Abstract
Importance
The Healthy Brain Initiative seeks to optimize brain health as we age. Free radical injury is an important effector of molecular and cellular stress in aging brain that derives from multiple sources.
Objective
Identify potentially modifiable risk factors associated with increased markers of brain oxidative stress.
Design, Setting, and Participants
Our study consisted of 320 research volunteers (178 women) aged 21 to 100 years old who were medically healthy and cognitively normal.
Measures
Free radical injury to brain was assessed using cerebrospinal fluid (CSF) F2-isoprostanes (IsoPs) and correlated with age, gender, race, cigarette smoking, body mass index (BMI), inheritance of the ε4 allele of apolipoprotein E gene (APOE), and cerebrospinal fluid biomarkers of Alzheimer’s disease (AD).
Results
CSF F2-IsoP concentration increased with age by approximately 10% from age 45 to 71 years in medically healthy cognitively normal adults. CSF F2-IsoP concentration increased by an average of >10% for every 5 kg/m2 increase in BMI. Current smokers had approximately three-fold greater effect than age on CSF F2-IsoPs. Women had greater average CSF F2-IsoP concentration than men at all ages after adjusting for other factors. Neither CSF AD biomarkers nor inheritance of APOE ε4 allele were associated with CSF F2-IsoP concentration in this group of medically healthy cognitively normal adults. Association between CSF F2-IsoP concentrations and race was not significant after controlling for effect of current smoking status.
Conclusions & Relevance
Our results are consistent with an age-related increase in free radical injury in human brain, and uniquely suggest that this form of injury may be greater in women than in men. Our results also highlighted two lifestyle modifications that would have even greater impact on suppressing free radical injury to brain than would suppressing processes of aging. These results inform efforts to achieve success in the Healthy Brain Initiative.
INTRODUCTION
The Healthy Brain Initiative 2013–20181 formulated by the Centers for Disease Control and Prevention and the Alzheimer’s Association is a challenge to researchers, health care providers, and public health officials to devise and deliver approaches that optimize brain health as we age. Free radical injury, an important effector of molecular and cellular stress in the central nervous system (CNS) as we age, derives from multiple sources including processes of aging, genetics, environmental factors, and latent Alzheimer’s disease (AD). Clarification of the sources of age-related free radical injury to CNS is important because those that are approachable through lifestyle modification or treatment offer an opportunity to optimize brain heath across the human life span. This study focuses on several potential drivers of age-related free radical injury in CNS in 320 research volunteers aged 21 to 100 years who were normal by medical examination and extensive cognitive testing by determining associations of genetic, environmental, demographic, and cerebrospinal fluid (CSF) AD biomarker data with CSF F2- isoprostanes (IsoPs), a widely-used biomarker of free radical injury to brain.2–9
METHODS
The institutional review boards of the participating institutions approved all procedures and all subjects provided written informed consent prior to study enrollment. 320 cognitively normal subjects were recruited from AD Centers from 2002–2009; were healthy by medical examination; had Mini-Mental State Examination scores ≥ 26, Clinical Dementia Rating (CDR) score = 0; and had no evidence or history of cognitive or functional decline. The neuropsychologic tests included in this study examined multiple aspects of cognition.10 The Wechsler Memory Scale-Revised Logical Memory Immediate and Delayed paragraph recall tasks measure verbal episodic memory.11,12 The Trail Making A task measures psychomotor speed, visuospatial function, and visual attention; Trail Making B adds a set-shifting element and captures executive function.13 Category fluency (animals) is a measure of semantic memory and language.14 CSF was collected in the morning after an overnight fast, frozen immediately, and stored at −80°C until quantified for F2-IsoPs, Aβ42, total Tau, and Tau-P181 as described previously.15,16 All CSF protein, glucose, and cell values were within normal ranges (not shown).3 Apolipoprotien E genotype (APOE) was determined by a restriction digest method.16
Linear regression was used to assess associations between CSF F2-IsoP concentration and age, gender, race (white versus non-white), current smoking status (yes versus no), BMI (per 5 kg/m2 increase), presence/absence of APOE ε4 allele, and CSF AD biomarker concentrations (Aβ42, total tau and tau-P181). Total tau and tau-P181 were strongly correlated (r=0.77, p<0.0001) and so to avoid colinearity, only tau was included in modeling. Age was modeled as a 3 degree restricted cubic spline summarized as average difference in CSF F2-IsoP concentrations in those aged 71 (75th percentile for age) compared to those aged 45 (25th percentile). Each study characteristic was modeled first as an independent variable with adjustment for age (Model 1). CSF AD biomarkers were added to age in Model 2. All other variables, including, gender, race, BMI, current smoking status, and APOE ε4 allele were then added to Model 2 to determine which had an independent association with CSF F2-IsoPs in the presence of all others (Model 3). Finally, an age by each potential effect modifier (gender, BMI, smoking status, or APOE ε4) interaction term was added to Model 3. All models were summarized using adjusted R2. Analyses were carried out using R version 2.15.217 using the rms package.18
RESULTS
Female (n=172) and male (n=148) subjects were matched well (Table 1). As expected, there was a positive association between CSF F2-IsoP concentration and age across the adult human life span (Table 2) that was independent of CSF AD biomarkers and other variables (Model 3). This association was approximately linear, although with some suggestion of accelerated increase in advanced age after adjustment for covariates in Model 3 (nonlinear trend p=0.05). Results from all three models estimated that CSF F2-IsoP concentration increased approximately 3 pg/ml, or about 10%, from age 45 to 71 years.
Table 1.
Characteristics of 320 cognitively normal, healthy volunteers stratified by gender
| Women (n=172) | Men (n=148) | Both (n=320) | |
|---|---|---|---|
| Age (yr) | 57.8 [1.3], 21–100 | 56.4 [1.6], 22–88 | 57.2 [1.0], 21–100 |
| Education (yr) | 15.8 [0.2], 10–22 | 16.5 [0.2], 10–27 | 16.1 [0.1], 10–27 |
| Caucasian (%) | 152 (88%) | 130 (88%) | 282 (88%) |
| APOE ε4 + (%) | 64 (37%) | 46 (31%) | 110 (34%) |
| BMI (kg/m2)^ | 25.8 [0.3], 18–41 | 26.4 [0.3], 20–37 | 26.1 [0.2], 18–41 |
| Current smoker (%) | 11 (6%) | 10 (7%) | 21 (7%) |
| CSF F2-IsoPs (pg/ml) | 30.6 [0.7], 13–60 | 28.8 [0.7], 11–65 | 29.8 [0.5], 11–65 |
| CSF Aβ42 (pg/ml)* | 334 [10], 60–829 | 322 [10], 91–786 | 328 [7], 60–829 |
| CSF Tau (pg/ml)* | 48.5 [1.0], 5–85 | 50.0 [1.2], 20–125 | 49.2 [0.8], 5–125 |
| CSF Tau-P181 (pg/ml)* | 31.8 [0.6], 2–63 | 32.1 [1.0], 10–98 | 31.9 [0.6], 2–98 |
Data are mean with [SE] and range, or number with (%).
using CDC standard weight status categories27 one (female) volunteer was underweight, 132 (80 female) volunteers were normal weight, 129 (59 female) volunteers were overweight, and 53 (32 female) volunteers were obese; BMI data was missing for one (male) volunteer.
adjusted for ELISA batch.
Table 2.
Associations between CSF F2-IsoP concentrations (pg/ml) and age, CSF AD biomarkers, and other genetic, environmental, and demographic variables in 320 cognitively normal subjects.
| Variables | Model 1 |
Model 2 R2 = 0.06 |
Model 3 R2 = 0.20 |
|---|---|---|---|
| β ± SE, p-value | β ± SE, p value | β ± SE, p value | |
| Age (change in pg/ml CSF F2-IsoPs from age 45 to age 71) | 3.0 ± 0.8, p=0.0009 R2 = 0.04 |
3.1 ± 0.8, p=0.0007 | 3.3 ± 0.8, p=0.0001 |
| CSF Aβ42 (per increase equivalent to the interquartile range, 240–386) | 1.7 ± 0.6, p=0.004, R2 = 0.06 | 1.3 ± 0.6, p=0.05 | 1.1 ± 0.6, p=0.07 |
| CSF total tau (per increase equivalent to the interquartile range, 40–57) | 1.5 ± 0.6, p=0.01 R2 = 0.05 |
0.9 ± 0.7, p=.19 | 0.8 ± 0.6, p=0.24 |
| Gender (female) | 1.8 ± 1.0, p=0.07 R2 = 0.04 |
2.3 ± 0.9, p=0.02 | |
| Race (non-Caucasian) | 3.4 ± 1.6, p=0.03 R2 = 0.05 |
1.1 ± 1.5, p=0.45 | |
| Current Cigarette Smoker (yes) | 9.4 ± 2.0, p<0.0001 R2 = 0.10 |
8.3 ± 1.9, p<0.0001 | |
| BMI (per 5 kg/m2 increase) | 3.3 ± 0.6 p<0.0001 R2 = 0.11 |
3.9 ± 0.7, p<0.0001 | |
| APOE ε4 allele (one or two) | 0.8 ± 1.1, p=0.46 R2 = 0.04 |
1.1 ± 1.0, p=0.29 |
Model 1 includes each variable separately and is adjusted only for age.
Model 2 includes both CSF AD biomarkers in addition to age.
Model 3 includes model 2 and gender, BMI, smoking status, and APOE ε4.
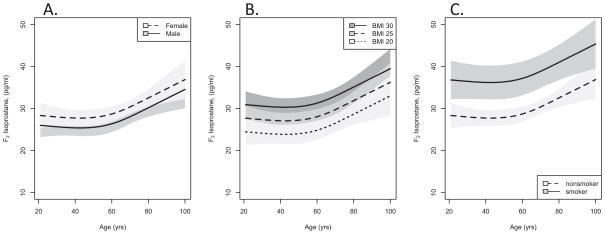
BMI and smoking were the strongest independent correlates of CSF F2-IsoP concentration (p< 0.0001), which increased by an average of >10% for every 5 kg/m2 increase in BMI (Model 3; Figure). Current cigarette smokers had significantly higher CSF F2-IsoP concentration than nonsmokers (Model 3; Figure) that was approximately three-fold greater than the effect of age. Women had greater average CSF F2-IsoP concentration than men after adjusting for other factors (Model 3; Figure), although the estimated difference between men and women was smaller than that observed for advancing age. None of these three variables was a significant effect modifier of the association between age and CSF F2-IsoP concentration (p=0.91, 0.29, or 0.11 for gender, BMI or current cigarette smoking). The difference in CSF F2-IsoP concentration by smoking status tended to be greatest in midlife; however, the number of current smokers in our study sample may be too few for detection of a significant effect modification.
Figure. CSF F2-IsoP concentrations versus age.
Predicted mean (lines) and 95% confidence intervals (shaded regions) for CSF F2-IsoP concentrations versus age in cognitively normal subjects across adult ages calculated from Model 3 and stratified by gender (A), BMI (B) and smoking status (C).
CSF AD biomarkers neither were associated with CSF F2-IsoP concentration after adjustment for age (Model 2) nor after adjustment for other co-variates (Model 3). Similarly, there was no effect on CSF F2-IsoP concentrations when stratified into those with versus without an APOE ε4 allele (p=0.29, Model 3). The association between CSF F2-IsoPs concentration and age did not differ by APOE ε4 status (age by ε4 allele interaction p=0.64).
Association between CSF F2-IsoP concentrations and race was complex and confounded by greater prevalence of cigarette smoking among non-Caucasians (24% for non-Caucasian versus 4% for Caucasians). Multivariable analysis (Model 3) did not detect a significant association between race and CSF F2-IsoP concentrations after controlling for effect of current smoking status.
DISCUSSION
Our results from a large group of adult research volunteers who were cognitively normal and in good health are consistent with an age-related increase in free radical injury in human brain, and suggest that this form of CNS injury may be slightly but consistently greater in women than in men. This difference is consistent with other reports of a sex difference in brain reserve and clinical manifestations of AD pathologic changes,19–21 although this association remains a point of discussion. Importantly our results also showed that both smoking and increased BMI, already know to be associated with increased free radical injury in peripheral organs,22,23 were also associated with increased CSF F2-isoprostanes. Thus avoidance of smoking and reducing BMI, in addition to their already established benefits, may also be beneficial in reducing molecular and cellular stress to our brains. Within the limitations of our study, our data do not implicate latent AD, inheritance of APOE ε4, or race as major contributors to this form of brain injury in healthy adults. Indeed, other factors likely are involved, as seen in our Model 3 results, where most of the F2-IsoP variability is not explained by the defined parameters. For example, obesity is associated with a variety of other abnormalities, including diabetes and dyslipidemia, which could contribute to or modify the observed effect. Finally, it is worth noting that while CSF F2-IsoPs are widely-used biomarkers of free radical injury,2–9 they also activate the thromboxane A2 receptor and thereby contribute to abnormal vasoconstriction in multiple organs, including cerebrum.24–26 Together, these results contribute to the evidence base necessary for achieving the long-term goal of the Healthy Brain Initiative: “to maintain or improve the cognitive performance of all adults.”
Acknowledgments
This work was generously supported by the NIH (P50AG05136, P30AG008017, P50AG005131) and the Nancy and Buster Alvord Endowment. These funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The corresponding author, Dr. Thomas J. Montine, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. We thank Dr. Kathleen Montine for editorial assistance.
Footnotes
Conflict of Interest Disclosures: None reported.
Author Contributions. Study conception and design: Peskind, Raskind, Montine. Acquisition, analysis, and interpretation of data: Peskind, Li, Shofer, Millard, Leverenz, Yu, Quinn, Galasko. Drafting of the manuscript: Li, Shofer, Millard, Montine. Critical revision of the manuscript for important intellectual content: Peskind, Leverenz, Yu, Raskind, Quinn, Galasko. Statistical analysis: Shofer, Millard. Obtained funding: Peskind, Raskind, Quinn, Galasko, Montine. Study supervision: Li, Leverenz, Yu
References
- 1.Alzheimer’s Association and Centers for Disease Control and Prevention. The Healthy Brain Initiative: The Public Health Road Map for State and National Partnerships, 2013–2018. Chicago, IL: Alzheimer’s Association; 2013. [Google Scholar]
- 2.Guest J, Grant R, Mori TA, Croft KD. Changes in oxidative damage, inflammation and [NAD(H)] with age in cerebrospinal fluid. PLoS One. 2014;9(1):e85335. doi: 10.1371/journal.pone.0085335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duits FH, Kester MI, Scheffer PG, et al. Increase in cerebrospinal fluid F2-isoprostanes is related to cognitive decline in APOE epsilon4 carriers. J Alzheimers Dis. 2013;36(3):563–570. doi: 10.3233/JAD-122227. [DOI] [PubMed] [Google Scholar]
- 4.Sbardella E, Greco A, Stromillo ML, et al. Isoprostanes in clinically isolated syndrome and early multiple sclerosis as biomarkers of tissue damage and predictors of clinical course. Mult Scler. 2013;19(4):411–417. doi: 10.1177/1352458512457721. [DOI] [PubMed] [Google Scholar]
- 5.Pomara N, Bruno D, Sarreal AS, et al. Lower CSF amyloid beta peptides and higher F2-isoprostanes in cognitively intact elderly individuals with major depressive disorder. Am J Psychiatry. 2012;169(5):523–530. doi: 10.1176/appi.ajp.2011.11081153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galasko DR, Peskind E, Clark CM, et al. Antioxidants for Alzheimer disease: a randomized clinical trial with cerebrospinal fluid biomarker measures. Arch Neurol. 2012;69(7):836–841. doi: 10.1001/archneurol.2012.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farias SE, Heidenreich KA, Wohlauer MV, Murphy RC, Moore EE. Lipid mediators in cerebral spinal fluid of traumatic brain injured patients. J Trauma. 2011;71(5):1211–1218. doi: 10.1097/TA.0b013e3182092c62. [DOI] [PubMed] [Google Scholar]
- 8.Mosconi L, Glodzik L, Mistur R, et al. Oxidative stress and amyloid-beta pathology in normal individuals with a maternal history of Alzheimer’s. Biol Psychiatry. 2010;68(10):913–921. doi: 10.1016/j.biopsych.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Korecka M, Clark CM, Lee VM, Trojanowski JQ, Shaw LM. Simultaneous HPLC-MS-MS quantification of 8-iso-PGF(2alpha) and 8,12-iso-iPF(2alpha) in CSF and brain tissue samples with on-line cleanup. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878(24):2209–2216. doi: 10.1016/j.jchromb.2010.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weintraub S, Salmon D, Mercaldo N, et al. The Alzheimer’s Disease Centers’ Uniform Data Set (UDS): the neuropsychologic test battery. Alzheimer Dis Assoc Disord. 2009;23(2):91–101. doi: 10.1097/WAD.0b013e318191c7dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wechsler D, Stone C. Manual: Wechsler Memory Scale. New York: Psychological Corporation; 1973. [Google Scholar]
- 12.Wechsler D. Wechsler Memory Scale-Revised. New York: Harcourt Brace Jovanovich; 1987. [Google Scholar]
- 13.Armitage S. An analysis of certain psychological tests used in the evaluation of brain injury. Psych Mono. 1946;60:1–48. [Google Scholar]
- 14.Morris JC, Heyman A, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39(9):1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 15.Milatovic D, VanRollins M, Li K, Montine KS, Montine TJ. Suppression of murine cerebral F2-isoprostanes and F4-neuroprostanes from excitotoxicity and innate immune response in vivo by alpha- or gamma-tocopherol. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;827(1):88–93. doi: 10.1016/j.jchromb.2005.03.037. [DOI] [PubMed] [Google Scholar]
- 16.Li G, Sokal I, Quinn JF, et al. CSF tau/Abeta42 ratio for increased risk of mild cognitive impairment: a follow-up study. Neurology. 2007;69(7):631–639. doi: 10.1212/01.wnl.0000267428.62582.aa. [DOI] [PubMed] [Google Scholar]
- 17.R Core Team . R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2012. [Google Scholar]
- 18.Harrell FE., Jr rms: Regression Modeling Strategies. R package version 3.6-0. 2012 [Google Scholar]
- 19.Perneczky R, Drzezga A, Diehl-Schmid J, Li Y, Kurz A. Gender differences in brain reserve : an (18)F-FDG PET study in Alzheimer’s disease. Journal of neurology. 2007;254(10):1395–1400. doi: 10.1007/s00415-007-0558-z. [DOI] [PubMed] [Google Scholar]
- 20.Barnes LL, Wilson RS, Bienias JL, Schneider JA, Evans DA, Bennett DA. Sex differences in the clinical manifestations of Alzheimer disease pathology. Archives of general psychiatry. 2005;62(6):685–691. doi: 10.1001/archpsyc.62.6.685. [DOI] [PubMed] [Google Scholar]
- 21.Musicco M. Gender differences in the occurrence of Alzheimer’s disease. Functional neurology. 2009;24(2):89–92. [PubMed] [Google Scholar]
- 22.Davi G, Guagnano MT, Ciabattoni G, et al. Platelet activation in obese women: role of inflammation and oxidant stress. JAMA. 2002;288(16):2008–2014. doi: 10.1001/jama.288.16.2008. [DOI] [PubMed] [Google Scholar]
- 23.Morrow JD, Frei B, Longmire AW, et al. Increase in circulating products of lipid peroxidation (F2-isoprostanes) in smokers. Smoking as a cause of oxidative damage. N Engl J Med. 1995 May 4;332(18):1198–1203. doi: 10.1056/NEJM199505043321804. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi K, Nammour TM, Fukunaga M, et al. Glomerular actions of a free radical-generated novel prostaglandin, 8-epi-prostaglandin F2 alpha, in the rat. Evidence for interaction with thromboxane A2 receptors. J Clin Invest. 1992;90(1):136–141. doi: 10.1172/JCI115826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Audoly LP, Rocca B, Fabre JE, et al. Cardiovascular responses to the isoprostanes iPF(2alpha)-III and iPE(2)-III are mediated via the thromboxane A(2) receptor in vivo. Circulation. 2000;101(24):2833–2840. doi: 10.1161/01.cir.101.24.2833. [DOI] [PubMed] [Google Scholar]
- 26.Hoffman SW, Moore S, Ellis EF. Isoprostanes: free radical-generated prostaglandins with constrictor effects on cerebral arterioles. Stroke. 1997;28(4):844–849. doi: 10.1161/01.str.28.4.844. [DOI] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention. [Accessed April 19, 2014, 2014.];Healthy Weight - It’s not a diet, it’s a lifestyle. 2014 http://www.cdc.gov/healthyweight/assessing/bmi/adult_bmi/