INTRODUCTION
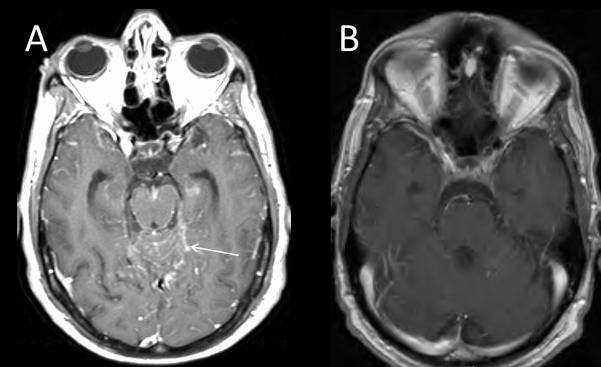
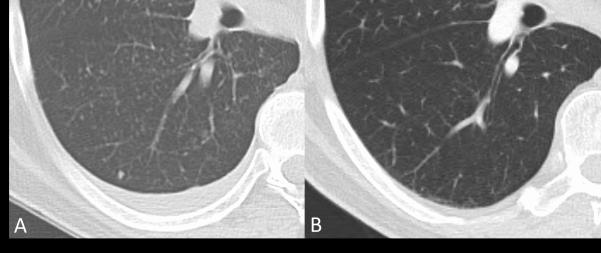
A 62 year old male former smoker presented emergently to an outside hospital with acute onset expressive aphasia of unknown etiology and was treated for stroke with tissue plasminogen activator infusion. Additional history revealed a 5 month history of headaches, weight loss, fatigue, poor work performance and episodic symptoms of significant confusion, expressive aphasia, dressing apraxia, gait imbalance, and hypersomnolence. Lumbar puncture revealed hypercellular cerebrospinal fluid (CSF, WBC 39 cells/mL) and increased protein (178g/dL). Computed tomography (CT) demonstrated a mass in the right lower lobe with innumerable ‘miliary’ nodules in bilateral lungs (figure 1A). A positron emitting tomography (PET)/CT and brain magnetic resonance imaging (MRI) with and without contrast re-demonstrated a hypermetabolic right lobe mass with no extrathoracic metastases and diffuse nodular leptomeningeal enhancement with extension along cranial nerves consistent with leptomeningeal metastases (figure 2A). Core needle biopsy of the right lobe nodule demonstrated a moderately differentiated adenocarcinoma that was CK7+ and TTF1+ by immunohistochemistry. Allele-specific real-time PCR EGFR mutational testing (Qiagen, Manchester, UK) revealed an in-frame deletion in exon 19 of EGFR. Dual ALK/ROS1 FISH was negative for either gene rearrangement. The patient was hospitalized for failure to thrive with an Eastern Cooperative Oncology Group (ECOG) performance status of 4 and was started on erlotinib (150 mg/day), dexamethasone and levetiracetam. Marked improvement in his neurologic symptoms occurred within weeks of initiating erlotinib, and restaging CT demonstrated marked decrease in the size of the primary tumor and miliary nodules (figure 1B). Restaging brain MRI showed significant decrease of leptomeningeal enhancement (figure 2B). At this point the patient’s neurological function had improved and he was no longer experiencing abrupt onset spells or seizure activity. He was living independently, driving, and was primarily responsible for all activities of daily living. Montreal Cognitive Assessment score improved from unable to perform to a normal score of 29 out of a possible 30 points. The patient remained on erlotinib with continued partial response in his intrathoracic disease with leptomeningeal progression by MRI and associated cognitive decline after 8 months of sustained clinical and radiographic response. Progression of the leptomeningeal disease caused a significant decline in the patient’s performance status including confusion, short-term memory loss, progressive ataxia, and urinary incontinence. He was determined to be an unsuitable candidate for brain radiation or systemic treatment. After approximately 8 months the dose of erlotinib was increased to 1500mg weekly in an attempt to achieve higher CNS penetration. Treatment was discontinued after 4 weeks of pulsatile high dose erlotinib and dexamethasone for edema related symptoms with no improvement in his neurological status. Hospice care was initiated, and shortly thereafter the patient passed away.
1).
Magnified axial Computed Tomography images of the chest (Panels A,B) demonstrating miliary disease burden within the right lower lobe of the lung at initial staging (Panel A). Additional representive axial image of the right lower lobe demostrating near complete resolution approximately 3 months after intiating erlotinib (Panel B).
2).
Axial T1 post-contrast images of Brain MRI with and without contrast (Panels A and B) demonstrating leptomeningeal enhancement in the subarachnoid spaces prior to erlotinib (A, arrow) with radiographic response approximately 4 months after initiating erlotinib (B).
DISCUSSION
EGFR tyrosine kinase inhibitors (TKIs, e.g. erlotinib, gefitinib, afatinib) are the preferred first line therapy for patients with activating mutations in EGFR due to superior efficacy and tolerability compared to standard chemotherapy.1-3 Penetration of erlotinib into the CNS is limited by the blood-brain barrier, with one pharmacokinetic trial of NSCLC patients with CNS metastasis reporting mean steady-state cerebrospinal fluid erlotinib (CSF) values of 54 ng/mL (standard deviation +/− 30ng/mL) which is equivalent to 5.1% (standard deviation +/−1 .9%) of the study cohort’s mean steady state plasma concentration. However, steady state CSF values of 54ng/mL well exceed known median inhibitory concentration of (IC50) of cell lines with sensitizing L858R and deletion 19 EGFR mutations treated with erlotinib.4
EGFR TKIs gefitinib and erlotinib have demonstrated activity against leptomeningeal metastases at standard dosages.5, 6 Furthermore, EGFR deletion 19 mutations may be predictive for improved outcomes for leptomeningeal metastasis as suggested by a recent cohort study that demonstrated that patients with EGFR deletion 19 mutations have improved clinical outcomes when compared to EGFR exon 21 mutation and EGFR wild-type cohorts with leptomeningeal metastasis. Median time to progression or symptom deterioration for patients with leptomeningeal metastasis was 7.8 months in the exon 19 deletion subgroup compared to 0.9 and 1.9 months in the wild type and exon 21 mutation cohorts, respectively.7
Where concerns regarding EGFR TKI penetration into the CNS exist or toxicity to standard daily dosages of erlotinib exist, pulsatile weekly erlotinib can improve both CSF drug concentration and CNS response.8, 9 While there was an attempt to recapture CNS response with pulsatile weekly erlotinib in our patient, it was proven unsuccessful with continued cognitive decline. It is unknown if the patient’s leptomeningeal relapse and continued progression was due inadequate delivery of erlotinib into the CNS or development of a secondary resistance mutation within the CNS. Isolated T790M resistance mutations within the CNS and different inter-patient mechanisms of resistance to EGFR inhibition (between the CNS and other areas of visceral metastasis) have been described in other case reports. 10
CONCLUSION
The presence of diffuse miliary pulmonary nodules has been associated with EGFR mutations and EGFR exon 19 deletion mutations specifically.11-13 Other analyses report an increased presence of a miliary pattern of CNS disease for NSCLC harboring a EGFR exon 19 deletion when compared to matched EGFR wild-type and EGFR exon 21 insertion mutations, suggesting an EGFR exon 19 specific tropism for growth and metastasis.14 This is the first report to describe a miliary pattern of intrapulmonary and leptomeningeal metastasis in the same patient and suggests that an increased probability of miliary-specific pattern of metastasis may exist for both organ systems in patients with EGFR exon 19 deletion mutation NSCLC. Larger series containing EGFR mutated NSCLC subpopulations and patterns of metastatic spread are needed to confirm this phenomenon.
We also describe a prolonged systemic and CNS response to standard dose erlotinib for greater than 8 months with near complete resolution of visceral disease. Lastly, the case report further emphasizes the safety, tolerability, and clinical benefit of erlotinib in a scenario where hospice was the sole other recommendation to almost complete return of previous functional status for an extended period of time. This underscores the notion that poor performance status and consideration for palliative care/hospice should not eliminate molecular testing from evaluation and treatment algorithms.
CLINICAL PRACTICE POINTS.
- EGFR positive non-small cell lung cancer (EGFR+ NSCLC) have improved clinical outcome with leptomeningeal carcinomatosis when compared to wild-type cohort.
- EGFR + NSCLC patients with leptomeningeal carcinomatosis can respond to both standard (150mg/d) and pulsatile high dose (1000-1500mg/week) erlotinib with improvement of cognitive function
- Patients with EGFR 19 deletion mutation NSCLC demonstrate a miliary pattern of pulmonary metastasis and propensity for leptomeningeal disease. This case report is the first to describe these linked phenomena.
Footnotes
CONFILICT OF INTEREST:
The above authors (SP, EMB, DLA, DD, RCD) state that they have no conflict of interest to disclose.
REFERENCES
- 1.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947–57. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 2.Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239–46. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 3.Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31(27):3327–34. doi: 10.1200/JCO.2012.44.2806. [DOI] [PubMed] [Google Scholar]
- 4.Kancha RK, von Bubnoff N, Peschel C, Duyster J. Functional analysis of epidermal growth factor receptor (EGFR) mutations and potential implications for EGFR targeted therapy. Clin Cancer Res. 2009;15(2):460–7. doi: 10.1158/1078-0432.CCR-08-1757. [DOI] [PubMed] [Google Scholar]
- 5.Nakamichi S, Kubota K, Horinouchi H, et al. Successful EGFR-TKI rechallenge of leptomeningeal carcinomatosis after gefitinib-induced interstitial lung disease. Jpn J Clin Oncol. 2013;43(4):422–5. doi: 10.1093/jjco/hyt012. [DOI] [PubMed] [Google Scholar]
- 6.Togashi Y, Masago K, Hamatani Y, et al. Successful erlotinib rechallenge for leptomeningeal metastases of lung adenocarcinoma after erlotinib-induced interstitial lung disease: a case report and review of the literature. Lung Cancer. 2012;77(2):464–8. doi: 10.1016/j.lungcan.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 7.Umemura S, Tsubouchi K, Yoshioka H, et al. Clinical outcome in patients with leptomeningeal metastasis from non-small cell lung cancer: Okayama Lung Cancer Study Group. Lung Cancer. 2012;77(1):134–9. doi: 10.1016/j.lungcan.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Clarke JL, Pao W, Wu N, Miller VA, Lassman AB. High dose weekly erlotinib achieves therapeutic concentrations in CSF and is effective in leptomeningeal metastases from epidermal growth factor receptor mutant lung cancer. J Neurooncol. 2010;99(2):283–6. doi: 10.1007/s11060-010-0128-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dhruva N, Socinski MA. Carcinomatous meningitis in non-small-cell lung cancer: response to high-dose erlotinib. J Clin Oncol. 2009;27(22):e31–2. doi: 10.1200/JCO.2008.21.0963. [DOI] [PubMed] [Google Scholar]
- 10.Scher KS, Saldivar JS, Fishbein M, Marchevsky A, Reckamp KL. EGFR-mutated lung cancer with T790M-acquired resistance in the brain and histologic transformation in the lung. J Natl Compr Canc Netw. 2013;11(9):1040–4. doi: 10.6004/jnccn.2013.0126. [DOI] [PubMed] [Google Scholar]
- 11.Wu SG, Hu FC, Chang YL, et al. Frequent EGFR mutations in nonsmall cell lung cancer presenting with miliary intrapulmonary carcinomatosis. Eur Respir J. 2013;41(2):417–24. doi: 10.1183/09031936.00006912. [DOI] [PubMed] [Google Scholar]
- 12.Laack E, Simon R, Regier M, et al. Miliary never-smoking adenocarcinoma of the lung: strong association with epidermal growth factor receptor exon 19 deletion. J Thorac Oncol. 2011;6(1):199–202. doi: 10.1097/JTO.0b013e3181fb7cf1. [DOI] [PubMed] [Google Scholar]
- 13.Togashi Y, Masago K, Kubo T, et al. Association of diffuse, random pulmonary metastases, including miliary metastases, with epidermal growth factor receptor mutations in lung adenocarcinoma. Cancer. 2011;117(4):819–25. doi: 10.1002/cncr.25618. [DOI] [PubMed] [Google Scholar]
- 14.Sekine A, Kato T, Hagiwara E, et al. Metastatic brain tumors from non-small cell lung cancer with EGFR mutations: distinguishing influence of exon 19 deletion on radiographic features. Lung Cancer. 2012;77(1):64–9. doi: 10.1016/j.lungcan.2011.12.017. [DOI] [PubMed] [Google Scholar]


