Abstract
Background
5-year survival after pelvic exenteration for gynecologic malignancies has been reported as high as 60%. The objective of this study was to determine overall survival (OS) after pelvic exenteration and evaluate factors impacting outcome.
Methods
A retrospective review of all women who underwent pelvic exenteration at our institution between February 1993 and December 2010 was performed. OS was defined as time from exenteration to date of death or last contact. Survival analysis was performed using the Kaplan Meyer method. Multivariate analysis was performed to determine the impact of clinical and pathologic factors on survival outcomes.
Results
One hundred sixty patients with gynecologic malignancy underwent pelvic exenteration. Five-year recurrence free survival (RFS) was 33% (95%CI 0.25 – 0.40). Factors which negatively impacted RFS included shorter treatment-free interval (p=.050), vulvar primary (p=.032), positive margins (p<.001), lymphovascular space invasion (LVSI, p<.001), positive lymph nodes (p<.001) and perineural invasion (p=0.030). In multivariate analysis, positive margins (p=.040), positive nodes (p<.001) and lymphovascular space invasion (LVSI, p=.003) retained a significant impact on RFS.
Five-year OS was 40% (95% CI 0.32 – 0.48). Factors which negatively impacted OS included vulvar primary (p=.04), positive margins (p<.001), LVSI (p<.001), positive lymph nodes (p<.001) and perineural invasion (p=.008). In multivariate analysis, positive nodes (p=.001) and LVSI (p=.001) retained a significant impact on OS.
Conclusion
Five-year OS after pelvic exenteration was 40%. Survival outcomes have not significantly improved despite improvements in technique and patient selection. Multiple non-modifiable factors at the time of exenteration are associated with poor survival.
Keywords: Pelvic Exenteration, Gynecologic Malignancy, Cervical Cancer, Endometrial Cancer, Vulvar Cancer, Overall Survival
Introduction
Pelvic exenteration, the en bloc removal of the pelvic organs, is indicated for central recurrent or persistent gynecologic cancer, including cervical, endometrial, vaginal, or vulvar cancer. While the surgery itself is associated with significant morbidity and mortality, for these patients there are no known alternative treatments that can result in cure(1). Since the initiation of pelvic exenteration, there have been multiple modifications to help decrease morbidity and improve patient satisfaction and outcome. These include antibiotic and thromboembolic prophylaxis, improved technology such as vessel sealing devices, and modifications in surgical technique(2). Modifications with the highest impact include options for urinary diversion (incontinent versus continent diversion) and vaginal reconstruction (modified vertical rectus abdominus myocutaneous flap or VRAM)(3). However, this surgery remains life changing; impacting physical, psychosocial and sexual function, as well as body image(4).
Although pelvic exenteration is intended to be curative, in the most recent literature the five-year overall survival has been reported between 30 - 60%(5-12). Preoperative imaging, intraoperative examination and lymph node sampling are currently utilized to select appropriate candidates to achieve a curative resection. Despite a maximal surgical effort, a large proportion of patients will recur and succumb to their disease(5). In the era of personalized therapy, there is a need to predict which patients are most likely to achieve cure from pelvic exenteration. Given improvement in upfront therapy over the last 20 years, the hypothesis of this study was that survival after exenteration may have changed. The objective of this study was to report survival rates after pelvic exenteration in the context of primary tumor site and define factors that predict survival.
Methods
A retrospective cohort study of all women who underwent pelvic exenteration between January 1993 and December 2010 at MD Anderson Cancer Center was performed after Institutional Review Board approval. Patients were identified through the institutional tumor registry and the departmental surgical database. Patient demographics, clinical and pathologic characteristics, treatment, surgical outcomes, disease status, and vital status were collected from the online medical record and medical charts. Patients with a new vaginal lesion greater than 10 years after diagnosis and treatment of cervical cancer were categorized as vaginal cancers. Exenterations were classified as total, anterior, or posterior.
Indications for exenteration consisted primarily of central recurrence of pelvic malignancy after primary surgery, chemotherapy, and/or radiation. Primary exenteration was performed in cases of locally advanced melanoma. Pelvic exenteration was not offered in the presence of known extrapelvic disease or lymph node involvement.
Postoperative morbidity was categorized as early (<60 days) or late (≥ 60 days), and included wound (separation, infection), infectious (sepsis, pneumonia, abscess), urinary (urostomy complications, ureteral injury/stricture, renal failure), gastrointestinal (obstruction, colostomy complications), or cardiovascular (thromboembolic disease, myocardial infarction) complications in addition to re-admissions or reoperations.
Summary statistics were used to describe the demographic and clinical characteristics of the entire population. Complications are displayed using frequencies and percentages. Five year recurrence-free survival (RFS) and overall survival (OS) were estimated using a Kaplan-Meier product-limit estimator and modeled with Cox proportional hazards regression. RFS was measured from the date of exenteration to the date of last contact, date of recurrence, or date of death. OS was measured from the date of exenteration to the date of last contact or death.
Particular factors impacting OS included in the models were age at diagnosis, body mass index (BMI), primary tumor, status of the margins, LVSI, and perineural invasion. The RFS model included an extra term for time from primary treatment to exenteration. RFS and OS analysis was conducted on the entire cohort and then subset analysis was performed on cervical cancer. The subset analysis included a category for primary treatment as radiation only, chemoradiation, and other primary treatment.
Using a 2-sided test with α = 0.05, the study had 80% power to detect a hazards ratio of 0.60 when the independent variable was binary with p = 0.5 and a hazards ratio of 0.58 when the independent variable was binary with p = 0.33. It had 80% power to detect a hazards ratio of 0.77 when the independent variable was normal with mean 0 and variance 1. All power calculations required that 70% of subjects experienced the event of interest.
For patients with negative margins, an optimal cut point on OS and RFS was examined numerous ways to determine if the length of the negative margin had an effect on survival. Summary statistics and percentiles were used to dichotomize margins and included in Cox proportional hazards models. In addition, the functional form of closest margins was examined by plotting the midpoint of specific percentiles on the x-axis and the log of the hazard ratio from the Cox proportional hazard models. Finally, receiver operating characteristic (ROC) analysis was utilized to examine each possible cut point of closest margins. The best cut point was defined to be the maximum sum of sensitivity and specificity from all cut points. Stata v12.1 (College Station, TX) was used to conduct all statistical analysis.
Results
Clinical and Surgical Characteristics
Pelvic exenteration was performed on 160 patients between 1993 and 2010. Of these, 110 (68.8%) had total, 34 (21.3%) anterior, and 16 (10.0%) posterior exenteration. Eighty nine percent (142) had a urinary reconstruction. Of those patients, the majority had an incontinent conduit (68.5%). Vaginal reconstruction was performed in 109 patients (68.1%). Of those 41.3% had VRAM and 53.2% had gracilis flaps. Median surgical time was 9.3 hours (range 4.0 – 15.5) and estimated blood loss was 2000 ml (range 280 – 16500).
Median age was 55 years and median BMI was 27.4 kg/m2. The median interval from prior treatment to exenteration was 1.6 years. Patient demographic and clinical features are described in Table 1.
Table 1.
Demographic and clinical characteristics of the population
| Characteristic | |
|---|---|
|
| |
| Age at exenteration, median years (range) | 55.4 (24.8 – 85.9) |
|
| |
| Body mass index, median kg/m2 (range) | 27.4 (15.1 – 50.9) |
|
| |
| Time from primary treatment to exenteration, median years (range) |
1.6 (0.0 – 40.3) |
|
| |
| Race, [n, (%)] | |
| White | 113 (70.6) |
| Hispanic | 36 (22.5) |
| Black | 10 (6.3) |
| Asian | 3 (1.9) |
|
| |
| Primary Tumor Location, [n, (%)] | |
| Cervix | 86 (53.8) |
| Vagina | 38 (23.8) |
| Vulva | 20 (12.5) |
| Uterus | 15 (9.4) |
| Other | 1 (0.6) |
The overall rate of complications was 94.4% (151/160). The frequency of early (< 60 days) and late (≥ 60 days) complications after pelvic exenteration is summarized in Table 2. Two patients died within 30 days of surgery resulting in a postoperative mortality rate of 1.3%. Eighty-five patients (55.2%) recurred with 34.9% of the recurrences in the pelvic region and 65.1% being distant recurrence.
Table 2.
Complications of pelvic exenteration
| Complication | Early (≤ 60 days) n, (%) |
Late (> 60 days) n, (%) |
|---|---|---|
| Wound Separation | 47 (29.4) | 3 (1.9) |
| Abscess | 26 (16.3) | 8 (5.0) |
| Sepsis | 14 (8.8) | 2 (1.3) |
| Pneumonia | 15 (9.4) | 2 (1.3) |
| Renal failure | 6 (3.8) | 8 (5.0) |
| Ureteral complication | 14 (8.8) | 26 (16.3) |
| Urinary diversion complication | 56 (35.0) | 21 (13.1) |
| Bowel obstruction | 16 (10.0) | 14 (8.8) |
| Colostomy complication | 11 (6.9) | 3 (1.9) |
| Flap complication | 25 (15.6) | 9 (5.6) |
| Fistula | 14 (8.8) | 14 (8.8) |
| Thromboembolic event | 3 (1.9) | 8 (5.0) |
| Myocardial infarction | 1 (0.6) | 1 (0.6) |
| Readmission within 60 days | 73 (45.6) | NA |
| Reoperation | 15 (9.4) | 3 (1.9) |
| Post-operative mortality | 2(1.3) | NA |
Survival after pelvic exenteration
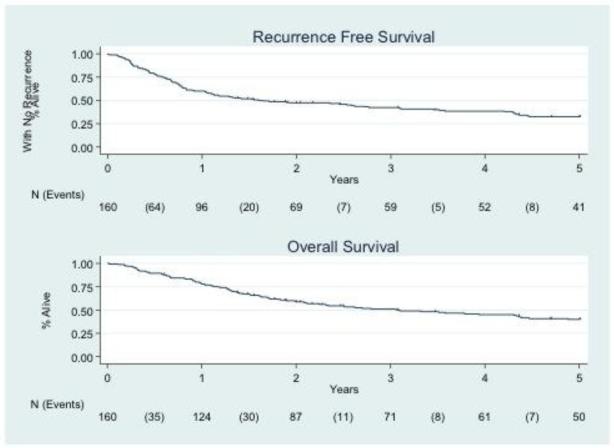
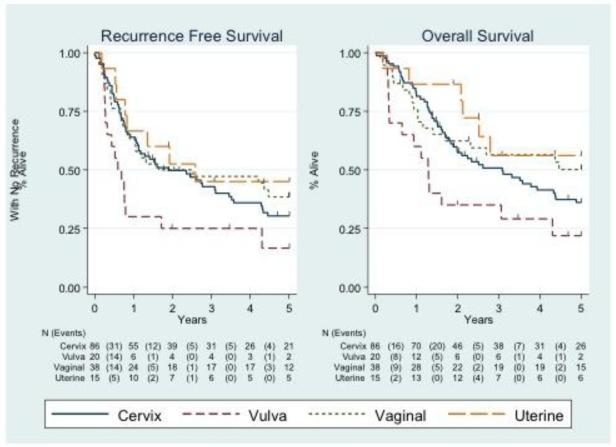
The five-year recurrence free survival and overall survival among the entire cohort was 0.33 (95% CI 0.25 – 0.40) and 0.40 (95% CI 0.32 – 0.48), respectively (Figure 1). Survival analysis was performed based on the primary cancer site (Figure 2). Five-year RFS by each cancer type are as follows: cervix (0.30; 95% CI: 0.21 – 0.41), vulva (0.17; 95% CI: 0.04 – 0.38), vaginal (0.39; 95% CI: 0.23 – 0.54), and uterine (0.45; 95% CI: 0.19 – 0.68). 5-year OS for the entire cohort was 0.40 (95% CI: 0.32 – 0.48). Five-year OS by each cancer type are as follows: cervix (0.36; 95% CI: 0.26 – 0.47), vulva (0.22; 95% CI: 0.06 – 0.43), vaginal (0.50; 95% CI: 0.33 – 0.65), and uterine (0.56; 95% CI: 0.27 – 0.78). Median follow up time for the entire cohort was 2.29 years. The median follow up time for patients remaining alive was 5 years. Nineteen cases were censored for overall survival before the 5 year endpoint.
Figure 1.
Five-year recurrence free (A) and overall survival (B) after pelvic exenteration of the entire cohort.
Figure 2.
Five-year recurrence-free survival and overall survival after pelvic exenteration by primary diagnosis.
Factors impacting RFS and OS after pelvic exenteration
Multivariate analysis of the clinical and pathologic factors that impact RFS and OS was performed. Table 3 demonstrates the univariate and multivariate analyses evaluating RFS. Treatment-free interval (p=.050), vulvar primary (p=.032), positive margins (p<.001), LVSI (p<.001), positive lymph nodes (p<.001) and perineural invasion (p=.030) were associated with poor RFS. In multivariate analysis, positive margins (p=.040), positive lymph nodes (p<.001), and LVSI (p=.003) retained a significant impact on RFS.
Table 3.
Analysis of factors impacting recurrence free survival in the whole cohort
| Effect | Univariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| HR | 95% LB |
95% UB |
p-value | HR | 95% LB |
95% UB |
p-value | |
|
| ||||||||
| Age at exenteration | 0.99 | 0.97 | 1.00 | 0.062 | 0.98 | 0.96 | 1.00 | 0.090 |
|
| ||||||||
| Body mass index | 0.99 | 0.96 | 1.02 | 0.501 | 1.00 | 0.96 | 1.03 | 0.819 |
|
| ||||||||
| Interval from treatment to exent |
0.97 | 0.94 | 1.00 | 0.050 | 0.97 | 0.93 | 1.01 | 0.162 |
|
| ||||||||
| Primary tumor (ref: Cervix) |
||||||||
| Vulva | 1.84 | 1.05 | 3.22 | 0.032 | 1.65 | 0.72 | 3.79 | 0.236 |
| Vagina | 0.88 | 0.54 | 1.42 | 0.594 | 1.26 | 0.60 | 2.62 | 0.538 |
| Uterine | 0.74 | 0.35 | 1.55 | 0.423 | 0.74 | 0.29 | 1.87 | 0.524 |
|
| ||||||||
| Positive margins | 2.64 | 1.66 | 4.22 | <0.001 | 1.80 | 1.03 | 3.16 | 0.040 |
|
| ||||||||
| Lymphovascular space invasion |
2.22 | 1.50 | 3.29 | <0.001 | 2.13 | 1.28 | 3.53 | 0.003 |
|
| ||||||||
| 0.85 | 0.50 | 1.45 | 0.550 | |||||
|
| ||||||||
| Perineural invasion | 1.60 | 1.05 | 2.45 | 0.030 | ||||
|
| ||||||||
| Positive lymph nodes |
3.39 | 1.83 | 6.27 | <0.001 | 4.84 | 2.34 | 10.01 | <0.001 |
OS was negatively impacted by vulvar primary (p=.04), positive margins (p<.001), LVSI (p<.001), positive lymph nodes (p<.006) and perineural invasion (p=.008). In multivariate analysis, positive lymph nodes (p=.001) and LVSI (p=.001) retained a significant impact on OS (Table 4).
Table 4.
Analysis of factors impacting overall survival in the whole cohort
| Effect | Univariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| HR | 95% LB |
95% UB |
p-value | HR | 95% LB |
95% UB |
p-value | |
|
| ||||||||
| Age at exenteration** |
0.99 | 0.97 | 1.01 | 0.215 | 1.00 | 0.98 | 1.02 | 0.771 |
|
| ||||||||
| Body mass index | 0.98 | 0.95 | 1.01 | 0.154 | 0.98 | 0.95 | 1.01 | 0.239 |
|
| ||||||||
| Primary tumor (ref: Cervix) |
||||||||
| Vulva | 1.83 | 1.03 | 3.25 | 0.040 | 1.21 | 0.57 | 2.59 | 0.615 |
| Vagina | 0.77 | 0.45 | 1.32 | 0.348 | 0.87 | 0.45 | 1.69 | 0.681 |
| Uterine | 0.58 | 0.25 | 1.36 | 0.213 | 0.41 | 0.14 | 1.20 | 0.105 |
|
| ||||||||
| Positive margins | 2.72 | 1.66 | 4.46 | <0.001 | 1.59 | 0.88 | 2.85 | 0.121 |
|
| ||||||||
| Lymphovascular space invasion |
2.62 | 1.72 | 3.97 | <0.001 | 2.29 | 1.39 | 3.79 | 0.001 |
|
| ||||||||
| Perineural invasion | 1.83 | 1.17 | 2.87 | 0.008 | 1.35 | 0.80 | 2.29 | 0.261 |
|
| ||||||||
| Positive lymph nodes |
3.16 | 1.63 | 6.13 | 0.001 | ||||
|
| ||||||||
|
2.94 |
1.59 |
5.46 |
<0.001 | |||||
Impact of “close margins” on overall survival
Multiple statistical models were performed to determine if distance of the closest margin had an impact on survival. Summary statistics and percentiles were used to dichotomize margins and included in Cox proportional hazards models. The 25th, 50th, and 75th percentiles were examined. None of these exploratory cut points were significant in the models for RFS or OS. Martingale residuals were also examined to explore possible cut points of interest and no cut points were identified (13).
The functional form of closest margins was examined by plotting the midpoint of specific percentiles on the x-axis and the log of the hazard ratio from the Cox proportional hazard models. No clear cut point could be identified for OS or RFS (data not shown). Next, a receiver operating characteristic (ROC) analysis was used to examine each possible cut point of closest margins. The best cut point was defined as the maximum sum of sensitivity and specificity from all cut points. Both area under the curve (AUC) for OS and RFS were approximately 0.5 indicating that predictive ability of survival (recurrence) is merely by chance (Supplementary Figure 1).
Survival after pelvic exenteration for cervical cancer
Given the large number of patients in our cohort who underwent pelvic exenteration for cervical cancer, a separate analysis of the clinical and pathologic factors impacting RFS and OS in this group was performed. On multivariate analysis the only factors that were significantly associated with shorter RFS were LVSI (p=0.031) and positive lymph nodes (p=0.021). The only factor that significantly impacted OS among patients with cervical cancer in the multivariate analysis was LVSI (p=0.015). Of those patients treated with primary radiation therapy, there was no difference in overall survival between patients treated with concurrent chemotherapy compared to those patients treated with radiation alone (1.8 vs. 4.3 months; p=0.263).
Discussion
Despite improvement in patient selection and surgical technique, overall survival after pelvic exenteration has not changed significantly from early estimates. In this study, the overall survival rate for all tumor types was 40% at 5 years, which lies within the range of what is reported in the literature (30 – 60%)(5-12). Although survival has improved with the addition of concurrent chemotherapy to primary radiation, there has been little corresponding improvement in the salvage setting(14-17). This study sought to determine if there were predictors of successful exenteration that may be utilized to guide patient selection.
Nearly every patient in this series experienced a complication after pelvic exenteration. Of those, 60% were potentially life-threatening events including infections, thromboembolic events, or reoperations. Our rate of long-term complications approached 70%, demonstrating the lasting impact of this surgery on those who survive. These rates are consistent with what has been reported by other institutions(5, 12, 18), and demonstrate the significant need for appropriate patient counseling and preparation. For highly morbid interventions, such as pelvic exenteration, there is a great need to identify those patients that have the most potential to benefit.
Patients in this cohort underwent pelvic exenteration for a variety of cancer indications. This study found that a diagnosis of vulvar cancer had lower 5-year overall survival compared to cervical cancer, which has been shown in other retrospective studies(7, 11). Interestingly, this study demonstrated improved 5-year overall survival in patients with vaginal and uterine cancer, although these differences did not reach significance in the multivariate model. Prior studies have typically shown worse survival in patients with vaginal cancer (7, 11). The improved success in this cancer type may be due to the study definition of vaginal cancer, which included patients with a new vaginal lesion greater than 10 years after diagnosis and treatment of cervical cancer.
Unfortunately, many of the features that correlate strongly with RFS and OS are pathologic features that are difficult to control or predict prior to exenterative surgery. Lymph node involvement was clearly associated with poor survival in this study. In general, this finding is supported by several single institution studies that have reported positive lymph nodes at the time of pelvic exenteration are associated with poor prognosis and survival(1, 6, 8, 11). Schmidt and colleagues found no difference in overall survival in the case of positive pelvic lymph nodes, however, presence of paraaortic lymph node metastasis negatively impacted survival(19). The potential impact of positive lymph nodes on survival supports the use of preoperative imaging and frozen section analysis to ensure negative lymph node status prior to the completion of the exenteration. While this institution routinely incorporates the use of PET/CT prior to exenteration in current practice, there was not a large enough cohort of patients who had a PET/CT included in this study to evaluate the clinical utility. Despite use of frozen section, a small group of patients with positive lymph nodes underwent exenteration resulting in poor outcomes. The presence of LVSI had a similar negative impact on both survival outcomes in our study. The presence of LVSI likely promotes worse survival through the potential for future recurrence in the lymph nodes.
Patients that had a longer interval of time between primary treatment and exenteration had a longer RFS in the entire patient cohort. Interestingly, time interval did not remain statistically significant in the multivariate. Length of time to exenteration has been assessed in several studies, typically in the range of 12 – 24 months, with variable results(5, 12). These varying results may be related to the number of patients included in the analysis or other confounding factors included in the multivariate model. Although the authors do not suggest that patients with a shorter interval between primary treatment and recurrence should not be offered an exenteration, this information may be used the preoperative setting to counsel patients about risk for recurrence.
Negative margins and complete surgical resection have long been known to correlate with improved survival after exenteration(1, 6). This study confirmed these findings, although the association was stronger in a multivariate model which included only node negative patients (data not shown). Among patients with negative margins, it would be ideal to identify a distance at which a patient had a higher risk of recurrence compared to others with larger negative margins. These patients with close margins could be offered additional post-operative therapy or closer and more frequent surveillance. Despite an attempt to assess close negative margins, an optimal cut point to predict RFS and OS was not identified. This may reflect the fact that margins are reported as negative or positive for tumor, but the distance of the closest margin is not routinely reported and was not available for all patients in this analysis.
In 1999, the National Cancer Institute issued a clinical alert regarding the importance of the addition of chemotherapy to radiotherapy for the treatment of locally advanced cervical cancer(14-17). It was hypothesized that a patient who recurred and required pelvic exenteration despite prior treatment with chemoradiation therapy would have worse outcome compared to those treated with radiation alone. Among patients treated with radiation in the upfront setting, there did not appear to be any difference in survival outcomes between patients treated with or without chemosensitization. This finding is similar to what McLean and colleagues found in a study of cervical cancer patients treated with radiation that subsequently underwent exenteration for treatment of recurrence(12).
To our knowledge, this report constitutes the largest single-institution description in the literature during the recent era of pelvic exenteration. The large number of patients allowed the use of multivariate models to reduce bias and clarify associations. Further, a large number of clinical and pathologic features were considered as potential factors in survival after exenteration. In addition, the study was conducted over a relatively short period of time, allowing for a clear look at the morbidity and mortality of this procedure in the era of modern instrumentation and imaging. Certainly, this reduced the potential for indeterminate survival results based on the variation in preoperative and intraoperative techniques that were employed for the surgery over time.
The limitations of this study primarily consist of those inherent to retrospective cohort analyses; the study was reliant on the medical record for documentation of surgical and survival outcomes. Although missing data were minimal, a close margin cut point was not able to be calculated due to inconsistency in reporting length of closest margin in the pathology report. Further, these results may not be generalizable to all institutions, as the data were generated at a large referral center and often included the highest risk cases.
In summary, this study found that recurrence free and overall survival after pelvic exenteration remain stable, despite marked improvement in the technology surrounding the surgery. Interestingly, there are multiple clinical and pathologic factors which can be used to appropriately counsel patients in regards to their risk of recurrence or death after exenteration. The development of a nomogram to predict survival outcomes would be an ideal tool to employ in the counseling of these patients. Careful counseling and consideration of appropriate candidates for pelvic exenteration remains essential in this patient population.
Supplementary Material
Supplementary Figure 1. Receiver operating characteristic curve to evaluate impact of close margins on overall survival.
Research Highlights.
5-year overall survival after pelvic exenteration was 40%.
Presence of LVSI and positive nodes at the time of exenteration were associated with poor survival.
Acknowledgments
Funding: K12 CA088084 K12 Calabresi Scholar Award, NIH 2P50 CA098258-06 SPORE in Uterine Cancer, NCI P30 CA016672
Footnotes
The authors of this manuscript have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This original research was presented in part at the 42nd Annual Meeting of the Society of Gynecologic Oncology, March 6-9, 2011, Orlando, FL.
References
- 1.Fleisch MC, Pantke P, Beckmann MW, Schnuerch HG, Ackermann R, Grimm MO, et al. Predictors for long-term survival after interdisciplinary salvage surgery for advanced or recurrent gynecologic cancers. Journal of surgical oncology. 2007 May 1;95(6):476–84. doi: 10.1002/jso.20686. [DOI] [PubMed] [Google Scholar]
- 2.Slomovitz BM, Ramirez PT, Frumovitz M, Soliman PT, Bevers M, Coleman RL, et al. Electrothermal bipolar coagulation for pelvic exenterations. Gynecologic oncology. 2006 Sep;102(3):534–6. doi: 10.1016/j.ygyno.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 3.Sood AK, Cooper BC, Sorosky JI, Ramirez PT, Levenback C. Novel modification of the vertical rectus abdominis myocutaneous flap for neovagina creation. Obstetrics and gynecology. 2005 Mar;105(3):514–8. doi: 10.1097/01.AOG.0000154158.41973.a2. [DOI] [PubMed] [Google Scholar]
- 4.Roos EJ, de Graeff A, van Eijkeren MA, Boon TA, Heintz AP. Quality of life after pelvic exenteration. Gynecologic oncology. 2004 Jun;93(3):610–4. doi: 10.1016/j.ygyno.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Sharma S, Odunsi K, Driscoll D, Lele S. Pelvic exenterations for gynecological malignancies: twenty-year experience at Roswell Park Cancer Institute. International journal of gynecological cancer: official journal of the International Gynecological Cancer Society. 2005 May-Jun;15(3):475–82. doi: 10.1111/j.1525-1438.2005.15311.x. [DOI] [PubMed] [Google Scholar]
- 6.Shingleton HM, Soong SJ, Gelder MS, Hatch KD, Baker VV, Austin JM., Jr. Clinical and histopathologic factors predicting recurrence and survival after pelvic exenteration for cancer of the cervix. Obstetrics and gynecology. 1989 Jun;73(6):1027–34. doi: 10.1097/00006250-198906000-00024. [DOI] [PubMed] [Google Scholar]
- 7.Benn T, Brooks RA, Zhang Q, Powell MA, Thaker PH, Mutch DG, et al. Pelvic exenteration in gynecologic oncology: a single institution study over 20 years. Gynecologic oncology. 2011 Jul;122(1):14–8. doi: 10.1016/j.ygyno.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Symmonds RE, Pratt JH, Webb MJ. Exenterative operations: experience with 198 patients. American journal of obstetrics and gynecology. 1975 Apr 1;121(7):907–18. doi: 10.1016/0002-9378(75)90908-4. [DOI] [PubMed] [Google Scholar]
- 9.Rutledge FN, Smith JP, Wharton JT, O’Quinn AG. Pelvic exenteration: analysis of 296 patients. American journal of obstetrics and gynecology. 1977 Dec 15;129(8):881–92. doi: 10.1016/0002-9378(77)90521-x. [DOI] [PubMed] [Google Scholar]
- 10.Goldberg GL, Sukumvanich P, Einstein MH, Smith HO, Anderson PS, Fields AL. Total pelvic exenteration: the Albert Einstein College of Medicine/Montefiore Medical Center Experience (1987 to 2003) Gynecologic oncology. 2006 May;101(2):261–8. doi: 10.1016/j.ygyno.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 11.Maggioni A, Roviglione G, Landoni F, Zanagnolo V, Peiretti M, Colombo N, et al. Pelvic exenteration: ten-year experience at the European Institute of Oncology in Milan. Gynecologic oncology. 2009 Jul;114(1):64–8. doi: 10.1016/j.ygyno.2009.03.029. [DOI] [PubMed] [Google Scholar]
- 12.McLean KA, Zhang W, Dunsmoor-Su RF, Shah CA, Gray HJ, Swensen RE, et al. Pelvic exenteration in the age of modern chemoradiation. Gynecologic oncology. 2011 Apr;121(1):131–4. doi: 10.1016/j.ygyno.2010.11.044. [DOI] [PubMed] [Google Scholar]
- 13.Therneau TM, Grambsch PM, Fleming TR. Martingale-Based Residuals for Survival Models. Biometrika. 1990 Mar;77(1):147–60. [Google Scholar]
- 14.Keys HM, Bundy BN, Stehman FB, Muderspach LI, Chafe WE, Suggs CL, 3rd, et al. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. The New England journal of medicine. 1999 Apr 15;340(15):1154–61. doi: 10.1056/NEJM199904153401503. [DOI] [PubMed] [Google Scholar]
- 15.Whitney CW, Sause W, Bundy BN, Malfetano JH, Hannigan EV, Fowler WC, Jr., et al. Randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stage IIB-IVA carcinoma of the cervix with negative paraaortic lymph nodes: a Gynecologic Oncology Group and Southwest Oncology Group study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 1999 May;17(5):1339–48. doi: 10.1200/JCO.1999.17.5.1339. [DOI] [PubMed] [Google Scholar]
- 16.Morris M, Eifel PJ, Lu J, Grigsby PW, Levenback C, Stevens RE, et al. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. The New England journal of medicine. 1999 Apr 15;340(15):1137–43. doi: 10.1056/NEJM199904153401501. [DOI] [PubMed] [Google Scholar]
- 17.Rose PG, Bundy BN, Watkins EB, Thigpen JT, Deppe G, Maiman MA, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. The New England journal of medicine. 1999 Apr 15;340(15):1144–53. doi: 10.1056/NEJM199904153401502. [DOI] [PubMed] [Google Scholar]
- 18.Berek JS, Howe C, Lagasse LD, Hacker NF. Pelvic exenteration for recurrent gynecologic malignancy: survival and morbidity analysis of the 45-year experience at UCLA. Gynecologic oncology. 2005 Oct;99(1):153–9. doi: 10.1016/j.ygyno.2005.05.034. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt AM, Imesch P, Fink D, Egger H. Indications and long-term clinical outcomes in 282 patients with pelvic exenteration for advanced or recurrent cervical cancer. Gynecologic oncology. 2012 Jun;125(3):604–9. doi: 10.1016/j.ygyno.2012.03.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Receiver operating characteristic curve to evaluate impact of close margins on overall survival.