Abstract
Importance
Amyotrophic lateral sclerosis (ALS) is a severe progressive disease that cannot be prevented or cured. Diet-derived long chain polyunsaturated fatty acids (PUFA) are incorporated in brain lipids and modulate oxidative and inflammatory processes, and could thus affect ALS risk and progression.
Objective
To examine the association between n-6 and n-3 PUFA consumption and ALS risk.
Design, setting, and participants
Longitudinal analyses based on 1,002,082 participants (479,114 women; 522,968 men) in five prospective cohorts: the National Institutes of Health-AARP Diet and Health Study, the Cancer Prevention Study II Nutrition Cohort, the Health Professionals Follow-up Study, the Multiethnic Cohort Study, and the Nurses’ Health Study. Diet was assessed via food frequency questionnaire developed or modified for each cohort. Participants were categorized into cohort-specific quintiles of intake of energy-adjusted dietary variables.
Main outcomes and measures
Cohort-specific multivariable-adjusted risk ratios (RR) of ALS incidence or death estimated by Cox proportional hazards regression and pooled using random-effects methods.
Results
A total of 995 ALS cases were documented during the follow-up. A greater n-3 PUFA intake was associated with a reduced risk of ALS – the pooled, multivariable-adjusted RR for the highest to the lowest quintile was 0.66 (95% CI: 0.53–0.81; P trend<0.001). Consumption of both α-linolenic acid (RR = 0.73; 95% CI: 0.59 to 0.89; P trend=0.003) and marine n-3 PUFAs (RR=0.84; 95% CI: 0.65–1.08, P trend=0.03) contributed to this inverse association. Intakes of n-6 PUFA were not associated with ALS risk.
Conclusion and relevance
Consumption of foods high in n-3 PUFAs may help prevent or delay onset of ALS.
INTRODUCTION
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disorder with few effective treatments and a disease pathogenesis that is poorly understood.1–3 Diet-derived polyunsaturated fatty acids (PUFA) in brain neural plasma membranes can modulate oxidative stress, excitotoxicity, and inflammation,4–6 mechanisms that have been implicated in the etiology of ALS and other neurodegenerative conditions.1–3 In particular, n-3 PUFAs have been found to have neuroprotective effects in animal models of aging6 and brain ischemia.7 Unexpectedly, however, pretreatment with high doses of eicosapentaenoic acid, a long chain n-3 PUFA, accelerated disease progression in a mouse model of ALS.8 It is unclear, however, to what extent this experimental result applies to human disease.
Data on the relation between PUFA intake and ALS risk are sparse. The results of two previous case-control studies9,10 suggested lower ALS risk among individuals with high PUFA intake; however, there are no prospective studies relating overall PUFA intake or n-3 PUFA intake to ALS risk. Therefore, we conducted a pooled analysis of nearly 1000 cases of ALS occurring in 5 large prospective cohort studies including the Health Professionals’ Follow-up Study (HPFS), the Nurses’ Health Study (NHS), the Cancer Prevention II-Nutrition Cohort (CPS-II Nutrition), the Multiethnic Cohort Study (MEC) and the National Institutes of Health-AARP Diet and Health Study (NIH-AARP), to assess whether specific dietary PUFAs or total dietary fat intake affects ALS risk.
METHODS
Study populations
The HPFS began in 1986 when 51,529 male health professionals aged 40 to 65 answered a mailed questionnaire pertaining to disease history and lifestyle characteristics.11 The NHS includes 121,700 registered female nurses and began in 1976 when these women aged 35 to 55 at baseline responded to a similar questionnaire.12 Follow-up of both studies continues through biennial questionnaires where participants in each cohort report disease occurrence and information on risk factors for chronic disease including dietary variables. The CPS-II Nutrition cohort consists of a subpopulation of the full CPS-II cohort and includes 86,404 men and 97,786 women aged 50 to 79 residing in 21 states with population based cancer surveillance.13,14 These men and women completed a mailed questionnaire in 1992 assessing various lifestyle and dietary factors. Updated exposure information was obtained in 1997 and biennially thereafter. The MEC cohort study is composed of 96,937 men and 118,843 women aged 45 to 75 with self-reported racial and ethnic backgrounds of African-American, Japanese-American, Latino, Native Hawaiian, and white.15 At the study baseline in 1993–1996, participants who were living primarily in Hawaii and California (Los Angeles) completed a lifestyle and disease history questionnaire; additional mailings were sent every 5 years subsequently. The NIH-AARP Diet and Health Study consists of 340,148 men and 227,021 women aged 50 to 71 residing in 6 states or 2 metropolitan areas that maintain high quality cancer registries and began in 1995–1996 when participants completed a mailed food frequency questionnaire.16 Of the original study population, approximately two-thirds completed a follow-up lifestyle questionnaire in 1996. All included studies were approved by the institutional review board at the institution where each study was conducted.
End-point definition
Follow-up of ALS in the CPS-II Nutrition, MEC, and NIH-AARP was through a search of the National Death Index. Vital status of the participants in these studies was determined by automated linkage with the National Death Index. The underlying and contributing causes of death were coded according to the International Classification of Diseases, Ninth Revision. All individuals with code 335.2 (motor neuron disease) listed as the underlying or contributing cause of death were considered to have had ALS. In a previous validation study,17 it was found that ALS was the primary diagnosis listed on death certificates in the majority of instances where code 335.2 was listed as a cause or contributory cause of death.17
In NHS and HPFS, incident ALS was also documented. In each biennial follow-up questionnaire, participants were asked to report a specific list of medically diagnosed conditions (initially not including ALS) and “any other major illness.” ALS was added to the list of specific conditions on the NHS questionnaires in 1992 onwards and on the HPFS questionnaires in 2000 onwards. We requested permission to contact the treating neurologist and for release of relevant medical records from participants who reported a diagnosis of ALS on the open question on major illnesses or on the specific question. Because of the rapidly progressive nature of the disease (median survival 1.5 to 3 years),15–17 many participants with ALS died before we could send the release request for medical records, so the request was sent to the closest family member. After obtaining permission, we asked the treating neurologists to complete a questionnaire to confirm the diagnosis of ALS and to rate the certainty of the diagnosis (definite, probable, or possible) and send medical records. Starting in 2004 the questionnaire was modified to include the El Escorial criteria. The final confirmation for our study purposes was made by a neurologist with experience in ALS diagnosis based on the review of medical records. We relied on the diagnosis made by the treating neurologist if the information in the medical record was insufficient or if it could not be obtained. Only participants with definite and probable ALS are included as cases in the primary analyses. When we were unable to confirm (i.e., obtain a copy of the medical record or the neurologist’s questionnaire) incident self-reported ALS, we classified the participant as having ‘possible ALS’ and excluded him or her from the primary analysis unless death occurred during follow-up and ALS was listed on the death certificate.
Assessment of diet and other covariates
We assessed participant diet using semi-quantitative food frequency questionnaires (FFQs) which were developed or modified specifically for each cohort.11,12,14–16,18 Participants reported habitual intake of each food on a scale ranging from never or less than 1 time per month to 6 servings or more per day. Nutrient intakes were then estimated by multiplying frequency of consumption by the specified portion size. Each study provided information on energy intake and macronutrient intakes including consumption of saturated, monounsaturated, and specific dietary PUFAs.
In the NIH-AARP and MEC studies, diet was assessed at baseline, whereas in the HPFS and NHS, diet was assessed at baseline and updated every 4 years. Because additional food items were added to NHS questionnaires after 1980, we considered 1984 as baseline. For CPS-II Nutrition, baseline for this study was in 1999, when dietary information on specific PUFA was assessed using a modified version of the FFQ used in HPFS and NHS. Individual validation and reproducibility studies for macronutrient intake including fat subtype were conducted for each dietary assessment instrument in subsets of participants from each study by comparing the FFQ estimates with intake estimated from multiple diet records (HPFS and NHS) or 24-hour recall (CPS-II Nutrition, MEC and NIH-AARP), and with the fatty acid composition of adipose or red cell membranes.11,18–24 In HPFS correlations for eicosapentaenoic acid (EPA) and n-6 fatty acids between reported FFQ intake and proportion in adipose tissue were 0.47 and 0.50, respectively.25 In NHS the correlations between the average of four 7 day diet records and baseline FFQ for total, saturated, monounsaturated, and polyunsaturated fats were 0.57, 0.68, 0.58 and 0.48, respectively,24 while correlation between FFQ estimated dietary PUFA and red blood cell concentration was 0.41 for EPA, 0.27 for linoleic acid, and 0.54 for docosaepentaenoic acid.26 In CPS-II Nutrition correlations between dietary assessment via FFQ with four 24-hour diet recalls ranged from 0.42 to 0.66 for dietary fat and fat subtypes.18 Correlations between FFQs administered in 1992 and 1997 were ≥ 0.54 for total dietary fat and fat subtype intake in men and in women.18 In MEC, energy-adjusted correlations for dietary fat intakes estimated from FFQs and three 24-hour dietary recalls ranged from 0.31 to 0.77.23 In NIH-AARP, energy-adjusted correlations for intakes of total, saturated, monounsaturated and polyunsaturated fat estimated from FFQs and two 24-hour recalls were ≥ 0.53 in both genders.27
Information on other covariates of interest including smoking status, height, weight, education level, and physical activity was collected at baseline for all cohorts.
Statistical analysis
54,756 (5.2%) persons with extreme energy intake (three standard deviations above or below the study-specific mean on a loge scale; roughly corresponding to total caloric <500 and ≥4500kcal/day) were excluded from the analysis. In the AARP, we excluded participants who reported serious illness at baseline (N=20,188, 3.6%). Person-years of follow-up were calculated from study baseline to the earliest of time of ALS symptom onset (in NHS and HPFS), death, loss to follow-up or the end of follow-up. End of follow-up was June 2008 for NHS, December 2008 for HPFS, December 2008 for CPS-II, and December 2007 for MEC and December 2008 for NIH-AARP.
Within each cohort, we energy-adjusted all nutrients using the residual method.30 We categorized participants into cohort-specific quintiles for all dietary fat variables as differences in intake across studies could reflect true dietary differences or reflect differences in dietary assessment. We calculated marine n-3 PUFAs as the sum of eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), and docosapentaenoic acid (DPA); because high correlations between intakes of theses PUFAs prevent assessment of their independent effects. We applied Cox proportional hazards regression stratified by age in years to calculate cohort-specific hazard ratios and associated 95% confidence intervals for each study separately. For CPS-II Nutrition, MEC and NIH-AARP analyses were conducted in men and women separately. We pooled estimates using DerSimonian and Laird methods for random effects and assessed heterogeneity using Q statistics.28 Within study specific multivariate Cox models, we adjusted for potential confounders, such as BMI (continuous), physical activity (approximate tertiles corresponding to low, medium and high activity levels), education level (<high school, high school, >high school), smoking status (never-smoker, past, current), vitamin E intake (quartiles), total major carotenoid intake (quartiles), as uniformly as possible across all studies.29–33 We tested for trend across all categorical analyses by modeling as a continuous covariate a new variable where participants in a certain category were assigned the median value for that category. To address the possibility that participants could be experiencing symptoms of ALS at the time of questionnaire completion, we conducted a lagged analysis where we excluded the first 4 years of follow-up in each cohort.
To address potential non-linearity, we assessed the effect of each PUFA on risk of ALS semi-parametrically using restricted cubic splines. To create these estimates, we pooled studies into a single data set and each spline model was stratified by study, gender, age and year of questionnaire. We additionally adjusted for a similar set of covariates as the study specific models using quintiles (total vitamin E, total carotenoid intake, BMI, physical activity, education level and energy intake).
We assessed effect modification by age (<median, ≥median age), smoking status (current or, non-smoker), sex, vitamin E intake (<median, ≥median intake), and low BMI (<22, ≥22). We performed sensitivity analyses using cumulative averages of intake of PUFA from available cohorts (NHS and HPFS); however, the results remain unchanged, so they are not presented.
In secondary analysis, we modeled fat subtypes as a percentage of total energy using nutrient-density models simultaneously adjusted for total energy intake, percentage of energy from protein, and percentage of energy from all other types of dietary fat.34 Coefficients from these models can be interpreted as the effect of substituting a specified percentage of energy from fat with the same percentage of energy from carbohydrates.34,35 We assessed nutrient densities continuously and pooled risk ratios using DerSimonian and Laird methods for random effects.
All statistical analyses were conducted using SAS (SAS Institute Inc., Cary, North Carolina), version 9.2 and graphics were generated using R software, version 2.11 (The R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
A total of 995 individuals with ALS were documented among 1,002,082 participants (479,114 women; 522,968 men) during study follow-up ranging from approximately 9 to 24 years. Gender-specific estimates of median dietary n-3 and n-6 PUFA intake were consistent across studies. For males, median n-3 PUFA intake ranged from 1.40 to 1.85 g/day and median n-6 PUFA intake ranged from 11.82 to 15.73 g/day. For females, median n-3 PUFA intake ranged from 1.14 to 1.43 g/day and median n-6 PUFA intake ranged from 8.94 to 12.01 g/day. The relation between n-3 PUFA intake and potential ALS risk factors is shown in Table 1 and the correlations between different PUFA in Supplemental Table e1.
Table 1.
Selected Age-adjusted* Characteristics
| No. of ALS cases (men/women) |
Baseline Cohort Size† |
Median length Of follow-up (years) |
Quintile of total n-3 fatty acid intake |
|||||
|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | ||||
| Nurses’ Health Study (1984 to 2008) | 81 (0/81) | 92,059 | 24 | |||||
| Age (years) | 61 (7) | 61 (7) | 61 (7) | 60 (7) | 60.72 (7) | |||
| Body mass index (kg/m2) | 26 (5) | 26 (5) | 27 (5) | 27 (5) | 27 (5) | |||
| Current smokers (%) | 15 | 13 | 12 | 12 | 13 | |||
| High physical activity level (%) | 27 | 29 | 30 | 30 | 29 | |||
| Total vitamin E intake (mg/day) | 68 (100) | 70 (101) | 72 (101) | 74 (103) | 79 (107) | |||
| Total major carotenoid intake (mg/day) | 13 (6) | 14 (7) | 15 (7) | 15 (7) | 16 (8) | |||
| Total n-6 fatty acid intake (g/day) | 7 (2) | 8 (2) | 8 (2) | 9 (2) | 10 (3) | |||
|
Health Professional’s Follow-up Study M (1986 to 2008) |
63 (63/0) | 51,529 | 21 | |||||
| Age (years) | 55 (10) | 54 (10) | 54 (10) | 54 (10) | 55 (10) | |||
| Body mass index (kg/m2) | 25 (3) | 25 (3) | 25 (3) | 26 (3) | 26 (7) | |||
| Current smokers (%) | 12 | 10 | 9 | 7 | 8 | |||
| High physical activity level (%) | 30 | 33 | 34 | 34 | 36 | |||
| Total vitamin E intake (mg/day) | 43 (80) | 47 (84) | 50 (85) | 50 (86) | 57 (93) | |||
| Total major carotenoid intake (mg/day) | 14 (7) | 16 (8) | 17 (8) | 19 (9) | 20 (11) | |||
| Total n-6 fatty acid intake (g/day) | 10 (3) | 11 (3) | 12 (3) | 13 (3) | 15 (4) | |||
|
Cancer Prevention Study – Nutrition Cohort (1999 to 2008) |
142 (71/71) | 151,347 | 9 | |||||
| Age (years) | 69 (3) | 69 (6) | 70 (6) | 70 (6) | 70.26 (6) | |||
| Body mass index (kg/m2) | 25 (5) | 27 (5) | 26 (6) | 26 (4) | 26.35 (4) | |||
| Current smokers (%) | 5 | 4 | 4 | 4 | 4 | |||
| High physical activity level (%) | 22 | 24 | 25 | 25 | 26 | |||
| < High school education (%) | 27 | 32 | 30 | 27 | 25 | |||
| Total vitamin E intake (mg/day) | 98 (99) | 96 (98) | 96 (98) | 96 (98) | 98 (98) | |||
| Total major carotenoid intake (mg/day) | 11 (5) | 12 (6) | 13 (6) | 13 (6) | 14 (7) | |||
| Total n-6 fatty acid intake (g/day) | 7 (2) | 9 (2) | 11 (2) | 12 (2) | 15 (4) | |||
|
Multiethnic Cohort Study (1993–1997 to 2007) |
140 (83/57) | 215,688 | 14 | |||||
| Age (years) | 61 (9) | 60 (9) | 60 (9) | 60 (9) | 59 (9) | |||
| Body mass index (kg/m2) | 25 (4) | 26 (5) | 26 (5) | 26 (5) | 26 (6) | |||
| Current smokers (%) | 14 | 14 | 15 | 17 | 18 | |||
| High physical activity level (%) | 25 | 26+ | 29 | 33 | 37 | |||
| < High school education (%) | 44 | 44 | ||||||
| Total vitamin E intake (mg/day) | 64 (126) | 61 (128) | 60 (122) | 59 (122) | 59 (122) | |||
| Total major carotenoid intake (mg/day) | 11 (8) | 11 (7) | 12 (7) | 12 (7) | 13 (8) | |||
| Total n-6 fatty acid intake (g/day) | 9 (2) | 12 (2) | 14 (2) | 16 (2) | 19 (3) | |||
| NIH-AARP Diet and Health Study | 568 (384/184) | 546,214 | 11 | |||||
| Age (years) | 61 (5) | 62 (5) | 62 (5) | 62 (5) | 62 (5) | |||
| Body mass index (kg/m2) | 27 (5) | 27 (5) | 27 (5) | 27 (5) | 28 (5) | |||
| Current smokers (%) | 12 | 12 | 11 | 12 | 12 | |||
| High physical activity level (%) | 20 | 19 | 19 | 19 | 19 | |||
| < High school education (%) | 29 | 27 | 26 | 24 | 23 | |||
| Total vitamin E intake (mg/day) | 87 (108) | 83 (106) | 80 (104) | 79 (103) | 80 (104) | |||
| Total major carotenoid intake (mg/day) | 15 (11) | 16 (10) | 17 (10) | 17 (10) | 18 (12) | |||
| Total n-6 fatty acid intake (g/day) | 9 (3) | 11 (3) | 13 (3) | 15 (3) | 18 (4) | |||
Values are means (SD) or percentages and are standardized to the age distribution of the specific study population
Rate is adjusted for age and gender.
Prior to exclusion for implausible energy intake
Value is not age-adjusted.
Overall, total n-3 PUFA intake was associated with a 34% reduced risk of ALS in the multivariable model comparing the highest to the lowest quintile (multivariable pooled relative risk (RR): 0.66; 95% CI: 0.53–0.81; P for trend<0.001; Table 2) and results were relatively consistent across studies (Figure 1). Total n-6 PUFA intake was not associated with ALS risk (multivariable pooled RR comparing the highest to lowest quintile: 0.88; 95% CI: 0.72–1.08; P for trend=0.22).
Table 2.
Multivariable-adjusted Relative Risks* of ALS according to quintile of baseline intake of n-3 and n-6 fatty acids
| Quintile 1 (ref) |
Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | Ptrend§ | Pheterogeneity¥ | |
|---|---|---|---|---|---|---|---|
| Total n-3 fatty acids (median, grams/day) | 0.94 | 1.21 | 1.43 | 1.68 | 2.11 | ||
| No. of cases | 246 | 200 | 199 | 195 | 154 | ||
| Age adjusted RR* (95% CI)† | 1.00 | 0.81 (0.67-0.98) | 0.78 (0.61-0.99) | 0.75 (0.57-0.99) | 0.62 (0.50-0.78) | <0.001 | 0.15 |
| Multivariable adjusted RR (95% CI)‡ | 1.00 | 0.83 (0.69-1.01) | 0.81 (0.64-1.03) | 0.78 (0.60-1.03) | 0.66 (0.53-0.81) | <0.001 | 0.21 |
| Total n-6 fatty acids (median, grams/day) | 7.65 | 10.14 | 12.20 | 14.51 | 18.38 | ||
| No. of cases | 221 | 197 | 202 | 188 | 186 | ||
| Age adjusted RR (95% CI)† | 1.00 | 0.90 (0.73-1.10) | 0.92 (0.72-1.17) | 0.87 (0.72-1.06) | 0.87 (0.72-1.07) | 0.15 | 0.36 |
| Multivariable adjusted RR (95% CI)‡ | 1.00 | 0.92 (0.75-1.13) | 0.94 (0.73-1.22) | 0.90 (0.74-1.10) | 0.88 (0.72-1.08) | 0.22 | 0.38 |
Adjusted for age (years) and sex
Adjusted for age (years), sex, smoking status (never, past, current), total vitamin E intake (quartiles), total major carotenoid intake (quartiles), body mass index (<23, 23–25, 25–30, ≥30), physical activity (low, average, high), education level (<high school, high school, >high school), and energy intake (quintiles).
RR: relative risk, CI, confidence interval
Ptrend calculated using median value for each quintile
Pheterogeneity is calculated form the Q-statistic and is used to quantify differences between studies
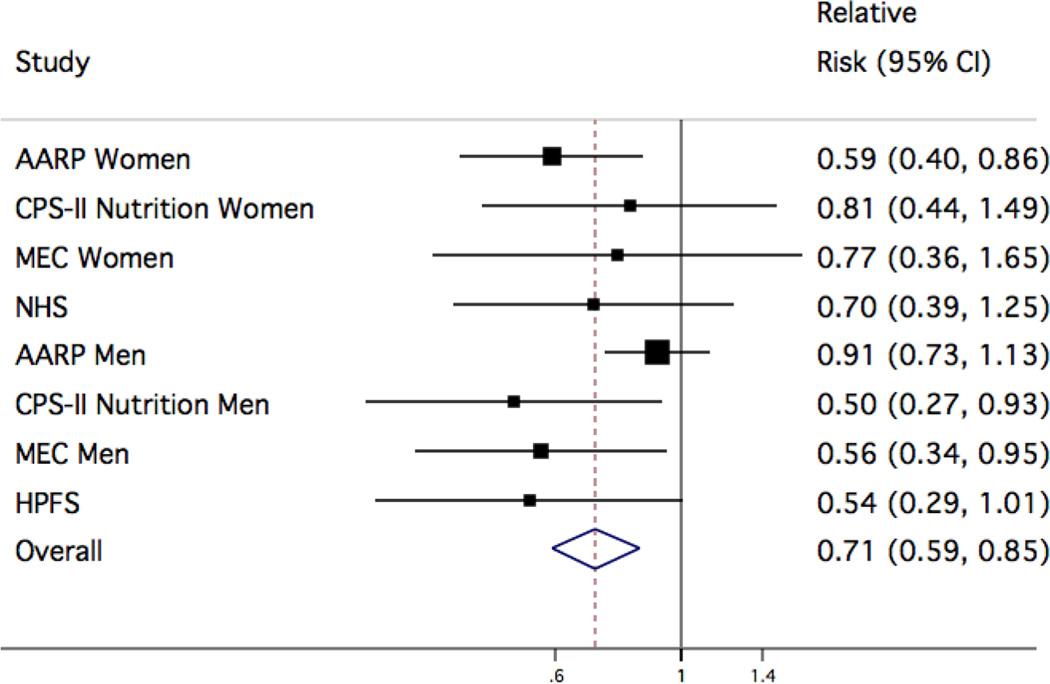
Figure 1. Study-specific and pooled multivariable relative risks (95% CI) of ALS for 1g/day increase in n-3 PUFA.
Study-specific and pooled multivariable relative risk (RR) and 95% confidence interval (CI) of amyotrophic lateral sclerosis for a 1g change in intake of n-3 PUFA. The squares and horizontal lines correspond to the study specific multivariable RR and 95% CI, respectively. The inverse of the variance is used to calculate study weight and is represented by the area of the square. The diamond displays the pooled multivariable RR and 95% CI. We observed no significant effect modification by gender (P=0.74).
Individually, intakes of α-linolenic acid (ALA; abbreviated as 18:3n-3) and marine n-3 PUFA were each associated with lower ALS risk (Table 3). The pooled multivariable RR comparing individuals in the highest quintile with those in the lowest was 0.73 for ALA (95% CI: 0.59–0.89; P for trend=0.003) and 0.84 for marine n-3 PUFA (95% CI: 0.65–1.08; P for trend=0.03). We also detected marginally non-significant inverse trends across quintiles of dietary arachidonic acid (AA) intake (abbreviated 20:4n-6; P for trend=0.07). However, upon control for total n-3 PUFA intake, AA was not associated with ALS. No such attenuation was observed for ALA or marine n-3 PUFA following adjustment for total n-6 intake. Linoleic acid intake appeared not to be associated with ALS risk.
Table 3.
Multivariable-adjusted Relative Risks* of ALS according to quintile of intake of individual fatty acids
| Quintile 1 (ref) |
Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | P trend§ | P het¥ | |
|---|---|---|---|---|---|---|---|
|
Linoleic acid (18:2n-6; median, grams) |
8.00 | 10.16 | 11.68 | 13.32 | 16.21 | ||
| No. of cases | 228 | 190 | 203 | 186 | 187 | ||
| Age adjusted RR* (95% CI)† | 1.00 | 0.83 (0.64–1.07) | 0.87 (0.67–1.14) | 0.84 (0.69–1.02) | 0.82 (0.65–1.04) | 0.09 | 0.18 |
| Multivariable adjusted RR (95% CI)‡ | 1.00 | 0.85 (0.62–1.10) | 0.90 (0.69–1.17) | 0.84 (0.67–1.05) | 0.83 (0.66–1.05) | 0.10 | 0.18 |
|
Arachidonic acid (20:4n-6; median, grams) |
0.07 | 0.08 | 0.10 | 0.13 | 0.18 | ||
| No. of cases | 229 | 225 | 194 | 173 | 175 | ||
| Age adjusted RR* (95% CI) † | 1.00 | 0.99 (0.81–1.21) | 0.87 (0.66–1.16) | 0.73 (0.51–1.03) | 0.80 (0.61–1.04) | 0.01 | 0.16 |
| Multivariable adjusted RR (95% CI)‡ | 1.00 | 1.00 (0.81–1.23) | 0.90 (0.67–1.20) | 0.76 (0.52–1.09) | 0.86 (0.64–1.12) | 0.07 | 0.12 |
|
α-Linolenic acid (18:3n-3; median, grams) |
0.82 | 1.06 | 1.27 | 1.50 | 1.93 | ||
| No. of cases | 235 | 202 | 211 | 184 | 162 | ||
| Age adjusted RR* (95% CI)† | 1.00 | 0.85 (0.67–1.08) | 0.89 (0.71–1.12) | 0.76 (0.58–1.00) | 0.70 (0.57–0.86) | 0.001 | 0.28 |
| Multivariable adjusted RR (95% CI)‡ | 1.00 | 0.88 (0.70–1.11) | 0.93 (0.74–1.17) | 0.79 (0.61–1.04) | 0.73 (0.59–0.89) | 0.003 | 0.34 |
| Marine n-3 (median, grams) | 0.04 | 0.07 | 0.11 | 0.17 | 0.30 | ||
| No. of cases | 195 | 218 | 224 | 193 | 164 | ||
| Age adjusted RR* (95% CI)† | 1.00 | 1.15 (0.94–1.39) | 1.15 (0.95–1.40) | 1.03 (0.81–1.30) | 0.82 (0.63–1.08) | 0.04 | 0.18 |
| Multivariable adjusted RR (95% CI)‡ | 1.00 | 1.16 (0.96–1.41) | 1.16 (0.95–1.41) | 1.04 (0.82–1.31) | 0.84 (0.65–1.08) | 0.03 | 0.28 |
Adjusted for age (years), sex, smoking status (never, past, current), total vitamin E intake (quartiles), total major carotenoid intake (quartiles), body mass index (<23, 23–25, 25–30, ≥30), physical activity (low, average, high), education level (<high school, high school, >high school), and energy intake (quintiles).
RR: relative risk, CI, confidence interval
Ptrend calculated using median value for each quintile
Pheterogeneity is calculated form the Q-statistic and is used to quantify differences between studies
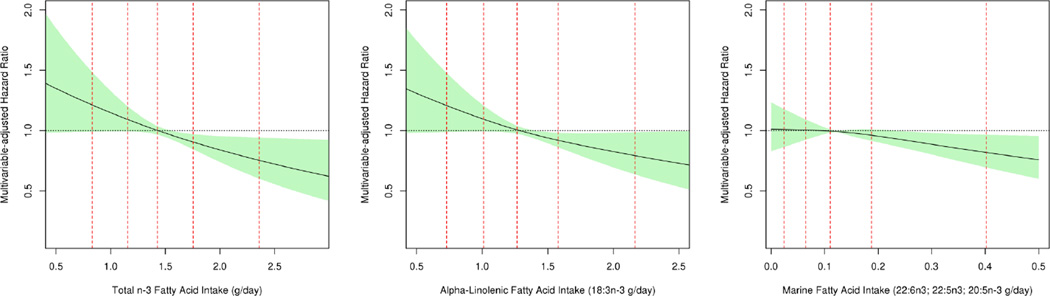
In semi-parametric analyses using restricted cubic splines, we detected significant inverse linear relationships between intake of total n-3 and ALA and marginally significant inverse trends for marine n-3 PUFA (Figure 2; for total n-3 P=0.0003; for ALA P=0.002; for marine n-3 P=0.055). Intakes of linoleic acid and AA were not associated with ALS in semi-parametric analyses (all P>0.20).
Figure 2. Multivariable adjusted relationship between total and individual long chain n-3 fatty acids with risk of ALS using restricted cubic splines.
For each fatty acid, the solid lines and shaded region represent the risk estimate and corresponding 95% CI, respectively. The dotted red vertical lines correspond to the 5th, 25th, 50th, 75th and 95th percentiles for each fatty acid and plotted lines represent changes in intake relative to the median intake of specified PUFA across studies. To create these estimates, studies were pooled into a single data set and stratified by study, gender, age and year of questionnaire. Each curve is additionally adjusted for smoking status (never, past, current), total vitamin E intake (quartiles), total major carotenoid intake (quartiles), body mass index (<23, 23–25, 25–30, ≥30), physical activity (low, average, high), education level (<high school, high school, >high school) and energy intake.
In the lagged analysis where we excluded the first 4 years of follow-up in each cohort, we also observed similar associations between total n-3 PUFA intake and ALA and ALS risk. The RR comparing the highest to the lowest quintile was 0.64 (95% CI: 0.51–0.81; P for trend=0.001) for total n-3 and 0.73 (95% CI: 0.58–0.92; P for trend=0.003) for ALA. Results were attenuated for marine n-3 (RR: 0.93; 95% CI: 0.72–1.20; P for trend=0.06).
Additional analyses were conducted using the percent energy from n-3 PUFA and from other sources as continuous variables. According to these analyses, adding 0.5% of energy from n-3 PUFA while maintaining a constant intake of n-6 fatty acids and reducing by an isocaloric amount the intake of other types of fat would reduce ALS risk by 34% (95% CI: 15 to 49%). Total baseline energy intake, or percent energy from total fat or other types of dietary fat were not associated with ALS risk.
We observed no significant evidence of effect modification by age, smoking, vitamin E supplement use, carotenoids, or BMI. Results were also unchanged when we adjusted for race or smoking using continuous pack-years or adjusted using continuous BMI in multivariable models.
DISCUSSION
In this pooled analysis of several large cohort studies with prospectively collected dietary information, we found that individuals with higher dietary intakes of n-3 PUFA had a markedly reduced risk of ALS. Both ALA, the main n-3 PUFA from vegetable sources, and marine n-3 PUFA contributed to this association, which appeared to be independent of n-6 intake.
Previous research of fat intake and ALS risk is sparse and results are inconsistent across studies. In a case-control study in Japan ALS risk was reported to decrease with increasing intakes of total fat, saturated fat, monounsaturated fat, and total PUFA, but no association was found with n-3 PUFA.10 Also, an inverse association between intake of total PUFA (separate results for n-6 and n-3 were not reported) was reported in a case-control study conducted in the Netherlands. 9 In contrast, an increased ALS risk across quartiles of intakes for total fat, saturated fat, cholesterol, and PUFA was reported in a case-control study in Washington State. .36 However, these studies may be vulnerable to combinations of recall and selection biases (where individuals without ALS do not represent the source population of individuals with ALS) when examining dietary exposures. Further, the case-control design cannot adequately account for the effects of disease status itself on diet.
Our current study also has noteworthy limitations. We use death rather than incidence in CPS Nutrition, MEC and NIH-AARP. Death is an imperfect proxy for incidence as it may bias results in favor of shorter survivors. However the majority of ALS patients rapidly progress (median survival of 1.5 to 3 years) and the observed consistent results in the lagged analysis suggests such bias minimally impacts our results.37–40 Previous research also indicated that 70–90% of ALS cases were identified using death certificates listing motor neuron disease as the cause of death allowing for potential misclassification of the outcome.41–43 Nevertheless, it’s possible that the lower risk of ALS deaths among individuals with higher n-3 PUFA intake in our cohort is in part due to a beneficial effect of high n-3 PUFA intake on survival of patients with ALS. This possibility, however, does not affect the main conclusion that high n-3 PUFA intake could be beneficial in delaying the onset or progression of ALS. Additionally, we did not have genetic or family history information and could not assess whether PUFA intake affects differently risk of sporadic or familial ALS. Some error in estimating nutrient intakes is also inevitable, either because of inaccurate reporting or because of changes in intake during the follow-up. Given the longitudinal design, error in measuring n-3 PUFA is most likely independent of future ALS risk, and will thus tend to weaken the evidence of a protective effect. On the other hand, some residual confounding due to errors in measuring other nutrients or confounding by unknown factors related to both ALS and reported n-3 PUFA intake cannot be excluded; however, confounding is unlikely to fully explain independent associations observed for both ALA and marine n-3 PUFA (which have different food sources and are only weakly correlated with each other).
Despite these limitations, our study has several strengths including the large number of participants and documented cases, validated dietary assessment methods, and extended follow-up in each of the cohorts. Another notable strength is each study’s prospective design, which is particularly important as most previous case-control used prevalent cases of ALS and may be vulnerable to recall bias, which is common in studies of dietary exposures. Additionally, our study is also likely to include a wide spectrum of patients with ALS, thus minimizing the selection bias that may occur in studies that recruit ALS patients from tertiary care centers.40
Biologic activity of PUFAs within the brain depends on the chain length as well as the number and position of the double bonds of the specific PUFA in question. Previous animal studies suggest ALA slows peroxidation activation of the binding of neural nuclear transcription factor kappaB (NF-κB) and reduces glutamate mediated excitotoxic damage and oxidative stress, possibly preventing neuronal cell death.44–47 Neuroprotective properties of ALA have also been noted in prolonging neuronal survival through reduction in immunoreactivity of proapototic proteins.48
In addition to individual biologic effects, ALA can be converted to EPA and eventually DHA via various fatty acid elongation enzymes (though the extent to which this occurs likely depends on an individual’s underlying direct DHA intake).5,49 Therefore, circulating levels of DHA and EPA available for uptake by the brain represent a combination of those derived from the diet and those biosynthesized in the liver from ALA. EPA and DHA themselves may have additional neuroprotective properties. In vivo studies have suggested that DHA potentially reduces levels of neural inflammation through prevention of microglial activation by pro-inflammatory cytokines or through production of anti-inflammatory and anti-oxidative neuroprotectin-D1 (NPD1).50,51 Both ALA and DHA are essential fatty acids derived from the diet, and endogenous synthesis of NDP1 is heavily dependent on sufficient levels of DHA. Therefore, moderate dietary intake of such n-3 PUFAs may present a modifiable means of promoting neuroprotection.
Overall, the results of our large prospective cohort study suggest that individuals with higher dietary intakes of total n-3 PUFA and ALA have a reduced risk of ALS. Further research, possibly including biomarkers of PUFA intake, should be pursued to confirm these findings and to determine whether high n-3 PUFA intake could be beneficial in individuals with ALS.
ACKNOWLEDGMENTS
Drs. Fitzgerald and Ascherio had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. We would like to acknowledge the cohorts participants and Leslie Unger for administrative support.
Funding: This work was supported by a grant from the National Institute of Neurological Diseases and Stroke (R01 NS045893), grants P01 CA87969 and P01 CA055075 from the National Cancer Institute, and a grant from the ALS Therapy Alliance Foundation. The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Financial disclosures: The authors have no relevant conflicts of interest.
REFERENCES
- 1.Cleveland DW, Rothstein JD. From Charcot to Lou Gehrig: deciphering selective motor neuron death in ALS. Nat. Rev. Neurosci. 2001;2:806–819. doi: 10.1038/35097565. [DOI] [PubMed] [Google Scholar]
- 2.Turner MR, et al. Controversies and priorities in amyotrophic lateral sclerosis. Lancet Neurol. 2013;12:310–322. doi: 10.1016/S1474-4422(13)70036-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferraiuolo L, Kirby J, Grierson AJ, Sendtner M, Shaw PJ. Molecular pathways of motor neuron injury in amyotrophic lateral sclerosis. Nat Rev Neurol. 2011;7:616–630. doi: 10.1038/nrneurol.2011.152. [DOI] [PubMed] [Google Scholar]
- 4.Lauritzen L, Hansen HS, Jørgensen MH, Michaelsen KF. The essentiality of long chain n-3 fatty acids in relation to development and function of the brain and retina. Prog. Lipid Res. 2001;40:1–94. doi: 10.1016/s0163-7827(00)00017-5. [DOI] [PubMed] [Google Scholar]
- 5.Rapoport SI, Ramadan E, Basselin M. Docosahexaenoic acid (DHA) incorporation into the brain from plasma, as an in vivo biomarker of brain DHA metabolism and neurotransmission. Prostaglandins Other Lipid Mediat. 2011;96:109–113. doi: 10.1016/j.prostaglandins.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang W, et al. Omega-3 polyunsaturated fatty acids in the brain: metabolism and neuroprotection. Front. Biosci. 2011;16:2653–2670. doi: 10.2741/3878. [DOI] [PubMed] [Google Scholar]
- 7.Huang WL, et al. A combination of intravenous and dietary docosahexaenoic acid significantly improves outcome after spinal cord injury. Brain. 2007;130:3004–3019. doi: 10.1093/brain/awm223. [DOI] [PubMed] [Google Scholar]
- 8.Yip PK, et al. The omega-3 fatty acid eicosapentaenoic acid accelerates disease progression in a model of amyotrophic lateral sclerosis. PLoS ONE. 2013;8:e61626. doi: 10.1371/journal.pone.0061626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Veldink JH, et al. Intake of polyunsaturated fatty acids and vitamin E reduces the risk of developing amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2007;78:367–371. doi: 10.1136/jnnp.2005.083378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okamoto K, et al. Nutritional status and risk of amyotrophic lateral sclerosis in Japan. Amyotroph Lateral Scler. 2007;8:300–304. doi: 10.1080/17482960701472249. [DOI] [PubMed] [Google Scholar]
- 11.Rimm EB, et al. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet. 1991;338:464–468. doi: 10.1016/0140-6736(91)90542-w. [DOI] [PubMed] [Google Scholar]
- 12.Colditz GA, Manson JE, Hankinson SE. The Nurses’ Health Study: 20-year contribution to the understanding of health among women. J Womens Health. 1997;6:49–62. doi: 10.1089/jwh.1997.6.49. [DOI] [PubMed] [Google Scholar]
- 13.Garfinkel L. Selection, follow-up, and analysis in the American Cancer Society prospective studies. Natl Cancer Inst Monogr. 1985;67:49–52. [PubMed] [Google Scholar]
- 14.Calle EE, et al. The American Cancer Society Cancer Prevention Study II Nutrition Cohort: rationale, study design, and baseline characteristics. Cancer. 2002;94:500–511. doi: 10.1002/cncr.10197. [DOI] [PubMed] [Google Scholar]
- 15.Kolonel LN, et al. A multiethnic cohort in Hawaii and Los Angeles: baseline characteristics. Am. J. Epidemiol. 2000;151:346–357. doi: 10.1093/oxfordjournals.aje.a010213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schatzkin A, et al. Design and serendipity in establishing a large cohort with wide dietary intake distributions : the National Institutes of Health-American Association of Retired Persons Diet and Health Study. Am. J. Epidemiol. 2001;154:1119–1125. doi: 10.1093/aje/154.12.1119. [DOI] [PubMed] [Google Scholar]
- 17.Sienko DG, Davis JP, Taylor JA, Brooks BR. Amyotrophic lateral sclerosis. A case-control study following detection of a cluster in a small Wisconsin community. Arch. Neurol. 1990;47:38–41. doi: 10.1001/archneur.1990.00530010046017. [DOI] [PubMed] [Google Scholar]
- 18.Flagg EW, Coates RJ, Calle EE, Potischman N, Thun MJ. Validation of the American Cancer Society Cancer Prevention Study II Nutrition Survey Cohort Food Frequency Questionnaire. Epidemiology. 2000;11:462–468. doi: 10.1097/00001648-200007000-00017. [DOI] [PubMed] [Google Scholar]
- 19.Rimm EB, et al. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am. J. Epidemiol. 1992;135:1114–1126. doi: 10.1093/oxfordjournals.aje.a116211. discussion 1127–1136. [DOI] [PubMed] [Google Scholar]
- 20.Hunter DJ, et al. Comparison of measures of fatty acid intake by subcutaneous fat aspirate, food frequency questionnaire, and diet records in a free-living population of US men. Am. J. Epidemiol. 1992;135:418–427. doi: 10.1093/oxfordjournals.aje.a116302. [DOI] [PubMed] [Google Scholar]
- 21.Hunter DJ, et al. Variability in portion sizes of commonly consumed foods among a population of women in the United States. Am. J. Epidemiol. 1988;127:1240–1249. doi: 10.1093/oxfordjournals.aje.a114916. [DOI] [PubMed] [Google Scholar]
- 22.Salvini S, et al. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol. 1989;18:858–867. doi: 10.1093/ije/18.4.858. [DOI] [PubMed] [Google Scholar]
- 23.Murphy SP, et al. Comparison of two instruments for quantifying intake of vitamin and mineral supplements: a brief questionnaire versus three 24-hour recalls. Am. J. Epidemiol. 2002;156:669–675. doi: 10.1093/aje/kwf097. [DOI] [PubMed] [Google Scholar]
- 24.Willett WC, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am. J. Epidemiol. 1985;122:51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 25.Hunter DJ, et al. Comparison of measures of fatty acid intake by subcutaneous fat aspirate, food frequency questionnaire, and diet records in a free-living population of US men. Am. J. Epidemiol. 1992;135:418–427. doi: 10.1093/oxfordjournals.aje.a116302. [DOI] [PubMed] [Google Scholar]
- 26.Sun Q, Ma J, Campos H, Hankinson SE, Hu FB. Comparison between plasma and erythrocyte fatty acid content as biomarkers of fatty acid intake in US women. Am J Clin Nutr. 2007;86:74–81. doi: 10.1093/ajcn/86.1.74. [DOI] [PubMed] [Google Scholar]
- 27.Thompson FE, et al. Performance of a food-frequency questionnaire in the US NIH-AARP (National Institutes of Health-American Association of Retired Persons) Diet and Health Study. Public Health Nutr. 2008;11:183–195. doi: 10.1017/S1368980007000419. [DOI] [PubMed] [Google Scholar]
- 28.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 29.Ascherio A, Weisskopf MG, O’Reilly EJ, Jacobs EJ, McCullough ML, Calle EE, Cudkowicz M, Thun MJ. Vitamin E intake and risk of amyotrophic lateral sclerosis. Ann Neurol. 2005;57(1):104–110. doi: 10.1002/ana.20316. [DOI] [PubMed] [Google Scholar]
- 30.Wang H, O'Reilly ÉJ, Weisskopf MG, Logroscino G, McCullough ML, Thun MJ, Schatzkin A, Kolonel LN, Ascherio A. Smoking and risk of amyotrophic lateral sclerosis: a pooled analysis of 5 prospective cohorts. Arch Neurol. 2011 Feb;68(2):207–13. doi: 10.1001/archneurol.2010.367. PMCID: PMC3319086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang H, O’Reilly EJ, Weisskopf MG, Logroscino G, McCullough ML, Thun MJ, Schatzkin A, Kolonel LN, Ascherio A. Vitamin E and risk of amyotrophic lateral sclerosis: a pooled analysis of five prospective cohorts. Am J Epidemiol. 2011 Mar 15;173(6):595–602. doi: 10.1093/aje/kwq416. PMCID: PMC3105261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fitzgerald KC, O'Reilly EJ, Fondell E, Falcone GJ, McCullough ML, Park Y, Kolonel LN, Ascherio A. Intakes of vitamin C and carotenoids and risk of amyotrophic lateral sclerosis: Pooled results from 5 cohort studies. Ann Neurol. 2013 Feb;73(2):236–45. doi: 10.1002/ana.23820. PMCID: PMC3608702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O'Reilly EJ, Wang H, Weisskopf MW, Fitzgerald KC, Falcone GJ, McCullough ML, Thun MJ, Park Y, Kolonel LN, Ascherio A. Premorbid Body Mass Index and Risk of Amyotrophic Lateral Sclerosis. Amyotroph Lateral Scler. 2013 Apr;14(3):205–11. doi: 10.3109/21678421.2012.735240. PMCID: PMC3615420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith-Warner SA, et al. Types of dietary fat and breast cancer: a pooled analysis of cohort studies. Int. J. Cancer. 2001;92:767–774. doi: 10.1002/1097-0215(20010601)92:5<767::aid-ijc1247>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 35.Willett WC. Nutritional Epidemiology. Oxford University Press; 1998. [Google Scholar]
- 36.Nelson LM, Matkin C, Longstreth WT, Jr, McGuire V. Population-based case-control study of amyotrophic lateral sclerosis in western Washington State II. Diet. Am. J. Epidemiol. 2000;151:164–173. doi: 10.1093/oxfordjournals.aje.a010184. [DOI] [PubMed] [Google Scholar]
- 37.Drory VE, Birnbaum M, Korczyn AD, Chapman J. Association of APOE epsilon4 allele with survival in amyotrophic lateral sclerosis. J. Neurol. Sci. 2001;190:17–20. doi: 10.1016/s0022-510x(01)00569-x. [DOI] [PubMed] [Google Scholar]
- 38.Magnus T, et al. Disease progression in amyotrophic lateral sclerosis: predictors of survival. Muscle Nerve. 2002;25:709–714. doi: 10.1002/mus.10090. [DOI] [PubMed] [Google Scholar]
- 39.Sorenson EJ, Stalker AP, Kurland LT, Windebank AJ. Amyotrophic lateral sclerosis in Olmsted County, Minnesota, 1925 to 1998. Neurology. 2002;59:280–282. doi: 10.1212/wnl.59.2.280. [DOI] [PubMed] [Google Scholar]
- 40.Del Aguila MA, Longstreth WT, Jr, McGuire V, Koepsell TD, van Belle G. Prognosis in amyotrophic lateral sclerosis: a population-based study. Neurology. 2003;60:813–819. doi: 10.1212/01.wnl.0000049472.47709.3b. [DOI] [PubMed] [Google Scholar]
- 41.Buckley J, et al. Motor neuron disease in England and Wales, 1959–1979. J. Neurol. Neurosurg. Psychiatr. 1983;46:197–205. doi: 10.1136/jnnp.46.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoffman PM, Brody JA. The reliability of death certificate reporting for amyotrophic lateral sclerosis. J Chronic Dis. 1971;24:5–8. doi: 10.1016/0021-9681(71)90053-1. [DOI] [PubMed] [Google Scholar]
- 43.O’Malley F, Dean G, Elian M. Multiple sclerosis and motor neurone disease: survival and how certified after death. J Epidemiol Community Health. 1987;41:14–17. doi: 10.1136/jech.41.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu H, et al. Dietary fish oil n-3 polyunsaturated fatty acids and alpha-linolenic acid differently affect brain accretion of docosahexaenoic acid and expression of desaturases and sterol regulatory element-binding protein 1 in mice. The Journal of Nutritional Biochemistry. 2010;21:954–960. doi: 10.1016/j.jnutbio.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 45.Kaltschmidt B, Kaltschmidt C. NF-κB in the Nervous System. Cold Spring Harb Perspect Biol. 2009;1 doi: 10.1101/cshperspect.a001271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blondeau N, Widmann C, Lazdunski M, Heurteaux C. Activation of the nuclear factor-kappaB is a key event in brain tolerance. J. Neurosci. 2001;21:4668–4677. doi: 10.1523/JNEUROSCI.21-13-04668.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pan H, et al. Alpha-linolenic acid is a potent neuroprotective agent against soman-induced neuropathology. Neurotoxicology. 2012;33:1219–1229. doi: 10.1016/j.neuro.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 48.Lang-Lazdunski L, Blondeau N, Jarretou G, Lazdunski M, Heurteaux C. Linolenic acid prevents neuronal cell death and paraplegia after transient spinal cord ischemia in rats. J. Vasc. Surg. 2003;38:564–575. doi: 10.1016/s0741-5214(03)00473-7. [DOI] [PubMed] [Google Scholar]
- 49.Gao F, et al. Whole-body synthesis-secretion rates of long-chain n-3 PUFAs from circulating unesterified alpha-linolenic acid in unanesthetized rats. J. Lipid Res. 2009;50:749–758. doi: 10.1194/jlr.D800056-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 50.Bazan NG, Molina MF, Gordon WC. Docosahexaenoic acid signalolipidomics in nutrition: significance in aging, neuroinflammation, macular degeneration, Alzheimer’s, and other neurodegenerative diseases. Annu. Rev. Nutr. 2011;31:321–351. doi: 10.1146/annurev.nutr.012809.104635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bazan NG. Neuroprotectin D1-mediated anti-inflammatory and survival signaling in stroke, retinal degenerations, and Alzheimer’s disease. J. Lipid Res. 2009;(50 Suppl):S400–S405. doi: 10.1194/jlr.R800068-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]