Abstract
G protein β3 (Gβ3) is an isoform of heterotrimeric G protein β subunits involved in transducing G protein coupled receptor (GPCR) signaling. Polymorphisms in Gβ3 (GNB3) are associated with many human disorders (e.g. hypertension, diabetes and obesity) but the role of GNB3 in these pathogeneses remains unclear. Here, Gβ3-null mice (GNB3−/−) were characterized to determine how Gβ3 functions to regulate blood pressure, body weight and metabolism. We found Gβ3 expression restricted to limited types of tissues, including the retina, several regions of brain and heart ventricles. Gβ3-deficient mice were normal as judged by body weight gain by age or by feeding with high-fat diet (HFD); glucose tolerance and insulin sensitivity; baseline blood pressure and angiotensin II infusion-induced hypertension. During tail-cuff blood pressure measurements, however, Gβ3-null mice had slower heart rates (~450 vs ~500 beats/min). This bradycardia was not observed in isolated and perfused Gβ3-null mouse hearts. Moreover, mouse hearts isolated from GNB3−/− and controls responded equivalently to muscarinic receptor- and β-adrenergic receptor-stimulated bradycardia and tachycardia, respectively. Since no difference was seen in isolated hearts, Gβ3 is unlikely to be involved directly in the GPCR signaling activity that controls heart pacemaker activity. These results demonstrate that although Gβ3 appears dispensable in mice for regulation of blood pressure, body weight and metabolic features associated with obesity and diabetes, Gβ3 may regulate heart rate.
Keywords: Heterotrimeric G protein, GNB3, GPCR, signalling, polymorphism, blood pressure, metabolism, obesity, diabetes
1. Introduction
Heterotrimeric G proteins transmit signals from G protein coupled receptors (GPCRs) to regulate diverse physiological processes, including metabolism, cardiovascular function and immune response [1, 2]. Dysregulation of GPCRs and G proteins is linked to various diseases, including hypertension, obesity, and diabetes [3-8]. G proteins are composed of Gα, Gβ and Gγ subunits; Gβ and Gγ form an obligate dimer and play a central role in G protein signaling [9]. Gβ and Gγ subunits each assume multiple isoforms, and are encoded by five distinct Gβ genes (Gβ1, Gβ2, Gβ3, Gβ4 and Gβ5) and 12 Gβ genes, respectively. The majority of Gβ and Gγ isoforms form a functional dimer when expressed in a heterologous expression system; however, some evidence suggests specific Gβ and Gγ isoforms perform distinct functions in vivo. For example, mice Gβ1−/− mice are embryonic lethal and display neural tube defects [10], while mice with a Gβ5 deficiency are viable but their vision and brain functions are impaired [11-13], suggesting the function of these two subunits is non-redundant in vivo. Likewise, Gγ1, Gγ3, Gγ5, Gγ7 and Gγ13 knockout mouse phenotypes are different, suggesting the various Gβ and Gγ couplings perform distinct functions [14-20]. Nevertheless, our understanding of the functional specificity of Gβ and Gγ subunits is far from complete, and the role of other Gβ and Gγ subunits have not yet been characterized in vivo by genetic manipulations in mice.
Of the five Gβ genes, Gβ3 is unique in that a number of genetic polymorphisms are known [21-24]. C825T is one of the most well characterized Gβ3 polymorphisms and is associated with a shorter, spliced variant, Gβ3s [24]. This polymorphism was initially identified in patients with essential hypertension; and later population-based association studies linked Gβ3s to diverse disorders, including obesity, diabetes, depression, and tumors [24-27]. For many of these studies, however, the results are inconsistent and in many cases contradictory [28-33].
The initial characterization of Gβ3s by Siffert's group suggested Gβ3s was a gain-of-function mutant with enhanced activity both in promoting GPCR-mediated Gα activation and stimulating Gγ effectors [22, 23]. We and others recently demonstrated, however, that Gβ3s is an unstable protein and unable to form a functional dimer with various Gγ subunits [34, 35]. These findings suggest any pathological effects associated with the Gβ3 C825T polymorphism are likely the consequence of Gβ3 inactivation. To understand the physiological function of Gβ3 in vivo, we characterized Gβ3 expression patterns and investigated the role of Gβ3 in regulating blood pressure, body weight and metabolism in Gβ3-null mice. Our results indicate that Gβ3 is expressed in limited types of tissues and does not play a major role in regulating blood pressure, body weight and metabolic features associated with obesity and diabetes, but may contribute to signaling involved in the control of heart rate.
2.Materials and methods
Mice
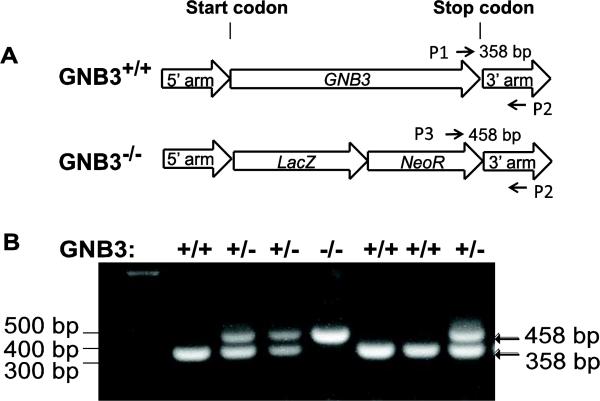
Heterozygous GNB3+/− C57BL/B6N mice were produced by the Knockout Mouse Project at the University of California, Davis. The entire coding region of GNB3 gene was replaced by the bacterial β-galactosidase (lacZ) gene through homologous recombination (Fig. 1A). As reported [36], we found it difficult to breed homozygous mice, so experimental mice were bred from heterozygous mice. Mouse genotyping was performed by PCR using the following oligonucleotides: Primer 1: 5' -GCAGCCTCTGTTCCACATACACTTCA-3'; primer 2: 5'-CTAGGAGCCACATCCATAGTCATGGG-3'; primer 3: 5'-TGGAACTGAGGAGGCTAGAGGAAGAG-3'. Amplification from the wild type genome with primers 1 and 2 produced a 358 bp product and the GNB3−/− genome with primers 3 and 2 produced a 458 bp product (Fig. 1B).
Figure 1. Generation and genotyping of Gβ3−/− mice.
A, schematic representation of the construct used to generate Gβ3−/− mice. The location of the primers used to distinguish wild-type (p1 and p2) and Gβ3−/− (p3 and p2) mice is indicated. B, representative images of genotyping results by PCR. +/+, wild-type; +/-, heterozygous; −/−, homozygous Gβ3 knockout.
Mice were housed in a standard 12:12 dark-light cycle with access to standard chow and water ad libitum, unless specified. Mice were weighed weekly 1 week after birth. In some studies, mice were fed with either normal chow (13.5% fat; LabDiet) or HFD (45% fat; D12492, Research Diets) ad libitum beginning at 10 weeks of age for up to 4 weeks, and body weight was monitored weekly. Animal experiments were approved by the University of Iowa Animal Care and Use Committee.
RNA isolation and quantitative PCR (q-PCR)
Total RNA was isolated from various tissues using TRIzol (Life Technologies) and treated with DNaseI (New England Biolab) according to the manufacturers’ instructions. First strand cDNA was synthesized using iScript cDNA synthesis kit (Bio-Rad); and q-PCR samples were run in a 25μl reaction (iQ SYBR green supermix, BioRad) using CFX96 q-PCR system (Bio-Rad). All samples were run in triplicate and normalized to the mouse housekeeping gene PPIB (peptidylprolyl isomerase B) using the ΔΔCt method [37]. Primers for mouse GNB1, GNB3 and PPIB are as follows: GNB1 forward: 5’-TCTGGGAATTATGTGGCCTG-3’; GNB1 reverse: TGATTGTCATCCAGGAACCG; GNB3 forward: 5’-TCTACAACCTCAAATCCCGC-3’; GNB3 reverse: 5’-TCTCAATGTCCCACAAGGC-3’; PPIB forward: 5’-GGAGATGGCACAGGAGGAA-3’; PPIB reverse: 5’-GCCCGTAGTGCTTCAGCTT-3’.
Detection of lacZ activity
LacZ activity was detected in whole mounts as described previously [38]. Briefly, 11.5 d embryonic offspring from breeding between GNB3+/− mice were dissected in cold phosphate buffered saline (PBS) and fixed in 4% paraformaldehyde/PBS for 1 hr at 4 °C. Embryos were washed three times in rinse buffer (5 mM EGTA, 0.01% deoxycholate, 0.02% NP40, 2 mM MgCl2, in PBS) at room temperature, and immersed in staining buffer (mM K3[Fe(CN)6], 5 mM K4[Fe(CN)6], 1 mg/ml X-gal, in rinse buffer) for up to 48 hours at 30 °C. For genotyping, the yolk sac surrounding the embryos was dissected and subjected to genomic DNA preparation and PCR.
To determine lacZ activity in mouse tissues, 20 μg of cell lysates were prepared from various tissues (using lysis buffer: 0.1 M Tris-HCl, pH 7.8, 0.5% Triton X-100) and incubated with o-nitrophenyl-β-D-galactopyranoside (0.4 mg/ml) in 300 μl of buffer (1 mM MgCl2, 45 mM β-mercapoethanol, 0.1 M sodium phosphate, pH 7.5) at 37 °C for 24 hr [39]. The reaction was stopped by adding Na2CO3 to a final concentration of 0.625 M. The optical density of the solution was read at a 420 nm wavelength, using a Biotek Synergy 4 microplate reader.
Resting metabolic rate
Resting metabolic rate was measured as previously described [40-42]. Briefly, O2 and CO2 exchange of mice during sleep at thermoneutrality (30°C) were measured using S-3A/II and CD-3A analyzers, respectively (AEI). Mass flow of effluent air was measured (Sensiron) and STP-corrected. Data were recorded and analyzed using a PowerLab (ADInstruments) and associated Chart software. Heat was estimated using the formula derived from Lusk [43];
where Heat is calculated as kcal/hr, VO2 represents STP-corrected rate of oxygen consumption (in mL/min), and the respiratory exchange ratio, RER, represents the ratio of carbon dioxide production to oxygen consumption (unitless).
Food intake and motor activity
The food intake and physical activity of mice were monitored by a Comprehensive Lab Animal Monitoring System (CLAMS; Columbus Instrument). For these experiments, male animals with matching body weight were individually housed for 4 days in metabolic cages and fed normal chow or a HFD. The amount and frequency of food intake were recorded and motor activity was monitored by infrared beam interruption.
Glucose tolerance and insulin sensitivity tests
Intraperitoneal glucose tolerance and insulin sensitivity tests were performed using 12 to14 week-old male mice. Mice first fasted for 5 hr and their baseline glucose level was measured from a drop of blood from a tail snip wound and Accu-chek active glucometers and test strips (Roche Diagnostics). Mice were then injected intraperitoneally with 100 μl of glucose (2 g/kg lean mass of mice) or human insulin (Eli Lilly and Company; 0.5 U/kg body mass of mice), and blood glucose levels monitored at 15, 30, 45, 60, 90, 120 and 180 min post-injection [44].
Blood pressure and heart rate measurement
Blood pressure and heart rate were measured using a BP-2000 tail-cuff blood pressure analysis system (Visitech Systems) as previously described [45]. Briefly, 14 to16 week-old mice were acclimated to warmed restraint boxes daily for 2 weeks. Once acclimated, 30 measurements of systolic blood pressure and heart rate were averaged from each animal daily for 2 weeks to assess baseline blood pressure. In some cases, Angiotensin II (AngII; Sigma Aldrich; 1μg/kg/min) was delivered to subsets of mice by osmotic minipump (model 1004, Alzet). After osmotic minipump implantation, pressures and heart rates were recorded daily for 10 days to assess drug effects.
Langendorff-Perfusion and Electrocardiography (ECG) measurements
ECG recording from Langendorff-perfused hearts was performed as described [46]. Hearts were excised from12 to14 week-old male mice and mounted on a modified Langendorff apparatus (HSE-HA perfusion systems, Harvard Apparatus). Retrograde aortic perfusion was performed at a constant pressure of 80 mm Hg with Krebs-Henseleit buffer (25 mM NaHCO3, 118 mM NaCl, 4.7 mM KCl, 1.2 mM MgSO4, 1.2 mM NaH2PO4, 2.5 mM CaCl2, 0.5 mM Na-EDTA, and 15 mM glucose, pH 7.4) oxygenated with 95% O2 and 5% CO2. After the heart was allowed to stabilize for 30 min, ECG recording of the perfused heart was continuously recorded with Ag+-AgCl electrodes. The hearts were perfused with 1 μM carbachol (CCh) for 10 min, washed for 20 min with buffer, followed by 5 nM isoproterenol for 10 min and then 1 μM carbachol (CCh) for another 5 min in the continued presence of isoproterenol.
Western blotting
Proteins were extracted from isolated tissues using modified RIPA buffer (50 mM Tris, pH7.5, 150 mM NaCl, 1 mM EDTA, 1% deoxycholate, 1% NP-40) containing various protease inhibitors, and quantified by the Bradford assay [47]. 50-100 μg proteins were resolved on 10% SDS-PAGE and probed with specific antibodies. Immunoblots were analyzed by an Odyssey infrared imaging system (LI-COR Biosciences).
Statistical analyses
Data are expressed as the mean ± SEM and analyzed by t test or ANOVA. Comparison between groups was analyzed by 2-tailed Student's t test or multifactor ANOVA, with differences considered significant at P < 0.05.
3 Results
3.1. Gβ3 is expressed at low levels in restricted tissues
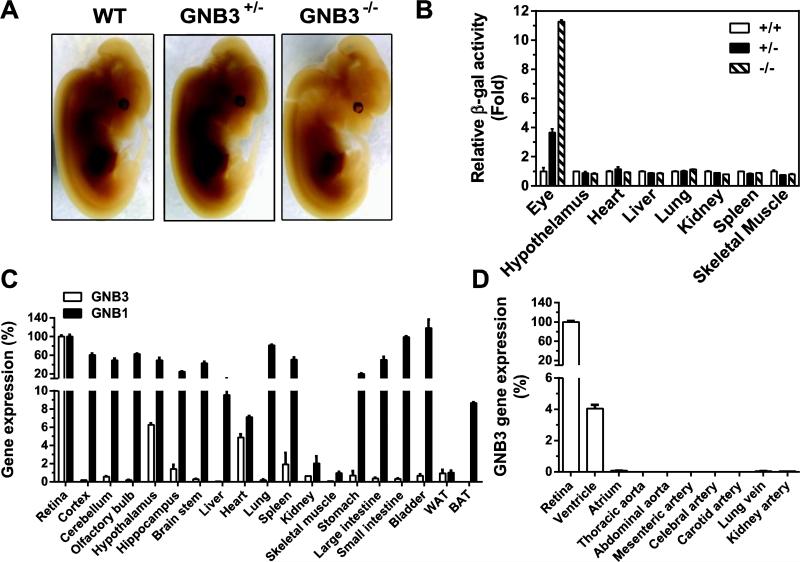
Various tissues have been shown to express Gβ3, as detected by Northern blot, Western blot and immunohistochemistry; however, in many cases, the probe specificity was not well characterized [36, 48, 49]. Since we know of no commercially available antibody that cleanly detects Gβ3, to examine the Gβ3 expression pattern, we took advantage of the GNB3−/− mice, in which the entire Gβ3 coding region is replaced with a β-galactosidase gene. This β-galactosidase gene is under the control of the endogenous Gβ3 promoter, so its expression can serve as a surrogate marker for Gβ3-expressing cells. Whole-mount analysis of E11.5 embryos, by lacZ staining, did not detect lacZ activity in any tissue, suggesting Gβ3 is not significantly expressed during early embryonic development (Fig. 2A). Similar results were found with whole-mount staining of isolated tissues from adult mice (not shown). A significant level of lacZ activity was, however, detected in tissue lysates prepared from the retina but not from other major organs (Fig. 2B), indicating the retina is the major site of Gβ3 expression. Consistent with these findings, qRT-PCR analysis showed that a majority of tissues, including cerebellum (1%), hypothalamus (6%), hippocampus (1.5%), brain stem (0.2%), ventricle (4%), spleen (2%), kidney (1%), stomach (1%), large intestine (0.2%), small intestine (0.2%), bladder (1%), white adipose tissue (WAT; 1.2%), only expressed a small percentage of Gβ3 transcripts in the retina (Fig. 2C, D). This is in contrast to Gβ1, which was expressed at a more comparable level in the retina and a majority of tissues examined (Fig. 2C). Moreover, in most tissues tested, Gβ1 transcripts were expressed at a level over 100 fold higher than Gβ3 transcripts (data not shown). Gβ3 transcripts were undetectable in many tissues, such as cortex, olfactory bulb, liver, lung, skeletal muscle, brown fat tissue (BAT) and large and small arteries and veins (Fig. 2B, C, D).
Figure 2. Gβ3 expression in various tissues.
A, lacZ staining of whole-mount embryos (E11.5). B, lacZ activity in tissue lysates prepared from 3 month-old mice with the indicated genotypes. C-D, expression of Gβ3 and Gβ1 transcripts in various tissues by qRT-PCR analysis. Data were normalized with PPIB transcripts and are expressed as percentage of Gβ3 transcript levels in the retina. N=3.
3.2. Gβ3 deficiency does not affect body weight, metabolism and metabolic features associated with obesity and diabetes
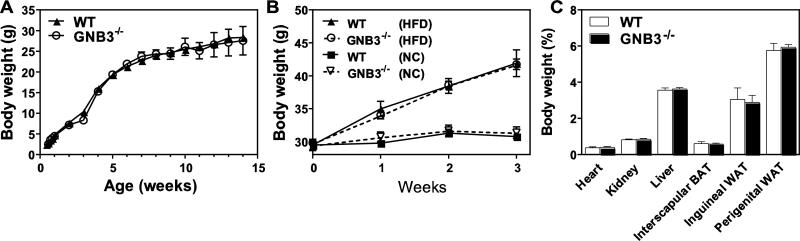
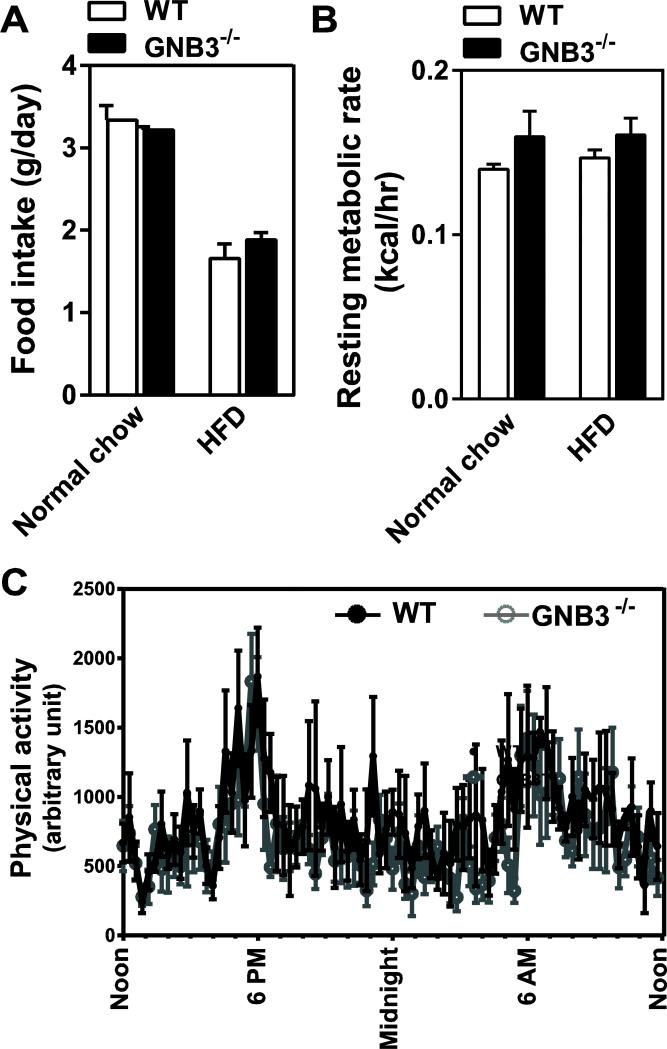
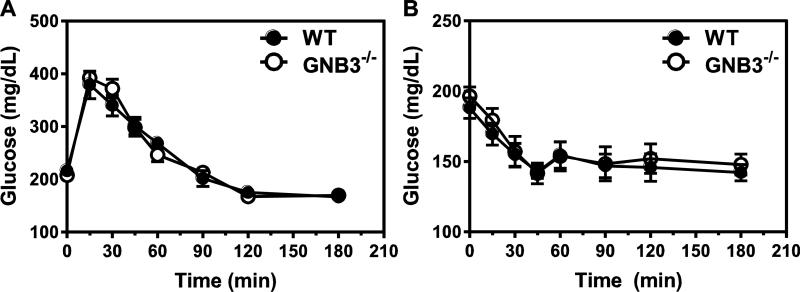
Gβ3 polymorphisms have been associated with obesity and diabetes [26, 50], and Gβ3 duplication implicated in a childhood obesity syndrome [51]. To determine if Gβ3 plays a role in body weight gain, we measured mouse body weight weekly in the control and Gβ3-null mice, beginning from two weeks after birth. No significant difference was observed in body mass between the wild-type and Gβ3−/− mice, regardless of sex (Fig. 3A and not shown). When fed a HFD, both the wild-type and Gβ3−/− mice gained weight at a similar rate (~ 4.1 g/week) (Fig. 3B), and their major organs had equivalent mass after mice had been fed with the HFD for three weeks (Fig. 3C). Over 7 consecutive days in metabolism cages, Gβ3−/− and wild-type mice consumed a similar amount of food, showed similar resting metabolic rates (as characterized by heat production) and had similar physical activity, regardless of whether they were fed with normal chow or HFD (Fig. 4A, B, C, and not shown). Gβ3−/− and wild-type mice also had the same level of fasting glucose, and exhibited the same glucose tolerance and insulin sensitivity (Fig. 5A, B). Together, these data indicate that in mice, Gβ3 does not play a major role in regulating body mass, resting metabolism or metabolic features associated with obesity and diabetes.
Figure 3. Gβ3 deficiency does not affect body weight.
A, measurement of mouse weight (male) by age under normal chow. WT n=4-30; Gβ3−/− n=4-30. B, measurement of mouse weight under normal (NC) or high fat diet (HFD). WT NC n=8; WT HFD n=6; Gβ3−/− NC n=8; Gβ3−/− HFD n=7. C, tissue masses of WT and Gβ3−/− mice. WT HFD n=4; Gβ3−/− HFD n=5.
Figure 4. Gβ3 ablation does not affect food intake, energy expenditure and physical activity.
A,food intake. N=10. B, resting metabolic rate at thermoneutrality. N=4. C, physical activity. WT n=5; Gβ3−/− n=6.
Figure 5. Gβ3 deficiency does not affect glucose tolerance and insulin sensitivity.
A, glucose tolerance test. WT n=6; Gβ3−/− n=8. B, insulin sensitivity test. WT n=8; Gβ3−/− n=8.
3.3. Gβ3 deficiency did not affect blood pressure control but caused bradycardia
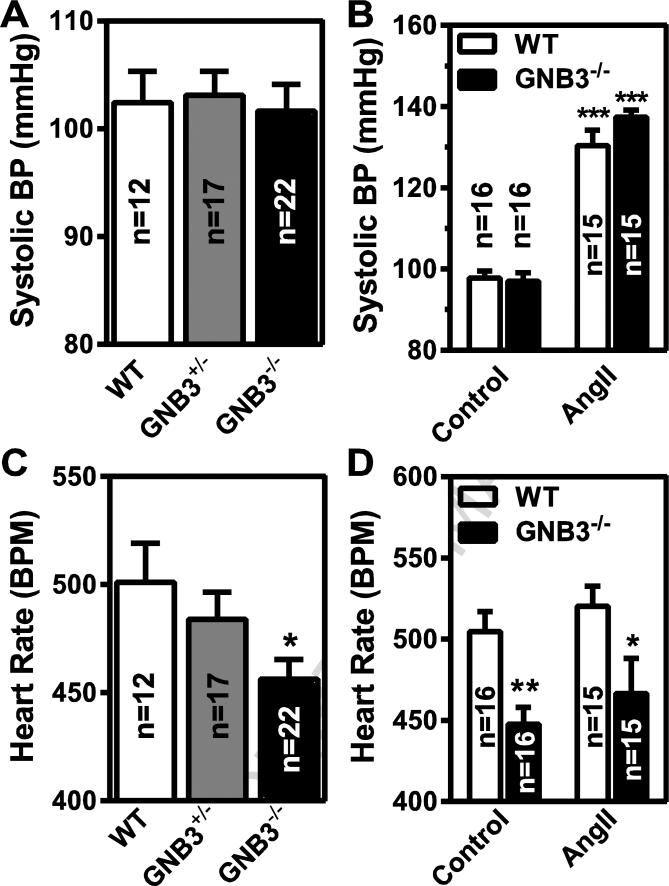
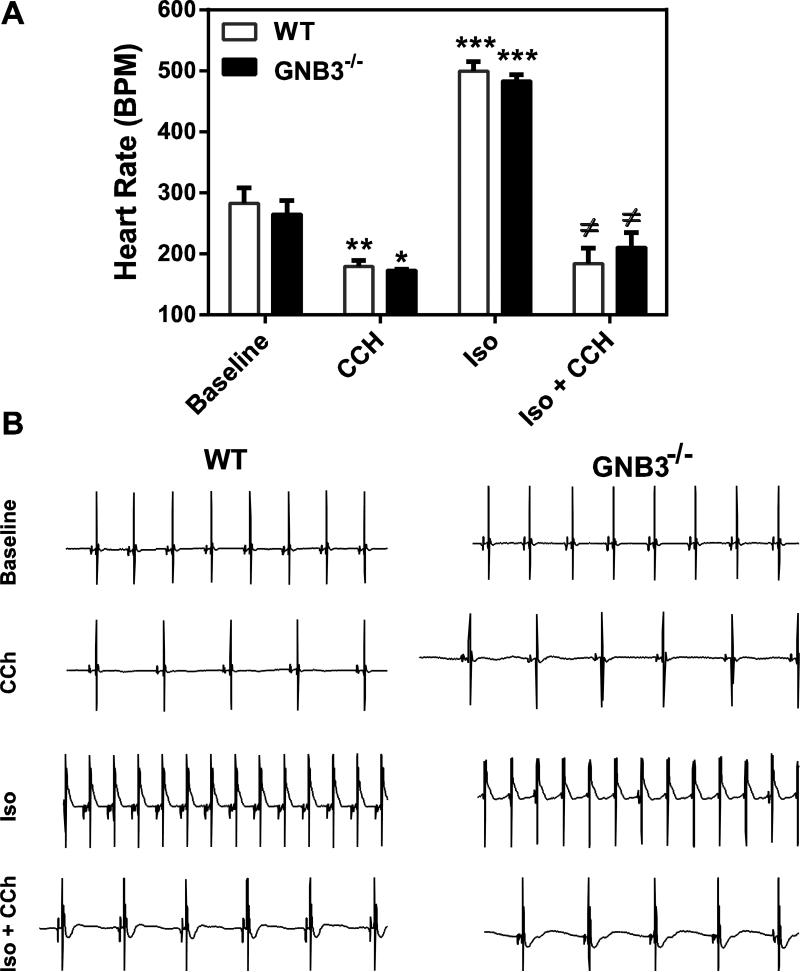
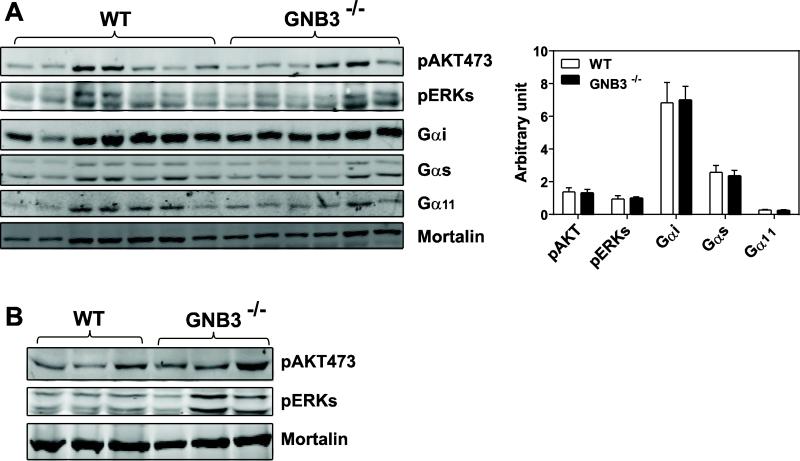
We next determined whether Gβ3 deficiency affects blood pressure and heart rate, since Gβ3 polymorphisms have been associated with hypertension and arrhythmia [24, 52]. The baseline systolic blood pressure was not significantly different between Gβ3−/− and wild-type mice, but the heart rates of Gβ3−/− mice were about 50 beats/min lower than that of the control (~450 vs. 500 beats/min, p<0.05; Fig 6A, C). Angiotensin II infusion similarly increased systolic blood pressure in the wild-type and Gβ3−/− mice (Fig. 6B), but did not alter the bradycardia observed in the Gβ3−/− mice (Fig. 6D). Interestingly, this bradycardia was not present in isolated perfused heart preparations of Gβ3−/− mice (Fig. 7A, B). Moreover, the isolated perfused hearts of Gβ3−/− mice were indistinguishable from those of controls in their responses to carbachol- and isoproterenol-induced bradycardia and tachycardia, respectively (Fig. 7A, B). These findings suggest the bradycardia in Gβ3-null mice is probably not triggered by intrinsic defects of G protein signaling in the heart. In supporting this notion, the activity of AKT and ERKs in the atrium and ventricle was not significantly different between Gβ3−/− and wild-type mice (Fig. 8A, B). Moreover, the expression levels of Gα subunits, including Gαi, Gαs and Gα11, in the atrium were not altered by Gβ3 deficiency (Fig. 8A).
Figure 6. Gβ3 ablation does not affect blood pressure control but causes bradycardia.
Systolic blood pressure (A) and heart rate (B) in control mice (control) and mice infused with angiotensin II ((AngII) for 10 days. The number of mice used in the studies is indicated in the graphs. *, ** p<0.05 and 0.01 versus control, respectively.
Figure 7. Gβ3 deficiency does not affect heart rates in isolated perfused heart.
A, Heart rates were determined from ECGs of isolated hearts at basal condition (basal), in the presence of 1 μM carbachol (CCh), 5 nM isoproterenol (Iso), or 5 nM isoproterenol plus 1 μM carbachol (Iso+CCh). WT n=5; Gβ3−/− n=4. *,**, *** p<0.05, 0.01 and 0.001 versus baseline, respectively; ≠ p<0.05 versus Iso. B, representative traces of ECGs are shown.
Figure 8. Gβ3 deficiency does not affect GPCR signaling or Gβ subunit expression.
Immunoblot analysis of the levels of phosphorylated AKT473 and ERKs (A, B), or Gαi, Gαs, and Gα11 (A) in the atrium (A) and ventricle (B) of the wild-type (WT) and GNB3−/− mice. Mortalin was used as a loading control. Each lane represents extracts from individual mouse. Quantification of protein expression (normalized by mortalin) in the atrium is also shown (A).
4. Discussion
Extensive studies have been conducted to elucidate the linkage of Gβ3 polymorphisms with multiple disorders, but little is known about the physiological functions of Gβ3 in vivo. In this study, we evaluated, for the first time, the effect of Gβ3 ablation on body weight, metabolism and blood pressure in mice. Moreover, we presented the first examination of endogenous expression of Gβ3 in various tissues. Overall, our results did not find a significant role for Gβ3 in regulating body weight, metabolic features associated with obesity and diabetes and blood pressure. The dispensable role of Gβ3 in regulating these functions may owe to its relatively low level of expression in restricted tissues, as compared to other Gβ isoforms. For example, Gβ3 was detected only in the retina when lacZ activity was used as a surrogate marker for its expression. Although Gβ3 transcripts were detected in a number of tissues when expression was measured by a more sensitive assay (qRT-PCR), Gβ3 transcript levels were at least 100 fold lower than Gβ1 levels. Moreover, Gβ3 expression was negligible in the major tissues important for metabolic and blood pressure regulation (i.e., liver, fat and blood vessels). Thus, the signaling function of G proteins in various tissues may be carried out primarily by other Gβ isoforms. Nevertheless, we cannot exclude the possibility that Gβ3 is expressed in a subset of cells in particular tissues and participates in G protein-mediated signaling cascades. Indeed, consistent with our findings that the retina is the major site of Gβ3 expression, Gβ3 was recently shown to be the major Gβ isoform expressed in retinal cones and ON bipolar cells and was shown to be critical for efficient light responses [36, 53]. Recent reports also indicate that transgenic mice carrying an extra copy of the Gβ3 gene gained more weight than wild-type mice, suggesting that under a pathological level of Gβ3 overexpression [51], Gβ3 may be important for weight-control signaling. We also cannot rule out the possibility that some Gβ3 functions may be compensated for by other Gβ isoforms, in response to lifelong loss of Gβ3 expression. Moreover, the tail-cuff plethysmography method may not be sensitive enough to detect subtle changes in blood pressure caused by Gβ3 deletion [54]. Nevertheless, given that both the control and Gβ3-null mice responded equally well to a large blood pressure increase induced by angiotensin II infusion, it is unlikely that Gβ3 plays a major role in blood pressure control, as previously proposed [24].
Interestingly, when measured by the tail-cuff method, Gβ3-null mice exhibited bradycardia, which persists under Angiotension II infusion-induced hypertension. Heart rate is normally determined by the pacemaker activity of sinoatrial node (SA node) located in the right atrium, and modified by autonomic regulation [55]. Activation of sympathetic systems increases heart rate by releasing catecholamine on to β-adrenergic receptors located on SA nodal cells [56]. In contrast, acetylcholine released from the vagus binds to muscarinic M2 receptors in SA nodal cells, which activates Gi/o proteins to release Gγ. Gγ binds to and activates G protein-coupled, inwardly rectifying K+ channels, leading to membrane hyperpolarization and bradycardia [57]. Thus, ablation of Gβ3 may decrease heart rate by altering the intrinsic automaticity of SA nodal cells, and/or autonomic control of SA nodal cells via β-adrenergic receptors and/or muscarinic M2 receptors. We found, however, that in the isolated perfused heart, neither the baseline heart rate nor a carbachol- or isoproterenol-induced decrease or increase in heart rate was affected by Gβ3 deficiency. Moreover, Gβ3 deficiency neither affected the activity of GPCR effectors, such as AKT and ERKs, nor the expression of Gα subunits in the atrium or ventricle. These results suggest Gβ3 functions outside of the heart to control heart rate. These findings are in contrast with previous suggestion that Gβ3 is directly involved in regulating atrial inwardly rectifying K+ channels [58], but consistent with the data that Gβ3 is not expressed in the atrium, where SA nodal cells are located. Nevertheless, it remains to be determined how exactly Gβ3 contributes to heart rate regulation. Since Gβ3 is expressed in several regions of brain, it could potentially participate in autonomic regulation of heart rate via GPCR-mediated signaling cascades in the central nervus system.
The initial discovery by Siffert et al. that hypertensive patients often harbored the Gβ3 C825T polymorphism has stimulated extensive followup studies to determine the association of Gβ3 polymorphisms with diverse human diseases [24, 26]. Many studies attempted to attribute the effect of Gβ3 C825T polymorphism to a shorter form of Gβ3, Gβ3s, which presumably has enhanced signaling activity, but the results of those studies have been inconsistent [22, 23, 34]. Very few of these studies, however, actually examined the expression and functionality of Gβ3s. We recently characterized the biochemical properties of Gβ3s and provided unambiguous evidence that unlike Gβ3, Gβ3s is a functionally inactive protein that fails to form a dimer with diverse Gγ subunits [35]. These findings indicate that any pathological effects of Gβ3 C825T polymorphism likely result from downregulation of Gβ3 function. In this study, our findings that Gβ3 ablation in mice does not affect weight, metabolism or blood pressure raises the question of whether any human disorders associated with the Gβ3 C825T polymorphism can be attributed to the function of Gβ3. Although we cannot rule out the possibility that Gβ3 may play a different role in human versus mouse, the function of Gβ3 bears further examination.
5. Conclusions
In conclusion, our results demonstrate that Gβ3 does not play a significant role in regulating body weight, metabolism or blood pressure, but may contribute to the signaling that regulates heart rate.
Highlights.
Gβ3 is expressed in restricted tissues
Ablation of Gβ3 in mice does not affect body weight, resting metabolisms, metabolic features associated with obesity and diabetes, and blood pressure
Gβ3–null mice exhibit bradycardia
Acknowledgements
The work was supported in part by National Institute of Health Grant GM094255 (to S.C.).
Abbreviations
- AngII
angiotensin II
- GPCR
G protein-coupled receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Oldham WM, Hamm HE. Heterotrimeric G protein activation by G-protein-coupled receptors. Nat Rev Mol Cell Biol. 2008;9:60–71. doi: 10.1038/nrm2299. [DOI] [PubMed] [Google Scholar]
- 2.Tesmer JJ. The quest to understand heterotrimeric G protein signaling. Nat Struct Mol Biol. 2010;17:650–652. doi: 10.1038/nsmb0610-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vassart G, Costagliola S. G protein-coupled receptors: mutations and endocrine diseases. Nat Rev Endocrinol. 2011;7:362–372. doi: 10.1038/nrendo.2011.20. [DOI] [PubMed] [Google Scholar]
- 4.Thathiah A, De Strooper B. The role of G protein-coupled receptors in the pathology of Alzheimer's disease. Nat Rev Neurosci. 2011;12:73–87. doi: 10.1038/nrn2977. [DOI] [PubMed] [Google Scholar]
- 5.Brinks HL, Eckhart AD. Regulation of GPCR signaling in hypertension. Biochim Biophys Acta. 2010;1802:1268–1275. doi: 10.1016/j.bbadis.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lappano R, Maggiolini M. G protein-coupled receptors: novel targets for drug discovery in cancer. Nat Rev Drug Discov. 2011;10:47–60. doi: 10.1038/nrd3320. [DOI] [PubMed] [Google Scholar]
- 7.Tiwari A. GPR43: an emerging target for the potential treatment of type 2 diabetes, obesity and insulin resistance. Curr Opin Investig Drugs. 2010;11:385–393. [PubMed] [Google Scholar]
- 8.Whalen EJ, Rajagopal S, Lefkowitz RJ. Therapeutic potential of beta-arrestin- and G protein-biased agonists. Trends Mol Med. 2011;17:126–139. doi: 10.1016/j.molmed.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smrcka AV. G protein betagamma subunits: central mediators of G protein-coupled receptor signaling. Cell Mol Life Sci. 2008;65:2191–2214. doi: 10.1007/s00018-008-8006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okae H, Iwakura Y. Neural tube defects and impaired neural progenitor cell proliferation in Gbeta1-deficient mice. Dev Dyn. 2010;239:1089–1101. doi: 10.1002/dvdy.22256. [DOI] [PubMed] [Google Scholar]
- 11.Krispel CM, Chen CK, Simon MI, Burns ME. Prolonged photoresponses and defective adaptation in rods of Gbeta5−/− mice. J Neurosci. 2003;23:6965–6971. doi: 10.1523/JNEUROSCI.23-18-06965.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang JH, Pandey M, Seigneur EM, Panicker LM, Koo L, Schwartz OM, Chen W, Chen CK, Simonds WF. Knockout of G protein beta5 impairs brain development and causes multiple neurologic abnormalities in mice. J Neurochem. 2011;119:544–554. doi: 10.1111/j.1471-4159.2011.07457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xie K, Ge S, Collins VE, Haynes CL, Renner KJ, Meisel RL, Lujan R, Martemyanov KA. Gbeta5-RGS complexes are gatekeepers of hyperactivity involved in control of multiple neurotransmitter systems. Psychopharmacology (Berl) 2012;219:823–834. doi: 10.1007/s00213-011-2409-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moon AM, Stauffer AM, Schwindinger WF, Sheridan K, Firment A, Robishaw JD. Disruption of G-protein gamma5 subtype causes embryonic lethality in mice. PLoS One. 2014;9:e90970. doi: 10.1371/journal.pone.0090970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwindinger WF, Mirshahi UL, Baylor KA, Sheridan KM, Stauffer AM, Usefof S, Stecker MM, Mirshahi T, Robishaw JD. Synergistic roles for G-protein gamma3 and gamma7 subtypes in seizure susceptibility as revealed in double knock-out mice. J Biol Chem. 2012;287:7121–7133. doi: 10.1074/jbc.M111.308395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwindinger WF, Borrell BM, Waldman LC, Robishaw JD. Mice lacking the G protein gamma3-subunit show resistance to opioids and diet induced obesity. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1494–1502. doi: 10.1152/ajpregu.00308.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwindinger WF, Giger KE, Betz KS, Stauffer AM, Sunderlin EM, Sim-Selley LJ, Selley DE, Bronson SK, Robishaw JD. Mice with deficiency of G protein gamma3 are lean and have seizures. Mol Cell Biol. 2004;24:7758–7768. doi: 10.1128/MCB.24.17.7758-7768.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwindinger WF, Betz KS, Giger KE, Sabol A, Bronson SK, Robishaw JD. Loss of G protein gamma 7 alters behavior and reduces striatal alpha(olf) level and cAMP production. J Biol Chem. 2003;278:6575–6579. doi: 10.1074/jbc.M211132200. [DOI] [PubMed] [Google Scholar]
- 19.Lobanova ES, Finkelstein S, Herrmann R, Chen YM, Kessler C, Michaud NA, Trieu LH, Strissel KJ, Burns ME, Arshavsky VY. Transducin gamma-subunit sets expression levels of alpha- and beta-subunits and is crucial for rod viability. J Neurosci. 2008;28:3510–3520. doi: 10.1523/JNEUROSCI.0338-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li F, Ponissery-Saidu S, Yee KK, Wang H, Chen ML, Iguchi N, Zhang G, Jiang P, Reisert J, Huang L. Heterotrimeric G protein subunit Ggamma13 is critical to olfaction. J Neurosci. 2013;33:7975–7984. doi: 10.1523/JNEUROSCI.5563-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosskopf D, Busch S, Manthey I, Siffert W. G protein beta 3 gene: structure, promoter, and additional polymorphisms. Hypertension. 2000;36:33–41. doi: 10.1161/01.hyp.36.1.33. [DOI] [PubMed] [Google Scholar]
- 22.Rosskopf D, Kielbik M, Manthey I, Bilmen G, Eisenhardt A, Siffert W. Characterization of the splice variant Gbeta3v of the human G-protein Gbeta3 subunit. Biochim Biophys Acta. 2003;1626:33–42. doi: 10.1016/s0167-4781(03)00035-6. [DOI] [PubMed] [Google Scholar]
- 23.Rosskopf D, Koch K, Habich C, Geerdes J, Ludwig A, Wilhelms S, Jakobs KH, Siffert W. Interaction of Gbeta3s, a splice variant of the G-protein Gbeta3, with Ggamma- and Galpha-proteins. Cell Signal. 2003;15:479–488. doi: 10.1016/s0898-6568(02)00140-7. [DOI] [PubMed] [Google Scholar]
- 24.Siffert W, Rosskopf D, Siffert G, Busch S, Moritz A, Erbel R, Sharma AM, Ritz E, Wichmann HE, Jakobs KH, Horsthemke B. Association of a human G-protein beta3 subunit variant with hypertension. Nat Genet. 1998;18:45–48. doi: 10.1038/ng0198-45. [DOI] [PubMed] [Google Scholar]
- 25.Sheu SY, Handke S, Brocker-Preuss M, Gorges R, Frey UH, Ensinger C, Ofner D, Farid NR, Siffert W, Schmid KW. The C allele of the GNB3 C825T polymorphism of the G protein beta3-subunit is associated with an increased risk for the development of oncocytic thyroid tumours. J Pathol. 2007;211:60–66. doi: 10.1002/path.2084. [DOI] [PubMed] [Google Scholar]
- 26.Siffert W. G protein polymorphisms in hypertension, atherosclerosis, and diabetes. Annu Rev Med. 2005;56:17–28. doi: 10.1146/annurev.med.56.082103.104625. [DOI] [PubMed] [Google Scholar]
- 27.Lopez-Leon S, Janssens AC, Gonzalez-Zuloeta Ladd AM, Del-Favero J, Claes SJ, Oostra BA, van Duijn CM. Meta-analyses of genetic studies on major depressive disorder. Mol Psychiatry. 2008;13:772–785. doi: 10.1038/sj.mp.4002088. [DOI] [PubMed] [Google Scholar]
- 28.Holmen OL, Romundstad S, Melien O. Association between the G protein beta3 subunit C825T polymorphism and the occurrence of cardiovascular disease in hypertensives: The Nord-Trondelag Health Study (HUNT) Am J Hypertens. 2010;23:1121–1127. doi: 10.1038/ajh.2010.121. [DOI] [PubMed] [Google Scholar]
- 29.Kato M, Wakeno M, Okugawa G, Fukuda T, Takekita Y, Hosoi Y, Azuma J, Kinoshita T, Serretti A. Antidepressant response and intolerance to SSRI is not influenced by G-protein beta3 subunit gene C825T polymorphism in Japanese major depressive patients. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1041–1044. doi: 10.1016/j.pnpbp.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 30.Plat AW, Stoffers HE, Klungel OH, van Schayck CP, de Leeuw PW, Soomers FL, Schiffers PM, Kester AD, Kroon AA. The contribution of six polymorphisms to cardiovascular risk in a Dutch high-risk primary care population: the HIPPOCRATES project. J Hum Hypertens. 2009;23:659–667. doi: 10.1038/jhh.2009.6. [DOI] [PubMed] [Google Scholar]
- 31.Pereira TV, Kimura L, Suwazono Y, Nakagawa H, Daimon M, Oizumi T, Kayama T, Kato T, Li L, Chen S, Gu D, Renner W, Marz W, Yamada Y, Bagos PG, Mingroni-Netto RC. Multivariate meta-analysis of the association of G-protein beta 3 gene (GNB3) haplotypes with cardiovascular phenotypes. Mol Biol Rep. 2014;41:3113–3125. doi: 10.1007/s11033-014-3171-0. [DOI] [PubMed] [Google Scholar]
- 32.Guo L, Zhang LL, Zheng B, Liu Y, Cao XJ, Pi Y, Li BH, Li JC. The C825T polymorphism of the G-protein beta3 subunit gene and its association with hypertension and stroke: an updated meta-analysis. PLoS One. 2013;8:e65863. doi: 10.1371/journal.pone.0065863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hayakawa T, Takamura T, Abe T, Kaneko S. Association of the C825T polymorphism of the G-protein beta3 subunit gene with hypertension, obesity, hyperlipidemia, insulin resistance, diabetes, diabetic complications, and diabetic therapies among Japanese. Metabolism. 2007;56:44–48. doi: 10.1016/j.metabol.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 34.Ruiz-Velasco V, Ikeda SR. A splice variant of the G protein beta 3-subunit implicated in disease states does not modulate ion channels. Physiol Genomics. 2003;13:85–95. doi: 10.1152/physiolgenomics.00057.2002. [DOI] [PubMed] [Google Scholar]
- 35.Sun Z, Runne C, Tang X, Lin F, Chen S. The Gbeta3 splice variant associated with the C825T gene polymorphism is an unstable and functionally inactive protein. Cell Signal. 2012;24:2349–2359. doi: 10.1016/j.cellsig.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dhingra A, Ramakrishnan H, Neinstein A, Fina ME, Xu Y, Li J, Chung DC, Lyubarsky A, Vardi N. Gbeta3 is required for normal light ON responses and synaptic maintenance. J Neurosci. 2012;32:11343–11355. doi: 10.1523/JNEUROSCI.1436-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 38.Krautzberger AM, Kosiol B, Scholze M, Schrewe H. Expression of vasorin (Vasn) during embryonic development of the mouse. Gene Expr Patterns. 2012;12:167–171. doi: 10.1016/j.gep.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 39.Smale ST. Beta-galactosidase assay. Cold Spring Harb Protoc. 2010. 2010 doi: 10.1101/pdb.prot5423. pdb prot5423. [DOI] [PubMed] [Google Scholar]
- 40.Burnett CM, Grobe JL. Direct calorimetry identifies deficiencies in respirometry for the determination of resting metabolic rate in C57Bl/6 and FVB mice. Am J Physiol Endocrinol Metab. 2013;305:E916–924. doi: 10.1152/ajpendo.00387.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burnett CMLGJ. Dietary effects on resting metabolic rate in C57BL/6 mice are differentially detected by indirect (O2/CO2 respirometry) and direct calorimetry. Molecular Metabolism. 2014 doi: 10.1016/j.molmet.2014.03.003. [In Press]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grobe JL, Grobe CL, Beltz TG, Westphal SG, Morgan DA, Xu D, de Lange WJ, Li H, Sakai K, Thedens DR, Cassis LA, Rahmouni K, Mark AL, Johnson AK, Sigmund CD. The brain Renin-angiotensin system controls divergent efferent mechanisms to regulate fluid and energy balance. Cell Metab. 2010;12:431–442. doi: 10.1016/j.cmet.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.G L. The elements of the science of nutrition. 4th edition. W.B. Saunders Company; Philadelphia, PA.: 1928. [Google Scholar]
- 44.Yokomizo H, Inoguchi T, Sonoda N, Sakaki Y, Maeda Y, Inoue T, Hirata E, Takei R, Ikeda N, Fujii M, Fukuda K, Sasaki H, Takayanagi R. Maternal high-fat diet induces insulin resistance and deterioration of pancreatic beta-cell function in adult offspring with sex differences in mice. Am J Physiol Endocrinol Metab. 2014;306:E1163–1175. doi: 10.1152/ajpendo.00688.2013. [DOI] [PubMed] [Google Scholar]
- 45.Littlejohn NK, Siel RB, Jr., Ketsawatsomkron P, Pelham CJ, Pearson NA, Hilzendeger AM, Buehrer BA, Weidemann BJ, Li H, Davis DR, Thompson AP, Liu X, Cassell MD, Sigmund CD, Grobe JL. Hypertension in mice with transgenic activation of the brain renin-angiotensin system is vasopressin dependent. Am J Physiol Regul Integr Comp Physiol. 2013;304:R818–828. doi: 10.1152/ajpregu.00082.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu Y, Gao Z, Chen B, Koval OM, Singh MV, Guan X, Hund TJ, Kutschke W, Sarma S, Grumbach IM, Wehrens XH, Mohler PJ, Song LS, Anderson ME. Calmodulin kinase II is required for fight or flight sinoatrial node physiology. Proc Natl Acad Sci U S A. 2009;106:5972–5977. doi: 10.1073/pnas.0806422106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 48.Ansari-Lari MA, Muzny DM, Lu J, Lu F, Lilley CE, Spanos S, Malley T, Gibbs RA. A gene-rich cluster between the CD4 and triosephosphate isomerase genes at human chromosome 12p13. Genome Res. 1996;6:314–326. doi: 10.1101/gr.6.4.314. [DOI] [PubMed] [Google Scholar]
- 49.Ryden M, Faulds G, Hoffstedt J, Wennlund A, Arner P. Effect of the (C825T) Gbeta(3) polymorphism on adrenoceptor-mediated lipolysis in human fat cells. Diabetes. 2002;51:1601–1608. doi: 10.2337/diabetes.51.5.1601. [DOI] [PubMed] [Google Scholar]
- 50.Siffert W, Rosskopf D, Erbel R. [Genetic polymorphism of the G-protein beta3 subunit, obesity and essential hypertension] Herz. 2000;25:26–33. doi: 10.1007/BF03044121. [DOI] [PubMed] [Google Scholar]
- 51.Goldlust IS, Hermetz KE, Catalano LM, Barfield RT, Cozad R, Wynn G, Ozdemir AC, Conneely KN, Mulle JG, Dharamrup S, Hegde MR, Kim KH, Angle B, Colley A, Webb AE, Thorland EC, Ellison JW, Rosenfeld JA, Ballif BC, Shaffer LG, Demmer LA. Unique Rare Chromosome Disorder Support G, Rudd MK, Mouse model implicates GNB3 duplication in a childhood obesity syndrome. Proc Natl Acad Sci U S A. 2013;110:14990–14994. doi: 10.1073/pnas.1305999110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schreieck J, Dostal S, von Beckerath N, Wacker A, Flory M, Weyerbrock S, Koch W, Schomig A, Schmitt C. C825T polymorphism of the G-protein beta3 subunit gene and atrial fibrillation: association of the TT genotype with a reduced risk for atrial fibrillation. Am Heart J. 2004;148:545–550. doi: 10.1016/j.ahj.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 53.Nikonov SS, Lyubarsky A, Fina ME, Nikonova ES, Sengupta A, Chinniah C, Ding XQ, Smith RG, Pugh EN, Jr., Vardi N, Dhingra A. Cones respond to light in the absence of transducin beta subunit. J Neurosci. 2013;33:5182–5194. doi: 10.1523/JNEUROSCI.5204-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Feng M, Whitesall S, Zhang Y, Beibel M, D'Alecy L, DiPetrillo K. Validation of volume-pressure recording tail-cuff blood pressure measurements. Am J Hypertens. 2008;21:1288–1291. doi: 10.1038/ajh.2008.301. [DOI] [PubMed] [Google Scholar]
- 55.Janssen BJ, Smits JF. Autonomic control of blood pressure in mice: basic physiology and effects of genetic modification. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1545–1564. doi: 10.1152/ajpregu.00714.2001. [DOI] [PubMed] [Google Scholar]
- 56.Adlam D, Herring N, Douglas G, De Bono JP, Li D, Danson EJ, Tatham A, Lu CJ, Jennings KA, Cragg SJ, Casadei B, Paterson DJ, Channon KM. Regulation of beta-adrenergic control of heart rate by GTP-cyclohydrolase 1 (GCH1) and tetrahydrobiopterin. Cardiovasc Res. 2012;93:694–701. doi: 10.1093/cvr/cvs005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lujan R, Marron Fernandez de Velasco E, Aguado C, Wickman K. New insights into the therapeutic potential of Girk channels. Trends Neurosci. 2014;37:20–29. doi: 10.1016/j.tins.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dobrev D, Wettwer E, Himmel HM, Kortner A, Kuhlisch E, Schuler S, Siffert W, Ravens U. G-Protein beta(3)-subunit 825T allele is associated with enhanced human atrial inward rectifier potassium currents. Circulation. 2000;102:692–697. doi: 10.1161/01.cir.102.6.692. [DOI] [PubMed] [Google Scholar]