Abstract
Adolescents’ peer experiences may have significant associations with biological stress-response systems, adding to or reducing allostatic load. This study examined relational victimization as a unique contributor to reactive hypothalamic–pituitary–adrenal (HPA) axis responses as well as friendship quality and behavior as factors that may promote HPA recovery following a stressor. A total of 62 adolescents (ages 12–16; 73% female) presenting with a wide range of life stressors and adjustment difficulties completed survey measures of peer victimization and friendship quality. Cortisol samples were collected before and after a lab-based interpersonally themed social stressor task to provide measures of HPA baseline, reactivity, and recovery. Following the stressor task, adolescents discussed their performance with a close friend; observational coding yielded measures of friends’ responsiveness. Adolescents also reported positive and negative friendship qualities. Results suggested that higher levels of adolescents’ relational victimization were associated with blunted cortisol reactivity, even after controlling for physical forms of victimization and other known predictors of HPA functioning (i.e., life stress or depressive symptoms). Friendship qualities (i.e., low negative qualities) and specific friendship behaviors (i.e., high levels of responsiveness) contributed to greater HPA regulation; however, consistent with theories of rumination, high friend responsiveness in the context of high levels of positive friendship quality contributed to less cortisol recovery. Findings extend prior work on the importance of relational victimization and dyadic peer relations as unique and salient correlates of adaptation in adolescence.
Among her many contributions to developmental psychopathology theory and research, Nicki Crick highlighted specific peer experiences that were relevant for understanding adjustment in youth. Perhaps her most well-known contributions were in the area of relational aggression and victimization. Aversive peer experiences had been studied for decades, mostly focused on physical acts among peers that might confer unique risks for adjustment difficulties (Olweus, 1977, 1978). However, it was a series of Crick’s seminal publications (e.g., 1995, 1996; Crick & Grotpeter, 1995, 1996) that helped to present a broadened definition of aggression and victimization into the mainstream. As compared to physical acts of peer aggression, relational aggression is characterized by behaviors that threaten one’s dyadic relationships (e.g., threatening friendship withdrawal or “silent treatments”) or social reputation within the larger peer group (e.g., gossip or social exclusion; Bjorkqvist, Lagerspetz, & Kaukiainen, 1992; Crick, 1995; Crick & Grotpeter, 1995; Galen & Underwood, 1997; Lagerspetz, Bjorkqvist, & Peltonen, 1988). Relational forms of aggression have distinct determinants and consequences from physical forms of aggression, and they appear to be relevant correlates of psychological adjustment across the age span (Mathieson & Crick, 2010; Ostrov & Godleski, 2010; Prinstein, Boergers, & Vernberg, 2001). An emerging area of research is the identification of relational victimization correlates after accounting for associations with physical victimization (Ostrov,2010; Sullivan, Farrell, & Kliewer, 2006).
Peer victimization is a significant source of social stress. Thus, it is important to consider peer victimization in the broader context of youth stress and stress responses. In particular, recent work has emphasized the need to understand biological stress-response systems as a potential source of vulnerability to psychopathology (Hostinar & Gunnar,2013). Perhaps most frequently examined has been the reactive stress response of the hypothalamic–pituitary–adrenal (HPA) axis. The HPA axis is activated when an environmental stressor is perceived. The HPA stress response begins with a release of corticotrophin-releasing hormone and arginine vasopressin from the hypothalamus, which causes the pituitary gland to release adrenocorticotropic hormone. In the final stages of the HPA stress response, the adrenocorticotropic hormone causes the adrenal glands to release glucocorticoids (Chrousos & Gold, 1992; Kaltas & Chrousos, 2007). Cortisol, one such glucocorticoid, often is sampled from saliva prior to, during, and following a stressor to provide indices of reactive HPA stress responses. Although diurnal cortisol assessments (i.e., cortisol patterns throughout the day) provide indication of general allostatic load, examinations of stress-induced changes in cortisol provide insight regarding individuals’ biological preparedness to respond to acute stress. Reactive HPA responses to stressors prepare an individual to behaviorally respond to stressors in several ways, including an increase in the availability and mobility of energy (i.e., glucose), increased blood circulation, and enhanced cognition (Sapolsky, Romero, & Munch, 2000). However, dysregulated HPA responses, including both hyper- and hyporeactive stress responses, are believed to reflect high allostatic load (i.e., the cumulative physiological and psychological effects of managing environmental stressors; McEwen & Stellar, 1993). High allostatic load is believed to diminish one’s cognitive and biological capacity to generate adaptive behavioral stress responses (Juster, McEwen, & Lupien,2009; McEwen, 1998, 2000). Though HPA recovery following a stressor is relatively understudied, the ability to return to baseline HPA functioning is important for preventing exhaustion of the stress-response system and subsequent increases in allostatic load (McEwen, 2000).
Numerous studies have examined social–psychological experiences that may be associated with dysregulated reactive HPA axis responses. For instance, findings indicate that a history of significant life stressors and current depressive symptoms are especially relevant predictors of hyperreactive or hyporeactive HPA stress responses, both of which indicate HPA dysregulation and high allostatic load (for reviews, see Guerry & Hastings, 2011; Lopez-Duran, Kovacs, & George,2009; Lupien, McEwen, Gunnar, & Heim, 2009; Miller, Chen, & Zhou, 2007). Fewer studies have considered environmental factors that may buffer or exacerbate atypical HPA responses to stress during adolescence. This is unfortunate given that adolescence is a time of potential recalibration or instantiation of stress-reactive biological responses (Ganzel & Morris, 2011), and the HPA axis is especially attuned to social stressors during this same developmental period (Stroud et al., 2009).
Relational victimization experiences may be an especially relevant predictor of adolescents’ reactive HPA stress responses, contributing to allostatic load and diminishing healthy stress responses. Although physical forms of peer victimization establish dominance and submission hierarchies, relational victimization is unique in its focus specifically on social inclusion and exclusion. Evolutionary and biological theories suggest that adolescence is accompanied by a unique set of responses to social inclusion threats. Evolutionary theory suggests that brain structure and function may have developed to signal threats to social inclusion (i.e., “social pain”) in the same manner (i.e., in the same brain regions) as for physical pain (Eisenberger & Lieberman, 2004; MacDonald & Leary, 2005) to ensure access to social resources. Several biological changes in adolescence also have unique implications for the processing of social stimuli. For instance, increased production of gonadal hormones during puberty leads to more oxytocin receptors in the amygdala and striatum; oxytocin has been linked with greater attention to social stimuli (Albert, Chein, & Steinberg, 2013). However, this increased attention to social stimuli is accompanied by increased sensitivity to signals of social exclusion. Asynchronous timing in brain maturation across the prefrontal cortex as compared to subcortical regions contributes to especially dysregulated responses to emotionally salient environmental experiences, including social exclusion stimuli specifically (Somerville, 2013). Developmental findings are consistent with these biological theories. Puberty is associated with more frequent affiliation with peers, increased positive affect when in the presence of peers, and especially pronounced distress when experiencing peer-related stressors (Brown, 2004; Conley & Rudolph, 2009). Because social interactions have substantial influence on adolescent development, adolescents’ HPA reactions in stressful social situations are especially important to consider. As such, in the current study, a social stressor task is used to evaluate the influence of prior and current peer experiences on HPA functioning.
Prior work has hypothesized that adolescents’ experiences with relational victimization are associated with dysregulated HPA functioning. Consistent with other work examining the influence of early life adversity on HPA functioning (e.g., Cicchetti & Rogosh, 2001), some findings suggest that victimized youth have lower diurnal levels of cortisol (Vaillancourt et al., 2008). However, results for differences in HPA reactivity to acute stress have been equivocal. Findings have alternatively suggested that victimized children and adolescents exhibit hyperreactive HPA stress responses (Kliewer, 2006), hyporeactive HPA stress responses (Ouellet-Morin, Danese, et al., 2011; Ouellet-Morin, Odgers, et al., 2011), or no associations between victimization and cortisol reactivity (Kliewer, Dibble, Goodman, & Sullivan, 2012; Knack, Jensen-Campbell, & Baum, 2011; Rudolph, Troop-Gordon, & Granger, 2010, 2011). In this study, a rigorous examination of the association between relational victimization and adolescents’ HPA stress responses to an in vivo social stressor was conducted by controlling for physical victimization, life stressors, pubertal status, and depressive symptoms as competing predictors. Given that relational victimization is a significant source of stress during adolescence, it was hypothesized that relational victimization would be associated with dysregulated HPA stress responses, as indicated by hypo- or hyperreactivity to the social stressor task.
Crick’s research focused not only on forms of aggressive behavior but also on the balance and interplay between both aversive and prosocial peer experiences. Many of her contributions pertained specifically to dyadic peer experiences, including the potential buffering role of positive friendship qualities for youth adjustment (e.g., Crick, Murray-Close, Marks, & Mohajeri-Nelson, 2009; Crick & Nelson, 2002; Kawabata, & Crick, 2008, 2011; Murray-Close, Ostrov, & Crick, 2007; Rockhill et al., 2007). According to stress-buffering models (e.g., Cohen & Wills, 1985), friendships can diminish the impact of stress on psychosocial functioning by providing emotional support and an outlet for generating potential resolutions to stress. Conversely, friendships that do not provide adequate support fail to act as a buffer, or even can exacerbate deleterious biopsychosocial responses to stress.
Understanding how friendships contribute to biological stress responses is an area that deserves much greater research attention (Hostinar & Gunnar, 2013). Recent preliminary findings suggest that the presence of a best friend following a negative experience may be associated with lower cortisol levels in children than among children who experiencing negative events without a best friend present (Adams, Santo, & Bukowski, 2011). These promising initial results offer important directions for further understanding the potential buffering role of friendships. For instance, it is unclear whether friends may buffer against stress reactivity or whether friends may facilitate rapid recovery from acute stress reactive responses. Moreover, it is unknown whether, apart from the presence of a friend, some friendship dimensions are especially potent for buffering biological stress responses. The effects of friendships require consideration of both specific friendship behaviors as well as the relationship quality in which these behaviors are embedded. Friendship quality is based on specific dynamics that may be classified within two orthogonal dimensions of positive (e.g., support and intimacy) and negative quality (e.g., conflict and antagonism). Positive friendship quality may buffer stress responses, as discussed above. Negative friendship quality, however, may serve as a source of stress (Furman & Buhrmester, 1985; Hostinar & Gunnar, 2013) and may decrease the availability of social support (Sandler, Miller, Short, & Wolchik, 1989), consequently increasing vulnerability to maladaptive responses. Much like peer victimization, friendships high in negative quality also could contribute to increased allostatic load, thereby decreasing youths’ biological capacity to respond to stressors (Byrd-Craven, Granger, & Auer, 2011). It was hypothesized that adolescents in friendships with high positive quality would demonstrate more adaptive HPA responses, while those in friendships with high negative qualities would experience HPA dysregulation. In addition, interaction effects between friendship quality and peer victimization on HPA axis responses were explored.
In terms of specific friendship behaviors, prior observational work suggests that specific friend behaviors, such as responsiveness, may increase the utility of friendships (Piehler & Dishion, 2007). A high level of a friend’s responsiveness immediately following the experience of a stressor may be related to more rapid stress recovery because this fundamental component of healthy communication indicates general attentiveness to the perspective of a conversational partner, which may increase perceived support. Note that specific friendship behaviors occur in the context of a relationship that itself may be characterized as globally supportive (i.e., high levels of positive friendship qualities) or adversarial (i.e., high levels of negative friendship qualities). Responsiveness in the context of a friendship high in positive qualities is hypothesized to offer the most potent buffering effects, as indicated by increased HPA recovery following the stressor task.
Thus, the current study examined associations among forms of peer victimization (i.e., relational and physical), friendships (behaviors and quality), and reactive HPA responses following an in vivo social stressor task (i.e., the Trier Social Stress Task; TSST). Hypotheses were examined within a clinically oversampled group of participants, allowing for an examination of processes among youth who exhibited a broad spectrum of prior life stressors and stress responses. Salivary cortisol was used as an index of HPA functioning prior to, during, and after the social stressor (i.e., baseline, reactivity, and recovery). Participants’ friends conversed with participants following the stressor task, and observational coding was conducted to friendship responsiveness. Given potential gender differences in the rates of peer victimization and friendship quality (Crick & Grotpeter,1995; Lagerspetz et al., 1988), exploratory analyses of gender differences were conducted. Several variables demonstrating prior associations with HPA stress responses were considered as covariates in primary analyses; these included age and pubertal stage (Shirtcliff et al., 2012; Stroud et al., 2009), gender (Schreiber et al., 2006; Shirtcliff & Essex, 2008), corticosteroid medications (Hastings, Fortier, Utendale, Simard, & Robaey, 2009), birth control (Bouma, Riese, Ormel, Verhulst, & Oldehinkel, 2009), depressive symptoms (Guerry & Hastings, 2011; Lopez-Duran et al., 2009), and negative life events (Lupien et al., 2009; Miller et al., 2007).
Method
Participants
Participants included 62 youth (73% female) at the adolescent transition, between the ages of 12 and 16 years (M = 14.70, SD = 1.33). Approximately 76% of participants self-identified as White/Caucasian, 8% African American, 8% Latino American, 5% Asian American, and 3% mixed or other ethnicity. Approximately 65% of adolescents lived in a two-parent household; 35% lived with a biological or adoptive mother only. Mothers’ highest level of education included 3% high school diploma/GED, 6% associate/trade degree, 29% some undergraduate college, 13% bachelor’s degree, 6% some graduate school, 26% master’s degree, and 16% doctoral degree. Participants and parents reported adolescents’ current use of a variety of medications including stimulants (n = 7), anticonvulsants (n = 2), antipsychotics (n = 9), antidepressants (n = 15), hypnotics (n = 1), antibiotics (n = 5), antihistamines (n = 2), pain relievers (n = 3), corticosteroids (n = 1), and birth control (n = 3). Exactly half of all participants reported no medication usage.
Study recruitment incorporated oversampling procedures to obtain a sample with a wide range of life stressors and adjustment difficulties. As such, referral sources included local inpatient units and outpatient clinics (n 13; 21%), community mental health agencies (n = 2; 3%), local high schools (n = 16; 26%), and mass e-mail advertisements (n = 31; 50%). Referred adolescents initially were screened for exclusion criteria (i.e., psychosis, mental retardation, or pervasive developmental disorder) that could substantially alter the validity of our assessment of social stress responses.
Procedure
Adolescents attended the laboratory-based assessment with a same-gender, same-aged (i.e., within 2 years) best friend and a primary caregiver. Adolescents first completed questionnaire data and then participated in an in vivo social stress-induction paradigm. Information about adolescents’ current medication usage was collected from parents and adolescents. Participants were instructed to refrain from usage of medications on the day of testing. Salivary cortisol samples were collected at scheduled intervals throughout the visit to provide markers of HPA functioning, as detailed below.
Social stressor task
Adolescents participated in the TSST during the laboratory assessment (e.g., Hastings, Zahn-Waxler, & Usher, 2007; Klimes-Dougan, Hastings, Granger, Usher, & Zahn-Waxler, 2001). Participants who had been acclimated to an observational setting were oriented toward a camera connected to a closed-circuit “feedback screen” displaying their own live image. Adolescents were instructed to face this camera and feedback screen while preparing (for 1 min) and subsequently delivering a 3-min speech. The explicit goal of the speech, as explained to participants, was to convince an audience of their peers (presumably watching the live video feed in a nearby room) that they should be selected to star in a fictional television show about teens’ ability to form and maintain friendships. Immediately prior to the adolescents’ delivery of the speech, an opposite-gender undergraduate research assistant entered the room, ostensibly to evaluate participants’ performance. Although this “observer” remained in the room at close proximity to the participant for the duration of the speech task, s/he was given instructions to focus on the feedback screen and withhold direct eye contact with the participant. At approximate intervals of 20 s, the observer was instructed to make a small mark on a clipboard in order to give the appearance of continuous evaluation.
Poststressor discussion with a friend
Within 15 min after completing the social stressor task, adolescents’ best friend entered the room in which the TSST took place. Adolescents were instructed to discuss the task and their performance with their friend for a total of 4 min. Adolescents and friends then completed questionnaires in separate rooms for the following 30 min.
Cortisol collection
Adolescents provided salivary cortisol samples using a passive drool procedure (see Klimes-Dougan et al., 2001) on three occasions throughout the baseline laboratory assessment described above: immediately prior to the speech task (following a 20-min relaxation period), 20 min postspeech, and 40 min postspeech. Cortisol reaches peak levels in human saliva approximately 20 min after the onset or peak of a stressor (e.g., Adam, Sutton, Doane, & Mineka,2008; Gunnar, Talge, & Herrera, 2009). Thus, the first sample provided a prestressor measure of cortisol (i.e., baseline), while the subsequent samples were representative of stressor and poststressor cortisol levels. Changes in cortisol levels from baseline to stressor represent cortisol “reactivity,” while comparisons of baseline and poststressor levels of cortisol are indicative of poststressor cortisol “recovery” (i.e., return to baseline).
Salivary samples were frozen for storage at −25 °C and then shipped on dry ice to Pennsylvania State University’s Behavioral Endocrinology Laboratory for assay (Salimetrics, PA). Samples were assayed for cortisol using a 510-k cleared high-sensitive enzyme immunoassay designed to assess adrenal function. This test, which uses 25 μl of saliva (for singlet determinations), has a lower limit sensitivity of 0.007 μg/dl and a range of sensitivity from 0.007 to 1.2 μg/dl.
Measures
Victimization
The victimization subscales of the Revised Peer Experiences Questionnaire (Prinstein et al., 2001) were used to assess overt and relational forms of victimization. This questionnaire includes 18 items that reflect different forms of victimization, including physical/overt and relational victimization, as well as adolescents’ receipt of prosocial peer behavior. Items for the physical/overt subscale assessed more direct forms of victimization such as being the recipient of physical or verbal aggression (e.g., “A teen hit, kicked, or pushed me in a mean way”), while items for the relational victimization subscale assessed being the recipient of direct or indirect social aggression (e.g., “A teen left me out of an activity or conversation that I really wanted to be included in”). Adolescents were asked to report how frequently they have been the recipient of each form of behavior (i.e., 1 = never, 2 = once or twice, 3 = a few times, 4 = about once a week, and 5 = a few times a week) over the past year. The Revised Peer Experiences Questionnaire has demonstrated acceptable psychometric properties in past research (De Los Reyes & Prinstein, 2004; Prinstein et al., 2001). For the purposes of the present study, only the overt (three items) and relational victimization (three items) subscales were included in analyses. Internal consistency of the two subscales was acceptable (overt, α = 0.77; relational, α = 0.61).
Friendship quality
Adolescents were asked to complete the Network of Relationships Inventory (Furman, 1998) to assess the quality of their relationship with the friend who accompanied them to the lab. This measure includes 30 items assessing eight domains of positive (i.e., companionship, instrumental aid, intimacy, nurturance, affection, admiration, reliable alliance, and emotional support) and two domains of negative (i.e., conflict and antagonism) friendship quality. Each domain is assessed with three items, and participants respond to each item using a 5-point Likert scale. Psychometric properties reported by Furman (1998) support the Network of Relationships Inventory as a reliable and valid measure of friendship quality. Internal consistency in the current sample was 0.96 for positive friendship quality and 0.79 for negative friendship quality.
Observational coding
Conversational responsiveness is a basic form of support that demonstrates attentiveness to, and engagement with, someone who is speaking. In the current study, an observational coding scheme for “responsiveness” (from the Peer Dyadic Mutuality Rating System; Piehler & Dishion, 2004) was implemented to provide a broad measure of the extent to which an adolescent’s friend verbally and non-verbally responded to the adolescent during the poststressor discussion task. Videotapes of adolescents’ poststressor discussion with a friend were separately coded by an advanced clinical psychology doctoral student and postdoctoral fellow, both blind to participants’ ratings on primary measures. Responses were evaluated jointly for nonverbal features (i.e., eye contact, displayed interest, and reciprocity of behavior and affect) and verbal features (i.e., relevant and timely comments). Responsiveness was evaluated based on general frequency of occurrence during the 5-min postspeech discussion. Coders trained for 1 month to code responsiveness, with regular meetings and rating comparisons. Coders rated friends’ level of responsiveness from 1 (rarely or never) to 7 (always or throughout). Reliability was determined using a novel subset (20%) of all tapes, which were randomly sampled. As in prior research using this same coding scheme (Piehler & Dishion,2007), reliability was evaluated allowing for 1-point disagreements on the 7-point scale. Upon establishing high interrater reliability (92% agreement), one coder coded the remaining tapes.
Covariate measures
Cortisol timing
Cortisol levels immediately following a stressor represent the sum of the acute cortisol response together with the basal cortisol level for that particular time of day. Thus, adolescent participants reported their time of awakening, and laboratory personnel recorded the times of cortisol collections. For each individual, a “cortisol timing” variable was computed representing the duration of time elapsed between the time of awakening and the time at which the first cortisol sample was collected.
Pubertal stage
A pictorial questionnaire was used to assess bodily changes associated with pubertal development, ranging from prepubertal (stage = 1) to postpubertal (stage = 5; Morris & Udry, 1980). Adolescents were presented with two sets of five serial line drawings and instructed to circle the picture that was closest to their stage of growth. For girls, drawings depicted stages of breast development and pubic hair growth. Boys rated their genital development and pubic hair growth. Ratings from this questionnaire have been highly correlated with physician assessments and are considered a valid estimation of pubertal stage (Dorn, Susman, Nottelmann, Inoff-Germain, & Chrousos, 1990; Morris & Udry,1980). Similar to prior studies (e.g., Negriff, Fung, & Trickett, 2008), the current study considered ratings of breast development (girls) and genital development (boys) to be most indicative of pubertal development associated with the hormonal changes characterizing the adolescent transition. These ratings were used to establish pubertal status for the current study.
Depressive symptoms
Adolescents reported depressive symptoms using the Mood and Feelings Questionnaire (MFQ; Costello & Angold, 1988). The MFQ consists of 33 items rated on a 3-point scale (0 = not true, 1 = sometimes true, and 2 = mostly true) designed for children and adolescents aged 8-18 years. The MFQ has demonstrated strong psychometric properties and has shown high convergent validity with diagnostic interviews for major depressive disorder (Angold, 1989; Wood, Kroll, Moore, & Harrington, 1995). Internal consistency in the current sample was excellent (α = 0.97).
Life events
Adolescents completed the Adolescent Life Events Questionnaire (ALEQ; Hankin & Abramson, 2002) to assess their experience of a range of negative experiences in a variety of domains (e.g., school problems, relationship difficulties, and family difficulties). The ALEQ includes 70 events that are often reported by adolescents, and respondents are asked to rate the occurrence (0/1, No/Yes) of events over the past 3 months. As in prior studies (e.g, Hankin, 2008; Hankin, Stone, & Wright, 2010), summed scores of occurrence were computed, with higher scores representing more negative life events. The psychometric properties of the ALEQ have been reported in previous studies (e.g., Hankin & Abramson,2002; Hankin et al., 2010) and suggest that the instrument has acceptable reliability and validity; internal consistency for the scale was good in the current sample (α = 0.93).
Data analytic plan
Descriptive statistics and correlations (or t values, for gender and medication usage) were computed for all study variables. Primary study analyses consisted of latent difference structural equation models for pre–post–post study designs (Willoughby, Vandergrift, Blair, & Granger, 2007). A logarithmic transformation was applied to cortisol values prior to model identification. An unconditional model was fit to the data to test for significant variances in latent factors specified for cortisol intercept, reactivity, and recovery. Next, an initial conditional model incorporated both forms of victimization (i.e., physical and relational), two domains of friendship quality (i.e., positive and negative), and one measure of observed friendship behavior (i.e., responsiveness) as predictors of the latent cortisol factors, while also accounting for multiple covariates, including friendship duration, cortisol timing, age, gender, pubertal stage, corticosteroid medication or birth control use, depressive symptoms, and negative life events.
A reduced model was computed incorporating only the primary variables of interest (i.e., victimization, friendship quality, and responsiveness) and covariates demonstrating significant associations with cortisol variables in the initial conditional model. Follow-up models also considered gender and suppression effects. All latent curve analyses were conducted using AMOS 19.0 utilizing maximum likelihood methods to account for missing observations.
Results
Descriptive statistics
Table 1 includes means, standard deviations, and intercorrelations among all primary variables. Consistent with prior work, depressive symptoms were associated positively with both overt and relational victimization. Overt victimization was correlated positively with life events and negative friendship quality. Observed friend’s responsiveness was negatively associated with friendship duration. A logarithmic transformation was applied to prestressor (M = 0.09, SD = 0.05), stressor (M = 0.12, SD = 0.09), and poststressor (M = 0.10, SD = 0.06) cortisol values. Log-transformed cortisol values showed nonsignificant zero-order correlations with all continuous study variables (|rs| < .24, ps > .07), except for cortisol timing (|rs| > .37, ps, < .001).
Table 1. Descriptive statistics and correlations for primary study variables.
| Variables | M | SD | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Age | 14.70 | 1.32 | — | ||||||||||
| 2. Pubertal stage | 4.04 | 0.81 | .65*** | — | |||||||||
| 3. Cortisol timing | 7.48 | 3.08 | −.06 | −.01 | — | ||||||||
| 4. Depressive symptoms | 0.47 | 0.47 | −.16 | .03 | .13 | — | |||||||
| 5. Life events | 23.86 | 10.38 | −.00 | .10 | .12 | .56*** | — | ||||||
| 6. Overt victimization | 1.29 | 0.65 | −.21 | .03 | .05 | .37** | .46*** | — | |||||
| 7. Relational victimization | 1.84 | 0.64 | −.27* | −.16 | .11 | .31* | .19 | .28* | — | ||||
| 8. Friendship duration | 57.24 | 46.10 | −.17 | −.01 | .05 | −.02 | −.15 | −.19 | .14 | — | |||
| 9. Positive friendship quality | 3.52 | 0.87 | −.04 | −.05 | .11 | .16 | −.00 | −.07 | .05 | .15 | — | ||
| 10. Negative friendship quality | 1.43 | 0.51 | −.06 | .02 | −.09 | .19 | .22 | .34** | .00 | −.06 | .09 | — | |
| 11. Observed responsiveness | 5.00 | 1.52 | .17 | .16 | .09 | .01 | −.06 | .26* | .00 | −.28* | −.03 | −.12 | — |
Note: Cortisol timing is the hours passed between awakening and prestressor cortisol sample; friendship duration is in months.
p < .05.
p < .01.
p < .001.
Gender and medication group differences on study variables were examined using independent samples t-test analyses. Girls reported significantly higher positive friendship quality and more advanced pubertal development than did boys, while boys reported greater overt victimization than did girls. Because corticosteroids and birth control have demonstrated associations with HPA functioning (Bouma et al.,2009; Hastings et al., 2009), usage of these medications was considered as a covariate for the current study. In the examination of medication differences, individuals reporting the use of corticosteroids and/or birth control were categorized into a “medication” group (N = 4), while all others were categorized into a “nonmedication” group. Results from t-test analyses revealed that participants in the medication group reported greater relational victimization and longer friendship duration. Given their significant associations with friendship and victimization constructs, gender and medication were included as covariates in the testing of primary hypotheses.
Prediction of HPA axis response
An unconditional latent difference model was first used to examine changes in cortisol levels over time. Latent variables for intercept, reactivity, and recovery were created using the framework outlined by Willoughby et al. (2007) for pre–post–post research designs. As explained by Willoughby et al. (2007), the unconditional latent difference model is identified, but it does not yield indices of overall model fit. The variances of the latent variables for cortisol intercept, reactivity, and recovery all were significant (p < .001).
An initial saturated conditional model was used to identify all covariates demonstrating significant associations with the latent cortisol factors. Cortisol timing, age, gender, medications, pubertal stage, depressive symptoms, life events, and friendship duration all were included as covariates in the model. Primary predictors in the model included victimization (overt and relational), friendship quality (positive and negative), and observed responsiveness. For observed responsiveness, a path was estimated only for the latent factor for cortisol recovery, corresponding to the time period in which friends interacted with target participants. For all other covariates and predictors, paths were estimated between each variable and the latent constructs for cortisol intercept, reactivity, and recovery. In this model, depressive symptoms were marginally associated with cortisol intercept (β = 0.28, p = .08). Cortisol timing was significantly associated with cortisol intercept (β = −0.38, p = .002) and reactivity (β = −0.24, p = .04), and marginally with recovery (β = −0.22, p = .06).
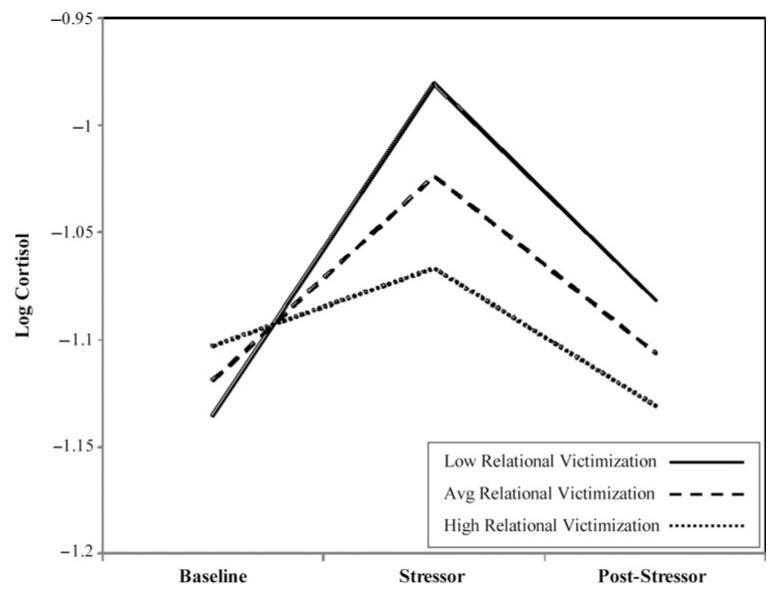
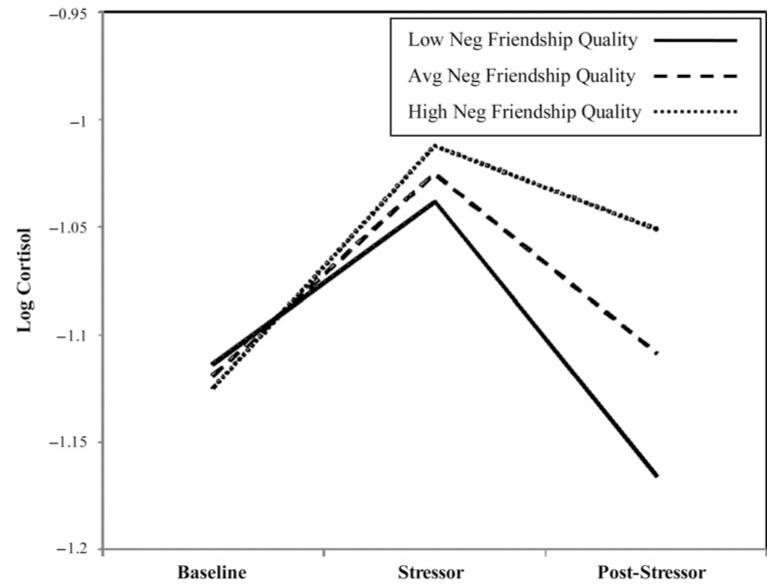
A reduced model was estimated to examine associations between primary predictors and the latent cortisol factors (i.e., intercept, reactivity, and recovery). In this model, paths among cortisol timing and all latent cortisol factors were retained. For depressive symptoms, a path was retained between depressive symptoms and cortisol intercept because this was the only marginally significant path revealed in the initial model. All other covariates were removed from the model to reduce suppression effects and increase the interpretability of findings. This model was an adequate fit to the data, χ2 (4) = 5.21, ns; χ2/df = 1.30; comparative fit index = 0.99; root mean square error of approximation = 0.07; Akaike information criterion = 127.21 (see Table 2 for model estimates). In this model, the association between depression and cortisol intercept was no longer marginally significant. Main effects for relational victimization on cortisol reactivity and cortisol recovery were observed. In addition, a main effect for negative friendship quality on cortisol recovery also was observed. Model implied trajectories of cortisol were plotted at low (−1 SD), mean, and high (+1 SD) levels of relational victimization (see Figure 1) and negative friendship quality (see Figure 2). Results indicated that higher levels of relational victimization were associated with a more hyporeactive cortisol response to the social stressor task. Although higher levels of relational victimization also were associated with greater cortisol recovery following the stressor, this effect should be interpreted in the context of the association between higher levels of victimization and blunted reactivity. In other words, higher levels of victimization were associated with lower reactivity and, thus, a quicker return to baseline. Results also indicated that higher levels of negative friendship quality were associated with reduced HPA recovery following the stressor task, predicting a slower return to baseline; this effect did not appear to be influenced by level of reactivity. A subsequent model tested gender differences using interactions between gender and each of the primary predictors. Though the interaction between gender and positive friendship quality significantly predicted cortisol recovery (β = −0.83, p < .001), independent testing of this effect for boys and girls revealed nonsignificant paths between positive friendship quality and cortisol recovery.
Table 2. Unstandardized and standardized regression weights for victimization, friendship quality, and observed responsiveness predicting latent difference scores for cortisol intercept, reactivity, and recovery, while accounting for covariates.
| Intercept |
Reactivity |
Recovery |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Predictors | b | SE | β | b | SE | β | b | SE | β |
| Cortisol timing | −0.03 | 0.01 | −0.39*** | −0.02 | 0.01 | −0.23* | −0.01 | 0.01 | −0.22† |
| Depressive symptoms | 0.09 | 0.06 | 0.16 | — | — | — | — | — | — |
| Overt victimization | 0.02 | 0.05 | 0.06 | −0.07 | 0.04 | −0.21 | −0.06 | 0.04 | −0.21 |
| Relational victimization | 0.03 | 0.05 | 0.07 | −0.09 | 0.04 | −0.28* | −0.07 | 0.03 | −0.24* |
| Positive friendship quality | −0.06 | 0.03 | −0.20 | 0.03 | 0.03 | 0.11 | −0.01 | 0.02 | 0.06 |
| Negative friendship quality | −0.01 | 0.06 | −0.02 | 0.03 | 0.04 | 0.09 | 0.10 | 0.04 | 0.31* |
| Responsiveness | — | — | — | — | — | — | 0.00 | 0.01 | 0.03 |
Note: For recovery, positive values indicate less return of cortisol to baseline levels following the stressor; negative values indicate greater return to baseline levels.
p < .06.
p < .05.
p < .001.
Figure 1.
Linear functions showing different degrees of cortisol reactivity by level of relational victimization (average = mean; high/low mean ±1 SD).
Figure 2.
Linear functions showing different degrees of cortisol recovery by level of negative friendship quality (average = mean; high/low mean ±1 SD).
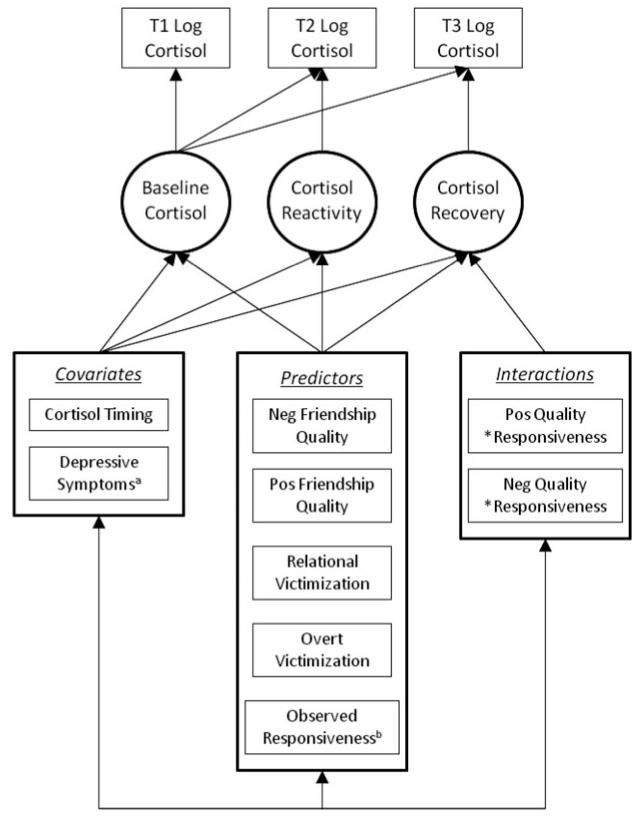
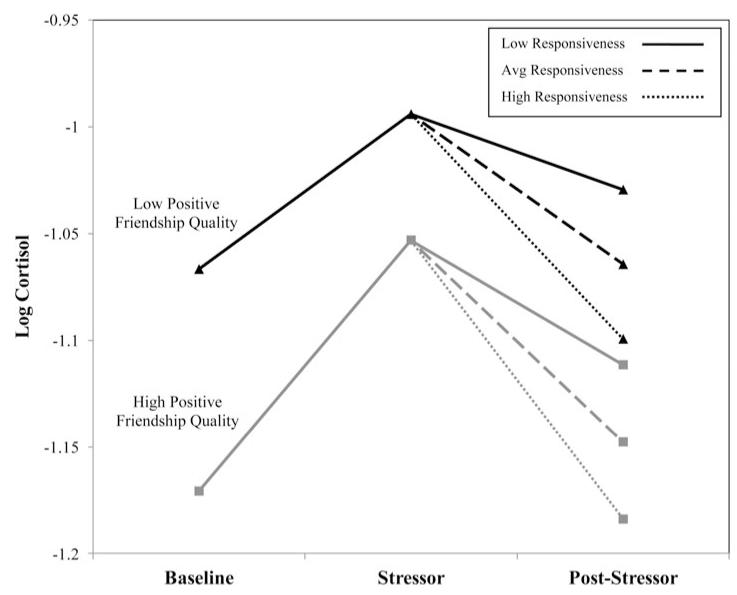
To examine whether friendship qualities interacted with specific friendship behavior to predict cortisol recovery, two interaction terms were generated (i.e., Responsiveness × Positive Friendship Quality; Responsiveness × Negative Friendship Quality). These two interaction terms were added to the reduced model (previously used to test for main effects) and paths were estimated between each interaction term and cortisol recovery (see Figure 3 for an illustration of the final model). This model was an adequate fit to the data, χ2 (8) = 6.27, ns; χ2/df = 0.78; comparative fit index = 1.00; root mean square error of approximation = 0.00; Akaike information criterion = 170.27 (see Table 3 for model estimates). In this model, the interaction between positive friendship quality and observed responsiveness significantly predicted cortisol recovery. This effect was plotted using model implied trajectories for low (−1 SD), mean, and high (+1 SD) levels of positive friendship quality and observed responsiveness (see Figure 4). Nonsignificant interactions were removed for probing to simplify interpretation of effects; effects remained unchanged with nonsignificant interactions removed. Under conditions of low positive friendship quality, high observed responsiveness was associated with greater cortisol recovery, while low responsiveness was associated with less cortisol recovery. Under conditions of high positive friendship quality, the effect was in the opposite direction. In supplementary models, gender differences and suppression effects were considered but results did not influence primary findings.1 Though interactions examining friendship constructs (quality and behaviors) as moderators of the association between peer victimization and HPA axis responses (reactivity and recovery) were tested in the model, these interactions yielded no significant findings and were not included in the final model.
Figure 3.
Final latent difference model with covariates, primary predictors, and interactions between friendship quality and proximal friend behavior (i.e., responsiveness) predicting latent constructs for baseline cortisol, cortisol reactivity, and cortisol recovery. All predictors were allowed to correlate with one another, as were the latent cortisol constructs. aIn this reduced model, depressive symptoms were only allowed to predict baseline cortisol. bResponsiveness was only allowed to predict cortisol recovery.
Table 3. Model testing interactions of established peer experiences (i.e., victimization and friendship quality) with observed responsiveness predicting cortisol recovery, while accounting for main effects and covariates.
| Intercept |
Reactivity |
Recovery |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Predictors | b | SE | β | b | SE | β | b | SE | β |
| Cortisol timing | −0.03 | 0.01 | −0.41*** | −0.01 | 0.01 | −0.21 | −0.01 | 0.01 | −0.23* |
| Depressive symptoms | 0.08 | 0.06 | 0.14 | — | — | — | — | — | — |
| Overt victimization | 0.03 | 0.05 | 0.07 | −0.07 | 0.04 | −0.21 | −0.15 | 0.18 | −0.51 |
| Relational victimization | 0.03 | 0.05 | 0.06 | −0.09 | 0.04 | −0.29* | −0.17 | 0.07 | −0.52* |
| Positive friendship quality | −0.06 | 0.03 | −0.21 | 0.03 | 0.03 | 0.11 | −0.12 | 0.06 | −0.57* |
| Negative friendship quality | −0.01 | 0.05 | −0.02 | 0.03 | 0.05 | 0.09 | 0.23 | 0.12 | 0.69* |
| Responsiveness | — | — | — | — | — | — | −0.12 | 0.06 | −0.96* |
| PosQual × Responsiveness | — | — | — | — | — | — | 0.03 | 0.01 | 1.02** |
| NegQual × Responsiveness | — | — | — | — | — | — | −0.02 | 0.02 | −0.46 |
p < .05.
p < .01.
p < .001.
Figure 4.
Linear functions showing different degrees of cortisol recovery by level of positive friendship quality and observed responsiveness (average = mean; high/low = mean ± 1 SD).
Discussion
This study considered peer victimization as a form of stress that may be associated with biological stress responses, and thus may contribute to allostatic load. This study also examined aspects of dyadic friendships (i.e., friendship quality and friend’s observed responsiveness behavior following an in vivo stressor) that may be associated with HPA axis responses. Consistent with theories regarding enhanced social orientation and sensitivity in adolescence, findings suggest that adolescents’ experiences with peers may be especially relevant for understanding biological stress responses. These findings have important implications not only for research examining allostasis but also for documenting the manner in which aversive peer experiences are broadly relevant for understanding youth adjustment. As such, this work offers a nice extension of Nicki Crick’s legacy.
In this study of 12- to 16-year-olds, many of whom already were experiencing significant life stressors and symptoms of psychopathology, prior experiences with relational victimization uniquely contributed to a blunted HPA axis response to an in vivo stressor. Given the adverse life experiences already endured by many in this sample, the effects of relational aggression are remarkable. Moreover, it is notable that significant findings emerged for relational while accounting for the effects of physical victimization. Relational victimization occurs more frequently during adolescence than in childhood (Crick et al., 2001), but it may also be uniquely relevant to the biological sensitivities of adolescence. Even in nonhuman species, adolescence is accompanied by an increased social orientation (e.g., Douglas, Varlinskaya, & Spear, 2004); within humans, actual or perceived social exclusion/rejection experiences trigger neurological threat detection centers (Ei-senberger & Lieberman, 2004; MacDonald & Leary, 2005). Relational victimization is characterized by a withdrawal of friendship support, gossip, or collusion among peers to ostracize. These experiences are consistent with the types of social rejection cues that activate socioaffective circuitry in functional magnetic resonance imaging studies (see Eisenberger,2011, for a review) and with pro-inflammatory responses in research on epigenetic expression (Cole et al., 2007; Miller, Maletic, & Raison, 2009). The use of an in vivo stressor paradigm extends prior research by demonstrating the importance of prior peer experiences as predictors of adolescents’ biological stress reactivity during acute stress.
Relational victimization was associated with blunted HPA reactivity in this study, extending prior work by Crick and her colleagues linking relational victimization to reduced diurnal cortisol levels in children (Murray-Close et al., 2007). Some evidence suggests that individuals with histories of chronic stress (characteristic of the adolescents in this study) may be more prone toward blunted HPA responses (e.g., Miller et al., 2007). Similar to other chronic stressors, relational victimization during adolescence may serve as a stable source of stress that frequently engages HPA stress responses, increasing allostatic load to the point that HPA responses become dysregulated. Blunted and heightened stress reactivity, and sustained HPA activation, have deleterious effects on one’s ability to carry out effective, or socially desirable, behavioral and affective responses to stressors (McEwen, 1998, 2000). Each of these markers of dysregulated HPA axis response also has been linked with related biological stress response processes (e.g., pro-inflammation) and with later psychopathology among youth (Calhoun et al., 2012; Hastings et al., 2011). In addition, adolescence may be a critical transition point where proximal and chronic symptom presentations correspond with different profiles of HPA dysregulation (Ruttle et al.,2011). Findings ultimately suggest that adolescents with high levels of relational victimization experiences may be poorly equipped to manage the biological demands required by future stressors, and therefore they may be less likely to utilize adaptive cognitive or behavioral responses to stress.
This study also suggests that dyadic peer experiences are relevant for understanding HPA axis recovery following a stressor. Specifically, this study offered two findings to support hypotheses regarding the ameliorative effects of friendships in the context of stress. First, lower levels of negative qualities (i.e., conflict and antagonism) within adolescents’ best friendships were associated with greater HPA recovery following an experimentally induced stressor. Second, for adolescents in low positive quality friendships, high friend responsiveness while adolescents discussed their stressful experience was associated with stronger HPA axis recovery. These findings suggest that adolescents’ friendships can confer biological benefits. This offers an exciting contribution to a large body of research considering the psychological correlates of dyadic friendship experiences (Schmidt & Bagwell,2007). Of note, friendship quality and behaviors did not moderate the association between peer victimization and HPA responses; it could be that connections between HPA responses and chronic experiences in the broader peer group are relatively substantial and less likely to be affected by a single dyadic friendship.
It is interesting that responsiveness in the context of a very high quality friendship was associated with less HPA axis recovery. As mentioned earlier, maintaining high cortisol reactions following a stressor could result in negative consequences such as increased allostatic load and decreased capacity to respond to future stressors (McEwen, 2000). Findings from this study may suggest possible deleterious friendship interactions that maintain high cortisol levels after a stressful social experience. One such friendship process may be “co-rumination,” which is characterized by a persistent focus on problems and negative affect within dyadic friendship interactions (Rose, 2002). Recent work suggests that trait ruminative thinking is associated with delayed cortisol recovery following a social stressor (Stewart, Mazurka, Bond, Wynne-Edwards, & Harkness, 2013). In addition, focus on negative affect during discussions of life problems with a friend is associated with heightened activation of the HPA system (Byrd-Craven et al., 2011). The extension of this result to co-ruminative processes offers an intriguing direction for future research. Overall, results offer important preliminary evidence to suggest that specific relationship qualities and specific types of dyadic behaviors following stress can change adolescents’ biological capacity to cope effectively. Applications of this idea to the study of other dyadic interactions in adolescents’ lives (e.g., parents, therapists, or teachers) are ripe with possibilities.
This study offered a rare opportunity to examine multiple peer experiences that may be associated with HPA stress responses in adolescence. The research was strengthened by using a sample that included a high proportion of adolescents referred from clinical settings, suggesting the relevance of the findings to youth who are vulnerable to psychopathology. The study also benefited from the use of multimethod approaches, including observational coding and objective indicators of stress responses. It was impressive that significant findings emerged despite a relatively small sample and reduced power to detect effects; however, a larger sample would provide greater power for testing higher order interactions (e.g., three-way interactions), which could offer additional insight as to other factors that play an important role the observed connections between HPA functioning and peer experiences. Given that the influence of victimization and friendships on adjustment may differ for adolescent boys and girls (Rose & Rudolph, 2006), future studies with more diverse samples should consider gender differences in connections between peer relations factors and biological stress responses. In addition, the utilization of peer reports could assist in determining whether findings are unique to adolescents’ internalized evaluations of peer victimization and friendship quality, or whether findings can be generalized to a more “true” assessment of their social status and peer relationships. Future observational work should continue to consider specific friend behaviors that may facilitate or diminish stress recovery. Specifically, it is important to disentangle and understand the multiple facets of high-quality friendships as they relate to stress responses. As discussed, co-rumination may be one process occurring within high-quality friendships that contradicts the stress-buffering hypothesis by maintaining heightened biological stress responses. Examining negative friend behaviors, such as criticism, during poststressor discussions also may clarify reasons why high negative quality friendships are associated with less recovery. Given that the current study employed a social stressor paradigm, it is possible that the observed effects are limited to social stress responses; additional work, implementing other types of stressor tasks (e.g., performance), is needed to determine if salient peer experiences influence stress responses in other contexts.
Further, though findings contribute a unique and important perspective on peer relations, the interpretation of findings are limited by the cross-sectional nature of the study. For instance, it is unclear whether peer experiences cause dysregulation of HPA stress responses, or whether maladaptive social behaviors resulting from biological dysregulation may influence how adolescents are treated by peers. The bidirectionality of peer experiences and HPA dysregulation may be especially important to consider in the context of friendships, given that adolescents’ maladaptive behavior could influence the development of positive and negative friend behaviors (e.g., support or criticism). For instance, an adolescent who has difficulty regulating stress reactions may be more likely to agitate a friend, thereby decreasing the likelihood that the friend will feel motivated to provide support. Longitudinal work considering the development of the HPA system and changes in peer experiences over time could clarify the directionality of findings. In addition, a longitudinal design would also offer opportunity to consider the chronicity of victimization and the stability of friendships as predictors of biological stress vulnerability. Both of these factors have demonstrated important connections with adjustment (Biebl, DiLalla, Davis, Lynch, & Shinn, 2011; Poulin & Chan, 2010) and may have unique associations with HPA functioning. Finally, longitudinal work is needed to determine how the influence of relational victimization and friendships on HPA stress responses may contribute to the development of psychopathology. Some work suggests that chronic stress may compromise the HPA axis’ role in other important biological tasks, such as down-regulating inflammation responses (Miller, Cohen, & Ritchey, 2002). For adolescents, salient peer experiences may serve an important role in establishing biological set points that increase or decrease vulnerability to psychopathology.
Nicki Crick’s research changed the way that scholars understand peer experiences. Her work broadened the repertoire of peer interactions that we now know can promote psychopathology in youth, and her contributions continue to advance future scientific discovery on the topics that were of greatest interest to her. This study demonstrated that relational victimization and dyadic friendship experiences may be associated with adolescents’ biological capacity to regulate interpersonal stress. The study of peer contributions to allostatic load may yield important insights into the unique role of social experiences in adolescent development.
Acknowledgments
This research was supported by a grant from the American Foundation of Suicide Prevention (to M.J.P.). Special thanks to Dan Bauer for his assistance with data analyses.
Footnotes
Supplementary two- and three-way gender interactions were considered; however, no significant associations with HPA recovery were revealed. Additional analyses also considered possible suppression effects by testing reduced models. All findings reported above remained significant in reduced models.
References
- Adam EK, Sutton JM, Doane LD, Mineka S. Incorporating hypothalamic–pituitary–adrenal axis measures into preventive interventions for adolescent depression: Are we there yet? Development and Psychopathology. 2008;20:975–1001. doi: 10.1017/S0954579408000461. doi:10.1017/S0954579408000461. [DOI] [PubMed] [Google Scholar]
- Adams RE, Santo JB, Bukowski WM. The presence of a best friend buffers the effects of negative experiences. Developmental Psychology. 2011;47:1786–1791. doi: 10.1037/a0025401. doi:10.1037/a0025401. [DOI] [PubMed] [Google Scholar]
- Albert D, Chein J, Steinberg L. The teenage brain: Peer influences on adolescent decision-making. Current Directions in Psychological Science. 2013;22:114–120. doi: 10.1177/0963721412471347. doi:10.1177/0963721412471347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angold A. Structured assessments of psychopathology in children and adolescents. In: Thompson C, editor. The instruments of psychiatric research. Wiley; Chichester: 1989. pp. 271–394. [Google Scholar]
- Biebl SJ, DiLalla LF, Davis EK, Lynch KA, Shinn SO. Longitudinal associations among peer victimization and physical and mental health problems. Journal of Pediatric Psychology. 2011;36:868–877. doi: 10.1093/jpepsy/jsr025. doi:10.1093/jpepsy/jsr025. [DOI] [PubMed] [Google Scholar]
- Bjorkqvist K, Lagerspetz KM, Kaukiainen A. Do girls manipulate and boys fight? Developmental trends in regard to direct and indirect aggression. Aggressive Behavior. 1992;18:117–127. doi:10.1002/1098-2337. [Google Scholar]
- Bouma EM, Riese H, Ormel J, Verhulst FC, Oldehinkel AJ. Adolescents’ cortisol responses to awakening and social stress: Effects of gender, menstrual phase and oral contraceptives. The TRAILS study. Psychoneuroendocrinology. 2009;34:884–893. doi: 10.1016/j.psyneuen.2009.01.003. doi:10.1016/j.psyneuen.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Brown B. Adolescents’ relationships with peers. In: Lerner R, Steinberg L, editors. Handbook of adolescent psychology. 2nd ed. Wiley; New York: 2004. pp. 363–394. [Google Scholar]
- Byrd-Craven J, Granger DA, Auer BJ. Stress reactivity to corumination in young women’s friendships: Cortisol, alpha-amylase, and negative affect focus. Journal of Social and Personal Relationships. 2011;28:469–487. doi:10.1177/0265407510382319. [Google Scholar]
- Calhoun CD, Franklin JC, Adelman CB, Guerry JD, Hastings PD, Nock MK, et al. Biological and cognitive responses to an in vivo interpersonal stressor: Longitudinal associations with adolescent depression. International Journal of Cognitive Therapy. 2012;5:283–299. doi:10.1521/ijct.2012.5.3.283. [Google Scholar]
- Chrousos GP, Gold PW. The concepts of stress and stress system disorders: Overview of physical and behavioral homeostasis. Journal of the American Medical Association. 1992;267:1244–1252. doi:10.1001/jama.1992.03480090092034. [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA. Diverse patterns of neuroendocrine activity in maltreated children. Development and Psychopathology. 2001;13:677–693. doi: 10.1017/s0954579401003145. doi:10.1017/S0954579401003145. [DOI] [PubMed] [Google Scholar]
- Cohen S, Wills TA. Stress, social support, and the buffering hypothesis. Psychological Bulletin. 1985;98:310–357. doi:10.1037/0033-2909.98.2.310. [PubMed] [Google Scholar]
- Cole SW, Hawkley LC, Arevalo JM, Sung CY, Rose RM, Cacioppo JT. Social regulation of gene expression in human leukocytes. Genome Biology. 2007;8:R189. doi: 10.1186/gb-2007-8-9-r189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley CS, Rudolph KD. The emerging sex difference in adolescent depression: Interacting contributions of puberty and peer stress. Development and Psychopathology. 2009;21:593–620. doi: 10.1017/S0954579409000327. doi:10.1017/S0954579409000327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello EJ, Angold A. Scales to assess child and adolescent depression: Checklists, screens, and nets. Journal of the American Academy of Child & Adolescent Psychiatry. 1988;27:726–737. doi: 10.1097/00004583-198811000-00011. doi:10.1097/00004583-198811000-00011. [DOI] [PubMed] [Google Scholar]
- Crick NR. Relational aggression: The role of intent attributions, feelings of distress, and provocation type. Development and Psychopathology. 1995;7:313–322. [Google Scholar]
- Crick NR. The role of overt aggression, relational aggression, and prosocial behavior in the prediction of children’s future social adjustment. Child Development. 1996;67:2317–2327. doi:10.2307/1131625. [PubMed] [Google Scholar]
- Crick NR, Grotpeter JK. Relational aggression, gender, and social–psychological adjustment. Child Development. 1995;66:710–722. doi: 10.1111/j.1467-8624.1995.tb00900.x. doi:10.2307/1131945. [DOI] [PubMed] [Google Scholar]
- Crick NR, Grotpeter JK. Children’s treatment by peers: Victims of relational and overt aggression. Development and Psychopathology. 1996;8:367–380. doi:10.1017/S0954579400007148. [Google Scholar]
- Crick NR, Murray-Close D, Marks PE, Mohajeri-Nelson N. Aggression and peer relationships in school-aged children: Relational and physical aggression in group and dyadic contexts. In: Rubin K, Bukowski W, Laursen B, editors. Handbook of peer interactions, relationships, and groups. Guilford Press; New York: 2009. pp. 287–302. [Google Scholar]
- Crick NR, Nelson DA. Relational and physical victimization within friendships: Nobody told me there’d be friends like these. Journal of Abnormal Child Psychology. 2002;30:599–607. doi: 10.1023/a:1020811714064. doi:10.1023/A:1020811714064. [DOI] [PubMed] [Google Scholar]
- Crick NR, Nelson DA, Morales JR, Cullerton-Sen C, Casas JF, Hickman SE. Relational victimization in childhood and adolescence. In: Juvonen J, Graham S, editors. Peer harassment in school: The plight of the vulnerable and victimized. Guilford Press; New York: 2001. pp. 196–214. [Google Scholar]
- De Los Reyes A, Prinstein MJ. Applying depression-distortion hypothesis to the assessment of peer victimization in adolescents. Journal of Clinical Child and Adolescent Psychology. 2004;33:325–335. doi: 10.1207/s15374424jccp3302_14. doi:10.1207/s15374424jccp3302_14. [DOI] [PubMed] [Google Scholar]
- Dorn LD, Susman EJ, Nottelmann ED, Inoff-Germain G, Chrousos GP. Perceptions of puberty: Adolescent, parent, and health care personnel. Developmental Psychology. 1990;26:322–329. doi:10.1037/0012-1649.26.2.322. [Google Scholar]
- Douglas LA, Varlinskaya EI, Spear LP. Rewarding properties of social interactions in adolescent and adult male and female rats: Impact of social versus isolate housing of subjects and partners. Developmental Psychobiology. 2004;3:153–162. doi: 10.1002/dev.20025. doi:10.1002/dev.20025. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI. Why rejection hurts: What social neuroscience has revealed about the brain’s response to social rejection. In: Decety J, Cacioppo J, editors. The handbook of social neuroscience. Oxford University Press; New York: 2011. pp. 586–598. [Google Scholar]
- Eisenberger NI, Lieberman MD. Why rejection hurts: A common neural alarm system for physical and social pain. Trends in Cognitive Sciences. 2004;8:294–300. doi: 10.1016/j.tics.2004.05.010. doi:10.1016/j.tics.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Furman W. The measurement of friendship perceptions: Conceptual and methodological issues. In: Bukowski W, Newcomb A, Hartup W, editors. The company they keep: Friendship in childhood and adolescence. Cambridge University Press; New York: 1998. pp. 41–65. [Google Scholar]
- Furman W, Buhrmester D. Children’s perceptions of the personal relationships in their social networks. Developmental Psychology. 1985;21:1016–1024. doi:10.1037/0012-1649.21.6.1016. [Google Scholar]
- Galen BR, Underwood MK. A developmental investigation of social aggression among children. Developmental Psychology. 1997;33:589–600. doi: 10.1037//0012-1649.33.4.589. doi:10.1037/0012-1649.33.4.589. [DOI] [PubMed] [Google Scholar]
- Ganzel BL, Morris PA. Allostasis and the developing human brain: Explicit consideration of implicit models. Development and Psychopathology. 2011;23:955–974. doi: 10.1017/S0954579411000447. doi:10.1017/S0954579411000447. [DOI] [PubMed] [Google Scholar]
- Guerry JD, Hastings PD. In search of HPA axis dysregulation in child and adolescent depression. Clinical Child and Family Psychology Review. 2011;14:135–160. doi: 10.1007/s10567-011-0084-5. doi:10.1007/s10567-011-0084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Talge NM, Herrera A. Stressor paradigms in developmental studies: What does and does not work to produce mean increases in salivary cortisol. Psychoneuroendocrinology. 2009;34:953–967. doi: 10.1016/j.psyneuen.2009.02.010. doi:10.1016/j.psyneuen.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL. Cognitive vulnerability-stress model of depression during adolescence: Investigating depressive symptom specificity in a multiwave prospective study. Journal of Abnormal Child Psychology. 2008;36:999–1014. doi: 10.1007/s10802-008-9228-6. doi:10.1007/s10802-008-9228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, Abramson LY. Measuring cognitive vulnerability to depression in adolescence: Reliability, validity, and gender differences. Journal of Clinical Child and Adolescent Psychology. 2002;31:491–504. doi: 10.1207/S15374424JCCP3104_8. doi:10.1207/153744202320802160. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Stone L, Wright PA. Corumination, interpersonal stress generation, and internalizing symptoms: Accumulating effects and transactional influences in a multiwave study of adolescents. Development and Psychopathology. 2010;22:217–235. doi: 10.1017/S0954579409990368. doi:10.1017/S0954579409990368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings PD, Fortier I, Utendale WT, Simard LR, Robaey P. Adrenocortical functioning in boys with attention-deficit/hyperactivity disorder: Examining subtypes of ADHD and associated comorbid conditions. Journal of Abnormal Child Psychology. 2009;37:565–578. doi: 10.1007/s10802-008-9292-y. doi:10.1007/s10802-008-9292-y. [DOI] [PubMed] [Google Scholar]
- Hastings PD, Shirtcliff EA, Klimes-Dougan B, Allison AL, Derose L, Kendziora KT, et al. Allostasis and the development of internalizing and externalizing problems: Changing relations with physiological systems across adolescence. Development and Psychopathology. 2011;23:1149–1165. doi: 10.1017/S0954579411000538. doi:10.1017/S0954579411000538. [DOI] [PubMed] [Google Scholar]
- Hastings PD, Zahn-Waxler C, Usher BA. Cardiovascular and affective responses to social stress in adolescents with internalizing and externalizing problems. International Journal of Behavioral Development. 2007;31:77–87. doi:10.1177/0165025407073575. [Google Scholar]
- Hostinar CE, Gunnar MR. Future directions in the study of social relationships as regulators of the HPA axis across development. Journal of Clinical Child and Adolescent Psychology. 2013;42:564–575. doi: 10.1080/15374416.2013.804387. doi:10.1080/15374416.2013.804387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juster RP, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neuroscience & Biobehavioral Reviews. 2009;35:2–16. doi: 10.1016/j.neubiorev.2009.10.002. doi:10.1016/j.neubiorev.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Kaltas GA, Chrousos GP. The neuroendocrinology of stress. In: Cacioppo J, Tassinary L, Berntson G, editors. Handbook of psychophysiology. Cambridge University Press; New York: 2007. pp. 303–318. [Google Scholar]
- Kawabata Y, Crick NR. The role of cross-racial/ethnic friendships in social adjustment. Developmental Psychology. 2008;44:1177–1183. doi: 10.1037/0012-1649.44.4.1177. doi:10.1037/0012-1649.44.4.1177. [DOI] [PubMed] [Google Scholar]
- Kawabata Y, Crick NR. The significance of cross-racial/ethnic friendships: Associations with peer victimization, peer support, sociometric status, and classroom diversity. Developmental Psychology. 2011;47:1763–1775. doi: 10.1037/a0025399. doi:10.1037/a0025399. [DOI] [PubMed] [Google Scholar]
- Kliewer W. Violence exposure and cortisol responses in urban youth. International Journal of Behavioral Medicine. 2006;13:109–120. doi: 10.1207/s15327558ijbm1302_2. doi:10.1207/s15327558ijbm1302_2. [DOI] [PubMed] [Google Scholar]
- Kliewer W, Dibble AE, Goodman KL, Sullivan TN. Physiological correlates of peer victimization and aggression in African American urban adolescents. Development and Psychopathology. 2012;24:637–650. doi: 10.1017/S0954579412000211. doi:10.1017/S0954579412000211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimes-Dougan B, Hastings PD, Granger DA, Usher BA, Zahn-Waxler C. Adrenocortical activity in at-risk and normally developing adolescents: Individual differences in salivary cortisol basal levels, diurnal variation, and responses to social challenges. Development and Psychopathology. 2001;13:695–719. doi: 10.1017/s0954579401003157. doi:10.1017/S0954579401003157. [DOI] [PubMed] [Google Scholar]
- Knack JM, Jensen-Campbell LA, Baum A. Worse than sticks and stones? Bullying is associated with altered HPA axis functioning and poorer health. Brain and Cognition. 2011;77:183–190. doi: 10.1016/j.bandc.2011.06.011. doi:10.1016/j.bandc.2011.06.011. [DOI] [PubMed] [Google Scholar]
- Lagerspetz KM, Bjorkqvist K, Peltonen T. Is indirect aggression typical of females? Gender differences in aggressiveness in 11- to 12-year-old children. Aggressive Behavior. 1988;14:403–414. doi:10.1002/1098-2337. [Google Scholar]
- Lopez-Duran NL, Kovacs M, George CJ. Hypothalamic–pituitary–adrenal axis dysregulation in depressed children and adolescents: A meta-analysis. Psychoneuroendocrinology. 2009;34:1271–1783. doi: 10.1016/j.psyneuen.2009.03.016. doi:10.1016/j.psyneuen.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience. 2009;10:434–445. doi: 10.1038/nrn2639. doi:10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- MacDonald G, Leary MR. Why does social exclusion hurt? The relationship between social and physical pain. Psychological Bulletin. 2005;131:202–223. doi: 10.1037/0033-2909.131.2.202. doi:10.1037/0033-2909.131.2.202. [DOI] [PubMed] [Google Scholar]
- Mathieson LC, Crick NR. Reactive and proactive subtypes of relational and physical aggression in middle childhood: Links to concurrent and longitudinal adjustment. School Psychology Review. 2010;39:601–611. [Google Scholar]
- McEwen BS. Stress, adaptation, and disease: Allostasis and allostatic load. Annals of the New York Academy of Sciences. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. doi:10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- McEwen BS. The neurobiology of stress: From serendipity to clinical relevance. Brain Research. 2000;886:172–189. doi: 10.1016/s0006-8993(00)02950-4. doi:10.1016/S0006-8993 (00)02950-4. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Stellar ES. Stress and the individual: Mechanisms leading to disease. Archives of Internal Medicine. 1993;153:2093–2101. doi:10.1001/archinte.1993.00410180039004. [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL. Inflammation and its discontents: The role of cytokines in the pathophysiology of major depression. Biological Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. doi:10.1016/j.biopsych.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic–pituitary–adrenocortical axis in humans. Psychological Bulletin. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. doi:10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Miller GE, Cohen S, Ritchey AK. Chronic psychological stress and the regulation of pro-inflammatory cytokines: A glucocorticoid-resistance model. Health Psychology. 2002;21:531–541. doi: 10.1037//0278-6133.21.6.531. doi:10.1037/0278-6133.21.6.531. [DOI] [PubMed] [Google Scholar]
- Morris NM, Udry JR. Validation of a self-administered instrument to assess stage of adolescent development. Journal of Youth and Adolescence. 1980;9:271–280. doi: 10.1007/BF02088471. doi:10.1007/BF02088471. [DOI] [PubMed] [Google Scholar]
- Murray-Close D, Ostrov JM, Crick NR. A short-term longitudinal study of growth of relational aggression during middle childhood: Associations with gender, friendship intimacy, and internalizing problems. Development and Psychopathology. 2007;19:187–203. doi: 10.1017/S0954579407070101. doi:10.1017/S09545794070. [DOI] [PubMed] [Google Scholar]
- Negriff S, Fung MT, Trickett PK. Self-rated pubertal development, depressive symptoms, and delinquency: Measurement issues and moderation by gender and maltreatment. Journal of Youth and Adolescence. 2008;37:736–746. doi: 10.1007/s10964-008-9274-y. doi:10.1007/s10964-008-9274-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olweus D. Aggression and peer acceptance in adolescent boys: Two short-term longitudinal studies of ratings. Child Development. 1977;48:1301–1313. doi:10.1111/1467-8624.ep10399372. [PubMed] [Google Scholar]
- Olweus D. Aggression in the schools. New York: Wiley. Ostrov, J. M. (2010). Prospective associations between peer victimization and aggression. Child Development. 1978;81:1670–1677. doi: 10.1111/j.1467-8624.2010.01501.x. doi:10.1111/j.1467-8624.2010.01501.x. [DOI] [PubMed] [Google Scholar]
- Ostrov JM, Godleski SA. Toward an integrated gender-linked model of aggression subtypes in early and middle childhood. Psychological Review. 2010;117:233–242. doi: 10.1037/a0018070. doi:10.1037/a0018070. [DOI] [PubMed] [Google Scholar]
- Ouellet-Morin I, Danese A, Bowes L, Shakoor S, Ambler A, Pariante CM, et al. A discordant monozygotic twin design shows blunted cortisol reactivity among bullied children. Journal of the American Academy of Child & Adolescent Psychiatry. 2011;50:574–582. doi: 10.1016/j.jaac.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellet-Morin I, Odgers CL, Danese A, Bowes L, Shakoor S, Papadopoulos AS, et al. Blunted cortisol responses to stress signal social and behavioral problems among maltreated/bullied 12-year-old children. Biological Psychiatry. 2011;70:1016–1023. doi: 10.1016/j.biopsych.2011.06.017. doi:10.1016/j.biopsych. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piehler TF, Dishion TJ. Peer Dyadic Mutuality Rating System. University of Oregon, Child and Family Center; 2004. Unpublished manuscript. [Google Scholar]
- Piehler TF, Dishion TJ. Interpersonal dynamics within adolescent friendships: Dyadic mutuality, deviant talk, and patterns of antisocial behavior. Child Development. 2007;78:1611–1624. doi: 10.1111/j.1467-8624.2007.01086.x. doi:10.1111/j.1467-8624.2007.01086.x. [DOI] [PubMed] [Google Scholar]
- Poulin F, Chan A. Friendship stability and change in childhood and adolescence. Developmental Review. 2010;30:257–272. doi:10.1016/j.dr.2009.01.001. [Google Scholar]
- Prinstein MJ, Boergers J, Vernberg EM. Overt and relational aggression in adolescents: Social-psychological adjustment of aggressors and victims. Journal of Clinical Child Psychology. 2001;30:479–491. doi: 10.1207/S15374424JCCP3004_05. doi:10.1207/S15374424JCCP3004_05. [DOI] [PubMed] [Google Scholar]
- Rockhill CM, Fan M, Katon WJ, McCauley E, Crick NR, Pleck JH. Friendship interactions in children with and without depressive symptoms: Observation of emotion during game-playing interactions and post-game evaluations. Journal of Abnormal Child Psychology. 2007;35:429–441. doi: 10.1007/s10802-007-9101-z. doi:10.1007/s10802-007-9101-z. [DOI] [PubMed] [Google Scholar]
- Rose AJ. Co-rumination in the friendships of girls and boys. Child Development. 2002;73:1830–1843. doi: 10.1111/1467-8624.00509. doi:10.1111/1467-8624.00509. [DOI] [PubMed] [Google Scholar]
- Rose AJ, Rudolph KD. A review of sex differences in peer relationship processes: Potential trade-offs for the emotional and behavioral development of girls and boys. Psychological Bulletin. 2006;132:98–131. doi: 10.1037/0033-2909.132.1.98. doi:10.1037/0033-2909.132.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph KD, Troop-Gordon W, Granger DA. Peer victimization and aggression: Moderation by individual differences in salivary cortisol and alpha-amylase. Journal of Abnormal Child Psychology. 2010;38:843–856. doi: 10.1007/s10802-010-9412-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph KD, Troop-Gordon W, Granger DA. Individual differences in biological stress responses moderate the contribution of early peer victimization to subsequent depressive symptoms. Psychopharmacology. 2011;214:209–219. doi: 10.1007/s00213-010-1879-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruttle PL, Shirtcliff EA, Serbin LA, Ben-Dat Fisher D, Stack DM, Schwartzman AE. Disentangling psychobiological mechanisms underlying internalizing and externalizing behaviors in youth: Longitudinal and concurrent associations with cortisol. Hormones and Behavior. 2011;59:123–132. doi: 10.1016/j.yhbeh.2010.10.015. doi:10.1016/j.yhbeh.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandler IN, Miller P, Short J, Wolchik SA. Social support as a protective factor for children in stress. In: Bell D, editor. Children’s social networks and social supports. Wiley; Oxford: 1989. pp. 277–307. [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Reviews. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. doi:10.1210/er.21.1.55. [DOI] [PubMed] [Google Scholar]
- Schmidt ME, Bagwell CL. The protective role of friendships in overtly and relationally victimized boys and girls. Merrill-Palmer Quarterly. 2007;53:439–460. doi:10.1353/mpq.2007.0021. [Google Scholar]
- Schreiber JE, Shirtcliff E, Van Hulle C, Lemery-Chalfant K, Klein MH, Kalin NH, et al. Environmental influences on family similarity in afternoon cortisol levels: Twin and parent-offspring designs. Psychoneuroendocrinology. 2006;31:1131–1137. doi: 10.1016/j.psyneuen.2006.07.005. doi:10.1016/j.psyneuen.2006.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Allison AL, Armstrong JM, Slattery MJ, Kalin NH, Essex MJ. Longitudinal stability and developmental properties of salivary cortisol levels and circadian rhythms from childhood to adolescence. Developmental Psychobiology. 2012;54:493–502. doi: 10.1002/dev.20607. doi:10.1002/dev.20607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Essex MJ. Concurrent and longitudinal associations of basal and diurnal cortisol with mental health symptoms in early adolescence. Developmental Psychobiology. 2008;50:690–703. doi: 10.1002/dev.20336. doi:10.1002/dev.20336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH. The teenage brain: Sensitivity to social evaluation. Current Directions in Psychological Science. 2013;22:121–127. doi: 10.1177/0963721413476512. doi:10.1177/0963721413476512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JG, Mazurka R, Bond L, Wynne-Edwards KE, Harkness KL. Rumination and impaired cortisol recovery following a social stressor in adolescent depression. Journal of Abnormal Child Psychology. 2013;41:1015–1026. doi: 10.1007/s10802-013-9740-1. doi:10.1007/s10802-013-9740-1. [DOI] [PubMed] [Google Scholar]
- Stroud LR, Foster E, Papandonatos GD, Handwerger K, Granger DA, Kivlighan KT, et al. Stress response and the adolescent transition: Performance versus peer rejection stressors. Development and Psychopathology. 2009;21:47–68. doi: 10.1017/S0954579409000042. doi:10.1017/S0954579409000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan TN, Farrell AD, Kliewer W. Peer victimization in early adolescence: Association between physical and relational victimization and drug use, aggression, and delinquent behaviors among urban middle school students. Development and Psychopathology. 2006;18:119–137. doi: 10.1017/S095457940606007X. doi:10.1017/S095457940606007X. [DOI] [PubMed] [Google Scholar]
- Vaillancourt T, Duku E, Decatanzaro D, Macmillan H, Muir C, Schmidt LA. Variation in hypothalamic–pituitary–adrenal axis activity among bullied and non-bullied children. Aggressive Behavior. 2008;34:294–305. doi: 10.1002/ab.20240. doi:10.1002/ab.20240. [DOI] [PubMed] [Google Scholar]
- Willoughby M, Vandergrift N, Blair C, Granger DA. A structural equation modeling approach for the analysis of cortisol data collected using pre-post-post designs. Structural Equation Modeling. 2007;14:125–145. doi:10.1207/s15328007sem1401_7. [Google Scholar]
- Wood A, Kroll L, Moore A, Harrington R. Properties of the Mood and Feelings Questionnaire (MFQ) in adolescent psychiatric outpatients: A research note. Journal of Child Psychology and Psychiatry. 1995;36:327–334. doi: 10.1111/j.1469-7610.1995.tb01828.x. doi:10.1111/j.1469-7610.1995.tb01828.x. [DOI] [PubMed] [Google Scholar]