Abstract
Nitric oxide (NO) has many biological roles (e.g., antimicrobial agent, promoter of angiogenesis, prevention of platelet activation, etc.) that make NO releasing materials desirable for a variety of biomedical applications. Localized NO release can be achieved from biomedical grade polymers doped with diazeniumdiolated dibutylhexanediamine (DBHD/N2O2) and poly(lactic-co-glycolic acid) (PLGA). In this study, the optimization of this chemistry to create film/patches that can be used to decrease microbial infection at wound sites is examined. Two polyurethanes with different water uptakes (Tecoflex SG-80A (6.2 ± 0.7 wt %) and Tecophillic SP-60D-20 (22.5 ± 1.1 wt%)) were doped with 25 wt% DBHD/N2O2 and 10 wt% of PLGA with various hydrolysis rates. Films prepared with the polymer that has the higher water uptake (SP-60D-20) were found to have higher NO release and for a longer duration than the polyurethane with lower water uptake (SG-80A). The more hydrophilic polymer enhances the hydrolysis rate of the PLGA additive, thereby providing a more acidic environment that increases the rate of NO release from the NO donor. The optimal NO releasing and control SG-80A patches were then applied to scald burn wounds that were infected with Acinetobacter baumannii. The NO released from these patches applied to the wounds is shown to significantly reduce the A. baumannii infection after 24 h (~4 log reduction). The NO release patches are also able to reduce the TGF-β levels, in comparison to controls, which can enhance reepithelialization, decrease scarring, and reduce migration of bacteria. The combined DBHD/N2O2 and PLGA-doped polymer patches, which could be replaced periodically throughout the wound healing process, demonstrate the potential to reduce risk of bacterial infection and promote the overall wound healing process.
Keywords: Antimicrobial, Burn Wounds, Diazeniumdiolates, Poly(lactic-co-glycolic acid), Nitric Oxide
1. Introduction
Bacterial infection and biofilm formation is a significant problem with a variety of biomedical devices that can lead to complications, increased medical costs, and increased morbidity [1]. Indwelling medical devices are responsible for more than one million hospital acquired infections, resulting in 99,000 deaths per year in the United States [2, 3]. Another significant area of infection is in wounds. More than one million burn injuries are reported annually in the US [4], resulting in 3,500 deaths per year [5]. Complications of wound infection are also significant and include delayed wound healing, tissue necrosis, spread of infection to the bloodstream and other organs, and transmission of wound-associated bacteria to other patients in hospitals [6]. Treatment of these infections often includes antibiotics and other antimicrobial agents, such as silver [7]. However, these materials often fail to prevent the infection and there is a growing concern for their use due to the emergence of bacterial resistance to antibiotics and antimicrobial agents [7-9]. Recent findings have also suggested that silver delays the wound-healing process and may have serious cytotoxic effects [10]. Acinetobacter baumannii is one such bacterial strain that has developed extensive antimicrobial resistance, and it also forms biofilms that are resistant to host defenses and antimicrobial treatment [11]. A. baumannii has been named a “new enemy” [12] due to large outbreaks in intensive care units [13-17] and also is a dominant organism isolated from wound infections (e.g., troops injured in Afghanistan and Iraq) [11, 18, 19].
Among its many biological roles, nitric oxide (NO) is known as a potent antimicrobial agent and an accelerant to the wound healing process [1]. Nitric oxide is endogenously synthesized by nitric oxide synthase enzymes (NOS): endothelial (eNOS), neuronal (nNOS), and inducible (iNOS). The iNOS is capable of producing high levels of NO [20] and micromolar concentrations of NO are known to have cytotoxic effects [21-23]. Reactive oxygen species (such as superoxide (O2−), hydrogen peroxide (H2O2), and hydroxyl radical (OH)) and reactive nitrogen species (such as NO, N2O3, and peroxynitrite (OONO−)) are generated by the iNOS and phagocyte oxidase pathways and are responsible for the antimicrobial effects observed due to their interactions with thiols, proteins, DNA, and lipids [20]. The broad-spectrum antibacterial properties of NO against a wide range of microbes have been demonstrated, showing that both gram-positive and gram-negative bacteria can be killed [24]. In addition, bacteria have the ability to form biofilms (communities of bacteria encased in a self-synthesized extracellular matrix), which is one of the mechanisms that bacteria use to survive in adverse environments [25-28]. Indeed, formation of biofilms protects bacteria from antiseptics, antibiotics, and host defenses, making the infections difficult to eradicate [29]. Evidence suggests that biofilms also play a role in wound infections, which may explain the chronic nature of many wounds infections and their resistance to antimicrobial therapy [30]. Low nM concentrations of NO have been shown to be efficient at dispersing biofilms of various bacterial strains [31-34]. Lu et al. also reported a 5 log reduction in Pseudomonas aeruginosa biofilms using diazeniumdiolate-functionalized chitosan oligosaccharides as the NO donor.[35] Therefore, NO releasing materials have great potential in biomedical applications, especially to reduce the risk of infection, promote wound healing, and improve biocompatibility of implantable medical devices [34, 36-38].
Due to the potential benefits of NO release, a wide variety of NO releasing polymers have been reported in the literature, and many of these are summarized in a recent review by Carpenter and Schoenfisch [39]. Materials with short durations of NO release may have potential wound healing applications due to the ease of replacing the material periodically throughout the wound healing process. The NO released from these materials may also decrease the risks of infected wounds, thereby reducing the wound healing time and repair chronic wounds [40]. Gaseous nitric oxide treatments and NO releasing materials have been used topically and shown to increase dermal blood flow, increase reepithelialization and angiogenesis, and accelerate wound repair; however, some of these studies have been conducted with uninfected wounds [41-45]. Previous studies have shown NO can be released from polymer films doped with diazeniumdiolate dibutylhexanediamine (DBHD/N2O2), which releases NO through proton or thermal driven mechanisms [46-49]. However, the loss of NO from DBHD/N2O2 creates free lipophilic amine species within the polymer that react with water, thereby increasing the pH within the polymer phase and effectively turning off the NO release. In a recent report, poly(lactic-co-glycolic) acid was used as an additive to promote and prolong the NO release from poly(vinyl chloride) films doped with DBHD/N2O2 [49, 50]. The ester linkages of the PLGA will hydrolyze in the presence of water, producing lactic and glycolic acids that can act as proton sources to promote the NO release from DBHD/N2O2-doped polymers. PLGAs can have varying hydrolysis rates, which is primarily determined by the copolymer ratio, the end group chemistry (either a free carboxylic acid or ester end group), and molecular weight. Lactate has been shown to enhance angiogenesis and accelerate wound healing [51], so any lactic acid monomers that leach from the NO releasing patches may also prove beneficial. Previous work with DBHD/N2O2-based films have primarily utilized hydrophobic polymers (e.g., PVC) as the base polymer [46, 49]. In this study, we compared the effects of the base polymer, in terms of their water uptake property, on the NO release from polymer films doped with DBHD/N2O2 and PLGA. The optimal formulation was then utilized to create NO releasing patches (and corresponding controls) that were applied to partial thickness scald burn wounds in a mouse model that were infected with A. baumannii to observe effects of such NO release patches on bacterial growth and TGF-β levels in the wounds after 24 h.
2. Materials and Methods
2.1. Materials
Tecoflex SG-80A and Tecophilic SP-60D-20 were purchased from Lubrizol Advanced Materials Inc. (Cleveland, OH). Anhydrous tetrahydrofuran (THF), anhydrous acetonitrile, sodium chloride, potassium chloride, sodium phosphate dibasic, and potassium phosphate monobasic were products of Sigma-Aldrich Chemical Company (St. Louis, MO). Poly(D,L-lactide-co-glycolide) 5050DLG1A (1-2 week hydrolysis rate), 5050DLG7E (1-2 month hydrolysis rate), and 6535DLG7E (3-4 month hydrolysis rate) were obtained from SurModics Pharmaceuticals Inc. (Birmingham, AL). N,N’-Dibutyl-1,6-hexanediamine (DBHD) was purchased from Alfa Aesar (Ward Hill, MA). DBHD/N2O2 was synthesized by treating DBHD with 80 psi NO gas purchased from Cryogenic Gases (Detroit, MI) at room temperature for 48 h, as previously described [46]. Phosphate buffered saline (PBS), pH 7.4, containing 138 mM NaCl, 2.7 mM KCl, and 10 mM sodium phosphate was used for all in vitro experiments.
2.2. Preparation of NO releasing films and patches
The focus of this study was to compare the effects of polymer water uptake on the NO release properties from DBHD/N2O2 and PLGA-doped within these base polymers (SG-80A or SP-60D-20). A variety of films (dia. = 2.5 cm) were prepared for the initial NO release profile studies, and ultimately larger patches (5 cm × 6 cm) were prepared for application on the mouse wounds. The PLGA additives used were 5050DLG1A (1-2 week hydrolysis rate), 5050DLG7E (1-2 month hydrolysis rate), and 6535DLG7E (3-4 month hydrolysis rate). The product names identify the copolymer ratio, inherent viscosity (used to determine the molecular weight), and the end group type (acid or ester), which are the main factors that determine the hydrolysis rate of the PLGA. For example, the 5050DLG7E is a PLGA with 50 mol% DL-lactide, 50 mol% glycolide, an inherent viscosity of 0.7 dLg−1, and has an ester end group (‘E’). A variety of NO releasing films were prepared via a solvent evaporation method using either SG-80A or SP-60D-20 polyurethanes as the base polymer, while keeping the amount of DBHD/N2O2 and PLGA constant at 25 wt% and 10 wt%, respectively. The NO releasing films consisting of 25 wt% DBHD/N2O2, 10 wt% PLGA, and 65 wt% polyurethane were prepared by dissolving 80 mg PLGA, 200 mg DBHD/N2O2, and 520 mg polyurethane in 5 mL THF. This solution was cast in Teflon rings (dia. = 2.5 cm) and cured under ambient conditions for 2 d. Disks (dia. = 0.9 cm) were cut from the parent films and dip-coated 4 times in a top-coat solution (550 mg of the respective polyurethane in 7.5 mL THF). Four top coat layers of the polymer (without additives) were added to minimize the amounts of DBHD/N2O2 and PLGA diffusing to the surface during this process. The polymer top coats were employed for three main reasons: (1) to prevent leaching of DBHD/N2O2; (2) to neutralize the surface charge; and (3) to yield a smoother finish to the surface.
The patches for the in vivo studies were prepared in a similar manner and consisted of SG-80A doped with 25 wt% DBHD/N2O2 and 10 wt% 5050DLG1A. The active layer of the NO releasing patches were prepared by dissolving 1300 mg SG-80A, 200 mg 5050DLG1A, and 500 mg DBHD/N2O2 in 20 mL THF. This solution was cast in a Teflon mold (5 cm × 6 cm) and dried under ambient conditions overnight. The control patches were prepared in a similar manner with 5050DLG1A and DBHD amine (non-diazeniumdiolate) in the active layer. The control active layer consisted of 1410 mg SG-80A, 200 mg 5050DLG1A, and 390 mg DBHD amine dissolved in 20 mL THF and then cast in rings as described above for active patches. The patches were dip coated 4 times using the corresponding top-coating solution (750 mg SG-80A in 20 mL THF).
All films and patches were dried under ambient conditions overnight after the top-coating, followed by vacuum drying for 48 h. The final films and patches had a total thickness of ~1000 μm (~600 μm active layer and ~200 μm top-coat), as measured using a Mitutoyo digital micrometer (Metron Precision, Inc.).
2.3 Polymer water uptake
SG-80A and SP-60D-20 polymer films were prepared by the solvent casting method. Polymer solutions consisting of 200 mg polymer dissolved in 5 mL THF were cast in Teflon rings (d = 2.5 cm). Disks (d = 0.9 cm) were cut from the parent films, weighed, and immersed in PBS buffer for 48 h at 37°C. The wet films were wiped dry and weighed again. The water uptake of the polymer films are reported in weight percent as follows: water uptake (wt%) = ((Wwet –Wdry)/Wdry) × 100, where Wwet and Wdry are the weights of the wet and dry films, respectively.
2.4. NO release measurements
Nitric oxide released from the NO release patches was measured using a Sievers chemiluminescence Nitric Oxide Analyzer (NOA) 280 (Boulder, CO). Films were placed in the sample vessel immersed in PBS (pH 7.4). Nitric oxide was continuously purged from the sample vessel and swept from the headspace using a N2 sweep gas into the chemiluminescence detection chamber. Patches for the in vivo studies were wrapped in a moist Kim wipe and Tegaderm dressing (which was replaced daily to mimic the moist environment of the wound) and tested for in vitro NO release at 37°C.
2.5. In vitro zone inhibition test
Overnight LB (Luria Bertani) broth grown A. baumannii ATCC 17978 culture was washed with 1 × PBS buffer three times by centrifugation, and was then resuspended in 1 × PBS buffer to make a final cell concentration of approximately 105 CFU/mL. For the zone of inhibition, 50 μL of the 105 CFU/mL solution was plated onto LB agar plates. NO releasing and control patches (1 cm ×1 1 cm square) were placed on the agar plates and incubated at 37°C for 24 h. The zone of inhibition was made by estimating the inhibition zone as circles and measuring the distance from the edge of the sample to the nearest bacterial colony. All the experiments were conducted in triplicate.
2.6. Partial thickness scald burn model in mice
Mouse burn model
The animal handling and surgical procedures used in this study were approved by the University Committee on the Use and Care of Animals (UCUCA) in accordance with university and federal regulations. A total of 9 female pathogen-free C57BL/6 mice (Harlan, Indianapolis, IN), 9-10 weeks old, weighing ~17-23 grams each were used in this study. Mice were housed in standard cages at the University’s Unit for Laboratory Animal Medicine Facility and were allowed to acclimate for 7 d after delivery prior to the experiment. The animals were kept on a 12 h light cycle and were provided with rodent chow (LabDiet 5001, PMI Int’l., Richmond, IN) and water ad libitum throughout the study. Pentobarbital (Nembutal, Ovation Pharmaceuticals,Inc., Deerfield, IL, manufactured by Hospira, Lake Forest, IL) was administered intraperatonially (50 mg/kg IP) for anesthesia. The eyes of the animals were covered with sterile Altalube (Altaire Pharmaceuticals, Aquebogue, NY). During the study, all mice were singly housed and all received 0.1 mg/kg buprenorphine (Buprenex; Reckitt Benckiser Pharmaceuticals Inc., Richmond, VA) subcutaneously (SQ) twice daily for post-burn pain control.
The skin over the lumbrosacral and back region of the mice was clipped using a 35-W model 5-55E electrical clipper (Oyster-Golden A-S, Head no.80, blade size 50). To create the burn, anesthetized mice were placed in an insulated, custom-made mold that exposes only the lumbrosacral and back region that is approximately 30% of the total body area (calculated using Meeh’s formula [52]). Partial thickness burns were achieved by exposure of the skin to 60 °C water for 18 s. The burn was then wiped with sterile gauze. The burn sites were immediately inoculated with A. baumannii bacteria (200 μL of 106 CFU/mL) and covered with Tegaderm dressing (3M, Minneapolis, MN). Because the wounds were approximately 10 cm2, 200 μL was an appropriate volume of bacteria solution to completely cover the wound site. The mice were returned to cages in a 37°C incubator until fully ambulatory. Each mouse was given a 1 mL injection of 5% Dextrose and Lactated Ringer’s Injection (Baxter) IP and another 500 μl injection SQ on the back of its hind leg.
Application of NO release and control patches
The A. baumannii infection was allowed to grow in the wounds for a 24 h period prior to the application of the patches. The mice were divided into 3 groups receiving the following treatments: control (DBHD + PLGA patch), NO releasing patch, and control (no patch). NO release and control patches were soaked in sterile saline for approximately 15 min before attachment. Soaking the patches in saline solution helps initiate the PLGA hydrolysis and NO release reactions. The Tegaderm dressing was removed, patches were applied to the wounds, and fresh Tegaderm was used to cover and hold the patch in place on the wound. The Tegaderm was removed and replaced for mice that did not receive the patch treatment.
Tissue collection
At the time-point for tissue harvest (24 h after NO release and control patch application) the mice were given IP injections of pentobarbital (100 mg/kg) and exsanguinated followed by a bilateral pneumothorax. Skin samples were collected for bacterial counts, slides/staining, and mRNA isolation for TGF-β PCR. For the bacterial counts, skin tissue samples were excised and weighed, homogenized in 2 mL of PBS for 30 s, and cultured to determine the number of living A. baumannii microorganisms. Plate counting was conducted with LB agar plates.
The relative TGF-β mRNA levels were determined using TGF-β1 and GAPDH primers previously reported [53, 54]. The skin samples were immediately frozen in liquid nitrogen and stored overnight at −80°C. Samples were thawed briefly and homogenized in TRizol (Invitrogen, Carlsbad, CA) and RNA was extracted according to the manufacturer’s instructions. RNA (2 μg) was utilized to make cDNA using the ABI High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). Two hundred ng of cDNA was used to perform PCR with iQ SYBRGreen Supermix (Bio-Rad, Hercules, CA) on an Eppendorf Mastercycler epgradient S realplex 4 thermocycler (Eppendorf North America, Hauppauge, NY). After an initial denaturation for 2 min at 95 °C, samples were subjected to 50 cycles of 95 °C for 20 s, annealing at 60°C for 30 s, and extension at 72 °C for 20 s.
2.7. Statistical analysis
Data are expressed as mean ± SEM (standard error of the mean). Comparison of results between the control and NO releasing patches were analyzed by a comparison of means using Student’s t-test. Values of p < 0.05 were considered statistically significant for all tests.
3. Results and Discussion
3.1 In vitro NO release measurements from films and patches
Diazeniumdiolates are a group of widely studied NO donor molecules that release NO through proton or thermal driven mechanisms [47-49]. Previous studies have shown NO can be released from polymer films doped with DBHD/N2O2 [46, 49]. Poly(lactic-co-glycolic) acid additives have been shown recently help promote and prolong the NO release from poly(vinyl chloride) films doped with DBHD/N2O2 [49]. The ester linkages of the PLGA will hydrolyze in the presence of water, producing lactic and glycolic acids that can act as a proton source to sustain the NO release from DBHD/N2O2-doped polymers. Previous work with DBHD/N2O2 films have primarily utilized hydrophobic polymers (e.g., PVC) as the base polymer [46, 49]; however, in this study we compared the effects of the base polymer, in terms of their water uptake property, on the NO release from combined DBHD/N2O2 and PLGA-doped films. In order to measure the water uptake of the two base polymers used in this study, films of the polymers were cast without any additives. The water uptake was then determined by the weight difference of the polymer film before and after soaking in PBS at 37°C for 48 h. As shown in Table 1, the Tecoflex SG-80A polymer is more hydrophobic and has a significantly lower water uptake than the Tecophillic SP-60D-20.
Table 1.
The water uptake of SG-80A and SP-60D-20 polyurethanes. Polymer films were weighed prior to immersing in PBS for 48 h at 37°C. The wet films were wiped dry and weighed again. The water uptake of the polymer films are reported in weight percent as follows: water uptake (wt%) = (Wwet – Wdry)/Wdry × 100, where Wwet and Wdry are the weights of the wet and dry films, respectively.
| Polymer | Water Uptake (wt%) |
|---|---|
| SG80A | 6.2 ± 0.7 |
| SP-60D-20 | 22.5 ± 1.1 |
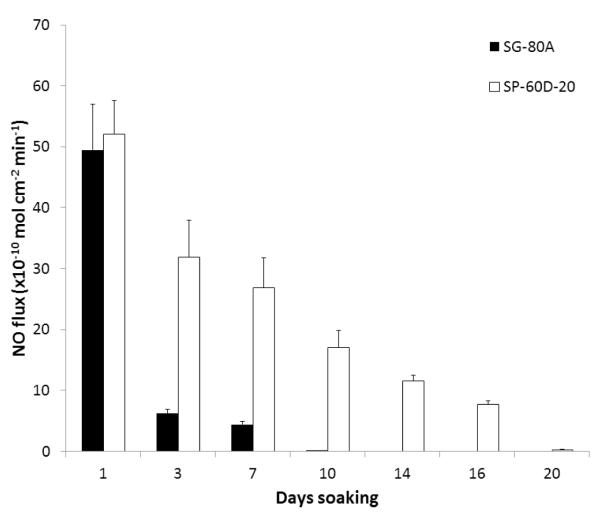
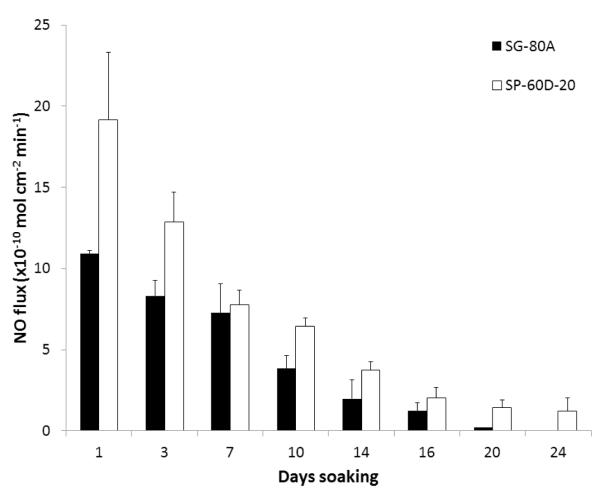
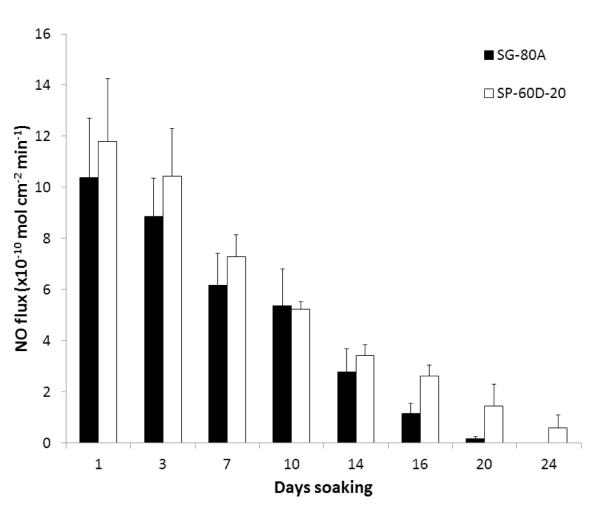
The NO releasing films and patches used in this study were prepared using a two layer configuration, top-coat and active coat, as shown in Fig. 1. The active coat was doped with 25 wt% DBHD/N2O2 and 10 wt% PLGA (either 5050DLG1A, 5050DLG7E, or 6535DLG7E). The NO release from these films was measured using a chemiluminescene NO analyzer at 37°C while immersed in PBS buffer. The films doped with 5050DLG1A have a significant burst of NO during the first day of soaking (see Fig. 2). This high initial burst can be attributed to the higher residual acid content (from the carboxylic end groups and residual monomers). The NO release rapidly decreases by day 3 for the SG-80A films, due to the low water uptake of this polymer, which slows the PLGA hydrolysis rate. The films made with SP-60D-20 continue to release higher levels of NO for up to 16 d. SP-60D-20 has a higher water uptake, which continues to allow water to diffuse into the film and promote PLGA hydrolysis and NO release. In contrast, the films doped with the ester-capped PLGAs (either 5050DLG7E or 6535DLG7E) do not exhibit an initial burst during the first day (Figs. 3 and 4). The SP-60D-20 based films have higher NO release that has a longer duration than the corresponding SG-80A based films. This trend is most noticeable in the films doped with PLGAs that have lower hydrolysis rates (5050DLG1A and 5050DLG7E). The higher water uptake of the SP-60D-20 polymer facilitates the PLGA hydrolysis, which then continues to promote NO release.
Fig. 1.
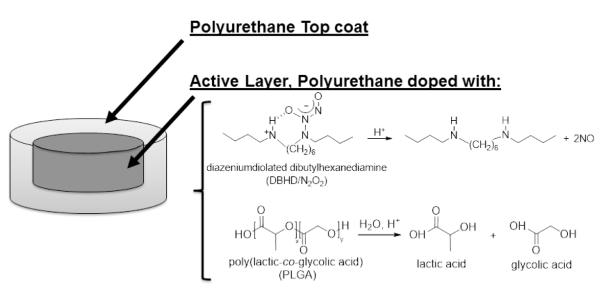
Diagram of the polyurethane (SG-80Aor SP-60D-20) based films/patches consisting of an active layer, doped with a lipophilic DBHD/N2O2 and PLGA additive, and a top coat of the corresponding polyurethane.
Fig. 2.
NO surface flux from SG-80A and SP-60D-20 films doped with 25 wt% DBHD/N2O2 and 10 wt% 5050DLG1A (1-2 week hydrolysis rate) PLGA additives. Films were incubated in PBS buffer at 37°C during the testing period. The data are means ± SEM (n=3).
Fig. 3.
NO surface flux from SG-80A and SP-60D-20 films doped with 25 wt% DBHD/N2O2 and 10 wt% 5050DLG7E (1-2 month hydrolysis rate) PLGA additives. Films were incubated in PBS buffer at 37°C during the testing period. The data are means ± SEM (n=3).
Fig. 4.
NO surface flux from SG-80A and SP-60D-20 films doped with 25 wt% DBHD/N2O2 and 10 wt% 6535DLG7E (3-4 month hydrolysis rate) PLGA additives. Films were incubated in PBS buffer at 37°C during the testing period. The data are means ± SEM (n=3).
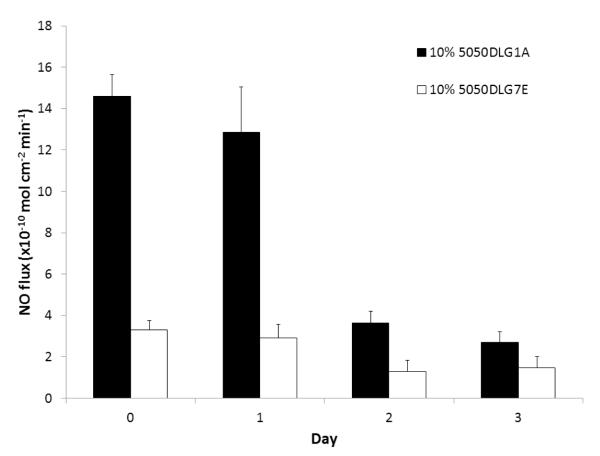
Although these NO releasing polymers have a wide variety of potential biomedical applications requiring various levels and duration of NO release [39], in this study we examined their potential use to reduce infection in burn wounds. For wound healing applications, the NO release from patches can have short durations because new patches could be applied daily. The polyurethanes used in this study are not typically used for wound healing applications, but we used these polyurethanes as an example of how to deliver NO to the wound site. The NO donor can be incorporated into other polymers as well for wound healing applications, but the polymers should not have too high a water uptake, otherwise there is risk of losing the free DBHD amine formed into the wound site. To choose the appropriate polymer composition for the in vivo studies, patches were prepared with Tecoflex SG-80A as the base polymer (due to the lower initial burst observed on the first day of soaking) and doped with 25 wt% DBHD/N2O2 and 10 wt% PLGA (either 5050DLG1A or 5050DLG7E). In order to mimic the moist environment of the wounds, NO releasing patches were wrapped with a moist Kim wipe and Tegaderm dressing, and tested at 37°C for their NO release. The patches doped with 5050DLG1A maintained a NO flux in the range of 12.8-14.5 × 10−10 mol cm−2 min−1 for the first 24 h under physiological conditions (Fig. 5). In contrast, the patches doped with the 5050DLG7E exhibit a significantly lower flux of ~3 × 10−10 mol cm−2 min−1 under the same moist conditions and this flux is also sustained for the first 24 h. The NO release from both patches begins to diminish on the third day due to the accumulation DBHD amine and the concomitant slower hydrolysis of the PLGA additive (from the reduced amount of water that diffuses into the polymer). For the in vivo studies, the SG-80A patch type doped with 25 wt% DBHD/N2O2 and 10 wt% 5050DLG1A was selected due to its higher NO release.
Fig. 5.
NO release from SG-80A patched doped with 25 wt% DBHD/N2O2 and 10 wt% of either 5050DLG1A (1-2 week hydrolysis rate) or 5050DLG7E (1-2 month hydrolysis rate) PLGA additives. Films were wrapped in moist Kim wipes and Tegederm dressing at 37°C during the testing period. The data are means ± SEM (n=3).
3.2. Effects of NO and control patches on zone inhibition and bacteria count in mouse burn model
The NO releasing (SG-80A doped with 25 wt% DBHD/N2O2 and 10 wt% 5050DLG1A) and control (SG-80A doped with 20 wt% DBHD amine and 10 wt% 5050DLG1A) used for the in vitro zone inhibition test and in vivo studies were prepared as described in Section 2.2. The control patches were prepared with equal moles of the DBHD amine, where the additional weight from the mass of the diaziniumdiolate NONO group was compensated by additional SG-80A, in order to observe the effects of the NO release vs. control. Prior to the in vivo experiments, the NO releasing and control patches were tested in vitro for their zone of inhibition. A. baumanii was spread on agar plates and the patches (1 cm × 1 cm square) were placed in the center of the plate. After a 24 h incubation at 37 °C, the NO release patch created a zone of inhibition that had a diameter of ca. 4.3 ± 0.9 cm. The control patch showed no zone of inhibition, where only the bacteria underneath the patch were slightly suppressed. This zone of inhibition test mimics the nutrient environment of the wound and demonstrates that the NO released from patches has the potential to diffuse in and around the wound site, and ultimately reduce the bacteria and infection.
3.3 Effects of NO and control patches on bacteria counts and TGF-β mRNA in mouse burn model
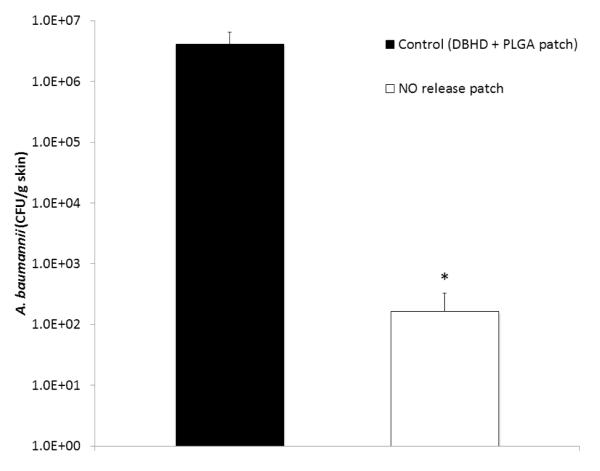
A mouse burn model was used to observe the effects of the NO releasing patch on an infected wound. Partial thickness scald burn wounds on mice were inoculated with A. baumannii and covered with Tegaderm dressing. The bacteria were allowed to grow in the wound for 24 h prior to the application of NO release patches, control patches, or control (no patch). After 24 h of patch or control treatment, skin tissue was harvested, homogenized in PBS, and grown on agar plates to assess the effects of NO on bacterial growth in the wounds. As shown in Fig. 6, the NO releasing patches significantly reduced the amount of A. baumannii bacteria present in the wounds after 24 h application (~ 4 log reduction) in comparison to the control patches (which did not have NO release). The wounds that receive the control treatment of no patch (only Tegaderm dressing covering the wound) had similar bacteria counts as the other control patch group (data not shown). Part of the reduced bacteria counts can be attributed to neutrophil infiltration, which happens in the case of all treatment groups in this study. However, the additional NO that is supplied by the NO releasing patches has the potential to improve the overall healing of the wound by reducing the infection and bacterial growth.
Fig. 6.
Plate counting of A. baumannii cells on the wounded skin of mice after 24 h application of SG-80A based NO releasing and control patches. NO releasing and control patches were applied to wounds 24 h after inoculation with A. baumannii. After 24 h, skin tissue was harvested, homogenized, serially diluted, and grown on agar plates. The data are means ± SEM (n=3). * = p <
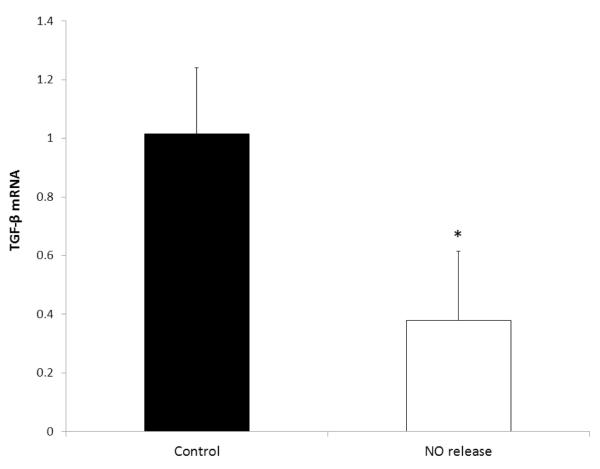
Burn wounds have been shown to have increased TGF-β mRNA levels, which contributes to immunosuppression [55], impairs humoral immunity (antibody generation) [56], and contributes to scar formation [57]. In this study, the harvested skin tissue was also assessed for the expression of TGF-β mRNA levels using RT-PCR. The wounds with the NO releasing patches created a significant reduction in TGF-β levels in comparison to the control patches (Fig. 7). This reduction may be due to the inhibition of T cell proliferation by NO [58]. Reduction of TGF-β has been shown to enhance reepithelialization, decrease post-burn scarring, and reduce trans-epithelial migration of bacteria [59, 60]. In addition to the promising bacteria and TGF-β results, the UM pathology report indicated that the NO releasing patches did not worsen the injury and indicated that there was less overall damage in the wounds in comparison to the controls. Nitric oxide has many biological roles, including reducing bacterial infection and decreasing TGF-β mRNA levels as addressed here, and both processes can be quite beneficial to the wound healing process [39]. The nitric oxide releasing patches used in this study could be replaced daily, in order to maintain consistent NO delivery to the wound site.
Fig. 7.
Expression of TGF-β mRNA after application of the SG-80A based NO releasing and control patches. RNA was extracted from the homogenized skin tissue and the expression of TGF-β was determined using RT-PCR and expressed as the ratio to that of untreated mice. The data are means ± SEM (n=3). * = p < 0.05.
4. Conclusions
In summary, this study demonstrates that the water uptake properties of the base polymer can be used to further control the NO release rates from polymeric films/patches doped with DBHD/N2O2 and PLGA. Films prepared with a more hydrophobic polyurethane (SG-80A) exhibit NO release that is lower and shorter in duration than the polyurethane with 20% water uptake (SP-60D-20). The more hydrophilic base polymer increases the rate of hydrolysis of the PLGA additive, supplying more protons locally within the polymeric phase that thereby increase the NO release rate. These new DBHD/N2O2 and PLGA-doped SG-80A patches demonstrate the potential to improve the healing of burn wounds by reducing the bacterial infection. Indeed, the NO released from the patches is clearly shown to be able to significantly reduce the A. baumannii infection after 24 h application to scald burn wounds. The NO release patches are also shown to be able to reduce the TGF-β levels, in comparison to controls, and this species has been reported to enhance reepithelialization, decrease scarring, and reduce migration of bacteria. The novel NO releasing patches developed here could be replaced frequently throughout the duration of the wound healing process in order to further promote and expedite the healthy wound healing process by maintaining exposure of the wound to a higher flux rate of NO. This study demonstrated that NO releasing polymers can be used to kill antibiotic resistant bacteria (A. baumannii) in burn wounds. A more elaborate study comparing the overall healing process of these new NO releasing polymers to appropriate controls, including silver-based materials, is a future direction and currently under investigation.
Acknowledgements
The authors declare this work is supported by the National Institutes of Health, Grants EB000783, and K25HL111213.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Halpenny GM, Mascharak PK. Emerging antimicrobial applications of nitric oxide (NO) and NO-releasing materials. Anti-Infect Agents Med Chem. 2010;9:187–97. [Google Scholar]
- [2].Darouiche RO. Treatment of infections associated with surgical implants. New Engl J Med. 2004;350:1422–9. doi: 10.1056/NEJMra035415. [DOI] [PubMed] [Google Scholar]
- [3].Klevens RM, Edwards JR, Richards CL, Horan TC, Gaynes RP, Pollock DA, et al. Estimating health care-associated infections and deaths in US hospitals, 2002. Public health reports. 2007;122:160. doi: 10.1177/003335490712200205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Runyan CW, Casteel C, Perkis D, Black C, Marshall SW, Johnson RM, et al. Unintentional injuries in the home in the United States: Part I: Mortality. Am J Prev Med. 2005;28:73–9. doi: 10.1016/j.amepre.2004.09.010. [DOI] [PubMed] [Google Scholar]
- [5].Horan JM, Mallonee S. Injury surveillance. Epidemiol Rev. 2003;25:24–42. doi: 10.1093/epirev/mxg010. [DOI] [PubMed] [Google Scholar]
- [6].Moran KA, Murray CK, Anderson EL. Bacteriology of blood, wound, and sputum cultures from non-US casualties treated in a combat support hospital in Iraq. Infect Control Hosp Epidemiol. 2008;29:981–4. doi: 10.1086/591034. [DOI] [PubMed] [Google Scholar]
- [7].Hospenthal DR, Murray CK, Andersen RC, Blice JP, Calhoun JH, Cancio LC, et al. Guidelines for the prevention of infection after combat-related injuries. J Trauma Acute Care Surg. 2008;64:S211–S20. doi: 10.1097/TA.0b013e318163c421. [DOI] [PubMed] [Google Scholar]
- [8].Fischbach MA, Walsh CT. Antibiotics for emerging pathogens. Science. 2009;325:1089–93. doi: 10.1126/science.1176667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Taubes G. The bacteria fight back. Science. 2008;321:356. doi: 10.1126/science.321.5887.356. [DOI] [PubMed] [Google Scholar]
- [10].Atiyeh BS, Costagliola M, Hayek SN, Dibo SA. Effect of silver on burn wound infection control and healing: review of the literature. Burns. 2007;33:139–48. doi: 10.1016/j.burns.2006.06.010. [DOI] [PubMed] [Google Scholar]
- [11].Dallo SF, Weitao T. Insights into acinetobacter war-wound infections, biofilms, and control. Adv Skin Wound Care. 2010;23:169–74. doi: 10.1097/01.ASW.0000363527.08501.a3. [DOI] [PubMed] [Google Scholar]
- [12].Towner K. Acinetobacter: an old friend, but a new enemy. J Hosp Infect. 2009;73:355–63. doi: 10.1016/j.jhin.2009.03.032. [DOI] [PubMed] [Google Scholar]
- [13].Beavers SF, Blossom DB, Wiemken TL, Kawaoka KY, Wong A, Goss L, et al. Comparison of risk factors for recovery of Acinetobacter baumannii during outbreaks at two Kentucky hospitals, 2006. Public Health Rep. 2009;124:868. doi: 10.1177/003335490912400615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Vorgias G, Iavazzo C, Makarova E, Akrivos T, Falagas ME. Infections caused by Acinetobacter baumannii susceptible only to polymyxin in a gynecologic oncology unit. Int J Gynaecol Obstet. 2009;105:264. doi: 10.1016/j.ijgo.2009.01.032. [DOI] [PubMed] [Google Scholar]
- [15].Supp DM, Gardner J, Klingenberg JM, Neely AN. Antibiotic resistance in clinical isolates of Acinetobacter baumannii, Pseudomonas aeruginosa, and Staphylococcus aureus does not impact sensitivity to human beta defensin 4. Burns. 2009;35:949–55. doi: 10.1016/j.burns.2009.02.016. [DOI] [PubMed] [Google Scholar]
- [16].Alişkan H, Colakoğlu S, Turunç T, Demiroğlu Y, Erdoğan F, Akin S, et al. Four years of monitoring of antibiotic sensitivity rates of Pseudomonas aeruginosa and Acinetobacter baumannii strains isolated from patients in intensive care unit and inpatient clinics. Mikrobiyol Nul. 2008;42:321. [PubMed] [Google Scholar]
- [17].Babik J, Bodnárová L, Sopko K. Acinetobacter: serious danger for burn patients. Acta Chir Plast. 2008;50:27–33. [PubMed] [Google Scholar]
- [18].Calhoun JH, Murray CK, FISDA M. Multidrug-resistant organisms in military wounds from Iraq and Afghanistan. Clin Orthop Relat Res. 2008;466:1356–62. doi: 10.1007/s11999-008-0212-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Keen EF, Robinson BJ, Hospenthal DR, Aldous WK, Wolf SE, Chung KK, et al. Incidence and bacteriology of burn infections at a military burn center. Burns. 2010;36:461–8. doi: 10.1016/j.burns.2009.10.012. [DOI] [PubMed] [Google Scholar]
- [20].Fang FC. Antimicrobial reactive oxygen and nitrogen species: concepts and controversies. Nat Rev Micro. 2004;2:820–32. doi: 10.1038/nrmicro1004. [DOI] [PubMed] [Google Scholar]
- [21].Denicola A, Freeman BA, Trujillo M, Radi R. Peroxynitrite reaction with carbon dioxide/bicarbonate: kinetics and influence on peroxynitrite-mediated oxidations. Arch Biochem Biophys. 1996;333:49–58. doi: 10.1006/abbi.1996.0363. [DOI] [PubMed] [Google Scholar]
- [22].Wink DA, Mitchell JB. Chemical biology of nitric oxide: insights into regulatory, cytotoxic, and cytoprotective mechanisms of nitric oxide. Free Radic Biol Med. 1998;25:434–56. doi: 10.1016/s0891-5849(98)00092-6. [DOI] [PubMed] [Google Scholar]
- [23].Fang FC. Mechanisms of nitric oxide-related antimicrobial activity. J Clin Invest. 1997;99:2818–25. doi: 10.1172/JCI119473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Raulli R, McElhaney-Feser G, Hrabie J, Cihlar R. Antimicrobial properties of nitric oxide using diazeniumdiolates as the nitric oxide donor. Recent Res Dev Microbiol. 2002;6:177–83. [Google Scholar]
- [25].Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM. Microbial biofilms. Annu Rev Microbiol. 1995;49:711–45. doi: 10.1146/annurev.mi.49.100195.003431. [DOI] [PubMed] [Google Scholar]
- [26].Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nature Rev Microbiol. 2004;2:95–108. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- [27].Parsek MR, Singh PK. Bacterial biofilms: an emerging link to disease pathogenesis. Annu Rev Microbiol. 2003;57:677–701. doi: 10.1146/annurev.micro.57.030502.090720. [DOI] [PubMed] [Google Scholar]
- [28].O’Toole G, Kaplan HB, Kolter R. Biofilm formation as microbial development. Annu Rev Microbiol. 2000;54:49–79. doi: 10.1146/annurev.micro.54.1.49. [DOI] [PubMed] [Google Scholar]
- [29].Fux C, Costerton J, Stewart P, Stoodley P. Survival strategies of infectious biofilms. TIM. 2005;13:34–40. doi: 10.1016/j.tim.2004.11.010. [DOI] [PubMed] [Google Scholar]
- [30].Wolcott RD, Ehrlich GD. Biofilms and chronic infections. JAMA. 2008;299:2682–4. doi: 10.1001/jama.299.22.2682. [DOI] [PubMed] [Google Scholar]
- [31].McDougald D, Rice SA, Barraud N, Steinberg PD, Kjelleberg S. Should we stay or should we go: mechanisms and ecological consequences for biofilm dispersal. Nat Rev Micro. 2011;10:39–50. doi: 10.1038/nrmicro2695. [DOI] [PubMed] [Google Scholar]
- [32].Barraud N, Storey MV, Moore ZP, Webb JS, Rice SA, Kjelleberg S. Nitric oxide-mediated dispersal in single- and multi-species biofilms of clinically and industrially relevant microorganisms. J Microbial Biotech. 2009;2:370–8. doi: 10.1111/j.1751-7915.2009.00098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Schmidt I, Steenbakkers PJ, op den Camp HJ, Schmidt K, Jetten MS. Physiologic and proteomic evidence for a role of nitric oxide in biofilm formation by Nitrosomonas europaea and other ammonia oxidizers. J Bacteriol. 2004;186:2781–8. doi: 10.1128/JB.186.9.2781-2788.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Cai W, Wu J, Xi C, Ashe Iii AJ, Meyerhoff ME. Carboxyl-ebselen-based layer-by-layer films as potential antithrombotic and antimicrobial coatings. Biomaterials. 2011;32:7774–84. doi: 10.1016/j.biomaterials.2011.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Lu Y, Slomberg DL, Schoenfisch MH. Nitric oxide-releasing chitosan oligosaccharides as antibacterial agents. Biomaterials. 2014;35:1716–24. doi: 10.1016/j.biomaterials.2013.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Nablo BJ, Rothrock AR, Schoenfisch MH. Nitric oxide-releasing sol–gels as antibacterial coatings for orthopedic implants. Biomaterials. 2005;26:917–24. doi: 10.1016/j.biomaterials.2004.03.031. [DOI] [PubMed] [Google Scholar]
- [37].Jen MC, Serrano MC, van Lith R, Ameer GA. Polymer-based nitric oxide therapies: recent Insights for biomedical applications. Adv Funct Mater. 2012;22:239–60. doi: 10.1002/adfm.201101707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Wold KA, Damodaran VB, Suazo LA, Bowen RA, Reynolds MM. Fabrication of biodegradable polymeric nanofibers with covalently attached NO donors. ACS Appl Mater Interfaces. 2012;4:3022–30. doi: 10.1021/am300383w. [DOI] [PubMed] [Google Scholar]
- [39].Carpenter AW, Schoenfisch MH. Nitric oxide release: Part II. Therapeutic applications. Chem Soc Rev. 2012;41:3742–52. doi: 10.1039/c2cs15273h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Lopez-Jaramillo P, Ruano C, Rivera J, Teran E, Salazar-Irigoyen R, Esplugues JV, et al. Treatment of cutaneous leishmaniasis with nitric-oxide donor. Lancet. 1998;351:1176–7. doi: 10.1016/s0140-6736(05)79119-4. [DOI] [PubMed] [Google Scholar]
- [41].Seabra AB, Pankotai E, Feher M, Somlai A, Kiss L, Biro L, et al. S-nitrosoglutathione-containing hydrogel increases dermal blood flow in streptozotocin-induced diabetic rats. Br J Dermatol. 2007;156:814–8. doi: 10.1111/j.1365-2133.2006.07718.x. [DOI] [PubMed] [Google Scholar]
- [42].Amadeu TP, Seabra AB, De Oliveira MG, Costa AMA. S-nitrosoglutathione-containing hydrogel accelerates rat cutaneous wound repair. J Eur Acad Dermatol. 2007;21:629–37. doi: 10.1111/j.1468-3083.2006.02032.x. [DOI] [PubMed] [Google Scholar]
- [43].Zhu HF, Wei XF, Bian K, Murad F. Effects of nitric oxide on skin burn wound healing. J Burn Care Res. 2008;29:804–14. doi: 10.1097/BCR.0b013e3181848119. [DOI] [PubMed] [Google Scholar]
- [44].Shekhter AB, Serezhenkov VA, Rudenko TG, Pekshev AV, Vanin AF. Beneficial effect of gaseous nitric oxide on the healing of skin wounds. Nitric Oxide. 2005;12:210–9. doi: 10.1016/j.niox.2005.03.004. [DOI] [PubMed] [Google Scholar]
- [45].Li Y, Lee PI. Controlled nitric oxide delivery platform based on S-nitrosothiol conjugated interpolymer complexes for diabetic wound healing. Mol Pharm. 2009;7:254–66. doi: 10.1021/mp900237f. [DOI] [PubMed] [Google Scholar]
- [46].Batchelor MM, Reoma SL, Fleser PS, Nuthakki VK, Callahan RE, Shanley CJ, et al. More lipophilic dialkyldiamine-based diazeniumdiolates: synthesis, characterization, and application in preparing thromboresistant nitric oxide release polymeric coatings. J Med Chem. 2003;46:5153–61. doi: 10.1021/jm030286t. [DOI] [PubMed] [Google Scholar]
- [47].Major TC, Brant DO, Reynolds MM, Bartlett RH, Meyerhoff ME, Handa H, et al. The attenuation of platelet and monocyte activation in a rabbit model of extracorporeal circulation by a nitric oxide releasing polymer. Biomaterials. 2010;31:2736–45. doi: 10.1016/j.biomaterials.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Davies KM, Wink DA, Saavedra JE, Keefer LK. Chemistry of the diazeniumdiolates. 2. Kinetics and mechanism of dissociation to nitric oxide in aqueous solution. J Amer Chem Soc. 2001;123:5473–81. doi: 10.1021/ja002899q. [DOI] [PubMed] [Google Scholar]
- [49].Handa H, Brisbois EJ, Major TC, Refahiyat L, Amoako KA, Annich GM, et al. In vitro and in vivo study of sustained nitric oxide release coating using diazeniumdiolate-doped poly(vinyl chloride) matrix with poly(lactide-co-glycolide) additive. J Mater Chem B. 2013;1:3578–87. doi: 10.1039/C3TB20277A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Handa H, Major TC, Brisbois EJ, Amoako KA, Meyerhoff ME, Bartlett RH. Hemocompatibility comparison of biomedical grade polymers using rabbit thrombogenicity model for preparing nonthrombogenic nitric oxide releasing surfaces. J Mater Chem B. 2014;2:1059–67. doi: 10.1039/C3TB21771J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Porporato PE, Payen VL, De Saedeleer CJ, Préat V, Thissen J-P, Feron O, et al. Lactate stimulates angiogenesis and accelerates the healing of superficial and ischemic wounds in mice. Angiogenesis. 2012;15:581–92. doi: 10.1007/s10456-012-9282-0. [DOI] [PubMed] [Google Scholar]
- [52].Gilpin D. Calculation of a new Meeh constant and experimental determination of burn size. Burns. 1996;22:607–11. doi: 10.1016/s0305-4179(96)00064-2. [DOI] [PubMed] [Google Scholar]
- [53].Menacho-Marquez M, García-Escudero R, Ojeda V, Abad A, Delgado P, Costa C, et al. The Rho exchange factors Vav2 and Vav3 favor skin tumor initiation and promotion by engaging extracellular signaling loops. PLoS Biol. 2013;11:e1001615. doi: 10.1371/journal.pbio.1001615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Risinger GM, Updike DL, Bullen EC, Tomasek JJ, Howard EW. TGF-β suppresses the upregulation of MMP-2 by vascular smooth muscle cells in response to PDGF-BB. Am J Physiol Cell Physiol. 2010;298:C191–C201. doi: 10.1152/ajpcell.00417.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Varedi M, Jeschke MG, Englander EW, Herndon DN, Barrow RE. Serum TGF-beta in thermally injured rats. Shock. 2001;16:380–2. doi: 10.1097/00024382-200116050-00010. [DOI] [PubMed] [Google Scholar]
- [56].Ishikawa K, Nishimura T, Meyer AA. The Effects of Transforming Growth Factor-beta Neutralization on Postburn Humoral Immunity. J Trauma Acute Care Surg. 2004;57:529–36. doi: 10.1097/01.ta.0000136306.53938.99. [DOI] [PubMed] [Google Scholar]
- [57].Tredget EE, Shankowsky HA, Pannu R, Nedelec B, Iwashina T, Ghahary A, et al. Transforming growth factor-β in thermally injured patients with hypertrophic scars: effects of interferon α-2b. Plast Reconstr Surg. 1998;102:1317–28. doi: 10.1097/00006534-199810000-00001. [DOI] [PubMed] [Google Scholar]
- [58].van der Veen RC. Nitric oxide and T helper cell immunity. Int Immunopharmacol. 2001;1:1491–500. doi: 10.1016/s1567-5769(01)00093-5. [DOI] [PubMed] [Google Scholar]
- [59].Beisswenger C, Coyne CB, Shchepetov M, Weiser JN. Role of p38 MAP kinase and transforming growth factor-β signaling in transepithelial migration of invasive bacterial pathogens. J Biol Chem. 2007;282:28700–8. doi: 10.1074/jbc.M703576200. [DOI] [PubMed] [Google Scholar]
- [60].Singer AJ, Huang SS, Huang JS, McClain SA, Romanov A, Rooney J, et al. A novel TGF-beta antagonist speeds reepithelialization and reduces scarring of partial thickness porcine burns. J Burn Care Res. 2009;30:329–34. doi: 10.1097/BCR.0b013e31819a6369. [DOI] [PubMed] [Google Scholar]