Abstract
Background
Non-small-cell lung cancer patients with malignant pleural effusion have a poor overall median survival (4.3 months). Vascular endothelial growth factor (VEGF) is a key regulator of pleural effusion production. It is unknown if pharmacological inhibition of VEGF signaling modifies the disease course of non-small-cell lung cancer patients with recurrent malignant pleural effusion. We report the final results of a single-arm phase II clinical trial of the VEGF receptor inhibitor vandetanib combined with intrapleural catheter placement in patients with non-small-cell lung cancer and recurrent malignant pleural effusion, to determine whether vandetanib reduces time to pleurodesis.
Material and Methods
Non-small-cell lung cancer patients with proven metastatic disease to the pleural space by pleural fluid cytology or pleural biopsy who required intrapleural catheter placement were eligible for enrollment. On the same day of the intrapleural catheter insertion, the patients were started on a daily oral dose of 300 mg vandetanib, for a maximum of 10 weeks. The primary endpoint was time to pleurodesis, with response rate as the secondary endpoint. Exploratory analyses included measurement of pleural fluid cytokines and angiogenic factors before and during therapy.
Results
Twenty eligible patients were included in the trial. Eleven patients completed 10 weeks of treatment. Median time to pleurodesis was 35 days (95% confidence interval 15, NA). Median time to pleurodesis in the historical cohort was 63 days (95% confidence interval 45, 86) when adjusted for ECOG performance status ≤ 2.
Conclusions
Vandetanib therapy was well tolerated; however it did not significantly reduce time to pleurodesis.
Introduction
Recurrent malignant pleural effusion (MPE) is a debilitating condition associated with significant morbidity and worsening of quality of life. The median overall survival time is short, changing only slightly by tumor site (breast cancer, 7.4 months; non-small cell lung cancer [NSCLC], 4.3 months; and ovarian cancer, 9.4 months (1)) and it appears to be associated with performance status (2).Therapy for MPE typically involves mechanical evacuation of the effusion to relieve dyspnea, as a palliative treatment. Different techniques are used to mechanically evacuate the effusion including repeated thoracentesis, tube thoracostomy, indwelling pleural catheter drainage, and pleurodesis.
Use of a chronic indwelling intrapleural catheter (IPC) was introduced over a decade ago as an alternative to pleurodesis for the management of MPE. IPC was found to be safe, equally effective, and it was associated with fewer hospitalization days and with lower costs when compared to pleurodesis achieved by tube thoracostomy and doxycycline in an outpatient setting (3, 4). Therefore, at our institution in recent years IPC placement has become common practice as first-line option in all patients with a recurrent and symptomatic MPE. Published data show that pleurodesis can be achieved in 40% to 70% of patients, with times to catheter removal ranging from 8 to 283 days, depending on the characteristics of the population studied and the strategy used to drain the pleural fluid (3, 5-8).
Several studies have examined the utility of intrapleural drug administration for management of MPE, however none of the studied drugs so far has reached clinical approval (9, 10)..
Vascular endothelial growth factor (VEGF), also known as vascular permeability factor, is considered one of the key regulators of pleural effusion pathophysiology (11), and high levels of VEGF have been found in diverse exudative effusions in patients with malignant and non-malignant disease (12-14). A direct relationship between VEGF production and pleural effusion formation was found in an animal model of lung cancer (15). Furthermore, transfection with an antisense VEGF gene reduced pleural effusion formation in a highly VEGF-expressing cell line, and transfection with sense VEGF gene to a cell line that did not produce pleural effusion resulted in effusion formation (15). Using the same animal model, Yano et al. induced a reduction in the formation of MPE by inhibiting VEGF receptor tyrosine kinase phosphorylation with vatalanib (PTK787; Novartis, Switzerland) (16). Another study demonstrated that fluid from pleural effusions and ascites from human patients activated human umbilical vein endothelial cell proliferation in vitro, and that the amount of activation correlated with the amount of VEGF in the fluid. Furthermore, the endothelial cell proliferation and activation was inhibited when the preparation was co-incubated with a VEGF receptor (VEGFR) tyrosine kinase inhibitor (17).
Vandetanib [N-(4-bromo-2-fluorophenyl)-6-methoxy-7-[1-methylpiperidin-4-yl)methoxy] quinazolin-4-amine] is a low-molecular-weight, orally bioavailable, multi-kinase inhibitor of VEGFR-2 (a kinase insert domain receptor), VEGFR-3, and epidermal growth factor receptor (EGFR) (18). Vandetanib has been shown to inhibit VEGF signaling, angiogenesis, and the growth of human tumor cell xenografts in nude mice (18). In addition, it has shown activity both in vitro and in vivo against tumor cells that expressed EGFR but not VEGFR-2 (19), as well as inhibition of pleural effusion in nude mice inoculated with human NSCLC adenocarcinoma cells (20).
Patients with locally advanced or metastatic NSCLC were randomized to receive docetaxel with placebo or with vandetanib after first-line chemotherapy failed. Treatment with vandetanib plus docetaxel significantly improved progression-free survival when compared to treatment with placebo plus docetaxel (21). However, vandetanib used as single agent did not show an overall survival advantage in another published randomized placebo-controlled phase III clinical trial (22).
The rationale of our trial was based on in vivo preclinical findings showing inhibition of MPE in an orthotopic mouse model of lung adenocarcinoma treated with vandetanib (23). However, it is currently unknown if pharmacological inhibition of VEGF signaling modifies the disease course of non-small-cell lung cancer patients with recurrent malignant pleural effusion.
We report the final results of a phase II clinical trial of vandetanib in addition to IPC placement in NSCLC patients with MPE. Our study tested the hypothesis that inhibition of VEGFR activation with vandetanib may decrease pleural fluid production in patients with NSCLC and recurrent MPEs, reducing the time to pleurodesis after IPC placement.
Methods
Calculation of Sample Size
This was a single-arm phase II study to evaluate the effect of vandetanib on the management of pleural effusions in NSCLC patients. The primary endpoint was time to pleurodesis after IPC insertion. Based on a historical cohort of 199 patients with NSCLC and metastatic pleural disease from our institution (5), the estimated median time to removal of catheter due to achievement of pleurodesis was calculated as 48 days. To calculate the sample size of the study, we estimated the hazard ratio between patients treated with vandetanib and the historical controls to be 2.0, assuming the median time to pleurodesis of patients treated with vandetanib would be 24 days. Using a log-rank test with a one-sided type I error of 5% and 90% power, we estimated we could detect differences with a cohort of 22 patients. The sample size was calculated with STPLAN v4.3 (MD Anderson, Houston, TX, USA). The protocol and the informed consent were approved by The University of Texas MD Anderson Cancer Center institutional review board (protocol 2005-0929).
Eligibility Criteria and Patient Selection
We included NSCLC patients who had been diagnosed with metastatic disease to the pleural space proven by pleural fluid cytology or pleural biopsy and who required IPC placement because of symptomatic recurrent pleural effusions between May 2007 and February 2010. Eligibility criteria included Eastern Cooperative Oncology Group (ECOG) performance status of ≤ 2; signed informed consent before any study-related procedures; age ≥ 18 years; and normal hematopoietic, hepatic, and renal functions.
Exclusion criteria included chemotherapy or other anticancer therapy in the 3 weeks before the study and previous diagnosis of other invasive cancers except for adequately treated basal cell carcinoma, skin squamous cell carcinoma, in situ cervical cancer, or other cancers from which the patient was disease-free for at least the 2 years before study enrollment. In addition, patients with pre-existing IPC placement for recurrent symptomatic MPE and with arrhythmias including corrected Q-to-T wave interval (QTc) prolongation and left bundle branch block were excluded. Concomitant use of the known potent inducers of cytochrome P450 3A4—rifampicin, phenytoin, carbamazepine, barbiturates, and St John's wort—were not allowed within 2 weeks of study or during the study. Palliative radiotherapy to extra-thoracic sites 2 weeks before study enrollment was allowed.
Treatment and Study Procedures
Patients enrolling in the study received oral vandetanib at a dose of 300 mg once a day for a maximum of 10 weeks. The study medication was started on the day of IPC insertion. Patients were instructed to drain the pleural fluid daily until they experienced chest discomfort or persistent cough or until fluid drainage resolved. Patients were asked to record the amount of pleural fluid drainage on a log provided to them. If daily drainage decreased to ≤ 150 mL daily for 3 consecutive days, patients were instructed to drain the pleural fluid every other day. While draining every other day, if the amount of pleural fluid obtained was ≤ 150 mL on three consecutive occasions, patients were evaluated clinically and roentgenographycally with posteroanterior and lateral chest radiographs. Patients who showed improvement of dyspnea, had drained ≤ 150 mL of pleural fluid every other day 3 times, had ≤ 50% re-accumulation of pleural fluid on chest radiographs, and had no need for further therapeutic interventions on the affected hemithorax were considered to have attained pleurodesis and their IPCs were removed.
Patients were evaluated at weeks 2, 6, and 10 by means of complete interim histories and physical examinations, including measurement of vital signs, ECOG performance status, and weight.
Study Endpoints
The primary endpoint of the trial was time to pleurodesis, calculated as the number of days between IPC insertion and removal. Secondary endpoints were rates of patient response to treatment and cytokine and angiogenic factor (CAF) profiles of pleural fluid at baseline and at subsequent time points (2, 6, and 10 weeks). Objective responses were evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST) as described in previous studies (24).
Toxicity
Adverse events were coded according to Common Terminology Criteria of Adverse Events (CTCAE), version 3. Dose modifications of vandetanib to levels −1 and −2 (200 and 100 mg/daily, respectively) were instituted in case of severe (grade 3) toxicity. For grade 4 toxicity, the dose of the study drug was withheld for up to 3 weeks, until the toxic effect was resolved to CTCAE grade 1 or disappeared.
Correlative Studies
We evaluated 63 different CAFs from pleural effusion obtained from consented patients enrolled in our trial. To our knowledge, this is the first study investigating CAF profiles in pleural effusion of NSCLC patients undergoing antiangiogenic therapy. We analyzed these CAFs in the pleural fluid at baseline, 2, 6, and 10 weeks, and correlated them with time to pleurodesis and objective responses. Samples were centrifuged at 1,100 × g for 15 minutes at 4 °C for separation of plasma and mono nuclear cell layers. Samples were stored at −70°C to −80°C. Before analysis, samples were thawe d overnight at 4 °C and centrifuged at 1,500 × g to remove debris. We measured pleural concentrations of 63 CAFs, at each of the four time points. We analyzed 59 factors with commercially available multiplexed bead suspension arrays (Bioplex, BioRad, Hercules, CA, USA). We analyzed osteopontin, CA-9 (carbonic anhydrase 9), Gas-6 and soluble VEGFR-2, using enzyme-linked immunosorbent assays (R&D Systems, Minneapolis, MN, USA) as previously reported (25), all per the manufacturer's instructions, using a BioPlex 200 machine (Bio-Rad) in the Thoracic Blood-Based Biomarkers Laboratory at The University of Texas MD Anderson Cancer Center. Each sample was analyzed in duplicate CAF concentrations from all time points for each patient in the same enzyme-linked immunosorbent assays and multiplexed bead suspension arrays to minimize interexperimental variability. Analytes for which > 25% of patients had non-detectable levels were not included in the subsequent analyses. We analyzed the plasma samples blinded to clinical outcome.
Mutation analysis was conducted for EGFR and KRAS genes on paraffin embedded cell blocks obtained from the patients pleural effusion specimens as previously described (26, 27).
Statistical Analysis
We used the Kaplan–Meier method to estimate time to pleurodesis and the log-rank test to compare the time to pleurodesis of the studied population with the historical cohort. CAF marker levels underwent log base 2 transformation before analysis to satisfy the normality assumption. The change of a CAF marker was calculated as the difference of CAF concentration at each time point from the baseline concentration on the log-transformed scale and Wilcoxon signed-rank test then to assess whether the change was significantly different from 0. The Cox proportional hazards analysis was applied to assess the effect of baseline CAF marker levels on the time to catheter removal.
We used SAS version 9.1 (SAS Institute, Cary, NC, USA) and S-Plus version 7.0 (Statistical Sciences, Seattle, WA, USA) to carry out the computations for all analyses. A p-value of ≤ 0.05 was considered statistically significant.
Results
Patients
The clinical trial was terminated before reaching the target patient accrual due to vandetanib unavailability. Slow accrual was a contributing factor to early termination. Twenty eligible patients were included in the trial between May 2007 and February 2010. The majority of the enrolled patients were of Caucasian origin (85%), current or former smokers (85%), with stage IIIB-IV NSCLC (65%), adenocarcinoma histology (90%) and ECOG performance status 1 (70%). Patients clinicopathologic characteristics are shown in Table 1. The median age was 72 years (range, 46 to 80 years).
Table 1.
Patients clinicopathological characteristics.
| Characteristics | No. (%) |
|---|---|
| Race | |
| African American | 2 (10) |
| Caucasian | 17 (85) |
| Hispanic | 1 (5) |
|
| |
| Disease stage* | |
| IA | 1 (5) |
| IB | 1 (5) |
| IIB | 1 (5) |
| IIIB | 5 (25) |
| IV | 8 (40) |
|
| |
| Disease histology | |
| Adenocarcinoma | 18 (90) |
| Squamous cell carcinoma | 1 (5) |
| Mixed | 1 (5) |
|
| |
| Performance status | |
| 1 | 14 (70) |
| 2 | 6 (30) |
|
| |
| Smoking history | |
| No | 3 (15) |
| Yes | 17 (85) |
|
| |
| Reason for IPC removal | |
| Pleurodesis | 16 (80) |
| Pleurodesis failure | 3 (15) |
| Death | 1 (5) |
|
| |
| No. prior therapies | |
| None | 13 (65) |
| 1 | 2 (10) |
| ≥2 | 5 (25) |
|
| |
| Best clinical response | |
| PR | 2 (10) |
| SD | 11 (55) |
| PD | 7 (35) |
|
| |
| Best radiologic response | |
| PR | 2 (10) |
| SD | 13 (65) |
| PD | 5 (25) |
No. = number; IPC = intrapleural catheter; PR = partial response; SD = stable disease; PD= progressive disease.
Stage at time of initial diagnosis.
Primary Endpoint: Time to Pleurodesis
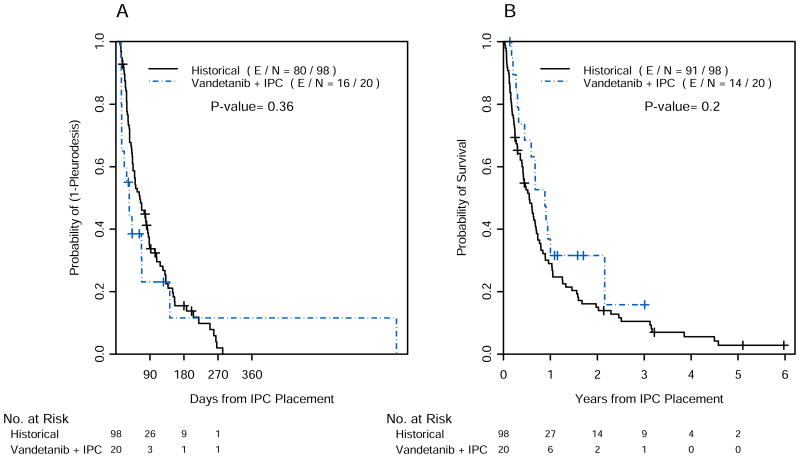
Eleven patients completed 10 weeks of treatment with vandetanib on protocol. Of the remaining nine patients who did not complete 10 weeks on protocol, 6 patients discontinued the study drug after 4 to 6 weeks due to disease progression and 3 patients between 3 and 5 weeks because of toxic effects, consisting of QTc prolongation, neurological symptoms, and uncontrolled atrial fibrillation. Reasons for IPC removal were: achievement of pleurodesis (80%), pleurodesis failure (15%) and death (5%) (Table 1). Median time to pleurodesis in the study group was 35 days (95% confidence interval [CI] 15, NA). We compared time to pleurodesis between treated group and historical cohort (48 days). Since the treated group included only patients with ECOG performace status ≤2, we calculated the median time to pleurodesis in our historical cohort including only patients with performance status ≤2 (63 days; 95% CI 45, 86). The point estimate for median time to pleurodesis in the study was lower than either of the point estimates that were used in the control group (48 or 63 days). However, given the broad confidence intervals, the difference was not statistically significant (Figure 1A).
Figure 1.
Time to pleurodesis (A) and overall survival (B) of patients enrolled in the study and historical controls. IPC= intrapleural catheter; E = events; N = number; += censoring times.
Median overall survival after IPC placement in the study group (10.6 months; 95% CI 7.1, NA) and in the historical control (6.6 months; 95% CI 5.0, 8.4) also was not statistically different (p = 0.2) (Figure 1B).
Secondary Endpoints
Objective Responses
Objective responses were evaluated by a dedicated radiologist who was blind to the patient clinicopathological characteristics. At 6 weeks from the start of the vandetanib therapy, two patients achieved a partial response, 13 patients had stable disease, and the remaining five patients had progressive disease (Table 1). Two of the 13 patients with radiological evidence of stable disease manifested worsening symptoms and were considered by the oncologist as having progressive disease (Table 1).
Correlative Studies
A detailed list of CAFs included in the assay and their baseline levels is shown in Table 2. Twenty pleural fluid samples collected at baseline and 18 samples collected at 2 weeks were available for statistical analysis. The number of pleural fluid samples collected at 6 and 10 weeks was insufficient for statistical correlations. When the baseline pleural fluid CAF profile was correlated with time to pleurodesis, high levels of human interleukin-1 receptor antagonist (IL-1 RA) showed a possible association with a lower probability of achieving pleurodesis after IPC placement (HR = 0.30; 95% CI 0.10, 0.92; p = 0.04). When baseline and 2-week pleural fluid CAF profiles were compared, a few CAFs showed a significantly increased or decreased level. However, we did not observe any significant association between changes in pleural effusion CAFs and time to pleurodesis or objective responses. Mutation analysis was conducted for EGFR gene on 20 tumor samples and for KRAS gene on 16 tumor samples. Results are described in Table 3. The two patients who responded to vandetanib had tumors that harbored EGFR sensitizing mutations: one had exon 21 L858R point mutation and the other had a 15 base-pair deletion in exon 19 (ΔE746_A750 GAA TTA AGA GAA GCA). Due to the small sample size of the EGFR- and KRAS -mutated cases, statistical analyses on time to pleurodesis and overall survival were not reported.
Table 2.
Baseline levels of cytokines and angiogenic factors.
| Characteristics | Median Value (min, max) (pg/mL) |
|---|---|
| Cytokine and Angiogenic Factors | |
|
| |
| Basic-FGF | 2.35 (-2.25, 4.97) |
| HGF | 11.28 (8.64, 14.35) |
| IL-6 | 13.40 (10.35, 15.17) |
| IL-8 | 7.72 (5.19, 10.01) |
| MCP-1 | 9.52 (4.22, 12.54) |
| IL-1a | 2.17 (-4.06, 3.61) |
| IL-1b | 3.95 (3.17, 4.39) |
| TNFα | 4.89 (2.28, 6.37) |
| VEGF | 10.66 (8.04, 12.75) |
| PDGF-bb | 4.06 (1.31, 6.51) |
| INF-a2 | 7.35 (6.46, 7.79) |
| INF-γ | 7.28 (3.81, 8.9) |
| IL-12p70 | 7.53 (5.71, 8.87) |
| IP-10 | 13.23 (9.59, 15.52) |
| MIG | 11.45 (10.09, 15.55) |
| IL-12P40 | 9.82 (7.48, 11.28) |
|
| |
| Markers of hypoxia | |
|
| |
| CA-9 | 11.01 (8.64, 13.36) |
| Osteopontin | 10.04 (6.92, 10.21) |
|
| |
| Hematopoietic growth factors | |
|
| |
| GM-CSF | 6.28 (0.73, 8.34) |
| G-CSF | 5.29 (4.07, 7.44) |
| M-CSF | 5.77 (3.45, 6.79) |
| SCF | 9.56 (8.46, 11.1) |
| Soluble VEGFR2 | 12.08 (11.13, 12.95) |
|
| |
| Other interleukins | |
|
| |
| IL-13 | 5.89 (4.27, 7.99) |
| IL-16 | 10.32 (9.06, 11.09) |
| IL-18 | 6.86 (5.48, 7.83) |
| IL-1 RA | 5.38 (0.58, 10.08) |
| IL-2 RA | 9.78 (9.19, 10.94) |
| IL-3 | 7.67 (5.72, 8.72) |
| IL-4 | 0.99 (-4.32, 4.19) |
| IL-5 | 2.47 (0.24, 6.09) |
| IL-7 | 3.50 (2.41, 5.47) |
| IL-9 | 5.12 (2.4, 8.57) |
| IL-10 | 4.98 (4.18, 8.61) |
| IL-15 | 6.12 (5.47, 6.33) |
| IL-17 | 4.09 (3.91, 6.06) |
|
| |
| Other cytokines and chemokines | |
|
| |
| IL-2 | 4.64 (4.57, 5.99) |
| MIP-1a | 5.08 (4.46, 5.86) |
| MIP-1b | 5.05 (1.53, 11.06) |
| Eotaxin | 6.77 (5.53, 8.31) |
| RANTES | 5.90 (3.22, 8.49) |
| CCL27/CTACK | 9.80 (8.73, 10.73) |
| CXCL1/GRO-Q | 7.33 (1.92, 11.48) |
| LIF | 5.51 (0.4, 7.17) |
| MCP-3L/CCL7 | 5.03 (-1.32, 6.45) |
| MIF | 9.92 (8.99, 12.67) |
| SDF-1a/CXCL-12 | 8.56 (7.66, 9.15) |
| SCGF-b | 17.41 (16.44, 18.05) |
|
| |
| Growth factors and receptors | |
|
| |
| EGFR | 11.64 (10.52, 12.17) |
| Amphiregulin | 9.74 (7.37, 13.39) |
| Epiregulin | 0.60 (-2.74, 5.13) |
| EGF | 2.43 (-1.64, 4.74) |
| Betacellulin | 2.05 (-1.29, 4.81) |
| b-NGF | 2.48 (-2.74, 3.71) |
| IGF-I | 7.01 (5.86, 8.18) |
| IGFBP-3 | 10.28 (9.14, 10.85) |
| TRAIL | 5.45 (3.6, 7.44) |
|
| |
| Coagulation proteins | |
|
| |
| Gas-6 | 14.03 (12.96, 14.36) |
|
| |
| Adhesion molecules | |
|
| |
| Tenascin | 12.17 (9.96, 14.33) |
| VCAM-1 | 16.71 (16.35, 17.61) |
| ICAM-1 | 17.37 (15.95, 19.06) |
| NGAL | 7.19 (5.69, 7.71) |
| PIGF | 9.62 (8.88, 11.35) |
Definition of abbreviations: CA-9= carbonic anhydrase 9; EGF = epidermal growth factor; EGFR = epidermal growth factor receptor; FGF = basic fibroblast growth factor; Gas-6 = growth arrest-specific 6; G-CSF = granulocyte colony-stimulating factor; HGF = hepatocyte growth factor; ICAM-1 = intercellular adhesion molecule 1; IL = interleukin; MMP = matrix metalloproteinase; IL-1RA = interleukin-1 receptor antagonist; INF = interferon; MIG = monokine induced by interferon gamma; IP-10 = interferon gamma– induced protein 10; LIF= leukemia inhibitory factor; MCP-3L/CCL7 = monocyte-specific chemokine-3; M-CSF = macrophage colony-stimulating factor; MCP-1 = macrophage chemoattractan protein-1; MIG = monokine induced by gamma interferon; MIF = macrophage migration inhibitory factor; MIP = macrophage inflammatory protein; NGAL = neutrophil gelatinase-associated lipocalin; PDGF= platelet derived growth factor; PIGF = phosphatidylinositol-glycan biosynthesis class F protein; SCGF-b=stem cell growth factor-beta; SCF = stem cell factor; SDF-1 = stromal cell-derived factor-1; TNF = tumor necrosis factor; TRAIL = TNF-related apoptosis-inducing ligand; VEGF = vascular endothelial growth factor; VEGFR2 = vascular endothelial growth factor.
Table 3.
Time to pleurodesis and median overall survival by EGFR- and KRAS-mutation analysis.
| Mutation status | No. | No. pleurodesis | Median time to pleurodesis, d (range) | Median OS, d (range) |
|---|---|---|---|---|
| EGFR-mutated | 4 | 3 | 405.5 (14, 743) | 291 (164, 787) |
| EGFR-wild type | 16 | 13 | 31.5 (13, 142) | 326 (62, 882) |
| KRAS-mutated | 5 | 3 | 42 (15, 42) | 343 (74, 789) |
| KRAS-wild type | 11 | 10 | 22 (13, 743) | 247 (62, 787) |
No.= number; OS= overall survival, d= days.
Toxicity
Cumulative toxic effects for the patients are shown in Table 4. The only grade 4 toxicity observed in the trial was hypomagnesemia, reported in one patient. The most common grade 3 toxic effects were fatigue, weight loss, diarrhea, constipation, prolonged QTc, hypertension, rash, dyspnea, hypoxia, infection without neutropenia, and hyperkalemia. All other toxic effects were grade 1 and 2.
Table 4. Cumulative toxicity.
| Toxicity | No. (%) of Patients | |||
|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
| Anemia | 1 (5) | |||
| Thrombocytopenia | 2 (10) | |||
| Prolonged PTT | 1 (5) | |||
| Fatigue | 2 (10) | 1 (5) | 4 (20) | |
| Weight loss | 4 (20) | 1 (5) | ||
| Anorexia | 2 (10) | |||
| Nausea | 3 (15) | |||
| Vomiting | 1 (5) | |||
| Diarrhea | 8 (40) | 3 (15) | 1 (5) | |
| Constipation | 1 (5) | |||
| Xerostomia | 2 (10) | |||
| Abdominal | 1 (5) | |||
| Distension/bloating | ||||
| Muscle weakness | 1 (5) | |||
| Prolonged QTc | 1 (5) | 2 (10) | 2 (10) | |
| Cardiac arrhythmia | 1 (5) | 1 (5) | 1 (5) | |
| Palpitations Hypertension | 1 (5) | 1 (5) | ||
| Premature ventricular contractions | 1 (5) | |||
| Acne | 2 (10) | |||
| Dry skin | 1 (5) | |||
| Erythema multiforme | 4 (20) | |||
| Rash/desquamation | 3 (15) | 1 (5) | ||
| Sweating | 1 (5) | |||
| Pneumothorax | 1 (5) | |||
| Dyspnea | 1 (5) | 1 (5) | ||
| Hypoxia | 1 (5) | |||
| Infection with normal ANC | 1 (5) | |||
| Hypomagnesemia | 4 (20) | 1 (5) | ||
| Hypokalemia | 3 (15) | 1 (5) | ||
| Hyponatremia | 1 (5) | |||
| Hypophosphatemia | 3 (15) | |||
| Hyperkalemia | 1 (5) | |||
| Dizziness | 1 (5) | |||
No.= number; ANC=absolute neutrophil count; PTT=partial thromboplastin time.
Discussion
We report the results of the first phase II clinical trial of a single-agent multitargeted tyrosine kinase inhibitor, vandetanib, in combination with placement of an indwelling IPC to control re-accumulation of pleural fluid in NSCLC patients with recurrent MPE. Vandetanib therapy did not significantly reduce time to pleurodesis.
The sample size estimation for this phase II trial was based on the median time of IPC removal of the historical control that included 199 NSCLC patients (5), which was calculated to be 48 days. However, in the final analysis, the median time to IPC removal in the historical cohort was adjusted to 63 days when we included only patients with NSCLC and ECOG performance status of ≤ 2, rendering the group more similar to the study cohort. The trial nearly met, but did not complete, the targeted sample size of 22 patients and was closed because of slow accrual and unavailability of the study drug. Twenty eligible patients were included in the final analysis.
Time to pleurodesis after IPC placement could be influenced by lung re-expansion at baseline. In our clinical trial, the effect of lung re-expansion on time to pleurodesis was not investigated prospectively, and patients with both good and poor lung re-expansion after IPC placement were included in the study. We retrospectively investigated whether this variable influenced our primary study endpoint. Seven patients had ≥ 80% lung re-expansion at the time of IPC insertion in our study, and their time to pleurodesis did not differ from that of the other patients.
Two patients whose tumors harbored an EGFR sensitizing mutation (10%) experienced a partial response from using single-agent vandetanib, and seven patients who achieved stable disease remained on the study drug beyond study completion. One patient whose tumor was EGFR-wild type received vandetanib for 16 months before experiencing disease progression. We are aware that EGFR mutations are known to increase the sensitivity of lung adenocarcinomas to EGFR-TK inhibitors, including vandetanib, and that they may have been involved in the MPE's responses to the study drug (28). Due to the small sample size of the EGFR-mutated and K-RAS-mutated samples, statistical conclusion could not be drawn on the time to IPC removal. In addition, other genetic abnormalities may have played a role in the response and disease stabilization obtained by those patients, including the recently discovered RET fusion, as RET tyrosine kinase is one of the targets of vandetanib (29).
High baseline levels of IL-1 RA were associated with a lower probability of achieving pleurodesis after IPC placement. However, we have to take into account that this association might have been the result of multiple testing and needs to be further investigated. IL-1 RA is an anti-inflammatory cytokine that competes with IL-1α and IL-1β in binding to IL-1 receptors without intrinsic effects (30). IL-1 RA has been shown to be secreted by pleural macrophages in regulating IL-1 mediated inflammatory reactions in pleural fluids of patients with tuberculosis, pneumonia and cancer (31). In addition, human bronchogenic carcinoma cells may contribute to the levels of IL-1 RA in pleural effusions (32). High levels of IL-1 RA in pleural effusion may be a marker of a low inflammatory response and therefore a low probability of achieving pleurodesis.
Among the secondary endpoints of our trial was vandetanib toxicity profile. The most frequent grade 3 or 4 side effects were fatigue and QTc prolongation, which are in line with already published data about single-agent vandetanib therapy (22).
In summary, although our treatment strategy did not provide the expected results, future studies addressing the potential benefit of a shorter time to pleurodesis using a combination of mechanical removal of fluid with IPC and the use of systemic targeted agents that affect the production of MPEs are warranted. These studies should consider adjusting for lung re-expansion and performance status after fluid removal, since these two factors appear to be the most influential ones when attempting pleurodesis.
Acknowledgments
This study was supported by the IMPACT (Imaging and Molecular Markers for Patients with Lung Cancer: Approaches with Molecular Targets, Complementary, Innovative and Therapeutic Modalities) Department of Defense grant, W81XWH-05-2-0027.
Footnotes
Conflict of Interest: Dr. Carlos A. Jimenez is the principal investigator for the intrapleural catheter daily versus three times a week drainage study (NCT00761618) funded by CareFusion Corporation.
Dr. Ignacio I. Wistuba received honoraria from AstraZeneca for services as a consultant.
All other authors declare no commercial interests to disclose that may be relevant to the topic of the manuscript and might be perceived as a real or potential conflict of interest.
Authors Contributions: Conception and design: CAJ, AO, JJL, WKH, RSH
Acquisition of data: CAJ, EMM, CMA, HTT, BM, IIW, SAF, LB, GAE, RCM
Analysis and Interpretation of data: CAJ, EM, DDL
Drafting manuscript: CAJ, EM
Critical revision of the manuscript for important intellectual content: CAJ, EM, AO, EMM, CMA, DDL, HTT, BM, IIW, SAF, LB, GAE, RCM, JJL, WKH, RSH
Final approval of the version to be published: CAJ, EM, AO, EMM, CMA, DDL, HTT, BM, IIW, SAF, LB, GAE, RCM, JJL, WKH, RSH
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sanchez-Armengol A, Rodriguez-Panadero F. Survival and talc pleurodesis in metastatic pleural carcinoma, revisited. Report of 125 cases. Chest. 1993 Nov;104(5):1482–5. doi: 10.1378/chest.104.5.1482. [DOI] [PubMed] [Google Scholar]
- 2.Burrows CM, Mathews WC, Colt HG. Predicting survival in patients with recurrent symptomatic malignant pleural effusions: an assessment of the prognostic values of physiologic, morphologic, and quality of life measures of extent of disease. Chest. 2000 Jan;117(1):73–8. doi: 10.1378/chest.117.1.73. [DOI] [PubMed] [Google Scholar]
- 3.Putnam JB, Jr, Light RW, Rodriguez RM, Ponn R, Olak J, Pollak JS, et al. A randomized comparison of indwelling pleural catheter and doxycycline pleurodesis in the management of malignant pleural effusions. Cancer. 1999 Nov 15;86(10):1992–9. [PubMed] [Google Scholar]
- 4.Putnam JB, Jr, Walsh GL, Swisher SG, Roth JA, Suell DM, Vaporciyan AA, et al. Outpatient management of malignant pleural effusion by a chronic indwelling pleural catheter. Annals of Thoracic Surgery. 2000 Feb;69(2):369–75. doi: 10.1016/s0003-4975(99)01482-4. [DOI] [PubMed] [Google Scholar]
- 5.Alinsonorin CY, J C, Ersoy YM, et al. Indwelling pleural catheters for management of recurrent malignant pleural effusions. American Journal of Respiratory and Critical Care Medicine. 2003;167:A901. [Google Scholar]
- 6.Tremblay A, Michaud G. Single-center experience with 250 tunnelled pleural catheter insertions for malignant pleural effusion. Chest. 2006 Feb;129(2):362–8. doi: 10.1378/chest.129.2.362. [DOI] [PubMed] [Google Scholar]
- 7.Jimenez CA, Mhatre AD, Martinez CH, Eapen GA, Onn A, Morice RC. Use of an indwelling pleural catheter for the management of recurrent chylothorax in patients with cancer. Chest. 2007 Nov;132(5):1584–90. doi: 10.1378/chest.06-2141. [DOI] [PubMed] [Google Scholar]
- 8.Tremblay A, Mason C, Michaud G. Use of tunnelled catheters for malignant pleural effusions in patients fit for pleurodesis. European Respiratory Journal. 2007 Oct;30(4):759–62. doi: 10.1183/09031936.00164706. [DOI] [PubMed] [Google Scholar]
- 9.North SA, Au HJ, Halls SB, Tkachuk L, Mackey JR. A randomized, phase III, double-blind, placebo-controlled trial of intrapleural instillation of methylprednisolone acetate in the management of malignant pleural effusion. Chest. 2003 Mar;123(3):822–7. doi: 10.1378/chest.123.3.822. [DOI] [PubMed] [Google Scholar]
- 10.Yoshida K, Sugiura T, Takifuji N, Kawahara M, Matsui K, Kudoh S, et al. Randomized phase II trial of three intrapleural therapy regimens for the management of malignant pleural effusion in previously untreated non-small cell lung cancer: JCOG 9515. Lung Cancer. 2007 Dec;58(3):362–8. doi: 10.1016/j.lungcan.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 11.Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. Journal of Clinical Oncology. 2002 Nov 1;20(21):4368–80. doi: 10.1200/JCO.2002.10.088. [DOI] [PubMed] [Google Scholar]
- 12.Cheng D, Rodriguez RM, Perkett EA, Rogers J, Bienvenu G, Lappalainen U, et al. Vascular endothelial growth factor in pleural fluid. Chest. 1999 Sep;116(3):760–5. doi: 10.1378/chest.116.3.760. [DOI] [PubMed] [Google Scholar]
- 13.Thickett DR, Armstrong L, Millar AB. Vascular endothelial growth factor (VEGF) in inflammatory and malignant pleural effusions. Thorax. 1999 Aug;54(8):707–10. doi: 10.1136/thx.54.8.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zebrowski BK, Yano S, Liu W, Shaheen RM, Hicklin DJ, Putnam JB, Jr, et al. Vascular endothelial growth factor levels and induction of permeability in malignant pleural effusions. Clinical Cancer Research. 1999 Nov;5(11):3364–8. [PubMed] [Google Scholar]
- 15.Yano S, Shinohara H, Herbst RS, Kuniyasu H, Bucana CD, Ellis LM, et al. Production of experimental malignant pleural effusions is dependent on invasion of the pleura and expression of vascular endothelial growth factor/vascular permeability factor by human lung cancer cells. American Journal of Pathology. 2000 Dec;157(6):1893–903. doi: 10.1016/S0002-9440(10)64828-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yano S, Herbst RS, Shinohara H, Knighton B, Bucana CD, Killion JJ, et al. Treatment for malignant pleural effusion of human lung adenocarcinoma by inhibition of vascular endothelial growth factor receptor tyrosine kinase phosphorylation. Clinical Cancer Research. 2000 Mar;6(3):957–65. [PubMed] [Google Scholar]
- 17.Verheul HM, Hoekman K, Jorna AS, Smit EF, Pinedo HM. Targeting vascular endothelial growth factor blockade: ascites and pleural effusion formation. Oncologist. 2000;5(Suppl 1):45–50. doi: 10.1634/theoncologist.5-suppl_1-45. [DOI] [PubMed] [Google Scholar]
- 18.Wedge SR, Ogilvie DJ, Dukes M, Kendrew J, Chester R, Jackson JA, et al. ZD6474 inhibits vascular endothelial growth factor signaling, angiogenesis, and tumor growth following oral administration. Cancer Research. 2002 Aug 15;62(16):4645–55. [PubMed] [Google Scholar]
- 19.Ciardiello F, Caputo R, Damiano V, Troiani T, Vitagliano D, Carlomagno F, et al. Antitumor effects of ZD6474, a small molecule vascular endothelial growth factor receptor tyrosine kinase inhibitor, with additional activity against epidermal growth factor receptor tyrosine kinase. Clinical Cancer Research. 2003 Apr;9(4):1546–56. [PubMed] [Google Scholar]
- 20.Matsumori Y, Yano S, Goto H, Nakataki E, Wedge SR, Ryan AJ, et al. ZD6474, an inhibitor of vascular endothelial growth factor receptor tyrosine kinase, inhibits growth of experimental lung metastasis and production of malignant pleural effusions in a non-small cell lung cancer model. Oncology Research. 2006;16(1):15–26. doi: 10.3727/000000006783981260. [DOI] [PubMed] [Google Scholar]
- 21.Herbst RS, Sun Y, Eberhardt WE, Germonpre P, Saijo N, Zhou C, et al. Vandetanib plus docetaxel versus docetaxel as second-line treatment for patients with advanced non-small-cell lung cancer (ZODIAC): a double-blind, randomised, phase 3 trial. Lancet Oncology. Jul;11(7):619–26. doi: 10.1016/S1470-2045(10)70132-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee JS, Hirsh V, Park K, Qin S, Blajman CR, Perng RP, et al. Vandetanib Versus Placebo in Patients With Advanced Non-Small-Cell Lung Cancer After Prior Therapy With an Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor: A Randomized, Double-Blind Phase III Trial (ZEPHYR) Journal of Clinical Oncology. 2012 Apr 1;30(10):1114–21. doi: 10.1200/JCO.2011.36.1709. [DOI] [PubMed] [Google Scholar]
- 23.Wu W, Onn A, Isobe T, Itasaka S, Langley RR, Shitani T, et al. Targeted therapy of orthotopic human lung cancer by combined vascular endothelial growth factor and epidermal growth factor receptor signaling blockade. Mol Cancer Ther. 2007 Feb;6(2):471–83. doi: 10.1158/1535-7163.MCT-06-0416. [DOI] [PubMed] [Google Scholar]
- 24.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000 Feb 2;92(3):205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 25.Hanrahan EO, Lin HY, Kim ES, Yan S, Du DZ, McKee KS, et al. Distinct patterns of cytokine and angiogenic factor modulation and markers of benefit for vandetanib and/or chemotherapy in patients with non-small-cell lung cancer. Journal of Clinical Oncology. 2010 Jan 10;28(2):193–201. doi: 10.1200/JCO.2009.22.4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Massarelli E, Varella-Garcia M, Tang X, Xavier AC, Ozburn NC, Liu DD, et al. KRAS mutation is an important predictor of resistance to therapy with epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancer. Clin Cancer Res. 2007 May 15;13(10):2890–6. doi: 10.1158/1078-0432.CCR-06-3043. [DOI] [PubMed] [Google Scholar]
- 27.Tang X, Shigematsu H, Bekele BN, Roth JA, Minna JD, Hong WK, et al. EGFR tyrosine kinase domain mutations are detected in histologically normal respiratory epithelium in lung cancer patients. Cancer Res. 2005 Sep 1;65(17):7568–72. doi: 10.1158/0008-5472.CAN-05-1705. [DOI] [PubMed] [Google Scholar]
- 28.Arao T, Fukumoto H, Takeda M, Tamura T, Saijo N, Nishio K. Small in-frame deletion in the epidermal growth factor receptor as a target for ZD6474. Cancer Research. 2004 Dec 15;64(24):9101–4. doi: 10.1158/0008-5472.CAN-04-2360. [DOI] [PubMed] [Google Scholar]
- 29.Kohno T, Ichikawa H, Totoki Y, Yasuda K, Hiramoto M, Nammo T, et al. KIF5B-RET fusions in lung adenocarcinoma. Nat Med. 2012 Mar;18(3):375–7. doi: 10.1038/nm.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arend WP. Interleukin 1 receptor antagonist. A new member of the interleukin 1 family. J Clin Invest. 1991 Nov;88(5):1445–51. doi: 10.1172/JCI115453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yanagawa H, Yano S, Haku T, Ohmoto Y, Sone S. Interleukin-1 receptor antagonist in pleural effusion due to inflammatory and malignant lung disease. European Respiratory Journal. 1996 Jun;9(6):1211–6. doi: 10.1183/09031936.96.09061211. [DOI] [PubMed] [Google Scholar]
- 32.Smith DR, Kunkel SL, Standiford TJ, Chensue SW, Rolfe MW, Orringer MB, et al. The production of interleukin-1 receptor antagonist by human bronchogenic carcinoma. American Journal of Pathology. 1993 Sep;143(3):794–803. [PMC free article] [PubMed] [Google Scholar]