cells.131 Instead anillin is targeted by the centralspindlin binding protein, Ect2, in mammalian cells.24 Collectively, the role of anillin during cytokinesis is likely to maintain all key components in close proximity, such as activated RhoA, actin, myosin, Citron kinase, septins, for efficient contraction and ingression ensuring that this stage of cytokinesis is robust.
Membrane abscission
Formation of midbody
The central spindle, also known as the spindle midzone, plays a key role in maintaining the segregated chromosomes separated during cytokinesis. If microtubules are depolymerized the newly forming nuclei return to the center of the cell and fuse.114 The minus end of microtubules that are locally nucleated in the midzone are coated with γ-tubulin.45,132 As the membrane ingresses, the midzone and associated microtubules are compressed, forming an intracellular bridge that is 1–1.5 µm in diameter whereby the cytoplasm of the nascent daughter cells remains connected. Compression of this region, as cytokinesis progresses, results in protein accumulation forming the phase-dense structure referred to as the Fleming Body, or as it is most commonly known the midbody ring. The mechanism of how cells transition from complete membrane ingression, i.e., contractile ring closure, to midbody ring formation and maturation are only starting to emerge. The first evidence has come from Drosophila. Time-lapse microscopy of Drosophila S2 cells revealed that anillin is required for this transition as depleted cells fail to complete the final stages of ingression and are unable to generate an intracellular bridge of the required thinness, i.e., 1–1.5 µm in diameter.103 Anillin acts as a linker protein connecting the actin-myosin contractile ring and septin filaments via its N- and C-terminus, respectively.102,103 Specifically, it is the removal and retention of anillin at the closing contractile ring and midbody ring, respectively, that corresponds with the transition from complete membrane ingression to midbody formation.102 This cycling of anillin is regulated by interactions with Sticky and septins, respectively.102
γ-tubulin is a core structural component of the midbody ring. Its depletion by anti-sense RNA causes cytokinesis failure due to dysmorphogenesis of the midbody structure.132 The midbody ring is thought to act as a scaffold platform for recruiting and/or activating signaling proteins that contribute to the regulation of abscission and post-mitotic cell fate.133-135 Proteomics and cell biology studies have indicated that a large number of proteins (>100) locate to the midbody ring itself or to flanking regions within the intracellular bridge.44,136 The molecular details of the role of these proteins, and the interaction between pathways involved in the abscission process, are only starting to emerge. Proteins that accumulate at the midbody ring and flanking regions within the intracellular bridge include those derived from (1) the central spindle such as kinesin-like motor proteins and chromosomal passenger proteins, that travel along the central spindle toward microtubule plus ends, accumulating in the midzone; (2) the cell equator such as MgcRacGAP, Ect2, and anillin; (3) the equatorial cortex such as RhoA and sept2; and (4) proteins previously located within the nascent daughter cells that traffic to the intracellular bridge upon formation such as Rab11 and ESCRT-III. Thus, in human cell lines several lines of evidence suggest that recruitment of key abscission proteins to the midbody are dependent on microtubules, e.g., depletion of γ-tubulin by anti-sense RNA disrupts microtubules associated with the intracellular bridge and causes cytokinesis failure.132 RNAi-mediated depletion of the microtubule bundling protein PRC1 causes abscission defects in HeLa cells.137 Different species may utilize alternate mechanisms as PRC1spd-1 (RNAi) C. elegans embryos do not form midbody microtubules but are still able to recruit midbody ring components such as MKLP.135
Scission
Following completion of membrane ingression and formation of the intracellular bridge, the abscission stage of cytokinesis is further characterized by a secondary ingression or thinning of the intracellular bridge to approximately 100 nm at a site on one side of the midbody ring. This is thought to be the site of abscission, which subsequently follows this secondary ingression.138-140 In addition to disassembly of the actin-myosin II contractile ring following membrane ingression, secondary ingression, and abscission also involve depolymerization of cortical actin located within the intracellular bridge. Inhibition of actin depolymerization within the intracellular bridge blocks cytokinesis completion.141 The molecular components and pathways required to disassemble cortical actin at this location are not well characterized. Nevertheless, the inactivation of RhoA within the intracellular bridge is thought to be one of the driving mechanisms.22,65 This is thought to involve the GAPs, MgcRacGAP and p50RhoGAP, as they both localize to the midbody during abscission. MgcRacGAP co-localizes at the midbody with Aurora B and RhoA, not Rac1. Here, it is phosphorylated at S387 by Aurora B functionally converting its GAP activity to RhoA from Rac1.142 Expression of a kinase-defective mutant of Aurora B disrupts cytokinesis and inhibits phosphorylation of MgcRacGAP at S387, but not its localization to the midbody. Overexpression of a phosphorylation-deficient MgcRacGAP-S387A mutant, but not phosphorylation-mimic MgcRacGAP-S387D mutant, prevents abscission and induces multinucleation. However, the idea that Aurora B-mediated phosphorylation of MgcRacGAP regulates its affinity toward RhoA and Rac1 is controversial as a recent study provided compelling evidence against this.84 Purified MgcRacGAP from metaphase and anaphase cells have different phosphorylation states and display equivalent activity toward Rac1 with no detectable activity toward RhoA.84 In this study, the phosphorylation-mimetic MgcRacGAP-S387D mutant causes a partial inactivation of Rac1 and Cdc42 activity. This finding is in line with other reports showing MgcRacGAP has little to no activity toward RhoA and has greater activity toward Rac1 and Cdc42.82 Other sites on MgcRacGAP are phosphorylated,84 with one of these fitting the Cdk1 consensus. These phosphorylation sites do not lie within the GAP domain and thus are likely to regulate MgcRacGAP activity indirectly possibly by regulating its mitotic localization, i.e., cytosol in metaphase to central spindle during anaphase. The primary RhoGAP targeting RhoA during abscission is therefore likely to be p50RhoGAP or an as yet unidentified GAP. Depletion of p50RhoGAP causes a delay in abscission and multinucleation.141 This cytokinesis failure phenotype correlated with an increase in cortical actin within the intracellular bridge,141 suggesting that actin reorganization/disassembly mediated by p50RhoGAP may potentially be via inhibiting RhoA activity locally for completion of abscission.
Secondary ingression and abscission also involve depolymerization of microtubules,138-140 trafficking of Rab11-FIP3 recycling endosomes to the midbody140,141,143,144 and subsequent recruitment of the ESCRT complexes.145,146 The ESCRT complex is involved in intralumenal vesicle formation and fission and consists of four proteins (ESCRT-0, -I, -II, -III) that function in a sequential manner. Together with the ESCRT interacting protein ALIX, they function at late endosomes to sort membrane proteins into the vesicular lumen to create multivesicular bodies (MVBs).147 ESCRT 0-II complexes recruit the cargo destined for these intralumenal vesicles. There are 11 ESCRT-III proteins in mammals and are often called charged MVB proteins (CHMPs). These proteins assemble into spiral, membrane-bound filaments required for intralumenal vesicle formation and scission. This filamentous assembly is block by cortical actin.148 ESCRT proteins are thought to play a similar role during cytokinesis as depletion of ESCRT proteins and ALIX by siRNA causes cytokinesis failure and these proteins are recruited to the midbody by Cep55.146,149 ALIX and TSG101 (ESCRT-I) accumulate at the midbody and recruit ESCRT-III proteins to this intracellular bridge region during late cytokinesis.138,145,146 ESCRT-III components have been associated with recruiting the microtubule severing enzyme spastin, which appears to be required for shearing the microtubules during abscission,138,150 and this most likely occurs after completion of the secondary ingression but prior to membrane scission. In a similar manner to its role in intralumenal vesicle formation and scission, ESCRT-III proteins polymerize to form 17 nm filaments within the intracellular bridge during cytokinesis, spiraling toward the secondary ingression site and driving membrane scission.139
Rab11-FIP3 endosomes traffic toward the intracellular bridge during the final stages of membrane ingression. The secondary ingression is also mediated by FIP3-endosome fusion and this is followed by recruitment of ESCRT-III to the abscission site. ESCRT-III proteins stabilize the secondary ingression site and act as the scission factor. RhoA signaling molecules have been associated with Rab11-FIP3 recycling endosomes and the ESCRT machinery for late stages of the abscission step. Using a combination of siRNA and proteomic analysis, MgcRacGAP was identified as a binding partner of FIP3 during cytokinesis.151 During the final stages of membrane ingression, Rab11-FIP3 endosomes traffic toward the midbody ring in a kinesin I-dependent manner. Once at the intracellular bridge, FIP3 binds MgcRacGAP and accumulates in the midbody. This association appears to be dependent on a dissociation of Ect2 from MgcRacGAP at the midbody as it shares the same binding region to FIP3.151,152 The mechanism of dissociation remains unknown but could involve dephosphorylation of MgcRacGAP80,81 or ubiquitin-mediated degradation of Ect2.153 During the secondary ingression, FIP3 endosomes deliver p50RhoGAP to the intracellular bridge to regulate depolymerization of the cortical actin network for abscission.141
In contrast to previous dogma, an active pool of RhoA exists at the midbody ring indicating that its role during abscission may not only be associated with cortical actin disassembly. Midbody ring localized RhoA is activated upon binding to FIP3/Rab11-containing endosomes.141 This localized pool of active RhoA is puzzling as cortical actin needs to be disassembled for abscission. This raises several questions: what is the role of RhoA-GTP during abscission and what are the responsible GEFs? Two candidate GEFs are Ect2 and leukemia-associated RhoGEF (LARG), which also concentrate at the midbody ring.154,155 The armadillo protein, p0071, is targeted to the midbody by the kinesin II KIF3b and once at the midbody ring associates with Ect2 and RhoA.154,156 Upon binding it stimulates Ect2-mediated activation of RhoA. Depletion of p0071 causes cytokinesis failure and multinucleation, which correlates with downregulation of Rho activity and reduced actin and phospho-myosin II at the midbody. In contrast to depletion of Ect2 and GEF-H1, depletion of LARG causes cells to spend a prolonged period of time in abscission without affecting any other earlier stages of mitosis.155 Thus, it is the only RhoA GEF whose function is specifically associated with the abscission stage of cytokinesis. Taken together, these studies provide tantalizing evidence to suggest that active RhoA plays a role during the abscission stage of cytokinesis. However, many questions are raised that future studies are likely to address: the temporal kinetics of inactivation and/or activation of RhoA, the molecular machinery that regulates this activity flux, and the role of active RhoA during abscission.
Rac is a negative regulator of cytokinesis
In contrast to RhoA and Cdc42, whose GTP-bound states fluctuate during mitosis and thus corresponds with their activity at specific locations and specific mitotic stages, GTP-Rac1 levels do not alter during mitosis (Fig. 1).3 Nevertheless, it appears as though inactivation of Rac1 is required during mitosis. The primary role of Rac1 is to stimulate membrane expansion at the leading edge of the cell157 to cause cell spreading and adhesion. It does this by promoting actin nucleation, specifically formation of branched actin filaments through activation of Arp2/3.158 Thus, the inactivation of Rac1 during mitosis appears to be particularly important during cytokinesis at the cell equator for correct formation and activity of the contractile ring for efficient membrane ingression. Indeed, FRET-based activity probes revealed that Rac1 activity is suppressed at the spindle midzone during anaphase and telophase.68 Overexpression of a constitutively active form of Rac1 causes multinucleation or failed cytokinesis in HeLa and RatA cells.84,159 In contrast, Rac1 RNAi has no effect on cell division in C. elegans,62,78,160 and D. melanogaster.83 Although the global GTP-Rac1 levels do not change throughout mitosis, FRET-based activity probes revealed that GTP-Rac1 levels are increased at the polar regions of the plasma membrane after telophase.68 Thus, Rac1 activity is suppressed at the cell equator during metaphase and anaphase to promote formation of RhoA/formin-dependent linear actin filament assembly thus restricting actin-myosin II contractility to a narrow zone for efficient membrane ingression. Whereas its activity at the polar regions after telophase would promote the formation of Arp2/3-dependent branched actin filaments to induce cell spreading and adhesion for completion of cytokinesis and re-entry into the next cell cycle. Identification of the GEF responsible for activating Rac1 at the polar regions will aid in our understand of the mechanisms of cell spreading and its relevance to completion of cytokinesis.
MgcRacGAP has been implicated as a key regulator of Rac1 during cytokinesis (Fig. 3). MgcRacGAP locates to the central spindle where it aids in recruitment of RhoA to the cell equator. However, biochemical analysis of MgcRacGAP shows that it has little to no activity toward RhoA and has greater activity toward Rac1 and Cdc4282 and genetic evidence in Drosophila indicates that Pebble (fly MgcRacGAP) inhibits Rac activity.83,84 Indeed, recent evidence has indicated that the GAP activity of MgcRacGAP is required during anaphase to inhibit Rac1-dependent effector pathways associated with control of cell spreading and adhesion in HeLa cells.84 This model is also supported by genetic evidence in C. elegans whereby MgcRacGAP downregulates Rac1 to block Rac1-mediated activation of the Arp2/3 complex.160 The spatial regulation of MgcRacGAP would therefore not result in Rac1 inactivation at the polar regions of the cell cortex to allow for cell elongation via the Pak1/2 and ARHGEF7 effectors. Rac1 activity as well as the cell adhesion marker vinculin are high in HeLa cells expressing a GAP-deficient MgcRacGAP mutant,159 and Rac1 RNAi in these cells can rescue the cytokinesis failure phenotype.84 These defects can also be rescued by depletion of the Rac1 effector proteins, ARHGEF7 and Pak1. Pak1 can also suppress MLCK by mediating its phosphorylation,159 and thus prevent activation of myosin II. As such, expression of a constitutively active form of Pak1 blocks cytokinesis in HeLa cells.159 Thus, MgcRacGAP-mediated inactivation of Rac1 at the site of ingression appears to have at least two roles: (1) block actin nucleation of branched filaments to promote a narrow zone of actin-myosin contractility and (2) prevent suppression of myosin II activity. This would collectively result in efficient actin-myosin II contractile activity to complete membrane ingression in a timely manner.
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
Acknowledgments
Apologies to all colleagues whose work could not be cited due to limited space. This work was supported by grants from the National Health and Medical Research Council (NH&MRC) of Australia, an NH&MRC Career Development Award Fellowship, Cancer Council NSW.
Glossary
Abbreviations:
- CSPP
centrosome/spindle pole-associated protein
- DAD
Diaphanous autoinhibitory domain
- GAP
GTPase-activating proteins
- GDI
guanine nucleotide-dissociation inhibitors
- GEF
guanine nucleotide-exchange factors
- ITSN-2
intersectin 2
- MBS
myosin binding subunit
- MLC
myosin light chain
- MLCK
myosin regulatory light chain kinase
- MR
midbody ring
- NuMA
nuclear mitotic apparatus
- PAK
p21-activated kinase
- SAC
spindle assembly checkpoint
- ROCK
Rho-kinase
References
- 1.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–35. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 2.Dransart E, Olofsson B, Cherfils J. RhoGDIs revisited: novel roles in Rho regulation. Traffic. 2005;6:957–66. doi: 10.1111/j.1600-0854.2005.00335.x. [DOI] [PubMed] [Google Scholar]
- 3.Oceguera-Yanez F, Kimura K, Yasuda S, Higashida C, Kitamura T, Hiraoka Y, Haraguchi T, Narumiya S. Ect2 and MgcRacGAP regulate the activation and function of Cdc42 in mitosis. J Cell Biol. 2005;168:221–32. doi: 10.1083/jcb.200408085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yasuda S, Oceguera-Yanez F, Kato T, Okamoto M, Yonemura S, Terada Y, Ishizaki T, Narumiya S. Cdc42 and mDia3 regulate microtubule attachment to kinetochores. Nature. 2004;428:767–71. doi: 10.1038/nature02452. [DOI] [PubMed] [Google Scholar]
- 5.Jordan SN, Canman JC. Rho GTPases in animal cell cytokinesis: an occupation by the one percent. Cytoskeleton (Hoboken) 2012;69:919–30. doi: 10.1002/cm.21071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirose K, Kawashima T, Iwamoto I, Nosaka T, Kitamura T. MgcRacGAP is involved in cytokinesis through associating with mitotic spindle and midbody. J Biol Chem. 2001;276:5821–8. doi: 10.1074/jbc.M007252200. [DOI] [PubMed] [Google Scholar]
- 7.Seguin L, Liot C, Mzali R, Harada R, Siret A, Nepveu A, Bertoglio J. CUX1 and E2F1 regulate coordinated expression of the mitotic complex genes Ect2, MgcRacGAP, and MKLP1 in S phase. Mol Cell Biol. 2009;29:570–81. doi: 10.1128/MCB.01275-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Su L, Agati JM, Parsons SJ. p190RhoGAP is cell cycle regulated and affects cytokinesis. J Cell Biol. 2003;163:571–82. doi: 10.1083/jcb.200308007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang YS, Maeda M, Okamoto M, Fujii M, Fukutomi R, Hori M, Tatsuka M, Ota T. Centrosomal localization of RhoGDIβ and its relevance to mitotic processes in cancer cells. Int J Oncol. 2013;42:460–8. doi: 10.3892/ijo.2012.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dao VT, Dupuy AG, Gavet O, Caron E, de Gunzburg J. Dynamic changes in Rap1 activity are required for cell retraction and spreading during mitosis. J Cell Sci. 2009;122:2996–3004. doi: 10.1242/jcs.041301. [DOI] [PubMed] [Google Scholar]
- 11.Kunda P, Pelling AE, Liu T, Baum B. Moesin controls cortical rigidity, cell rounding, and spindle morphogenesis during mitosis. Curr Biol. 2008;18:91–101. doi: 10.1016/j.cub.2007.12.051. [DOI] [PubMed] [Google Scholar]
- 12.Matthews HK, Delabre U, Rohn JL, Guck J, Kunda P, Baum B. Changes in Ect2 localization couple actomyosin-dependent cell shape changes to mitotic progression. Dev Cell. 2012;23:371–83. doi: 10.1016/j.devcel.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maddox AS, Burridge K. RhoA is required for cortical retraction and rigidity during mitotic cell rounding. J Cell Biol. 2003;160:255–65. doi: 10.1083/jcb.200207130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mali P, Wirtz D, Searson PC. Interplay of RhoA and motility in the programmed spreading of daughter cells postmitosis. Biophys J. 2010;99:3526–34. doi: 10.1016/j.bpj.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gavet O, Pines J. Progressive activation of CyclinB1-Cdk1 coordinates entry to mitosis. Dev Cell. 2010;18:533–43. doi: 10.1016/j.devcel.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hara T, Abe M, Inoue H, Yu LR, Veenstra TD, Kang YH, Lee KS, Miki T. Cytokinesis regulator ECT2 changes its conformation through phosphorylation at Thr-341 in G2/M phase. Oncogene. 2006;25:566–78. doi: 10.1038/sj.onc.1209078. [DOI] [PubMed] [Google Scholar]
- 17.Niiya F, Tatsumoto T, Lee KS, Miki T. Phosphorylation of the cytokinesis regulator ECT2 at G2/M phase stimulates association of the mitotic kinase Plk1 and accumulation of GTP-bound RhoA. Oncogene. 2006;25:827–37. doi: 10.1038/sj.onc.1209124. [DOI] [PubMed] [Google Scholar]
- 18.Chalamalasetty RB, Hümmer S, Nigg EA, Silljé HH. Influence of human Ect2 depletion and overexpression on cleavage furrow formation and abscission. J Cell Sci. 2006;119:3008–19. doi: 10.1242/jcs.03032. [DOI] [PubMed] [Google Scholar]
- 19.Kamijo K, Ohara N, Abe M, Uchimura T, Hosoya H, Lee JS, Miki T. Dissecting the role of Rho-mediated signaling in contractile ring formation. Mol Biol Cell. 2006;17:43–55. doi: 10.1091/mbc.E05-06-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim JE, Billadeau DD, Chen J. The tandem BRCT domains of Ect2 are required for both negative and positive regulation of Ect2 in cytokinesis. J Biol Chem. 2005;280:5733–9. doi: 10.1074/jbc.M409298200. [DOI] [PubMed] [Google Scholar]
- 21.Tatsumoto T, Xie X, Blumenthal R, Okamoto I, Miki T. Human ECT2 is an exchange factor for Rho GTPases, phosphorylated in G2/M phases, and involved in cytokinesis. J Cell Biol. 1999;147:921–8. doi: 10.1083/jcb.147.5.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yüce O, Piekny A, Glotzer M. An ECT2-centralspindlin complex regulates the localization and function of RhoA. J Cell Biol. 2005;170:571–82. doi: 10.1083/jcb.200501097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saito S, Liu XF, Kamijo K, Raziuddin R, Tatsumoto T, Okamoto I, Chen X, Lee CC, Lorenzi MV, Ohara N, et al. Deregulation and mislocalization of the cytokinesis regulator ECT2 activate the Rho signaling pathways leading to malignant transformation. J Biol Chem. 2004;279:7169–79. doi: 10.1074/jbc.M306725200. [DOI] [PubMed] [Google Scholar]
- 24.Frenette P, Haines E, Loloyan M, Kinal M, Pakarian P, Piekny A. An anillin-Ect2 complex stabilizes central spindle microtubules at the cortex during cytokinesis. PLoS One. 2012;7:e34888. doi: 10.1371/journal.pone.0034888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Su KC, Takaki T, Petronczki M. Targeting of the RhoGEF Ect2 to the equatorial membrane controls cleavage furrow formation during cytokinesis. Dev Cell. 2011;21:1104–15. doi: 10.1016/j.devcel.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 26.Helmke KJ, Heald R, Wilbur JD. Interplay between spindle architecture and function. Int Rev Cell Mol Biol. 2013;306:83–125. doi: 10.1016/B978-0-12-407694-5.00003-1. [DOI] [PubMed] [Google Scholar]
- 27.Rieder CL. Kinetochore fiber formation in animal somatic cells: dueling mechanisms come to a draw. Chromosoma. 2005;114:310–8. doi: 10.1007/s00412-005-0028-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Musacchio A, Salmon ED. The spindle-assembly checkpoint in space and time. Nat Rev Mol Cell Biol. 2007;8:379–93. doi: 10.1038/nrm2163. [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez-Fraticelli AE, Vergarajauregui S, Eastburn DJ, Datta A, Alonso MA, Mostov K, Martín-Belmonte F. The Cdc42 GEF Intersectin 2 controls mitotic spindle orientation to form the lumen during epithelial morphogenesis. J Cell Biol. 2010;189:725–38. doi: 10.1083/jcb.201002047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–69. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- 31.Jaffe AB, Kaji N, Durgan J, Hall A. Cdc42 controls spindle orientation to position the apical surface during epithelial morphogenesis. J Cell Biol. 2008;183:625–33. doi: 10.1083/jcb.200807121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kotak S, Gönczy P. Mechanisms of spindle positioning: cortical force generators in the limelight. Curr Opin Cell Biol. 2013;25:741–8. doi: 10.1016/j.ceb.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 33.Sütterlin C, Colanzi A. The Golgi and the centrosome: building a functional partnership. J Cell Biol. 2010;188:621–8. doi: 10.1083/jcb.200910001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sütterlin C, Polishchuk R, Pecot M, Malhotra V. The Golgi-associated protein GRASP65 regulates spindle dynamics and is essential for cell division. Mol Biol Cell. 2005;16:3211–22. doi: 10.1091/mbc.E04-12-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kodani A, Sütterlin C. The Golgi protein GM130 regulates centrosome morphology and function. Mol Biol Cell. 2008;19:745–53. doi: 10.1091/mbc.E07-08-0847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kodani A, Kristensen I, Huang L, Sütterlin C. GM130-dependent control of Cdc42 activity at the Golgi regulates centrosome organization. Mol Biol Cell. 2009;20:1192–200. doi: 10.1091/mbc.E08-08-0834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ban R, Irino Y, Fukami K, Tanaka H. Human mitotic spindle-associated protein PRC1 inhibits MgcRacGAP activity toward Cdc42 during the metaphase. J Biol Chem. 2004;279:16394–402. doi: 10.1074/jbc.M313257200. [DOI] [PubMed] [Google Scholar]
- 38.Gundersen GG. Evolutionary conservation of microtubule-capture mechanisms. Nat Rev Mol Cell Biol. 2002;3:296–304. doi: 10.1038/nrm777. [DOI] [PubMed] [Google Scholar]
- 39.Bader JR, Vaughan KT. Dynein at the kinetochore: Timing, Interactions and Functions. Semin Cell Dev Biol. 2010;21:269–75. doi: 10.1016/j.semcdb.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin SL, Qi ST, Sun SC, Wang YP, Schatten H, Sun QY. PAK1 regulates spindle microtubule organization during oocyte meiotic maturation. Front Biosci.(Elite.Ed) 2010; 2:1254-1264. [DOI] [PubMed] [Google Scholar]
- 41.Maroto B, Ye MB, von Lohneysen K, Schnelzer A, Knaus UG. P21-activated kinase is required for mitotic progression and regulates Plk1. Oncogene. 2008;27:4900–8. doi: 10.1038/onc.2008.131. [DOI] [PubMed] [Google Scholar]
- 42.Lagana A, Dorn JF, De Rop V, Ladouceur AM, Maddox AS, Maddox PS. A small GTPase molecular switch regulates epigenetic centromere maintenance by stabilizing newly incorporated CENP-A. Nat Cell Biol. 2010;12:1186–93. doi: 10.1038/ncb2129. [DOI] [PubMed] [Google Scholar]
- 43.Glotzer M. The molecular requirements for cytokinesis. Science. 2005;307:1735–9. doi: 10.1126/science.1096896. [DOI] [PubMed] [Google Scholar]
- 44.Skop AR, Liu H, Yates J, 3rd, Meyer BJ, Heald R. Dissection of the mammalian midbody proteome reveals conserved cytokinesis mechanisms. Science. 2004;305:61–6. doi: 10.1126/science.1097931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Julian M, Tollon Y, Lajoie-Mazenc I, Moisand A, Mazarguil H, Puget A, Wright M. gamma-Tubulin participates in the formation of the midbody during cytokinesis in mammalian cells. J Cell Sci. 1993;105:145–56. doi: 10.1242/jcs.105.1.145. [DOI] [PubMed] [Google Scholar]
- 46.Gromley A, Jurczyk A, Sillibourne J, Halilovic E, Mogensen M, Groisman I, Blomberg M, Doxsey S. A novel human protein of the maternal centriole is required for the final stages of cytokinesis and entry into S phase. J Cell Biol. 2003;161:535–45. doi: 10.1083/jcb.200301105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gromley A, Yeaman C, Rosa J, Redick S, Chen CT, Mirabelle S, Guha M, Sillibourne J, Doxsey SJ. Centriolin anchoring of exocyst and SNARE complexes at the midbody is required for secretory-vesicle-mediated abscission. Cell. 2005;123:75–87. doi: 10.1016/j.cell.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 48.Lordier L, Jalil A, Aurade F, Larbret F, Larghero J, Debili N, Vainchenker W, Chang Y. Megakaryocyte endomitosis is a failure of late cytokinesis related to defects in the contractile ring and Rho/Rock signaling. Blood. 2008;112:3164–74. doi: 10.1182/blood-2008-03-144956. [DOI] [PubMed] [Google Scholar]
- 49.Gao Y, Smith E, Ker E, Campbell P, Cheng EC, Zou S, Lin S, Wang L, Halene S, Krause DS. Role of RhoA-specific guanine exchange factors in regulation of endomitosis in megakaryocytes. Dev Cell. 2012;22:573–84. doi: 10.1016/j.devcel.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burgess DR, Chang F. Site selection for the cleavage furrow at cytokinesis. Trends Cell Biol. 2005;15:156–62. doi: 10.1016/j.tcb.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 51.Glotzer M. Cleavage furrow positioning. J Cell Biol. 2004;164:347–51. doi: 10.1083/jcb.200310112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Danowski BA. Fibroblast contractility and actin organization are stimulated by microtubule inhibitors. J Cell Sci. 1989;93:255–66. doi: 10.1242/jcs.93.2.255. [DOI] [PubMed] [Google Scholar]
- 53.Pletjushkina OJ, Rajfur Z, Pomorski P, Oliver TN, Vasiliev JM, Jacobson KA. Induction of cortical oscillations in spreading cells by depolymerization of microtubules. Cell Motil Cytoskeleton. 2001;48:235–44. doi: 10.1002/cm.1012. [DOI] [PubMed] [Google Scholar]
- 54.Ren XD, Kiosses WB, Schwartz MA. Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 1999;18:578–85. doi: 10.1093/emboj/18.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Canman JC, Bement WM. Microtubules suppress actomyosin-based cortical flow in Xenopus oocytes. J Cell Sci. 1997;110:1907–17. doi: 10.1242/jcs.110.16.1907. [DOI] [PubMed] [Google Scholar]
- 56.Werner M, Munro E, Glotzer M. Astral signals spatially bias cortical myosin recruitment to break symmetry and promote cytokinesis. Curr Biol. 2007;17:1286–97. doi: 10.1016/j.cub.2007.06.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Canman JC, Cameron LA, Maddox PS, Straight A, Tirnauer JS, Mitchison TJ, Fang G, Kapoor TM, Salmon ED. Determining the position of the cell division plane. Nature. 2003;424:1074–8. doi: 10.1038/nature01860. [DOI] [PubMed] [Google Scholar]
- 58.Rappaport R. Establishment of the mechanism of cytokinesis in animal cells. Int Rev Cytol. 1986;105:245–81. doi: 10.1016/S0074-7696(08)61065-7. [DOI] [PubMed] [Google Scholar]
- 59.Bringmann H, Hyman AA. A cytokinesis furrow is positioned by two consecutive signals. Nature. 2005;436:731–4. doi: 10.1038/nature03823. [DOI] [PubMed] [Google Scholar]
- 60.Dechant R, Glotzer M. Centrosome separation and central spindle assembly act in redundant pathways that regulate microtubule density and trigger cleavage furrow formation. Dev Cell. 2003;4:333–44. doi: 10.1016/S1534-5807(03)00057-1. [DOI] [PubMed] [Google Scholar]
- 61.Drechsel DN, Hyman AA, Hall A, Glotzer M. A requirement for Rho and Cdc42 during cytokinesis in Xenopus embryos. Curr Biol. 1997;7:12–23. doi: 10.1016/S0960-9822(06)00023-6. [DOI] [PubMed] [Google Scholar]
- 62.Jantsch-Plunger V, Gönczy P, Romano A, Schnabel H, Hamill D, Schnabel R, Hyman AA, Glotzer M. CYK-4: A Rho family gtpase activating protein (GAP) required for central spindle formation and cytokinesis. J Cell Biol. 2000;149:1391–404. doi: 10.1083/jcb.149.7.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kishi K, Sasaki T, Kuroda S, Itoh T, Takai Y. Regulation of cytoplasmic division of Xenopus embryo by rho p21 and its inhibitory GDP/GTP exchange protein (rho GDI) J Cell Biol. 1993;120:1187–95. doi: 10.1083/jcb.120.5.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Piekny A, Werner M, Glotzer M. Cytokinesis: welcome to the Rho zone. Trends Cell Biol. 2005;15:651–8. doi: 10.1016/j.tcb.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 65.Bement WM, Benink HA, von Dassow G. A microtubule-dependent zone of active RhoA during cleavage plane specification. J Cell Biol. 2005;170:91–101. doi: 10.1083/jcb.200501131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nishimura Y, Yonemura S. Centralspindlin regulates ECT2 and RhoA accumulation at the equatorial cortex during cytokinesis. J Cell Sci. 2006;119:104–14. doi: 10.1242/jcs.02737. [DOI] [PubMed] [Google Scholar]
- 67.Yonemura S, Hirao-Minakuchi K, Nishimura Y. Rho localization in cells and tissues. Exp Cell Res. 2004;295:300–14. doi: 10.1016/j.yexcr.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 68.Yoshizaki H, Ohba Y, Kurokawa K, Itoh RE, Nakamura T, Mochizuki N, Nagashima K, Matsuda M. Activity of Rho-family GTPases during cell division as visualized with FRET-based probes. J Cell Biol. 2003;162:223–32. doi: 10.1083/jcb.200212049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mishima M, Kaitna S, Glotzer M. Central spindle assembly and cytokinesis require a kinesin-like protein/RhoGAP complex with microtubule bundling activity. Dev Cell. 2002;2:41–54. doi: 10.1016/S1534-5807(01)00110-1. [DOI] [PubMed] [Google Scholar]
- 70.Somers WG, Saint R. A RhoGEF and Rho family GTPase-activating protein complex links the contractile ring to cortical microtubules at the onset of cytokinesis. Dev Cell. 2003;4:29–39. doi: 10.1016/S1534-5807(02)00402-1. [DOI] [PubMed] [Google Scholar]
- 71.Matuliene J, Kuriyama R. Role of the midbody matrix in cytokinesis: RNAi and genetic rescue analysis of the mammalian motor protein CHO1. Mol Biol Cell. 2004;15:3083–94. doi: 10.1091/mbc.E03-12-0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mollinari C, Kleman JP, Jiang W, Schoehn G, Hunter T, Margolis RL. PRC1 is a microtubule binding and bundling protein essential to maintain the mitotic spindle midzone. J Cell Biol. 2002;157:1175–86. doi: 10.1083/jcb.200111052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lekomtsev S, Su KC, Pye VE, Blight K, Sundaramoorthy S, Takaki T, Collinson LM, Cherepanov P, Divecha N, Petronczki M. Centralspindlin links the mitotic spindle to the plasma membrane during cytokinesis. Nature. 2012;492:276–9. doi: 10.1038/nature11773. [DOI] [PubMed] [Google Scholar]
- 74.Birkenfeld J, Nalbant P, Bohl BP, Pertz O, Hahn KM, Bokoch GM. GEF-H1 modulates localized RhoA activation during cytokinesis under the control of mitotic kinases. Dev Cell. 2007;12:699–712. doi: 10.1016/j.devcel.2007.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Asiedu M, Wu D, Matsumura F, Wei Q. Centrosome/spindle pole-associated protein regulates cytokinesis via promoting the recruitment of MyoGEF to the central spindle. Mol Biol Cell. 2009;20:1428–40. doi: 10.1091/mbc.E08-01-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Asiedu M, Wu D, Matsumura F, Wei Q. Phosphorylation of MyoGEF on Thr-574 by Plk1 promotes MyoGEF localization to the central spindle. J Biol Chem. 2008;283:28392–400. doi: 10.1074/jbc.M801801200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wu D, Asiedu M, Adelstein RS, Wei Q. A novel guanine nucleotide exchange factor MyoGEF is required for cytokinesis. Cell Cycle. 2006;5:1234–9. doi: 10.4161/cc.5.11.2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Loria A, Longhini KM, Glotzer M. The RhoGAP domain of CYK-4 has an essential role in RhoA activation. Curr Biol. 2012;22:213–9. doi: 10.1016/j.cub.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Miller AL, Bement WM. Regulation of cytokinesis by Rho GTPase flux. Nat Cell Biol. 2009;11:71–7. doi: 10.1038/ncb1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Burkard ME, Maciejowski J, Rodriguez-Bravo V, Repka M, Lowery DM, Clauser KR, Zhang C, Shokat KM, Carr SA, Yaffe MB, et al. Plk1 self-organization and priming phosphorylation of HsCYK-4 at the spindle midzone regulate the onset of division in human cells. PLoS Biol. 2009;7:e1000111. doi: 10.1371/journal.pbio.1000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wolfe BA, Takaki T, Petronczki M, Glotzer M. Polo-like kinase 1 directs assembly of the HsCyk-4 RhoGAP/Ect2 RhoGEF complex to initiate cleavage furrow formation. PLoS Biol. 2009;7:e1000110. doi: 10.1371/journal.pbio.1000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Touré A, Dorseuil O, Morin L, Timmons P, Jégou B, Reibel L, Gacon G. MgcRacGAP, a new human GTPase-activating protein for Rac and Cdc42 similar to Drosophila rotundRacGAP gene product, is expressed in male germ cells. J Biol Chem. 1998;273:6019–23. doi: 10.1074/jbc.273.11.6019. [DOI] [PubMed] [Google Scholar]
- 83.D’Avino PP, Savoian MS, Glover DM. Mutations in sticky lead to defective organization of the contractile ring during cytokinesis and are enhanced by Rho and suppressed by Rac. J Cell Biol. 2004;166:61–71. doi: 10.1083/jcb.200402157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bastos RN, Penate X, Bates M, Hammond D, Barr FA. CYK4 inhibits Rac1-dependent PAK1 and ARHGEF7 effector pathways during cytokinesis. J Cell Biol. 2012;198:865–80. doi: 10.1083/jcb.201204107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zanin E, Desai A, Poser I, Toyoda Y, Andree C, Moebius C, Bickle M, Conradt B, Piekny A, Oegema K. A conserved RhoGAP limits M phase contractility and coordinates with microtubule asters to confine RhoA during cytokinesis. Dev Cell. 2013;26:496–510. doi: 10.1016/j.devcel.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Maupin P, Pollard TD. Arrangement of actin filaments and myosin-like filaments in the contractile ring and of actin-like filaments in the mitotic spindle of dividing HeLa cells. J Ultrastruct Mol Struct Res. 1986;94:92–103. doi: 10.1016/0889-1605(86)90055-8. [DOI] [PubMed] [Google Scholar]
- 87.Ikebe M, Koretz J, Hartshorne DJ. Effects of phosphorylation of light chain residues threonine 18 and serine 19 on the properties and conformation of smooth muscle myosin. J Biol Chem. 1988;263:6432–7. [PubMed] [Google Scholar]
- 88.Komatsu S, Yano T, Shibata M, Tuft RA, Ikebe M. Effects of the regulatory light chain phosphorylation of myosin II on mitosis and cytokinesis of mammalian cells. J Biol Chem. 2000;275:34512–20. doi: 10.1074/jbc.M003019200. [DOI] [PubMed] [Google Scholar]
- 89.Yamakita Y, Yamashiro S, Matsumura F. In vivo phosphorylation of regulatory light chain of myosin II during mitosis of cultured cells. J Cell Biol. 1994;124:129–37. doi: 10.1083/jcb.124.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Scholey JM, Taylor KA, Kendrick-Jones J. Regulation of non-muscle myosin assembly by calmodulin-dependent light chain kinase. Nature. 1980;287:233–5. doi: 10.1038/287233a0. [DOI] [PubMed] [Google Scholar]
- 91.Field CM, al-Awar O, Rosenblatt J, Wong ML, Alberts B, Mitchison TJ. A purified Drosophila septin complex forms filaments and exhibits GTPase activity. J Cell Biol. 1996;133:605–16. doi: 10.1083/jcb.133.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kinoshita M, Kumar S, Mizoguchi A, Ide C, Kinoshita A, Haraguchi T, Hiraoka Y, Noda M. Nedd5, a mammalian septin, is a novel cytoskeletal component interacting with actin-based structures. Genes Dev. 1997;11:1535–47. doi: 10.1101/gad.11.12.1535. [DOI] [PubMed] [Google Scholar]
- 93.Kinoshita M, Field CM, Coughlin ML, Straight AF, Mitchison TJ. Self- and actin-templated assembly of Mammalian septins. Dev Cell. 2002;3:791–802. doi: 10.1016/S1534-5807(02)00366-0. [DOI] [PubMed] [Google Scholar]
- 94.Nagata K, Kawajiri A, Matsui S, Takagishi M, Shiromizu T, Saitoh N, Izawa I, Kiyono T, Itoh TJ, Hotani H, et al. Filament formation of MSF-A, a mammalian septin, in human mammary epithelial cells depends on interactions with microtubules. J Biol Chem. 2003;278:18538–43. doi: 10.1074/jbc.M205246200. [DOI] [PubMed] [Google Scholar]
- 95.Surka MC, Tsang CW, Trimble WS. The mammalian septin MSF localizes with microtubules and is required for completion of cytokinesis. Mol Biol Cell. 2002;13:3532–45. doi: 10.1091/mbc.E02-01-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Xie H, Surka M, Howard J, Trimble WS. Characterization of the mammalian septin H5: distinct patterns of cytoskeletal and membrane association from other septin proteins. Cell Motil Cytoskeleton. 1999;43:52–62. doi: 10.1002/(SICI)1097-0169(1999)43:1<52::AID-CM6>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 97.Zhang J, Kong C, Xie H, McPherson PS, Grinstein S, Trimble WS. Phosphatidylinositol polyphosphate binding to the mammalian septin H5 is modulated by GTP. Curr Biol. 1999;9:1458–67. doi: 10.1016/S0960-9822(00)80115-3. [DOI] [PubMed] [Google Scholar]
- 98.Neufeld TP, Rubin GM. The Drosophila peanut gene is required for cytokinesis and encodes a protein similar to yeast putative bud neck filament proteins. Cell. 1994;77:371–9. doi: 10.1016/0092-8674(94)90152-X. [DOI] [PubMed] [Google Scholar]
- 99.Nguyen TQ, Sawa H, Okano H, White JG. The C. elegans septin genes, unc-59 and unc-61, are required for normal postembryonic cytokineses and morphogenesis but have no essential function in embryogenesis. J Cell Sci. 2000;113:3825–37. doi: 10.1242/jcs.113.21.3825. [DOI] [PubMed] [Google Scholar]
- 100.Kinoshita M. The septins. Genome Biol. 2003;4:236. doi: 10.1186/gb-2003-4-11-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Oegema K, Savoian MS, Mitchison TJ, Field CM. Functional analysis of a human homologue of the Drosophila actin binding protein anillin suggests a role in cytokinesis. J Cell Biol. 2000;150:539–52. doi: 10.1083/jcb.150.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.El Amine N, Kechad A, Jananji S, Hickson GR. Opposing actions of septins and Sticky on Anillin promote the transition from contractile to midbody ring. J Cell Biol. 2013;203:487–504. doi: 10.1083/jcb.201305053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kechad A, Jananji S, Ruella Y, Hickson GR. Anillin acts as a bifunctional linker coordinating midbody ring biogenesis during cytokinesis. Curr Biol. 2012;22:197–203. doi: 10.1016/j.cub.2011.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dobbelaere J, Barral Y. Spatial coordination of cytokinetic events by compartmentalization of the cell cortex. Science. 2004;305:393–6. doi: 10.1126/science.1099892. [DOI] [PubMed] [Google Scholar]
- 105.Schmidt K, Nichols BJ. A barrier to lateral diffusion in the cleavage furrow of dividing mammalian cells. Curr Biol. 2004;14:1002–6. doi: 10.1016/j.cub.2004.05.044. [DOI] [PubMed] [Google Scholar]
- 106.Li F, Higgs HN. The mouse Formin mDia1 is a potent actin nucleation factor regulated by autoinhibition. Curr Biol. 2003;13:1335–40. doi: 10.1016/S0960-9822(03)00540-2. [DOI] [PubMed] [Google Scholar]
- 107.Alberts AS. Identification of a carboxyl-terminal diaphanous-related formin homology protein autoregulatory domain. J Biol Chem. 2001;276:2824–30. doi: 10.1074/jbc.M006205200. [DOI] [PubMed] [Google Scholar]
- 108.Otomo T, Otomo C, Tomchick DR, Machius M, Rosen MK. Structural basis of Rho GTPase-mediated activation of the formin mDia1. Mol Cell. 2005;18:273–81. doi: 10.1016/j.molcel.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 109.Rose R, Weyand M, Lammers M, Ishizaki T, Ahmadian MR, Wittinghofer A. Structural and mechanistic insights into the interaction between Rho and mammalian Dia. Nature. 2005;435:513–8. doi: 10.1038/nature03604. [DOI] [PubMed] [Google Scholar]
- 110.Watanabe N, Madaule P, Reid T, Ishizaki T, Watanabe G, Kakizuka A, Saito Y, Nakao K, Jockusch BM, Narumiya S. p140mDia, a mammalian homolog of Drosophila diaphanous, is a target protein for Rho small GTPase and is a ligand for profilin. EMBO J. 1997;16:3044–56. doi: 10.1093/emboj/16.11.3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tominaga T, Sahai E, Chardin P, McCormick F, Courtneidge SA, Alberts AS. Diaphanous-related formins bridge Rho GTPase and Src tyrosine kinase signaling. Mol Cell. 2000;5:13–25. doi: 10.1016/S1097-2765(00)80399-8. [DOI] [PubMed] [Google Scholar]
- 112.Kasahara K, Nakayama Y, Nakazato Y, Ikeda K, Kuga T, Yamaguchi N. Src signaling regulates completion of abscission in cytokinesis through ERK/MAPK activation at the midbody. J Biol Chem. 2007;282:5327–39. doi: 10.1074/jbc.M608396200. [DOI] [PubMed] [Google Scholar]
- 113.Guha M, Zhou M, Wang YL. Cortical actin turnover during cytokinesis requires myosin II. Curr Biol. 2005;15:732–6. doi: 10.1016/j.cub.2005.03.042. [DOI] [PubMed] [Google Scholar]
- 114.Straight AF, Cheung A, Limouze J, Chen I, Westwood NJ, Sellers JR, Mitchison TJ. Dissecting temporal and spatial control of cytokinesis with a myosin II Inhibitor. Science. 2003;299:1743–7. doi: 10.1126/science.1081412. [DOI] [PubMed] [Google Scholar]
- 115.Zang JH, Spudich JA. Myosin II localization during cytokinesis occurs by a mechanism that does not require its motor domain. Proc Natl Acad Sci U S A. 1998;95:13652–7. doi: 10.1073/pnas.95.23.13652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dean SO, Rogers SL, Stuurman N, Vale RD, Spudich JA. Distinct pathways control recruitment and maintenance of myosin II at the cleavage furrow during cytokinesis. Proc Natl Acad Sci U S A. 2005;102:13473–8. doi: 10.1073/pnas.0506810102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dean SO, Spudich JA. Rho kinase’s role in myosin recruitment to the equatorial cortex of mitotic Drosophila S2 cells is for myosin regulatory light chain phosphorylation. PLoS One. 2006;1:e131. doi: 10.1371/journal.pone.0000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Madaule P, Eda M, Watanabe N, Fujisawa K, Matsuoka T, Bito H, Ishizaki T, Narumiya S. Role of citron kinase as a target of the small GTPase Rho in cytokinesis. Nature. 1998;394:491–4. doi: 10.1038/28873. [DOI] [PubMed] [Google Scholar]
- 119.Kosako H, Yoshida T, Matsumura F, Ishizaki T, Narumiya S, Inagaki M. Rho-kinase/ROCK is involved in cytokinesis through the phosphorylation of myosin light chain and not ezrin/radixin/moesin proteins at the cleavage furrow. Oncogene. 2000;19:6059–64. doi: 10.1038/sj.onc.1203987. [DOI] [PubMed] [Google Scholar]
- 120.Yamashiro S, Totsukawa G, Yamakita Y, Sasaki Y, Madaule P, Ishizaki T, Narumiya S, Matsumura F. Citron kinase, a Rho-dependent kinase, induces di-phosphorylation of regulatory light chain of myosin II. Mol Biol Cell. 2003;14:1745–56. doi: 10.1091/mbc.E02-07-0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, et al. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–8. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- 122.Kawano Y, Fukata Y, Oshiro N, Amano M, Nakamura T, Ito M, Matsumura F, Inagaki M, Kaibuchi K. Phosphorylation of myosin-binding subunit (MBS) of myosin phosphatase by Rho-kinase in vivo. J Cell Biol. 1999;147:1023–38. doi: 10.1083/jcb.147.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yokoyama T, Goto H, Izawa I, Mizutani H, Inagaki M. Aurora-B and Rho-kinase/ROCK, the two cleavage furrow kinases, independently regulate the progression of cytokinesis: possible existence of a novel cleavage furrow kinase phosphorylates ezrin/radixin/moesin (ERM) Genes Cells. 2005;10:127–37. doi: 10.1111/j.1365-2443.2005.00824.x. [DOI] [PubMed] [Google Scholar]
- 124.Bassi ZI, Verbrugghe KJ, Capalbo L, Gregory S, Montembault E, Glover DM, D’Avino PP. Sticky/Citron kinase maintains proper RhoA localization at the cleavage site during cytokinesis. J Cell Biol. 2011;195:595–603. doi: 10.1083/jcb.201105136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gai M, Camera P, Dema A, Bianchi F, Berto G, Scarpa E, Germena G, Di Cunto F. Citron kinase controls abscission through RhoA and anillin. Mol Biol Cell. 2011;22:3768–78. doi: 10.1091/mbc.E10-12-0952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Watanabe S, De Zan T, Ishizaki T, Narumiya S. Citron kinase mediates transition from constriction to abscission through its coiled-coil domain. J Cell Sci. 2013;126:1773–84. doi: 10.1242/jcs.116608. [DOI] [PubMed] [Google Scholar]
- 127.Hickson GR, O’Farrell PH. Rho-dependent control of anillin behavior during cytokinesis. J Cell Biol. 2008;180:285–94. doi: 10.1083/jcb.200709005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Piekny AJ, Glotzer M. Anillin is a scaffold protein that links RhoA, actin, and myosin during cytokinesis. Curr Biol. 2008;18:30–6. doi: 10.1016/j.cub.2007.11.068. [DOI] [PubMed] [Google Scholar]
- 129.Prokopenko SN, Brumby A, O’Keefe L, Prior L, He Y, Saint R, Bellen HJ. A putative exchange factor for Rho1 GTPase is required for initiation of cytokinesis in Drosophila. Genes Dev. 1999;13:2301–14. doi: 10.1101/gad.13.17.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhao WM, Fang G. Anillin is a substrate of anaphase-promoting complex/cyclosome (APC/C) that controls spatial contractility of myosin during late cytokinesis. J Biol Chem. 2005;280:33516–24. doi: 10.1074/jbc.M504657200. [DOI] [PubMed] [Google Scholar]
- 131.Gregory SL, Ebrahimi S, Milverton J, Jones WM, Bejsovec A, Saint R. Cell division requires a direct link between microtubule-bound RacGAP and Anillin in the contractile ring. Curr Biol. 2008;18:25–9. doi: 10.1016/j.cub.2007.11.050. [DOI] [PubMed] [Google Scholar]
- 132.Shu HB, Li Z, Palacios MJ, Li Q, Joshi HC. A transient association of gamma-tubulin at the midbody is required for the completion of cytokinesis during the mammalian cell division. J Cell Sci. 1995;108:2955–62. doi: 10.1242/jcs.108.9.2955. [DOI] [PubMed] [Google Scholar]
- 133.Ettinger AW, Wilsch-Bräuninger M, Marzesco AM, Bickle M, Lohmann A, Maliga Z, Karbanová J, Corbeil D, Hyman AA, Huttner WB. Proliferating versus differentiating stem and cancer cells exhibit distinct midbody-release behaviour. Nat Commun. 2011;2:503. doi: 10.1038/ncomms1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kuo TC, Chen CT, Baron D, Onder TT, Loewer S, Almeida S, Weismann CM, Xu P, Houghton JM, Gao FB, et al. Midbody accumulation through evasion of autophagy contributes to cellular reprogramming and tumorigenicity. Nat Cell Biol. 2011;13:1214–23. doi: 10.1038/ncb2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Green RA, Mayers JR, Wang S, Lewellyn L, Desai A, Audhya A, Oegema K. The midbody ring scaffolds the abscission machinery in the absence of midbody microtubules. J Cell Biol. 2013;203:505–20. doi: 10.1083/jcb.201306036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Steigemann P, Gerlich DW. Cytokinetic abscission: cellular dynamics at the midbody. Trends Cell Biol. 2009;19:606–16. doi: 10.1016/j.tcb.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 137.Mollinari C, Kleman JP, Saoudi Y, Jablonski SA, Perard J, Yen TJ, Margolis RL. Ablation of PRC1 by small interfering RNA demonstrates that cytokinetic abscission requires a central spindle bundle in mammalian cells, whereas completion of furrowing does not. Mol Biol Cell. 2005;16:1043–55. doi: 10.1091/mbc.E04-04-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Elia N, Sougrat R, Spurlin TA, Hurley JH, Lippincott-Schwartz J. Dynamics of endosomal sorting complex required for transport (ESCRT) machinery during cytokinesis and its role in abscission. Proc Natl Acad Sci U S A. 2011;108:4846–51. doi: 10.1073/pnas.1102714108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Guizetti J, Schermelleh L, Mäntler J, Maar S, Poser I, Leonhardt H, Müller-Reichert T, Gerlich DW. Cortical constriction during abscission involves helices of ESCRT-III-dependent filaments. Science. 2011;331:1616–20. doi: 10.1126/science.1201847. [DOI] [PubMed] [Google Scholar]
- 140.Schiel JA, Park K, Morphew MK, Reid E, Hoenger A, Prekeris R. Endocytic membrane fusion and buckling-induced microtubule severing mediate cell abscission. J Cell Sci. 2011;124:1411–24. doi: 10.1242/jcs.081448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Schiel JA, Simon GC, Zaharris C, Weisz J, Castle D, Wu CC, Prekeris R. FIP3-endosome-dependent formation of the secondary ingression mediates ESCRT-III recruitment during cytokinesis. Nat Cell Biol. 2012;14:1068–78. doi: 10.1038/ncb2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Minoshima Y, Kawashima T, Hirose K, Tonozuka Y, Kawajiri A, Bao YC, Deng X, Tatsuka M, Narumiya S, May WS, Jr., et al. Phosphorylation by aurora B converts MgcRacGAP to a RhoGAP during cytokinesis. Dev Cell. 2003;4:549–60. doi: 10.1016/S1534-5807(03)00089-3. [DOI] [PubMed] [Google Scholar]
- 143.Fielding AB, Schonteich E, Matheson J, Wilson G, Yu X, Hickson GR, Srivastava S, Baldwin SA, Prekeris R, Gould GW. Rab11-FIP3 and FIP4 interact with Arf6 and the exocyst to control membrane traffic in cytokinesis. EMBO J. 2005;24:3389–99. doi: 10.1038/sj.emboj.7600803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wilson GM, Fielding AB, Simon GC, Yu X, Andrews PD, Hames RS, Frey AM, Peden AA, Gould GW, Prekeris R. The FIP3-Rab11 protein complex regulates recycling endosome targeting to the cleavage furrow during late cytokinesis. Mol Biol Cell. 2005;16:849–60. doi: 10.1091/mbc.E04-10-0927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Carlton JG, Agromayor M, Martin-Serrano J. Differential requirements for Alix and ESCRT-III in cytokinesis and HIV-1 release. Proc Natl Acad Sci U S A. 2008;105:10541–6. doi: 10.1073/pnas.0802008105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Morita E, Sandrin V, Chung HY, Morham SG, Gygi SP, Rodesch CK, Sundquist WI. Human ESCRT and ALIX proteins interact with proteins of the midbody and function in cytokinesis. EMBO J. 2007;26:4215–27. doi: 10.1038/sj.emboj.7601850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Raiborg C, Stenmark H. The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature. 2009;458:445–52. doi: 10.1038/nature07961. [DOI] [PubMed] [Google Scholar]
- 148.Hanson PI, Roth R, Lin Y, Heuser JE. Plasma membrane deformation by circular arrays of ESCRT-III protein filaments. J Cell Biol. 2008;180:389–402. doi: 10.1083/jcb.200707031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Carlton JG, Martin-Serrano J. Parallels between cytokinesis and retroviral budding: a role for the ESCRT machinery. Science. 2007;316:1908–12. doi: 10.1126/science.1143422. [DOI] [PubMed] [Google Scholar]
- 150.Connell JW, Lindon C, Luzio JP, Reid E. Spastin couples microtubule severing to membrane traffic in completion of cytokinesis and secretion. Traffic. 2009;10:42–56. doi: 10.1111/j.1600-0854.2008.00847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Simon GC, Prekeris R. Mechanisms regulating targeting of recycling endosomes to the cleavage furrow during cytokinesis. Biochem Soc Trans. 2008;36:391–4. doi: 10.1042/BST0360391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Simon GC, Schonteich E, Wu CC, Piekny A, Ekiert D, Yu X, Gould GW, Glotzer M, Prekeris R. Sequential Cyk-4 binding to ECT2 and FIP3 regulates cleavage furrow ingression and abscission during cytokinesis. EMBO J. 2008;27:1791–803. doi: 10.1038/emboj.2008.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Liot C, Seguin L, Siret A, Crouin C, Schmidt S, Bertoglio J. APC(cdh1) mediates degradation of the oncogenic Rho-GEF Ect2 after mitosis. PLoS One. 2011;6:e23676. doi: 10.1371/journal.pone.0023676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Keil R, Kiessling C, Hatzfeld M. Targeting of p0071 to the midbody depends on KIF3. J Cell Sci. 2009;122:1174–83. doi: 10.1242/jcs.045377. [DOI] [PubMed] [Google Scholar]
- 155.Martz MK, Grabocka E, Beeharry N, Yen TJ, Wedegaertner PB. Leukemia-associated RhoGEF (LARG) is a novel RhoGEF in cytokinesis and required for the proper completion of abscission. Mol Biol Cell. 2013;24:2785–94. doi: 10.1091/mbc.E12-07-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wolf A, Keil R, Götzl O, Mun A, Schwarze K, Lederer M, Hüttelmaier S, Hatzfeld M. The armadillo protein p0071 regulates Rho signalling during cytokinesis. Nat Cell Biol. 2006;8:1432–40. doi: 10.1038/ncb1504. [DOI] [PubMed] [Google Scholar]
- 157.Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–10. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 158.Pollard TD. Regulation of actin filament assembly by Arp2/3 complex and formins. Annu Rev Biophys Biomol Struct. 2007;36:451–77. doi: 10.1146/annurev.biophys.35.040405.101936. [DOI] [PubMed] [Google Scholar]
- 159.Yoshizaki H, Ohba Y, Parrini MC, Dulyaninova NG, Bresnick AR, Mochizuki N, Matsuda M. Cell type-specific regulation of RhoA activity during cytokinesis. J Biol Chem. 2004;279:44756–62. doi: 10.1074/jbc.M402292200. [DOI] [PubMed] [Google Scholar]
- 160.Canman JC, Lewellyn L, Laband K, Smerdon SJ, Desai A, Bowerman B, Oegema K. Inhibition of Rac by the GAP activity of centralspindlin is essential for cytokinesis. Science. 2008;322:1543–6. doi: 10.1126/science.1163086. [DOI] [PMC free article] [PubMed] [Google Scholar]