Abstract
Background
Obsessive–compulsive disorder (OCD) is a common, heritable neuropsychiatric disorder, hypothetically underpinned by dysfunction of brain cortical–striatal–thalamic–cortical (CSTC) circuits; however, the extent of brain functional abnormalities in individuals with OCD is unclear, and the genetic basis of this disorder is poorly understood. We determined the whole brain functional connectivity patterns in patients with OCD and their healthy first-degree relatives.
Methods
We used resting-state fMRI to measure functional connectivity strength in patients with OCD, their healthy first-degree relatives and healthy controls. Whole brain functional networks were constructed by measuring the temporal correlations of all brain voxel pairs and further analyzed using a graph theory approach.
Results
We enrolled 39 patients with OCD, 20 healthy first-degree relatives and 39 healthy controls in our study. Compared with healthy controls, patients with OCD showed increased functional connectivity primarily within the CSTC circuits and decreased functional connectivity in the occipital cortex, temporal cortex and cerebellum. Moreover, patients with OCD and their first-degree relatives exhibited overlapping increased functional connectivity strength in the bilateral caudate nucleus, left orbitofrontal cortex (OFC) and left middle temporal gyrus.
Limitations
Potential confounding factors, such as medication use, heterogeneity in symptom clusters and comorbid disorders, may have impacted our findings.
Conclusion
Our preliminary results suggest that patients with OCD have abnormal resting-state functional connectivity that is not limited to CSTC circuits and involves abnormalities in additional large-scale brain systems, especially the limbic system. Moreover, resting-state functional connectivity strength abnormalities in the left OFC, bilateral caudate nucleus and left middle temporal gyrus may be neuroimaging endophenotypes for OCD.
Introduction
Obsessive–compulsive disorder (OCD), which affects 2%–3% of the general population, is a common debilitating disorder characterized by persistent intrusive thoughts (obsessions) and/or repetitive behaviours (compulsions).1 Despite the high morbidity associated with OCD, the pathophysiology of the disorder remains unclear. Evidence from epidemiologic studies has demonstrated significant familial aggregation of OCD, with a nearly 8-fold higher risk of OCD symptoms developing in first-degree relatives of OCD probands than in the general population.2 Recent data suggest that intrinsic brain activity may be under genetic control,3 and there has been an increased interest in exploring neuroimaging endophenotypes to bridge the gap between genetic predispositions and the clinical symptoms.
Resting-state fMRI is a promising imaging technique that can be used to detect abnormalities in spontaneous neuronal activity.4 In recent years, most OCD studies have demonstrated resting-state functional connectivity abnormalities within the cortical–striatal–thalamic–cortical (CSTC) circuits, including the orbitofrontal cortex (OFC), anterior cingulate cortex (ACC), thalamus, putamen and caudate nucleus.5–7 Furthermore, the strength of the functional connectivity in the resting state between the caudate nucleus and OFC has been reported to predict clinical symptom severity in patients with OCD.5 The CSTC circuits have extensive connectivity to numerous cortical and subcortical regions, and dysregulation of the connectivity within CSTC circuits is thought to be associated with impaired executive performance, inability to inhibit cognition and behaviour and enhanced error monitoring processes in patients with OCD.8,9 Despite these advances, an important question that remains unanswered is whether or not OCD-related functional changes during the resting state occur only within the CSTC circuits. It is worth noting that most of the previous resting-state fMRI studies in patients with OCD have used a seed-based approach. A seed-based approach is useful to detect regionally specific hypotheses of brain function, but has limited power to detect connectivity patterns not predicted a priori.7 Recently, several lines of evidence from neuroimaging studies have indicated that there are functional abnormalities in large-scale networks outside CSTC circuits, including the parietal, temporal, insular and occipital regions.10,11 In the present study, we applied a novel approach to analyze resting-state fMRI data using graph theory to identify brain regions displaying high-degree centrality of connectivity, which represents cortical hubs in the whole brain networks. In contrast to traditional seeding approaches, these methods do not require a priori selection of a particular region and test for whole brain connectivity in every brain region.
Another important question pertains to whether or not there are neuroimaging endophenotypes during the resting state in patients with OCD. Two previous structural studies demonstrated that patients with OCD and their first-degree relatives exhibited grey and white matter abnormalities in common regions, including the OFC and the striatum, suggesting that there may be structural endophenotypes representing markers of increased genetic risk for OCD.12,13 In addition, a recent task-based fMRI study showed abnormally reduced activation of the OFC during reversal learning not only in patients with OCD, but also in their healthy first-degree relatives.14 These structural and functional findings directly support the existence of underlying neuroimaging endophenotypes and provide insight into the pathophysiology of OCD. To our knowledge, however, no study published to date has assessed brain function in healthy first-degree relatives of patients with OCD during the resting state, which is a vital step in the identification of putative neuroimaging endophenotypes.
To address these questions, we applied resting-state fMRI to investigate the whole brain fundamental functional alterations in patients with OCD and their healthy first-degree relatives. Given that several previous neuroimaging studies showed abnormal functional integration outside the CSTC circuits in patients with OCD, we hypothesized that these patients would show functional connectivity abnormalities in some regions outside the CSTC circuits (e.g., the parietal, temporal and occipital regions). More importantly, based on previous findings regarding structural and functional abnormalities in the OFC and striatum in first-degree relatives of patients with OCD, we hypothesized that patients and their first-degree relatives would show overlapping resting-state functional connectivity disruption in these regions.
Methods
Participants
We recruited patients with OCD from the Department of Clinical Psychology at the Southwest Hospital, Chongqing, China, between September 2010 and December 2012. The diagnosis of OCD was established by 2 qualified psychiatrists (W.Q. and J.-W.G.) using the Structured Clinical Interview according to DSM-IV criteria. Comorbid nonpsychotic mood and anxiety disorders were not considered to be exclusion criteria if OCD was the primary clinical diagnosis. The healthy first-degree relatives of OCD patients were recruited by approaching patients in the clinic, and the relatives were enrolled if they did not have current or past depression, other major physical or neurologic illnesses, substance abuse or axis I disorders. We recruited healthy controls among hospital staff and by advertisements on the Internet. Exclusion criteria for all participants were history of other psychiatric or neurologic illnesses, history of drug or alcohol abuse, pregnancy, serious physical illness and contraindications to MRI. Conventional MRIs (T1- and T2-weighted images) were inspected by 2 experienced neuroradiologists (J.W. and H.-T.L.).
We assessed the severity of OCD symptoms using the Yale–Brown Obsessive Compulsive Scale (Y-BOCS).15,16 Current symptoms of depression and anxiety were assessed using the 17-item Hamilton Rating Scale for Depression (HAM-D) and the 14-item Hamilton Rating Scale for Anxiety (HAM-A), respectively.17,18 The first-degree relatives of patients with OCD and healthy controls were screened using the Structured Clinical Interview for DSM-IV (non-patient edition) to ascertain that there was no personal history of psychiatric and neurologic illnesses. The Medical Ethics Committee of Third Military Medical University (Chongqing, China) approved the study protocol, and all participants gave written informed consent according to the Declaration of Helsinki.
Data acquisition
We acquired all images using a 3.0 T MRI system (TIM Trio; Siemens) with an 8-channel phased array head coil. During the MRI scans, all participants were instructed to relax with their eyes closed and lie still without moving. The resting-state functional images were acquired using an echo-planar-imaging (EPI) sequence as follows: 36 axial slices with a slice thickness of 4 mm and no slice gap, repetition time 2000 ms, echo time 30 ms, flip angle 90°, field of view 256 × 256 mm2, matrix 64 × 64 and isotropic voxel 4 × 4 × 4 mm3. For each participant, the fMRI scanning lasted for 480 seconds, and 240 volumes were obtained.
Image preprocessing
Image preprocessing was carried out using SPM8 software (www.fil.ion.ucl.ac.uk/spm/). The first 10 volumes were discarded for steady state magnetization and stabilization of participant status. The EPI images were slice-timing corrected to the middle slice acquired in time and then realigned and resliced to correct for head motion with a mean volume created. The head motion of all participants during resting-state fMRI acquisition was observed, and data were discarded if the translation exceeded 2 mm or if rotation exceeded 2° (see the Appendix, available at jpn.ca). Next, all functional data were normalized to Montreal Neurological Institute (MNI) space by applying transformation parameters obtained by segmentation of the structural images, and each voxel was resampled to isotropic 3 × 3 × 3 mm. Further preprocessing included linear trend removal and temporal band-pass filtering (0.01–0.08 Hz), which we used to reduce the effects of low-frequency drift and high-frequency physiologic noise. Finally, we removed spurious or regionally nonspecific variance, including the 6-parameter rigid body head motion, global brain average signals and average signals from the cerebrospinal fluid and white matter, from the data using linear regression. Removal of the global signal caused a shift in the distribution of correlation coefficients such that there were approximately equal numbers of positive and negative correlations, making interpretation of the sign of the correlation ambiguous;19,20 therefore, we restricted our explorations to positive correlations, as in previous studies.21,22
Whole brain functional connectivity analysis
The method used to calculate the functional connectivity has been fully described in previous studies.21–24 Briefly, we subjected preprocessed functional runs to voxel-based whole brain correlation analysis (i.e., correlation of each voxel’s blood oxygen level–dependent [BOLD] time series to every other voxel’s BOLD time series), resulting in a Pearson correlation coefficient (r) matrix. The computation was constrained within a grey matter mask, which was generated by thresholding (a threshold of 0.2) a prior grey matter probability map in SPM8. Then we computed the functional connectivity strength (FCS) of the given voxel using the following equation:22 Svoxel (i) = rij > r0, where rij is the correlation coefficient between voxel i and voxel j, r0 is a threshold that is set to eliminate weak correlations possibly arising from signal noise (r0 = 0.25 in this study) and rij is converted to zij using Fisher z transformation when calculating FCS. Before statistical analysis, all individual functional connectivity strength maps were spatially smoothed with a 6 mm Gaussian kernel using SPM8. In general, the brain voxels with higher FCS values usually indicate central roles in the functional integrity of the whole brain networks. This measure has been widely used to examine the importance of the brain voxels of large-scale brain intrinsic connectivity networks.22,25,26
Seed-based functional connectivity analysis
To investigate more detailed connectivity alteration patterns associated with the identified hubs, we conducted a seed-based interregional correlation analysis. We selected the regions with abnormal FCS values in patients with OCD and their first-degree relatives as regions of interest (ROIs). All analytic methods were the same as most previous resting-state functional connectivity studies (see the Appendix for details).
Statistical analysis
We compared distributions of age, years of education and sex among the groups by conducting 1-way analysis of variance (ANOVA) and the χ2 test using SPSS software version 18.0. Statistical tests on the functional connectivity maps across groups were performed using a voxel-based, 1-way analysis of covariance (ANCOVA) with post hoc t tests for age, sex and years of education as covariates of no interest. Statistical inferences were made with a voxel-level threshold of p < 0.05 after family-wise error correction for multiple comparisons. For clusters identified in the ANCOVA, we performed follow-up between-group voxel-wise t tests to characterize group differences using the same thresholds within a mask showing group differences from the ANCOVA analysis. Finally, to determine the association between functional connectivity strength and clinical characteristics (Y-BOCS score, HAM-D score, HAM-A score and disease duration), we conducted a voxel-based multiple linear regression analysis in the patients with OCD within regions showing significant functional connectivity strength differences compared to healthy controls, with age, sex and years of education as confounding factors.
Results
Participants
We recruited 40 patients with OCD, 20 healthy first-degree relatives and 40 healthy controls. All participants were right-handed. Of the original participants, 1 woman in the OCD group and 1 man in the control group were excluded from the final analysis; the patient was excluded because of excessive head motion during MRI scanning (> 2 mm in translation) and the control was excluded owing to an incidental finding on conventional MRIs (intracranial arachnoid cyst). The final sample consisted of 39 patients with OCD, 20 healthy first-degree relatives and 39 healthy controls. The 3 groups were well-matched for age (F2,95 = 1.352, p = 0.27), sex (χ22 = 0.096, p = 0.95) and years of education (F2,95 = 0.078, p = 0.93; Table 1). No patients in this study met the criteria for major depressive disorder, Tourette syndrome or other tic-related conditions. Comorbid lifetime diagnoses for the patients with OCD included social phobia (n = 4), dysthymic disorder (n = 2), panic disorder (n = 2), social phobia (n = 4) and generalized anxiety disorder (n = 1). Of the 39 patients with OCD, 33 were drug-naive and 6 had a history of treatment with selective serotonin reuptake inhibitors (fluoxetine [20–40 mg/d]; mean duration of treatment, 62.3 ± 46.5 [range, 10–180] d). Four of the 6 patients had stopped medication for 14–30 days, and 2 patients had stopped medication more than 1 month before entering the study. None of the participants had taken any medications that might affect the central nervous system for at least 2 weeks before MRI scans.
Table 1.
Demographic and clinical characteristics of the study sample
| Group; mean ± SD* | |||||
|---|---|---|---|---|---|
|
|
|||||
| Characteristics | OCD, n = 39 | Relatives, n = 20 | Control, n = 39 | F2,95 | p value |
| Sex, male:female | 20:19 | 10:10 | 20:19 | 0.096 | 0.95 |
| Age, yr | 26.6 ± 9.8 | 29.5 ± 8.6 | 26.0 ± 6.3 | 1.352 | 0.27 |
| Education, yr | 12.6 ± 3.3 | 12.9 ± 2.9 | 13.1 ± 3.2 | 0.078 | 0.93 |
| Disease duration, mo | 45.3 ± 37.3 | — | — | — | — |
| HAM-D total score | 6.5 ± 5.9 | — | — | — | — |
| HAM-A total score | 10.2 ± 3.6 | — | — | — | — |
| Y-BOCS total score | 26.7 ± 6.1 | — | — | — | — |
HAM-A = Hamilton Rating Scale for Anxiety; HAM-D = Hamilton Rating Scale for Depression; OCD = obsessive–compulsive disorder; SD = standard deviation; Y-BOCS = Yale–Brown Obsessive Compulsive Scale.
Unless otherwise indicated.
Whole-brain functional connectivity analysis
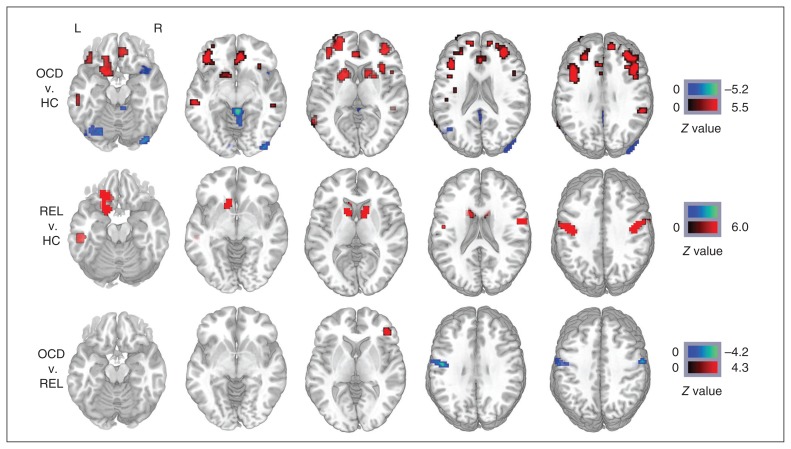
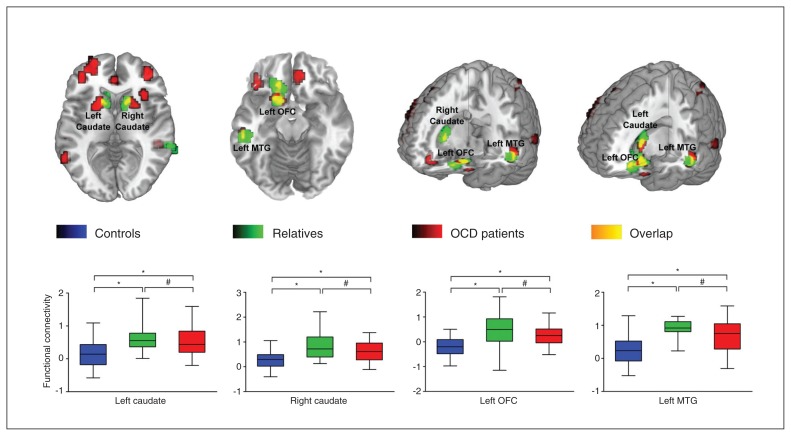
Our ANCOVA analysis revealed significant group differences in FCS values, primarily in the bilateral prefrontal cortex, cingulate cortex, caudate nucleus, putamen, temporal lobe, parietal lobe and occipital lobe. Compared with the healthy controls, patients with OCD had significantly increased FCS values in the bilateral OFC, ACC, caudate nucleus, putamen, middle frontal gyrus, middle temporal gyrus, parietal lobule and right insula and decreased FCS values in the bilateral occipital lobe, cerebellum and right superior temporal gyrus. Compared with the healthy controls, the first-degree relatives of patients with OCD had significantly increased FCS values primarily in the left OFC, bilateral caudate nucleus, precentral gyrus and left middle temporal gyrus. Compared with the first-degree relatives, patients with OCD had significantly increased FCS values in the right inferior frontal gyrus and decreased FCS values in the bilateral precentral gyrus (Fig. 1). More detailed information about the clusters in the group comparisons is presented in the Appendix. Notably, compared with healthy controls, patients with OCD and their first-degree relatives showed overlapping increased FCS values in the left OFC, bilateral caudate nucleus and left middle temporal gyrus; the patients did not differ significantly from their relatives in these regions. Figure 2 quantifies the change in FCS values in the affected regions. Correlation analyses in patients with OCD revealed no significant associations between the functional connectivity strength in abnormal brain regions and the Y-BOCS score, HAM-D score, HAM-A score or disease duration.
Fig. 1.
Whole brain difference of functional connectivity strength maps among 3 groups. The colour bar indicates the statistical significance threshold. The left side of the image corresponds to the left hemispheric regions. Map thresholds were set at p < 0.05 with multiple comparisons. HC = healthy controls; L = left; OCD = obsessive–compulsive disorder; R = right; REL = first-degree relatives of patients with OCD.
Fig. 2.
The overlapping regions of abnormal functional connectivity strength in patients with obsessive–compulsive disorder (OCD) and their healthy first-degree relatives. The box plot represents connectivity strength values of the 3 groups at the peak of the overlapping regions. Blue denotes the healthy control group, green denotes the first-degree relative group or abnormal brain regions in first-degree relatives, red denotes patients with OCD or abnormal brain regions in these patients, and yellow denotes the overlapping regions of abnormal functional connectivity strength regions between patients with OCD and their healthy first-degree relatives. *p < 0.001; #p > 0.05. MTG = middle temporal gyrus; OFC = orbitofrontal cortex.
Seed ROI-based functional connectivity analysis
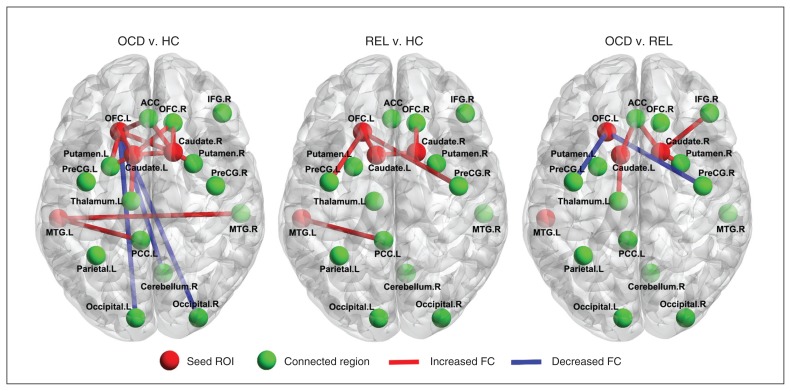
To investigate more detailed connectivity alteration patterns associated with the identified hubs, we performed a seed ROI-based interregional correlation analysis. We selected the left OFC, bilateral caudate and left middle temporal gyrus as seed ROIs because of the significantly decreased whole brain FCS in patients with OCD and their relatives compared with healthy controls. Figure 3 shows interregional connectivity with the 4 seed regions (all p < 0.05, corrected). Compared with healthy controls, the patients with OCD and their first-degree relatives had increased functional connectivity, primarily within the CSTC circuits, including the functional connectivity between the bilateral caudate and OFC, and between the left middle temporal gyrus and left posterior cingulate cortex. More detailed information about the clusters in the group comparisons is presented in the Appendix.
Fig. 3.
Abnormal interregional correlations in the patients with obsessive–compulsive disorder (OCD) and their first-degree relatives. Red denotes the brain regions with increased functional connectivity (FC) and blue denotes the brain regions with reduced functional connectivity. Map thresholds were set at p < 0.05 with multiple comparisons. ACC = anterior cingulate cortex; HC = healthy controls; IFG = inferior frontal cortex; L = left; MTG = middle temporal gyrus; OFC = orbitofrontal cortex; PCC = posterior cingulate cortex; PreCG = precentral gyrus; R = right; REL = first-degree relatives of patients with OCD; ROI = region of interest.
Discussion
We used resting-state fMRI to study whole-brain functional connectivity patterns in patients with OCD, their unaffected first-degree relatives and healthy controls. The primary finding was that patients with OCD had network alterations that were not limited to the CSTC circuits and involved alterations in additional brain systems, including the parietal lobe, occipital lobe, temporal lobe and insula. Moreover, our findings also showed that patients with OCD and their first-degree relatives had an overlapping increased resting-state functional connectivity primarily in the left OFC, bilateral caudate nucleus and left middle temporal gyrus, suggesting that these regions are neuroimaging endophenotypes representing markers of increased genetic risk for OCD. These findings may not only improve our understanding of the pathophysiologic characteristics of OCD, but may also be helpful in further highlighting heritable neuroimaging endophenotypes of OCD.
The CSTC circuits have extensive connectivity to many cortical and subcortical regions, and the dysregulation of these connectivities may contribute to the disturbances of emotion, behaviour and cognition in patients with OCD.27 Convergent evidence from neuroimaging studies and neuropsychologic studies suggests that abnormalities of CSTC circuits may be at the root of the pathogenesis of OCD.5,28,29 Consistent with this hypothesis, aberrant functional activity in CSTC circuits has been reported in task-based and resting-state fMRI studies, although results have been inconsistent, with reports of increased and decreased functional activity. For example, the striatum, as a crucial region in CSTC circuits, was reported to have increased functional activity,30 normal functional activity,31 or decreased functional activity32,33 in patients with OCD. Our results confirmed that patients with OCD had increased functional connectivity strength in the bilateral striatum (caudate nucleus and putamen) and other regions within CSTC circuits (bilateral OFC and ACC) in the resting state. We speculate that the increased functional connectivity strength in CSTC circuits may cause dysfunction of information transmission and functional integration, which can bring about obsessive–compulsive symptoms, such as the weakening of inhibitory ability and repetitive behaviour.
In addition, we observed changed functional connectivity in large-scale networks outside CSTC circuits, including the temporal cortex, occipital cortex, cingulate gyrus, insula and cerebellum, and these abnormalities did not exist in first-degree relatives. In fact, most of these regions belong to the limbic system. The limbic system is tightly connected to the prefrontal cortex, and the prefrontal-limbic system has been recognized to be involved in affective processing, decision-making and regulation of memories.34 In recent years, evidence from neuroimaging studies has suggested that the pathogenesis of OCD may also be related to functional abnormalities within other widely distributed large-scale systems, including the parietal, temporal and occipital regions.10,35,36 In addition, a recent meta-analysis of studies on OCD demonstrated abnormalities in large-scale networks outside CSTC circuits in patients with OCD.37 Our findings support the existence of systems-level pathology in this disorder, particularly in the limbic system. Future experiments are expected to combine different modalities to clarify the involvement of dysfunction of large-scale networks in the pathophysiology of OCD.
Interestingly, our patients with OCD and their first-degree relatives had increased FCS values in the left OFC, bilateral caudate nucleus and left middle temporal gyrus compared to healthy controls, but patients did not differ significantly from their relatives in these regions. Additional seed ROI–based analyses demonstrated that patients with OCD and their first-degree relatives exhibited increased functional connectivity between the bilateral caudate and OFC and between the left middle temporal gyrus and left posterior cingulate cortex. The presence of these abnormalities in patients with OCD and their healthy first-degree relatives suggests that dysfunction of these regions may not be due to treatment and other secondary environmental factors; it is more likely to be due to neuroimaging endophenotypes for OCD.
The OFC is central to our understanding of OCD endophenotypes because structural and functional alterations of this region are the most frequently reported findings in neuroimaging studies.6,14,38 Chamberlain and colleagues14 demonstrated that patients with OCD as well as their unaffected first-degree relatives have abnormal activation of the OFC, suggesting that such abnormalities may reflect an underlying vulnerability marker of illness, which is highly consistent with our findings. We speculate that the functional alterations in the OFC that we observed might influence information transmission and functional integration and lead to a decrease in response inhibition for patients with OCD and their first-degree relatives. This can be confirmed by some studies that reported impaired cognitive flexibility and motor inhibition in patients with OCD and their first-degree relatives.39 The caudate nucleus projects to the OFC, ACC and thalamus and is thought to play a central role in the CSTC circuits. Dysfunction of the caudate in patients with OCD is considered to lead to deficits in thalamic filtering, which in turn lead to excessive activity in OFC.40 A number of previous studies have demonstrated that the caudate nucleus exhibits abnormalities in functional activation41–43 and grey matter morphology.44–46 Our findings suggest that functional alterations in the bilateral caudate nucleus might underlie implicit information processing deficits, whereas hyperactivity in the OFC leads to a decrease in response inhibition for patients with OCD and their first-degree relatives. The middle temporal gyrus is thought to be related to several cognitive processes, including language and semantic memory processing and multimodal sensory integration.47,48 The middle temporal gyrus is thought to be related to several cognitive processes, including language and semantic memory processing and multimodal sensory integration.47,48 This region has been implicated in the neuroanatomic model for OCD predominantly through functional connections with the OFC, ventral prefrontal cortex and dorsolateral prefrontal cortex. In recent years, functional deficits in cognitive domains (i.e., language, semantic memory, visuoconstructive abilities) have been reported in patients with OCD and their first-degree relatives.49 Thus, we speculate that dysfunction of the middle temporal gyrus may be related to functional deficits in cognitive domains found in these patients and their relatives and may also play an important role in the gene-related endophenotype of OCD.
Compared with first-degree relatives, patients with OCD showed increased functional connectivity strength in the right middle frontal gyrus. This region belongs to the dorsolateral prefrontal cortex (DLPFC) and is connected to the OFC, thalamus and caudate nucleus. An important function of the DLPFC is executive functions, such as working memory, cognitive flexibility, planning, inhibition and abstract reasoning.50 Our findings indicate that the increased functional connectivity in the DLPFC may be a state marker that is related to explicit clinical symptoms and may indicate the presence of a therapeutic target for OCD. In the present study, we also observed increased functional connectivity strength in the bilateral precentral gyrus in the relatives of patients with OCD compared with healthy controls and the patients themselves. It is possible that the increased functional connectivity in the precentral gyrus in the relatives of patients with OCD may reflect a compensatory mechanism, such that additional areas are recruited to compensate for the genetic deficiencies. In the present study, we showed that relatives of patients with OCD had abnormal functional connectivity, primarily within CSTC circuits; it is likely that the compensatory mechanism may “cover” the functional deficits of CSTC circuits in this group.
Limitations
There were several limitations to our study. First, there are currently many strategies that can be used to analyze resting-state whole brain network data, such as small-world and modularity. We focused mainly on the nodal functional connectivity strength (weighted degree centrality) because it is very difficult to compute other graph attributes in a huge network with 67 632 nodes owing to a high computational load.22 In the future, it would be very interesting to compute these graph attributes with the help of high-performance computing systems. Second, 6 patients with OCD had a history of treatment with psychotropic medication; although these patients had a minimum 2-week medication washout period before MRI scans, we could not completely rule out the influence of medicine on brain function. Further studies using large, treatment-naive patients or longitudinal studies are warranted to confirm our preliminary results. Third, patients in the present study were heterogeneous in major symptom dimensions, and several structural and functional studies have shown that there are distinct neural substrates under different major symptom dimensions of OCD;51,52 however, we could not further divide the patients into different subgroups owing to the limited sample size. Future studies that focus on the different symptoms or subtypes of OCD may help to further the understanding of the pathophysiology of the disorder. Fourth, we did not obtain HAM-D and HAM-A scores for the relatives of patients with OCD or the healthy controls. However, no participants in this study met the criteria for major depressive disorder, and no significant correlations between the functional connectivity values and HAM-D or HAM-A scores were found in patients with OCD. We could not completely exclude the possibility that our results were influenced by the current comorbid symptoms of the participants. Further investigations are warranted to confirm our preliminary results after controlling for the comorbid depression and anxiety ratings of the participants on the HAM-D and HAM-A scales.
Conclusion
Using resting-state fMRI, our findings demonstrated that patients with OCD have abnormal resting-state functional connectivity that is not limited to CSTC circuits and involves abnormalities in the limbic system. Moreover, we identified similar functional connectivity abnormalities within CSTC circuits in patients with OCD and their healthy first-degree relatives. Such brain-based neuroimaging endophenotypes may be helpful in the search for an underlying genetic predisposition to OCD. Future experiments are expected to combine different modalities to provide more information about OCD.
Acknowledgements
This study was supported by the National Nature Science Foundation of China (grant no. 81171283). We thank all the participants for their contribution to this study.
Footnotes
Competing interests: None declared.
Contributors: J.-M. Hou and H.-T. Li designed the study. J.-M. Hou, W.-J. Wu, W. Zhang, L.-H. Song, J.-Wang, D.-Q. Zhou, B. Xie, M. He and W. Qu acquired the data, which J.-M. Hou, M.-Zhao, H.-T. Li and B. Xie analyzed. J.-M. Hou, H.-T. Li, W.-J. Wu, W. Zhang, L.- H. Song and B. Xie wrote the article, which all authors reviewed and approved for publication.
References
- 1.Horwath E, Weissman MM. The epidemiology and cross-national presentation of obsessive-compulsive disorder. Psychiatr Clin North Am. 2000;23:493–507. doi: 10.1016/s0193-953x(05)70176-3. [DOI] [PubMed] [Google Scholar]
- 2.Pauls DL, Alsobrook JP, II, Goodman W, et al. A family study of obsessive-compulsive disorder. Am J Psychiatry. 1995;152:76–84. doi: 10.1176/ajp.152.1.76. [DOI] [PubMed] [Google Scholar]
- 3.Hasler G, Northoff G. Discovering imaging endophenotypes for major depression. Mol Psychiatry. 2011;16:604–19. doi: 10.1038/mp.2011.23. [DOI] [PubMed] [Google Scholar]
- 4.Biswal B, Yetkin FZ, Haughton VM, et al. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–41. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 5.Harrison BJ, Soriano-Mas C, Pujol J, et al. Altered corticostriatal functional connectivity in obsessive-compulsive disorder. Arch Gen Psychiatry. 2009;66:1189–200. doi: 10.1001/archgenpsychiatry.2009.152. [DOI] [PubMed] [Google Scholar]
- 6.Beucke JC, Sepulcre J, Talukdar T, et al. Abnormally high degree connectivity of the orbitofrontal cortex in obsessive-compulsive disorder. JAMA Psychiatry. 2013;70:619–29. doi: 10.1001/jamapsychiatry.2013.173. [DOI] [PubMed] [Google Scholar]
- 7.Anticevic A, Hu S, Zhang S, et al. Global resting-state functional magnetic resonance imaging analysis identifies frontal cortex, striatal, and cerebellar dysconnectivity in obsessive-compulsive disorder. Biol Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.10.021. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van den Heuvel OA, Veltman DJ, Groenewegen HJ, et al. Frontal-striatal dysfunction during planning in obsessive-compulsive disorder. Arch Gen Psychiatry. 2005;62:301–9. doi: 10.1001/archpsyc.62.3.301. [DOI] [PubMed] [Google Scholar]
- 9.Maltby N, Tolin DF, Worhunsky P, et al. Dysfunctional action monitoring hyperactivates frontal-striatal circuits in obsessive–compulsive disorder: an event-related fMRI study. Neuroimage. 2005;24:495–503. doi: 10.1016/j.neuroimage.2004.08.041. [DOI] [PubMed] [Google Scholar]
- 10.Zhang T, Wang J, Yang Y, et al. Abnormal small-world architecture of top-down control networks in obsessive-compulsive disorder. J Psychiatry Neurosci. 2011;36:23–31. doi: 10.1503/jpn.100006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stern ER, Welsh RC, Gonzalez R, et al. Subjective uncertainty and limbic hyperactivation in obsessive-compulsive disorder. Hum Brain Mapp. 2013;34:1956–70. doi: 10.1002/hbm.22038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Menzies L, Achard S, Chamberlain SR, et al. Neurocognitive endophenotypes of obsessive-compulsive disorder. Brain. 2007;130:3223–36. doi: 10.1093/brain/awm205. [DOI] [PubMed] [Google Scholar]
- 13.Menzies L, Williams GB, Chamberlain SR, et al. White matter abnormalities in patients with obsessive-compulsive disorder and their first-degree relatives. Am J Psychiatry. 2008;165:1308–15. doi: 10.1176/appi.ajp.2008.07101677. [DOI] [PubMed] [Google Scholar]
- 14.Chamberlain SR, Menzies L, Hampshire A, et al. Orbitofrontal dysfunction in patients with obsessive-compulsive disorder and their unaffected relatives. Science. 2008;321:421–2. doi: 10.1126/science.1154433. [DOI] [PubMed] [Google Scholar]
- 15.Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry. 1989;46:1006–11. doi: 10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- 16.Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive Compulsive Scale. II. Validity. Arch Gen Psychiatry. 1989;46:1012–6. doi: 10.1001/archpsyc.1989.01810110054008. [DOI] [PubMed] [Google Scholar]
- 17.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–5. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 19.Murphy K, Birn RM, Handwerker DA, et al. The impact of global signal regression on resting state correlations: Are anti-correlated networks introduced? Neuroimage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weissenbacher A, Kasess C, Gerstl F, et al. Correlations and anticorrelations in resting-state functional connectivity MRI: a quantitative comparison of preprocessing strategies. Neuroimage. 2009;47:1408–16. doi: 10.1016/j.neuroimage.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 21.Buckner RL, Sepulcre J, Talukdar T, et al. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer’s disease. J Neurosci. 2009;29:1860–73. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang L, Dai Z, Peng H, et al. Overlapping and segregated resting-state functional connectivity in patients with major depressive disorder with and without childhood neglect. Hum Brain Mapp. 2013 doi: 10.1002/hbm.22241. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zuo XN, Ehmke R, Mennes M, et al. Network centrality in the human functional connectome. Cereb Cortex. 2012;22:1862–75. doi: 10.1093/cercor/bhr269. [DOI] [PubMed] [Google Scholar]
- 24.He Y, Wang J, Wang L, et al. Uncovering intrinsic modular organization of spontaneous brain activity in humans. PLoS ONE. 2009;4:e5226. doi: 10.1371/journal.pone.0005226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fransson P, Aden U, Blennow M, et al. The functional architecture of the infant brain as revealed by resting-state fMRI. Cereb Cortex. 2011;21:145–54. doi: 10.1093/cercor/bhq071. [DOI] [PubMed] [Google Scholar]
- 26.Cole MW, Pathak S, Schneider W. Identifying the brain’s most globally connected regions. Neuroimage. 2010;49:3132–48. doi: 10.1016/j.neuroimage.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 27.Stein DJ, Goodman WK, Rauch SL. The cognitive-affective neuroscience of obsessive-compulsive disorder. Curr Psychiatry Rep. 2000;2:341–6. doi: 10.1007/s11920-000-0079-2. [DOI] [PubMed] [Google Scholar]
- 28.Welch JM, Lu J, Rodriguiz RM, et al. Corticostriatal synaptic defects and OCD-like behaviours in Sapap3-mutant mice. Nature. 2007;448:894–900. doi: 10.1038/nature06104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Figee M, Luigjes J, Smolders R, et al. Deep brain stimulation restores frontostriatal network activity in obsessive-compulsive disorder. Nat Neurosci. 2013;16:386–7. doi: 10.1038/nn.3344. [DOI] [PubMed] [Google Scholar]
- 30.Saxena S, Brody AL, Maidment KM, et al. Cerebral glucose metabolism in obsessive-compulsive hoarding. Am J Psychiatry. 2004;161:1038–48. doi: 10.1176/appi.ajp.161.6.1038. [DOI] [PubMed] [Google Scholar]
- 31.Yang T, Cheng Y, Li H, et al. Abnormal regional homogeneity of drug-naive obsessive-compulsive patients. Neuroreport. 2010;21:786–90. doi: 10.1097/WNR.0b013e32833cadf0. [DOI] [PubMed] [Google Scholar]
- 32.Remijnse PL, Nielen MM, van Balkom AJ, et al. Reduced orbitofrontal-striatal activity on a reversal learning task in obsessive-compulsive disorder. Arch Gen Psychiatry. 2006;63:1225–36. doi: 10.1001/archpsyc.63.11.1225. [DOI] [PubMed] [Google Scholar]
- 33.Rubin RT, Villanueva-Meyer J, Ananth J, et al. Regional xenon 133 cerebral blood flow and cerebral technetium 99m HMPAO uptake in unmedicated patients with obsessive-compulsive disorder and matched normal control subjects. Determination by highresolution single-photon emission computed tomography. Arch Gen Psychiatry. 1992;49:695–702. doi: 10.1001/archpsyc.1992.01820090023004. [DOI] [PubMed] [Google Scholar]
- 34.Roxo MR, Franceschini PR, Zubaran C, et al. The limbic system conception and its historical evolution. ScientificWorldJournal. 2011;11:2428–41. doi: 10.1100/2011/157150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Del Casale A, Kotzalidis G, Rapinesi C, et al. Functional neuroimaging in obsessive-compulsive disorder. Neuropsychobiology. 2011;64:61–85. doi: 10.1159/000325223. [DOI] [PubMed] [Google Scholar]
- 36.Hou J, Wu W, Lin Y, et al. Localization of cerebral functional deficits in patients with obsessive-compulsive disorder: a resting-state fMRI study. J Affect Disord. 2012;138:313–21. doi: 10.1016/j.jad.2012.01.022. [DOI] [PubMed] [Google Scholar]
- 37.Menzies L, Chamberlain SR, Laird AR, et al. Integrating evidence from neuroimaging and neuropsychological studies of obsessive-compulsive disorder: the orbitofronto-striatal model revisited. Neurosci Biobehav Rev. 2008;32:525–49. doi: 10.1016/j.neubiorev.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Szeszko PR, Christian C, Macmaster F, et al. Gray matter structural alterations in psychotropic drug-naive pediatric obsessive- compulsive disorder: an optimized voxel-based morphometry study. Am J Psychiatry. 2008;165:1299–307. doi: 10.1176/appi.ajp.2008.08010033. [DOI] [PubMed] [Google Scholar]
- 39.Chamberlain SR, Fineberg NA, Menzies LA, et al. Impaired cognitive flexibility and motor inhibition in unaffected first-degree relatives of patients with obsessive-compulsive disorder. Am J Psychiatry. 2007;164:335–8. doi: 10.1176/appi.ajp.164.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Graybiel AM, Rauch SL. Toward a neurobiology of obsessive-compulsive disorder. Neuron. 2000;28:343–7. doi: 10.1016/s0896-6273(00)00113-6. [DOI] [PubMed] [Google Scholar]
- 41.Hansen ES, Hasselbalch S, Law I, et al. The caudate nucleus in obsessive-compulsive disorder. Reduced metabolism following treatment with paroxetine: a PET study. Int J Neuropsychopharmacol. 2002;5:1–10. doi: 10.1017/S1461145701002681. [DOI] [PubMed] [Google Scholar]
- 42.Kang DH, Jang JH, Han JY, et al. Neural correlates of altered response inhibition and dysfunctional connectivity at rest in obsessive-compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2013;40:340–6. doi: 10.1016/j.pnpbp.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 43.Breiter HC, Rauch SL, Kwong KK, et al. Functional magnetic resonance imaging of symptom provocation in obsessive-compulsive disorder. Arch Gen Psychiatry. 1996;53:595–606. doi: 10.1001/archpsyc.1996.01830070041008. [DOI] [PubMed] [Google Scholar]
- 44.Robinson D, Wu H, Munne RA, et al. Reduced caudate nucleus volume in obsessive-compulsive disorder. Arch Gen Psychiatry. 1995;52:393–8. doi: 10.1001/archpsyc.1995.03950170067009. [DOI] [PubMed] [Google Scholar]
- 45.Narayanaswamy JC, Jose DA, Kalmady SV, et al. Clinical correlates of caudate volume in drug-naive adult patients with obsessive-compulsive disorder. Psychiatry Res. 2013;212:7–13. doi: 10.1016/j.pscychresns.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 46.Zarei M, Mataix-Cols D, Heyman I, et al. Changes in gray matter volume and white matter microstructure in adolescents with obsessive-compulsive disorder. Biol Psychiatry. 2011;70:1083–90. doi: 10.1016/j.biopsych.2011.06.032. [DOI] [PubMed] [Google Scholar]
- 47.Onitsuka T, Shenton ME, Salisbury DF, et al. Middle and inferior temporal gyrus gray matter volume abnormalities in chronic schizophrenia: an MRI study. Am J Psychiatry. 2004;161:1603–11. doi: 10.1176/appi.ajp.161.9.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Visser M, Jefferies E, Embleton KV, et al. Both the middle temporal gyrus and the ventral anterior temporal area are crucial for multimodal semantic processing: distortion-corrected fMRI evidence for a double gradient of information convergence in the temporal lobes. J Cogn Neurosci. 2012;24:1766–78. doi: 10.1162/jocn_a_00244. [DOI] [PubMed] [Google Scholar]
- 49.Rajender G, Bhatia MS, Kanwal K, et al. Study of neurocognitive endophenotypes in drug-naive obsessive-compulsive disorder patients, their first-degree relatives and healthy controls. Acta Psychiatr Scand. 2011;124:152–61. doi: 10.1111/j.1600-0447.2011.01733.x. [DOI] [PubMed] [Google Scholar]
- 50.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 51.Harrison BJ, Pujol J, Cardoner N, et al. Brain corticostriatal systems and the major clinical symptom dimensions of obsessive-compulsive disorder. Biol Psychiatry. 2013;73:321–8. doi: 10.1016/j.biopsych.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 52.van den Heuvel OA, Remijnse PL, Mataix-Cols D, et al. The major symptom dimensions of obsessive-compulsive disorder are mediated by partially distinct neural systems. Brain. 2009;132:853–68. doi: 10.1093/brain/awn267. [DOI] [PubMed] [Google Scholar]