Abstract
Background
Neuroimaging research has traditionally explored fear and anxiety in response to discrete threat cues (e.g., during fear conditioning). However, anxiety is a sustained aversive state that can persist in the absence of discrete threats. Little is known about mechanisms that maintain anxiety states over a prolonged period. Here, we used a robust translational paradigm (threat of shock) to induce sustained anxiety. Recent translational work has implicated an amygdala–prefrontal cortex (PFC) circuit in the maintenance of anxiety in rodents. To explore the functional homologues of this circuitry in humans, we used a novel paradigm to examine the impact of sustained anticipatory anxiety on amygdala–PFC intrinsic connectivity.
Methods
Task-independent fMRI data were collected in healthy participants during long-duration periods of shock anticipation and safety. We examined intrinsic functional connectivity.
Results
Our study involved 20 healthy participants. During sustained anxiety, amygdala activity was positively coupled with dorsomedial PFC (DMPFC) activity. High trait anxiety was associated with increased amygdala–DMPFC coupling. In addition, induced anxiety was associated with positive coupling between regions involved in defensive responding, and decreased coupling between regions involved in emotional control and the default mode network.
Limitations
Inferences regarding anxious pathology should be made with caution because this study was conducted in healthy participants.
Conclusion
Findings suggest that anticipatory anxiety increases intrinsic amygdala–DMPFC coupling and that the DMPFC may serve as a functional homologue for the rodent prefrontal regions by sustaining anxiety. Future research may use this defensive neural context to identify bio-markers of risk for anxious pathology and target these circuits for therapeutic intervention.
Introduction
While fear is a short-duration defensive response to clearly identifiable impending danger, anxiety is a more sustained feeling of apprehension or dread about uncertain future threat.1 A wealth of basic science studies in animals and neuroimaging studies in humans indicate that the amygdala plays a key role in threat detection, fear learning and fear expression. However, while the amygdala is critical to initiate defensive responses to threat, its activation is of short duration and habituates rapidly, even when the behavioural manifestation of fear persists.2 This raises the question of the neural system involved in the maintenance of sustained defensive responses. A potential mechanism by which persistent brain states are maintained is via an interactive system of synchronization.3,4 Based on animal models that suggest the involvement of a prefrontal mechanism in this protracted response,2 we hypothesized a pivotal role for the dorsomedial prefrontal cortex (DMPFC) in the face of prolonged uncertain threat (i.e., anxiety). Specifically, we suggest that the DMPFC sustains defensive readiness during anxiety by maintaining synchronized activity with structures, including the amygdala, that are responsible for the signs and symptoms of anxiety.
Fear mechanisms can be studied in animals using fear conditioning procedures. Firing of amygdala neurons in response to a conditioned stimulus have substantially shorter duration than conditioned responses,5,6 such as freezing, suggesting that while the amygdala initiates defensive responses, it does not maintain them. Similarly, in humans the amygdala is only transiently associated with the expression of defensive responses but does not seem to be involved in their maintenance.7,8 This raises the question of which mechanism underlies the maintenance of anxiety responses.
The search for structures involved in the maintenance of defensive states has identified sites in the medial prefrontal cortex. In rodents, the prelimbic cortex tracks the behavioural manifestation of anxiety (i.e., freezing).2 In nonhuman primates the dorsal anterior cingulate cortex (dACC), which has similar anatomy and connectivity to that of the rodent prelimbic cortex,9,10 is also engaged in defensive response expression to uncertain threat.11 In humans, there is no known functional homologue of the prelimbic cortex, although evidence suggests that dorsal portions of the PFC (Brodmann areas [BA] 8 and 9 and dorsal regions of BA 32) are involved in fear learning,12 threat appraisal13,14 and maintenance of anxiety-related behavioural biases, such as heightened attention to emotionally negative stimuli.15 Further, serotonergic excitation of the amygdala–DMPFC circuit, accompanied by the amplification of negative behavioural biases, suggests that this circuit may be a prime target for pharmacological intervention.16 Critically, these findings indicate that communication between the DMPFC and amygdala may be integral to both modulating and preserving anxiety state over time. Although promising, this work does not directly address the neural substrates of sustained anticipatory anxiety in the absence of external stimuli. Further research is therefore necessary to examine involvement of this circuit in the maintenance of anxiety. In addition, much of the neuroimaging research on anxiety has been conducted in the context of behavioural tasks, rather than in the emotional context of the anxious state itself. In this line of research, discrete cues are typically used to indicate imminent threat (e.g., fear conditioning paradigms; for a review see Büchel and Dolan17), or short-duration periods of unpredictable threat are used to induce anxiety during a behavioural task (e.g., threat of shock paradigms; for a review see Robinson and colleagues18). These approaches are limited in that responses to discrete threat cues may not model clinically relevant sustained anxiety.1
To address these limitations, a task-independent approach was adopted to induce sustained anxiety using a well- validated shock anticipation paradigm.1 Current interest in task-independent slow neural connectivity signal fluctuations (e.g., resting-state paradigms) centres on the belief that they may represent some intrinsic form of brain functional connectivity within a discrete neuroanatomical system.19 Although intrinsic correlations are enduring phenomena and often viewed as somewhat endogenous, they are also influenced by changes in active brain states20 and are sensitive to both pharmacological manipulations21 and subjective experience.22 Further, the temporally extended nature of intrinsic connectivity paradigms provides a perfect venue with which to examine the influence of a sustained state on the baseline milieu of the brain.
Previous intrinsic connectivity research has either focused on resting state connectivity associated with random inter-subject variability in state anxiety in the absence of explicit threat,23 connectivity associated with a specific clinical disorder,24 or between-subject differences in connectivity following a stressful event (e.g., an aversive movie),25 but not during stress induction itself. In short, the amygdala–DMPFC connectivity associated with a sustained anxious state versus sustained safety has yet to be explored.
Here we implemented a within-subject resting state paradigm coupled with a translational method of anxiety induction (unpredictable threat of shock) that serves as a robust model of anxiety disorders.18,26 A within-subject design provided an effective method for evaluating neural changes within the same individuals and allowed us to uniquely compare connectivity during a sustained anxious state and a baseline state.
The main aim of the study was to examine the effect of induced anxiety on amygdala–PFC connectivity. Based on the reviewed evidence, we predicted that anticipatory anxiety would increase amygdala–DMPFC coupling. A second aim was to examine amygdala and DMPFC connectivity across the whole brain in order to identify additional circuitry influenced by these structures. Neural systems supporting hypervigilance,22 interoceptive awareness,27 apprehension,28 defensive readiness29 and aversive bias15 are engaged when an individual is anxious, and the literature suggests that prefrontal and amygdala interactions may play an important role in orchestrating these responses. We also sought to explore whether emotional control (e.g., ventromedial PFC; vmPFC30) systems were spontaneously engaged during threat processing. Previous research has shown that individuals may spontaneously initiate top–down emotion regulation processes, suggesting that these regions may be engaged in the threatening context (for a review see Mauss and colleagues31). Specifically, we predicted that during anticipatory anxiety, signal in the amygdala and DMPFC seeds would be more positively coupled with structures that mediate defensive readiness (e.g., insula, thalamus, basal ganglia) and those that support a salience network (e.g., insula, ACC32) than during safety and less negatively coupled with regions implicated in emotional control (e.g., vmPFC30) and regions typically coupled during baseline (i.e., those involved in the default mode network [DMN], such as the precuneus and posterior cingulate). Ultimately, we sought to determine if positive DMPFC–amygdala coupling is associated with sustained anticipatory anxiety and whether such coupling is dependent on vulnerability to anxiety disorders (i.e., trait anxiety).
Methods
Participants
We recruited individuals with normal or corrected-to-normal vision for participation in the study from 2010 to 2011. Upon arrival, recruits completed an intake evaluation consisting of a physical exam, urine screen and a Structured Clinical Interview for assessing DSM-IV Axis I psychiatric diagnoses.33 Exclusion from the study was based on the following criteria: acute or chronic medical condition, past or current psychiatric disorders, use of psychoactive medications or illicit drugs, lifetime history of alcohol or drug dependence, current pregnancy or breastfeeding, structural brain abnormalities on MRI, or MRI contraindications. We also excluded individuals because of excessive movement, physiologic equipment failure, or incomplete fMRI data. All participants provided written informed consent and received compensation for taking part in the study. The Combined Neuroscience Institutional Review Board of the National Institutes of Health approved our study protocol.
Stimuli and apparatus
We used a Digitimer constant current stimulator (DS7A; Digitimer) to present shocks. Shocks were administered to the top of the left foot using 2 Ag/AgCl electrodes (6 mm). Shock intensity was determined individually during a workup procedure performed in the scanner before scanning. Participants were told that the shock level could vary 70%–130% from the original level selected. This technique was used to introduce additional unpredictability of the threat and has been shown to induce a sustained anxious state.34,35 No shocks were actually delivered during the resting state runs.
Sustained anxiety procedure
Two 6-minute runs were used to assess neural connectivity during anticipatory anxiety and safety. During 1 run, the word “THREAT” was presented on the screen, and participants were told that they could be shocked at any time. During another run (counterbalanced), the word “SAFE” was presented on the screen, and participants were told that they would not receive any shocks and were completely safe. Participants were asked to lay still, clear their minds, keep their eyes open and focus on the word that was presented on the screen for the entire run. Anxiety ratings on a scale of 0 (no anxiety) to 9 (extreme anxiety) were verbally collected immediately after each run to determine the internal state of participants during the previous scan. We used the Spielberger Trait Anxiety Inventory (STAI, Form Y36) to assess trait anxiety.
Image acquisition
Functional MRIs were acquired on a GE Signa HDXT 3 T 940 scanner equipped with an 8-channel coil array. Ambient lights were turned off to limit visual distraction. Both 6-minute runs (180 sagittal volumes, with parallel imaging to increase coverage) had identical imaging parameters: repetition time (TR) 2000 ms, echo time (TE) 30 ms, slice thickness 3.5 mm, flip angle 90°, field of view (FOV) 22 × 22 cm, matrix 64 × 64. The first 5 volumes from each run were discarded to allow for magnetization equilibrium before image acquisition. The structural T1-weighted image was acquired using a magnetization prepared rapid gradient echo sequence: flip angle 10°, TR 7200 ms, TE 3000 ms, slice thickness 1.0 mm, slice spacing 0 mm, inversion time 450 ms, FOV 24 × 24 cm, matrix 224 × 224.
Physiologic data acquisition
We measured respiration volume using a bellows belt placed around the diaphragm, and we measured heart rhythm using a pulse oximeter cuff placed around the right index finger. Respiration and pulse data were sampled at 50 Hz.
Image preprocessing
All data were processed in Analysis of Functional Neuro-Images37 and FreeSurfer,38 using the ANATICOR approach to remove local artifacts in the echo planar imaging signal and to reduce the distance-dependent bias39 (i.e., increased proximal and decreased distal correlations that result from artifacts in the data) identified by Power and colleagues40 without introducing the problems associated with global signal regression.41 At the single-subject level potential artifacts were addressed by truncating signal spikes, modelling physiologic noise using cardiac and respiratory phase, and modelling movement using 6 rigid-body motion parameter regressors. We discarded data in cases of excessive motion (centre of mass deviation > 1.5 mm). After slice-time correction, the anatomy and second time series acquired were registered to the first time series. The aligned anatomy was processed through FreeSurfer’s automated pipeline in order to reconstruct the cortical surface area and conduct whole-brain parcellation. White matter and lateral ventricle masks were extracted and eroded 1 voxel in each direction (to reduce partial volume effects). In the final regression model we entered the following regressors: local estimates of eroded white matter and ventricle signal, motion parameters and respiration and cardiac signal. In cases where erosion of the ventricle mask eliminated the mask entirely, it was not included in the analysis for these participants. Variance explained by these nuisance regressors was discarded, and the residual variance (assumed to be an estimate of the true grey matter signal) was kept as the variable of interest. The residuals were bandpass filtered from 0.01 to 0.1 Hz, converted to Talairach space, and smoothed with a 6 mm full-width at half- maximum Gaussian kernel.
Seed-based functional connectivity analysis
Amygdala and DMPFC regions of interest seeds were created so that signal in these regions could be correlated with signal in the rest of the brain. The right amygdala seed was selected based on each participant’s FreeSurfer parcellation, and each mask was verified against the corresponding anatomy. The bilateral DMPFC seed was selected based on a meta-analysis of instructed fear studies (i.e., studies that induced anticipatory anxiety).12 An 8 mm sphere was centred on the DMPFC cluster maximum (Montreal Neurological Institute [MNI] space x, y, z = 0, 16, 36) and converted to Talairach space (x, y, z = −1, 10, 38) using the icbm2tal transform implemented in GingerALE.42,43 The fMRI signal was extracted from both seeds for the threat and safe runs. The correlation analysis consisted of quadratic fitting of the baseline and drift, whole-brain correlation with the extracted time series and transformation of the output using Fisher z in preparation for group analysis. At the group level, we performed a t test on the individual participants’ z scores for the threat and safe runs using the amygdala seed and the DMPFC seed. These maps were then entered into a second t test to examine differences in amygdala connectivity during threat versus safe runs (addressing the question, “What are the areas with which the amygdala shows differential connectivity as a function of induced anxiety?”) and differences in DMPFC connectivity during threat versus safe runs (addressing the question, “What are the areas with which the DMPFC shows differential connectivity as a function of induced anxiety?”). In addition to the whole-brain connectivity analysis performed with the amygdala and DMPFC seeds, we specifically assessed coupling between the amygdala and DMPFC by comparing a correlation analysis on the time course extracted from each seed during anticipatory anxiety and safety (addressing the question, “Does sustained anxiety modify amygdala–DMPFC connectivity?”). All maps were thresholded using a false discovery rate correction at q < 0.05 and restricted to clusters of 20 voxels or larger.
Results
Participants
Thirty-one healthy right-handed individuals (16 men) were recruited and received compensation for participation in the study. We excluded data from our analyses for 8 participants because of excessive movement, for 2 because of physiologic equipment failure and for 1 because of incomplete fMRI data. The final group consisted of 20 adults (13 men; mean age 28 [range 22–37] yr).
Anxiety manipulation check
All participants reported that they expected to receive shocks and were significantly more anxious during the threat run than the safe run (anxiety during threat run: mean 5.6 ± 1.9; anxiety during safe run: 1.9 ± 1.5; t19 = 8.10, p < 0.001).
Neural connectivity during anticipatory anxiety versus safety
In preparation for comparison between the 2 states, we first identified the connectivity during threat of shock and safety separately by conducting a simple correlation using the right amygdala and DMPFC as seeds for each run. During the anticipatory anxiety period, right amygdala activity was positively coupled with activity in the DMPFC, dACC, posterior cingulate, bilateral insula and orbitofrontal cortex (OFC); DMPFC activity was positively coupled with activity in the bilateral amygdala (confirming the correlation between these regions with the amygdala as a seed), dACC, bilateral basal ganglia (caudate, putamen), insula and paracentral lobule. In contrast, during the safety period, right amygdala activity was positively coupled with activity in the vmPFC, posterior cingulate, precuneus and superior frontal gyrus. Similar to threat, during safety DMPFC activity was positively coupled with activity in the ACC, bilateral basal ganglia (caudate, putamen) and insula, but not the amygdala.
To examine the differential connectivity during threat of shock versus safety, using the latter as a baseline measure to address connectivity changes induced by anxiety, we conducted a t test comparing connectivity during anxious (threat run) and safety (safe run) states for the amygdala and DMPFC seed (see Table 1 for significant clusters). During anticipatory anxiety in comparison to safety, right amygdala activity was positively coupled with activity in the DMPFC, bilateral insula, bilateral basal ganglia and thalamus (Fig. 1). In contrast, right amygdala activity was negatively coupled with activity in the vmPFC, bilateral inferior temporal gyrus (ITG), posterior cingulate and precuneus. Activity in the DMPFC during threat versus safe runs was positively correlated with activity in the right amygdala, left insula, bilateral basal ganglia and dACC (Fig. 2). In contrast, activity in the DMPFC was negatively correlated with activity in left ITG.
Table 1.
Whole-brain regions showing significant differences in connectivity with the seed regions in the contrast threat > safe
| Seed (direction); location | Talairach coordinates; x, y, z | Peak t value | Voxels | ||
|---|---|---|---|---|---|
| Amygdala (Pos) | |||||
| L basal ganglia | 23 | −3 | −4 | 3.1 | 188 |
| R basal ganglia | −31 | 5 | −4 | 2.5 | 167 |
| R thalamus | −7 | −11 | 12 | 2.8 | 105 |
| R middle frontal gyrus | −41 | −47 | 18 | 3.2 | 64 |
| L thalamus | 15 | 21 | 2 | 3.1 | 47 |
| R insula | −37 | 15 | 14 | 3.5 | 44 |
| R OFC | −13 | −53 | −12 | 3.0 | 41 |
| L insula | 41 | 17 | 8 | 3.3 | 38 |
| L parahippocampal gyrus | 19 | 37 | 6 | 3.7 | 37 |
| R middle occipital gyrus | −39 | 71 | 6 | 3.0 | 30 |
| L OFC | 7 | −49 | −12 | 3.0 | 27 |
| R DMPFC | −5 | −23 | 46 | 2.5 | 21 |
| Amygdala (Neg) | |||||
| R vmPFC | −5 | −23 | −12 | −2.8 | 48 |
| R parahippocampal gyrus | −25 | 11 | −26 | −3.1 | 43 |
| L fusiform gyrus | 37 | 21 | −22 | −3.3 | 35 |
| R precuneus | −7 | 69 | 28 | −3.2 | 29 |
| L ITG | 39 | −1 | −28 | −2.8 | 27 |
| DMPFC (Pos) | |||||
| L thalamus | 9 | 3 | 12 | 3.0 | 164 |
| R anterior cingulate gyrus | −3 | −19 | 32 | 3.7 | 84 |
| R middle cingulate | −3 | 5 | 46 | 3.3 | 66 |
| R OFC | −37 | −31 | −10 | 4.0 | 34 |
| L insula | 49 | 7 | 12 | 3.5 | 21 |
| R amygdala | −13 | 1 | −16 | 2.9 | 20 |
| DMPFC (Neg) | |||||
| R ITG | 55 | 37 | −16 | −4.4 | 256 |
| L posterior cingulate | 5 | 31 | 34 | −4.0 | 110 |
| L parahippocampal gyrus/inferior temporal gyrus | 31 | 15 | −26 | −5.3 | 81 |
| L posterior cingulate | −1 | 33 | 32 | −3.1 | 58 |
| R cuneus | −3 | 81 | 6 | −3.7 | 42 |
| L parahippocampal gyrus | 17 | 1 | −24 | −4.9 | 27 |
DMPFC = dorsomedial prefrontal cortex; ITG = inferior temporal gyrus; L = left; Neg = negative; OFC = orbitofrontal cortex; Pos = positive; R = right; vmPFC = ventromedial prefrontal cortex.
False discovery rate–corrected at q < 0.05.
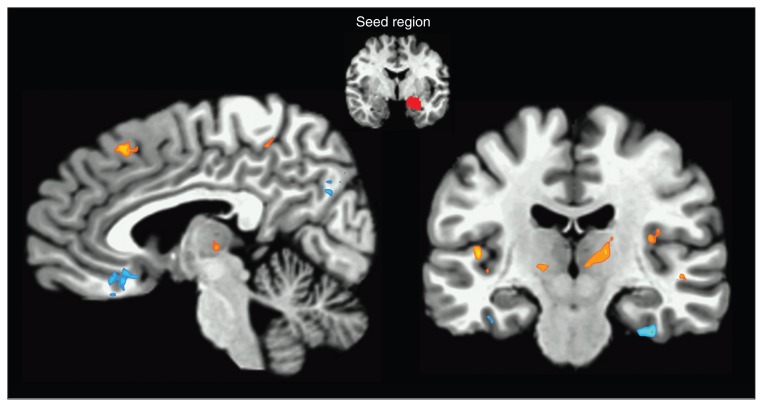
Fig. 1.
Right amygdala seed functional connectivity. The statistical map illustrates areas that showed increased positive coupling with the amygdala (orange) and decreased coupling with the amygdala (blue) during anticipatory anxiety as compared with safety. Areas with significant increases in positive coupling include the medial prefrontal cortex, insula, thalamus and basal ganglia. Areas with significant decreases in coupling include the ventromedial prefrontal cortex, inferior temporal gyrus and precuneus. Sagittal view x = −5; Coronal view y = 18. Images are in Talairach space, neurologic convention, and thresholded at q < 0.05, false discovery rate–corrected.
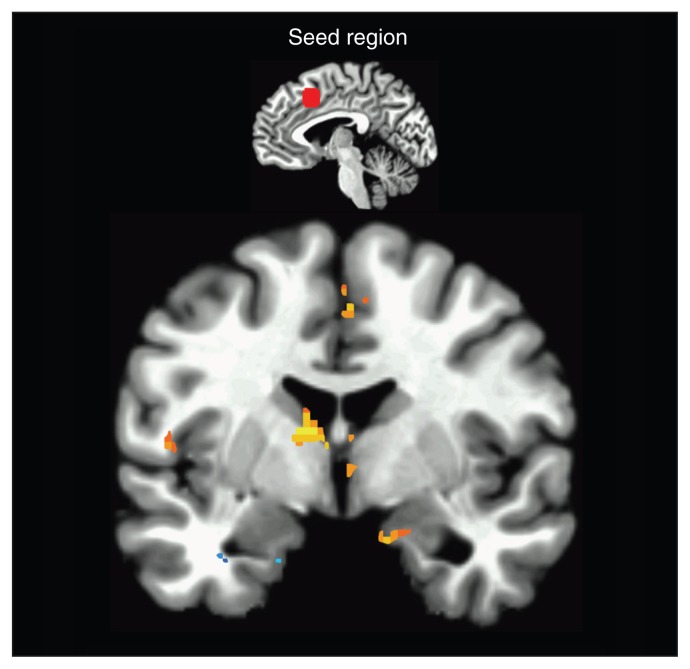
Fig. 2.
Dorsomedial prefrontal cortex (DMPFC) seed functional connectivity. The statistical map illustrates areas that showed increased positive coupling with the DMPFC (orange) and decreased coupling with the DMPFC (blue) during anticipatory anxiety compared with safety. Areas with significant increases in positive coupling include the amygdala, anterior cingulate cortex, insula and basal ganglia. The inferior temporal gyrus showed significant decreases in coupling with DMPFC. Coronal view y = 5. Images are in Talairach space, neurologic convention, and thresholded at q < 0.05, false discovery rate–corrected.
Trait anxiety predicts increased amygdala–DMPFC coupling
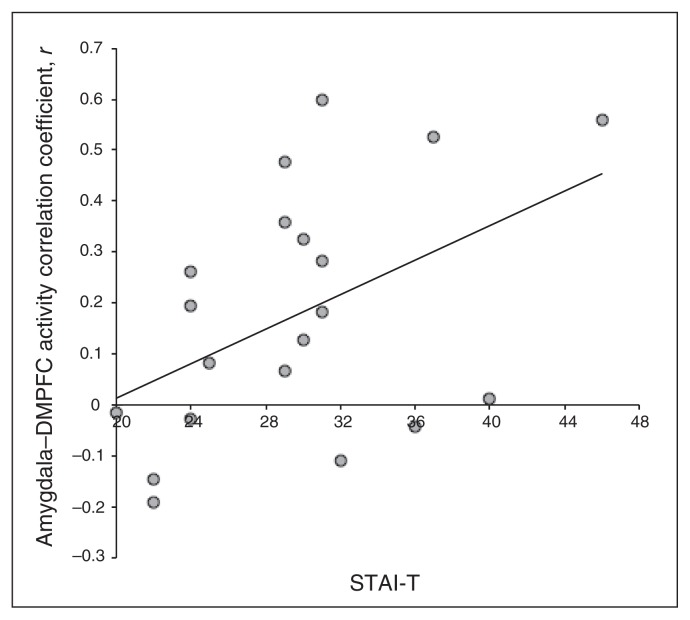
Previous research12,15,44 and results from the present study suggest that the amygdala and DMPFC are integrally involved in a neural circuit that supports anxiety. To examine the potential impact of dispositional anxiety on amygdala and DMPFC coupling, we conducted Pearson product- moment correlation analyses between amygdala–DMPFC connectivity and trait anxiety. During sustained anticipatory anxiety, positive amygdala–DMPFC coupling was positively correlated with trait anxiety (r = 0.457, p = 0.043; Fig. 3). In contrast, when participants were safe from shock, amygdala–DMPFC coupling was not related to trait anxiety (r = 0.103, p = 0.67). Further, a Steiger Z-test demonstrated that the association between trait anxiety and amygdala–DMPFC coupling was significantly stronger in the threat versus the safe condition (Z17 = 2.07, p = 0.039).36
Fig. 3.
Dispositional anxiety predicts positive coupling between the amygdala and dorsomedial prefrotnal cortex (DMPFC). Trait anxiety was positively correlated with amygdala–DMPFC coupling (r = 0.457, p = 0.037). STAI-T = State Trait Anxiety Inventory Trait.
Supplemental psychophysiological analysis
To address the possibility that we excluded potentially meaningful MRI signal (v. artifact) by regressing out pulse and respiration data, we conducted a supplemental analysis of the physiologic data to determine if there were obvious differences in the mean signal between threat and safe conditions. Paired-sample t tests demonstrated that mean pulse (t19 = −1.5, p = 0.14) and respiration (t19 = 0.39, p = 0.70) did not differ between threat and safe conditions.
Discussion
Our findings show that anticipatory anxiety increases intrinsic amygdala–DMPFC coupling, indicating that these 2 regions work in concert to support anxious responding. This finding is consistent with the idea that the DMPFC serves as a functional homologue for the prelimbic cortex in rodents15 by sustaining anxiety, potentially priming the amygdala for a rapid response to threat. Defensive responses to threat (e.g., acoustic startle reflex) are known to rely at least partially on the amygdala;45 however, the evidence that these responses are protracted while amygdala firing is transient46 suggests that the amygdala does not act alone. In addition, lesion studies47,48 find mixed support for the amygdala as a critical component in defensive responding, which may reflect the fact that it is the ongoing medial PFC–amygdala communication that is critical to sustained experience of anxiety.
Our finding of increased amygdala–DMPFC coupling during induced anxiety is a replication and extension of research showing that state anxiety, without anxiety induction, disrupts baseline negative coupling between these 2 brain regions in healthy controls.23 Specifically, Kim and colleagues23 found negative amygdala–DMPFC coupling at the group level, but they also reported a positive correlation between this coupling and state anxiety, which reflected the fact that as state anxiety increased (probably owing to the scanner environment), the amygdala–DMPFC connectivity also increased, changing from negative coupling to no coupling or positive coupling (see supplementary Figure 1 in the study by Kim and colleagues23 for the curve of the association between state anxiety and amygdala–DMPFC coupling). The present finding of no coupling in the safe condition is not necessarily inconsistent with these effects; instead of an anxiety-related shift from negative coupling to neutral (i.e., no coupling) or positive amygdala–DMPFC coupling, here we see a shift from no coupling to positive coupling. Both sets of findings suggest a move away from negative and toward positive synchronization between these 2 regions. When anxiety becomes more salient and consuming, as in the threat condition, DMPFC activity becomes increasingly synchronized with activity in the amygdala, suggesting that anxiety underlies the increase in positive coupling.
In addition to the amygdala–DMPFC circuit, we found that anticipatory anxiety is associated with increased positive coupling between regions involved in an affective, cognitive physiologic and motor defensive response, and decreased coupling between affective regions and regions involved in emotional control and other regions in the DMN. Specifically, compared with safety, during anticipatory anxiety right amygdala activity was positively coupled with activity in the DMPFC, bilateral insula, bilateral basal ganglia and thalamus. The nodes in this network (Fig. 4) each play an important role in the different aspects of anxiety, such as orienting attention (amygdala49), controlling autonomic responding (amygdala, insula,50 thalamus51), amplifying affective biases (DMPFC15), appraising threat (DMPFC12), evaluating affective value (OFC), heightening interoceptive awareness (insula27), orchestrating necessary cardiovascular changes (insula52), priming motor function (insula,53 basal ganglia, thalamus54) and increasing alertness (thalamus, PFC55). In contrast, right amygdala activity was negatively coupled with activity in the vmPFC, which is implicated in emotional control via downregulation of amygdala activity,30 bilateral ITG (a region involved in reorienting attention to control emotion)56 and precuneus (a central hub of the DMN).57 Reduced or negative coupling with the DMN during anticipatory anxiety suggests that this network may not be involved in the cognitive aspects of an anxious response (e.g., worry). Although the DMN is associated with a focus on internal thoughts and anticipatory anxiety may increase activity in this network, anticipation of shock could also be seen as a cognitive task, and thus anticipatory anxiety may decrease activity in this network. Previous research has shown that DMN activity is reduced when individuals engage in a concurrent task (e.g., working memory58). Together, these findings suggest that anticipatory anxiety may act as a concurrent task, engaging physiologic and cognitive networks while disengaging from the baseline DMN.
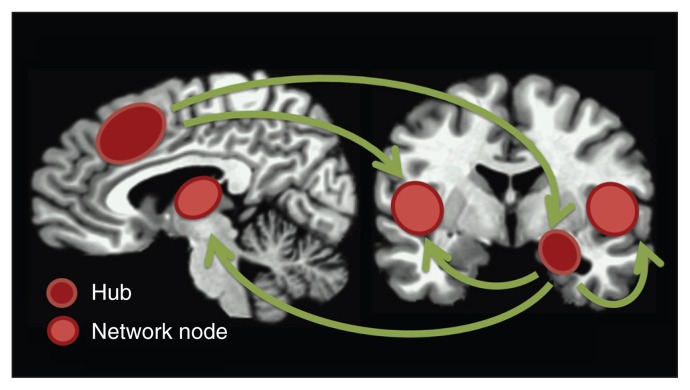
Fig. 4.
Proposed anticipatory anxiety network. Schematic illustration of the amygdala and prefrontal cortex (encompassing the dorsomedial prefrontal cortex and dorsal anterior cingulate cortex) as hubs in a network associated with anticipatory anxiety. Other central components of the network include the bilateral insula and thalamus. These regions are known to support affective, cognitive and autonomic nervous system changes, and in concert they create a state of readiness to respond to potential threat.
Activity in the DMPFC during anticipatory anxiety versus safety was positively correlated with activity in areas involved in the expression of anxiety (bilateral amygdala1), autonomic and interoceptive changes (insula50,52), motor planning (left basal ganglia54) and increasing negative biases and affective evaluation (dACC,15 OFC56). Similar to the amygdala coupling results, the DMPFC was negatively correlated with activity in the ITG, suggesting that emotional control processes may have been active.56
Looking beyond the group level, PFC–amygdala coupling during anticipatory anxiety was also driven by interparticipant variability in trait anxiety, suggesting that such a coupling constitutes a potential vulnerability marker for pathology. This hypothesis is consistent with reports that increases in sustained metabolic activity in the DMPFC is a risk factor for posttraumatic stress disorder.59 The finding that increased amygdala–PFC communication is linearly associated with increased dispositional anxiety reinforces the idea that anxiety is better conceptualized as a continuous versus a categorical dimension. By identifying neural circuitry that varies along a parallel continuum, the spectrum of anxiety from adaptive to maladaptive can be described by underlying biology, as suggested by the Research Domain Criteria initiative,60 which promotes a classification framework for mental disorders that is rooted in biology. Studies investigating patient groups can illuminate the tipping point where impairment is manifested in this circuit; once identified, such circuits may serve as biomarkers and candidate targets for more efficacious pharmacological interventions. The mechanisms of current treatment methods like selective serotonin reuptake inhibitors, which are known to modulate this circuit,16 will be better understood and potentially optimized to target this discrete circuit. Interventions may eventually include techniques like transcranial magnetic stimulation, which can be used to increase61 or decrease62 activity in proximal and distal neural circuits.63 This type of intervention could preserve certain aspects of the adaptive response to threat by indirectly rather than directly targeting the amygdala while downregulating the maladaptive features of anticipatory anxiety that contribute to an exaggerated response.
What is the potential role of amygdala–DMPFC coupling? One possibility is that it serves to prime the amygdala. In other words, the DMPFC may maintain the amygdala in a state of readiness. This priming could be the mechanism by which the amygdala can react rapidly to explicit threat cues during a sustained anxiety state without showing a sustained level of activation.5 It was recently reported that shock anticipation increases attention bias for threat (fearful faces) and that this effect was also mediated by increased DMPFC–amygdala coupling.12 One possible interpretation of these results is that DMPFC–amygdala coupling may serve to keep the amygdala in a primed state during uncertain threat and then drive or amplify amygdala reactivity when an explicit threat is encountered.
Limitations
Among the strengths of the present study is the use of a robust translational sustained anxiety induction paradigm that enabled us to study functional connectivity changes in anxiety in a within-subjects design. However, such coupling may not reflect anatomic coupling. Yet it is important to note that when Hebbian principles (neurons that fire together wire together) are applied on a larger scale, functional connectivity may indeed suggest anatomic connectivity. The DMPFC has both direct64 and indirect10 reciprocal anatomic connections to the amygdala, with the indirect connections stemming from the dACC (which is a richly interconnected extension of the DMPFC65). Taken together, this evidence suggests that there may be viable projections to and from the DMPFC to the amygdala. One limitation of the study was the small sample size, which reduced the power to detect individual differences. As such, our correlational findings should be interpreted with this in mind. A second potential limitation is that our decision to reduce spurious artifacts in our data by controlling for regularity in physiologic signals may have inadvertently excluded true neural signal from our analysis. However, a supplemental examination of pulse and respiration signal suggests that the means did not differ between threat and safe conditions. Further, although there are many data to support the claim that emotions like anxiety have discrete physiologic signatures, these signatures are not drawn directly from pulse and respiration data, particularly at such a low sampling rate (paradigms optimized for this type of psychophysiological data acquisition typically sample at 4–20 times the rate used here66,67). Differentiation of emotion states based on physiologic data require complex pattern classification using a broad range of signals, including respiratory sinus arrhythmia and heart rate variability.66 Anxiety in particular has been shown to exhibit a discrete physiologic signature based on a combination of electrocardiography parameters.67,68 Accordingly, it is unlikely that our artifact reduction technique suppressed a substantial amount of critical neural signal because anxiety-related neural signal associated with these physiologic states likely follows a more complex pattern than pulse or respiration signals reveal. Given that our sample consisted solely of healthy individuals, we also acknowledge that our results primarily reflect normal changes in the face of threat. Yet the variability in connectivity observed when trait anxiety is taken into account suggests that individuals on the higher end of the range may show patterns that are more similar to patient populations if anxiety is indeed a continuous trait.
Conclusion
Unlike fear, anxiety is a dimensional construct that is pathologically manifested in a wide range of psychiatric disorders. Our sustained anticipatory anxiety paradigm allowed us to uniquely identify intrinsic DMPFC–amygdala network associated with this construct. In sum, our findings show that anticipatory anxiety increases coupling between regions involved in both adaptive and maladaptive responses to threat, creating a specific emotional, cognitive and physiologic signature. The identification and description of this circuitry is crucial because it can serve as a target for the detection of pathology in anxiety disorders and as a target for the development of more effective pharmacological interventions.
Acknowledgements
We thank Hang Joon Jo for help with ANATI-COR implementation, and Richard Reynolds for assistance with AFNI analysis.
Footnotes
Competing interests: This research was supported by the Intramural Research Program of the National Institutes of Mental Health (grant # MH002798). The author(s) declare that, except for income received from the primary employer, no financial support or compensation has been received from any individual or corporate entity over the past 3 years for research or professional service and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest.
Contributors: All authors designed the study, acquired and analyzed the data, wrote the article and reviewed and approved the final version for publication.
References
- 1.Davis M, Walker DL, Miles L, et al. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35:105–35. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burgos-Robles A, Vidal-Gonzalez I, Quirk GJ. Sustained conditioned responses in prelimbic prefrontal neurons are correlated with fear expression and extinction failure. J Neurosci. 2009;29:8474–82. doi: 10.1523/JNEUROSCI.0378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buzsáki G, Watson BO. Brain rhythms and neural syntax: implications for efficient coding of cognitive content and neuropsychiatric disease. Dialogues Clin Neurosci. 2012;14:345–67. doi: 10.31887/DCNS.2012.14.4/gbuzsaki. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ward LM. Synchronous neural oscillations and cognitive processes. Trends Cogn Sci. 2003;7:553–9. doi: 10.1016/j.tics.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 5.Paré D, Collins D. Neuronal correlates of fear in the latteral amygdala: multiple extracellular recordings in conscious cats. J Neurosci. 2000;20:2701–10. doi: 10.1523/JNEUROSCI.20-07-02701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quirk GJ, Repa JC, LeDoux JE. Fear conditioning enhances short latency auditory responses of lateral amygdala neurons: parallel recordings in the freely behaving rat. Neuron. 1995;15:1029–39. doi: 10.1016/0896-6273(95)90092-6. [DOI] [PubMed] [Google Scholar]
- 7.Phelps EA, O’Connor KJ, Gatenby JC, et al. Activation of the left amygdala to a cognitive representation of fear. Nat Neurosci. 2001;4:437–41. doi: 10.1038/86110. [DOI] [PubMed] [Google Scholar]
- 8.Cheng DT, Richards J, Helmstetter FJ. Activity in the human amygdala corresponds to early, rather than late period autonomic responses to a signal for shock. Learn Mem. 2007;14:485–90. doi: 10.1101/lm.632007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McDonald AJ. Cortical pathways to the mammalian amygdala. Prog Neurobiol. 1998;55:257–332. doi: 10.1016/s0301-0082(98)00003-3. [DOI] [PubMed] [Google Scholar]
- 10.Ghashghaei HT, Hilgetag CC, Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage. 2007;34:905–23. doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Livneh U, Paz R. Amygdala-prefrontal synchronization underlies resistance to extinction of aversive memories. Neuron. 2012;75:133–42. doi: 10.1016/j.neuron.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 12.Mechias M-L, Etkin A, Kalisch R. A meta-analysis of instructed fear studies: Implications for conscious appraisal of threat. Neuroimage. 2010;49:1760–8. doi: 10.1016/j.neuroimage.2009.09.040. [DOI] [PubMed] [Google Scholar]
- 13.Kalisch R, Wiech K, Critchley HD, et al. Levels of appraisal: a medial prefrontal role in high-level appraisal of emotional material. Neuroimage. 2006;30:1458–66. doi: 10.1016/j.neuroimage.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 14.Kalisch R, Wiech K, Critchley HD, et al. Anxiety reduction through detachment: subjective, physiological, and neural effects. J Cogn Neurosci. 2005;17:874–83. doi: 10.1162/0898929054021184. [DOI] [PubMed] [Google Scholar]
- 15.Robinson OJ, Charney DR, Overstreet C, et al. The adaptive threat bias in anxiety: amygdala-dorsomedial prefrontal cortex coupling and aversive amplification. Neuroimage. 2012;60:523–9. doi: 10.1016/j.neuroimage.2011.11.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robinson OJ, Overstreet C, Allen PS, et al. The role of serotonin in the neurocircuitry of negative affective bias: serotonergic modulation of the dorsal medial prefrontal-amygdala ‘aversive amplification’ circuit. Neuroimage. 2013;78C:217–23. doi: 10.1016/j.neuroimage.2013.03.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Büchel C, Dolan RJ. Classical fear conditioning in functional neuroimaging. Curr Opin Neurobiol. 2000;10:219–23. doi: 10.1016/s0959-4388(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 18.Robinson O, Vytal K, Cornwell B, et al. The impact of anxiety upon cognition: translational perspectives. Front Hum Neurosci. 2013;7:203. doi: 10.3389/fnhum.2013.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deco G, Jirsa VK, McIntosh AR. Emerging concepts for the dynamical organization of resting-state activity in the brain. Nat Rev Neurosci. 2011;12:43–56. doi: 10.1038/nrn2961. [DOI] [PubMed] [Google Scholar]
- 20.Somerville LH, Wagner DD, Wig GS, et al. Interactions between transient and sustained neural signals support the generation and regulation of anxious emotion. Cereb Cortex. 2013;23:49–60. doi: 10.1093/cercor/bhr373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCabe C, Mishor Z. Antidepressant medications reduce subcortical–cortical resting-state functional connectivity in healthy volunteers. Neuroimage. 2011;57:1317–23. doi: 10.1016/j.neuroimage.2011.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Somerville LH, Whalen PJ, Kelley WM. Human bed nucleus of the stria terminalis indexes hypervigilant threat monitoring. Biol Psychiatry. 2010;68:416–24. doi: 10.1016/j.biopsych.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim MJ, Gee DG, Loucks RA, et al. Anxiety dissociates dorsal and ventral medial prefrontal cortex functional connectivity with the amygdala at rest. Cereb Cortex. 2011;21:1667–73. doi: 10.1093/cercor/bhq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hahn A, Stein P, Windischberger C, et al. Reduced resting-state functional connectivity between amygdala and orbitofrontal cortex in social anxiety disorder. Neuroimage. 2011;56:881–9. doi: 10.1016/j.neuroimage.2011.02.064. [DOI] [PubMed] [Google Scholar]
- 25.van Marle HJF, Hermans EJ, Qin S. Fernandez Gn. Enhanced resting-state connectivity of amygdala in the immediate aftermath of acute psychological stress. Neuroimage. 2010;53:348–54. doi: 10.1016/j.neuroimage.2010.05.070. [DOI] [PubMed] [Google Scholar]
- 26.Grillon C, Ameli R, Woods SW, et al. Fear-potentiated startle in humans: effects of anticipatory anxiety on the acoustic blink reflex. Psychophysiology. 1991;28:588–95. doi: 10.1111/j.1469-8986.1991.tb01999.x. [DOI] [PubMed] [Google Scholar]
- 27.Critchley HD, Wiens S, Rotshtein P, et al. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7:189–95. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- 28.Engels AS, Heller W, Mohanty A, et al. Specificity of regional brain activity in anxiety types during emotion processing. Psychophysiology. 2007;44:352–63. doi: 10.1111/j.1469-8986.2007.00518.x. [DOI] [PubMed] [Google Scholar]
- 29.Vytal K, Cornwell B, Arkin N, et al. Describing the interplay between anxiety and cognition: From impaired performance under low cognitive load to reduced anxiety under high load. Psychophysiology. 2012;49:842–52. doi: 10.1111/j.1469-8986.2012.01358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quirk GJ, Garcia R, Gonzalez-Lima F. Prefrontal mechanisms in extinction of conditioned fear. Biol Psychiatry. 2006;60:337–43. doi: 10.1016/j.biopsych.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 31.Mauss IB, Bunge SA, Gross JJ. Automatic emotion regulation. Soc Personal Psychol Compass. 2007;1:146–67. [Google Scholar]
- 32.Bonnelle V, Ham TE, Leech R, et al. Salience network integrity predicts default mode network function after traumatic brain injury. Proc Natl Acad Sci U S A. 2012;109:4690–5. doi: 10.1073/pnas.1113455109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.First MB, Gibbon M, Spitzer RL, et al. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) Research version. New York (NY): New York State Psychiatric Institute; 1995. [Google Scholar]
- 34.Grillon C, Baas JM, Lissek S, et al. Anxious responses to predictable and unpredictable aversive events. Behav Neurosci. 2004;118:916–24. doi: 10.1037/0735-7044.118.5.916. [DOI] [PubMed] [Google Scholar]
- 35.Grillon C, Davis M. Fear-potentiated startle conditioning in humans: explicit and contextual cue conditioning following paired vs. unpaired training. Psychophysiology. 1997;34:451–8. doi: 10.1111/j.1469-8986.1997.tb02389.x. [DOI] [PubMed] [Google Scholar]
- 36.Spielberger CD, Gorsuch RL, Lushene R, et al. Manual for the State-Trait Anxiety Inventory. Palo Alto (CA): Consulting Psychologist Press; 1983. [Google Scholar]
- 37.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 38.Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–55. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 39.Jo HJ, Gotts SJ, Reynolds RC, et al. Effective preprocessing procedures virtually eliminate distance-dependent motion artifacts in resting state fMRI. J Appl Math. 2013;2013:1–9. doi: 10.1155/2013/935154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Power JD, Barnes KA, Snyder AZ, et al. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–54. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jo HJ, Saad ZS, Simmons WK, et al. Mapping sources of correlation in resting state FMRI, with artifact detection and removal. Neuroimage. 2010;52:571–82. doi: 10.1016/j.neuroimage.2010.04.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lancaster JL, Tordesillas-Gutiérrez D, Martinez M, et al. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum Brain Mapp. 2007;28:1194–205. doi: 10.1002/hbm.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Laird AR, Robinson JL, McMillan KM, et al. Comparison of the disparity between Talairach and MNI coordinates in functional neuroimaging data: validation of the Lancaster transform. Neuroimage. 2010;51:677–83. doi: 10.1016/j.neuroimage.2010.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hitchcock JM, Sananes CB, Davis M. Sensitization of the startle reflex by footshock: blockade by lesions of the central nucleus of the amygdala or its efferent pathway to the brainstem. Behav Neurosci. 1989;103:509–18. doi: 10.1037//0735-7044.103.3.509. [DOI] [PubMed] [Google Scholar]
- 46.Goosens KA, Maren S. NMDA receptors are essential for the acquisition, but not expression, of conditional fear and associative spike firing in the lateral amygdala. Eur J Neurosci. 2004;20:537–48. doi: 10.1111/j.1460-9568.2004.03513.x. [DOI] [PubMed] [Google Scholar]
- 47.Weike AI, Hamm AO, Schupp HT, et al. Fear conditioning following unilateral temporal lobectomy: dissociation of conditioned startle potentiation and autonomic learning. J Neurosci. 2005;25:11117–24. doi: 10.1523/JNEUROSCI.2032-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liang KC, Melia KR, Campeau S, et al. Lesions of the central nucleus of the amygdala, but not the paraventricular nucleus of the hypothalamus, block the excitatory effects of corticotropin- releasing factor on the acoustic startle reflex. J Neurosci. 1992;12:2313–20. doi: 10.1523/JNEUROSCI.12-06-02313.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anderson AK, Phelps EA. Lesions of the human amygdala impair enhanced perception of emotionally salient events. Nature. 2001;411:305–9. doi: 10.1038/35077083. [DOI] [PubMed] [Google Scholar]
- 50.Benarroch EE. The central autonomic network: functional organization, dysfunction, and perspective. Mayo Clin Proc. 1993;68:988–1001. doi: 10.1016/s0025-6196(12)62272-1. [DOI] [PubMed] [Google Scholar]
- 51.Buchanan SL, Powell DA. Cingulothalamic and prefrontal control of autonomic function. In: Vogt BA, Gabriel M, editors. Neurobiology of cingulate cortex and limbic thalamus. New York (NY): Springer; 1993. pp. 381–414. [Google Scholar]
- 52.Critchley HD, Elliott R, Mathias CJ, et al. Neural activity relating to generation and representation of galvanic skin conductance responses: a functional magnetic resonance imaging study. J Neurosci. 2000;20:3033–40. doi: 10.1523/JNEUROSCI.20-08-03033.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chollet F, DiPiero V, Wise RJ, et al. The functional anatomy of motor recovery after stroke in humans: a study with positron emission tomography. Ann Neurol. 1991;29:63–71. doi: 10.1002/ana.410290112. [DOI] [PubMed] [Google Scholar]
- 54.Alexander GE. Basal ganglia-thalamocortical circuits: their role in control of movements. J Clin Neurophysiol. 1994;11:420–31. [PubMed] [Google Scholar]
- 55.Thomas M, Sing H, Belenky G, et al. Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res. 2000;9:335–52. doi: 10.1046/j.1365-2869.2000.00225.x. [DOI] [PubMed] [Google Scholar]
- 56.Ochsner KN, Ray RD, Cooper JC, et al. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23:483–99. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 57.Fransson P, Marrelec G. The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: evidence from a partial correlation network analysis. Neuroimage. 2008;42:1178–84. doi: 10.1016/j.neuroimage.2008.05.059. [DOI] [PubMed] [Google Scholar]
- 58.Hampson M, Driesen NR, Skudlarski P, et al. Brain connectivity related to working memory performance. J Neurosci. 2006;26:13338–43. doi: 10.1523/JNEUROSCI.3408-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shin LM, Lasko NB, Macklin ML, et al. Resting metabolic activity in the cingulate cortex and vulnerability to posttraumatic stress disorder. Arch Gen Psychiatry. 2009;66:1099–107. doi: 10.1001/archgenpsychiatry.2009.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Insel T, Cuthbert BN, Garvey MA, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167:748–51. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- 61.Di Lazzaro V, Pilato F, Dileone M, et al. The physiological basis of the effects of intermittent theta burst stimulation of the human motor cortex. J Physiol. 2008;586:3871–9. doi: 10.1113/jphysiol.2008.152736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Di Lazzaro V, Pilato F, Saturno E, et al. Theta-burst repetitive transcranial magnetic stimulation suppresses specific excitatory circuits in the human motor cortex. J Physiol. 2005;565:945–50. doi: 10.1113/jphysiol.2005.087288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Baeken C, De Raedt R, Van Schuerbeek P, et al. Right prefrontal HF-rTMS attenuates right amygdala processing of negatively valenced emotional stimuli in healthy females. Behav Brain Res. 2010;214:450–5. doi: 10.1016/j.bbr.2010.06.029. [DOI] [PubMed] [Google Scholar]
- 64.Ghashghaei HT, Barbas H. Pathways for emotion: interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience. 2002;115:1261–79. doi: 10.1016/s0306-4522(02)00446-3. [DOI] [PubMed] [Google Scholar]
- 65.Barbas H. Anatomic basis of cognitive-emotional interactions in the primate prefrontal cortex. Neurosci Biobehav Rev. 1995;19:499–510. doi: 10.1016/0149-7634(94)00053-4. [DOI] [PubMed] [Google Scholar]
- 66.Rainville P, Bechara A, Naqvi N, et al. Basic emotions are associated with distinct patterns of cardiorespiratory activity. Int J Psychophysiol. 2006;61:5–18. doi: 10.1016/j.ijpsycho.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 67.Blechert J, Lajtman M, Michael T, et al. Identifying anxiety states using broad sampling and advanced processing of peripheral physiological information. Biomed Sci Instrum. 2006;42:136–41. [PubMed] [Google Scholar]
- 68.Blechert J, Michael T, Grossman P, et al. Autonomic and respiratory characteristics of posttraumatic stress disorder and panic disorder. Psychosom Med. 2007;69:935–43. doi: 10.1097/PSY.0b013e31815a8f6b. [DOI] [PubMed] [Google Scholar]