Abstract
Here we report the synthesis of storable N-phenyl-carbamate palladacycles that showed robust reactivity in the cross-coupling reaction with an alkyne-encoded protein with second-order rate constantapproaching 19 770 ± 930 M−1 s−1.
Because of their excellent functional group tolerance and compatibility with biological systems, palladium-mediated cross coupling reactions have attracted a lot of interests recently as a bioconjugation tool both in vitro and in vivo.1–5 For example, we reported the development of the palladium–N,N-dimethylamino-pyrimidine complex mediated Sonogashira cross-coupling for selective functionalization of a homopropargylglycine (Hpg)-encoded protein (Ub-Hpg) in aqueous medium and inside E. coli cells.6 Chen and co-workers reported the ligand-free Pd(NO3)2-mediated Sonogashira cross-coupling and its application in labeling an alkyne-encoded protein in gram-negative Shigella bacteria.7 In both cases, a two-step ‘pre-activation’ procedure was employed in which the arylpalladium(II) complex was formed in situ prior to incubation with the alkyne-encoded proteins. While the reactions were generally clean, the arylpalladium(II) complexes were unstable and prone to decompose over time in PBS buffer. As an alternative strategy, Myers and co-workers reported the preparation of storable arylpalladium(II) complexes through decarboxylative palladation and the use of the complexes in Heck-type bioconjugation reactions in aqueous medium.8 Inspired by this work, we turned our attention to identify stable, yet reactive, palladium complexes for selectively functionalizing the alkyne-encoded proteins.
Palladacycles are usually identified as the reactive intermediates in palladium-mediated reactions 9, including C-H functionalization reactions,10, 11 and have been used as precatalysts,12–14 sensors,15 chiral auxiliaries, optical, and electronic devices.16 Recently, we reported the first example of using the acetanilide palladacycles to functionalize the Hpg-encoded ubiquitin protein (Ub-Hpg).17 These palladacycles exhibited improved stability and good ligation efficiency with the Ub-Hpg compared with the in situ generated arylpalladium(II) complex.6, 7 However, the conversions were modest for palladacycles containing BODIPY or fluorescein fluorophore. For in vivo applications, the low concentrations of the alkyne-encoded substrates (usually nM to low μM) demands high bioconjugation efficiency.18, 19 Herein, we report the discovery of the N-phenyl-carbamate palladacycles for robust cross-coupling with a terminal alkyne-encoded protein. To our knowledge, this is the first report of this type of palladacycles and their use as storable organometallic reagents for biological applications.
Our previously study showed the acetanilide palladacycles. e.g., 1 and 2 in Table 1, were stable in PBS, yet retained selective ligation reactivity to alkyne group.17 We reasoned that this reactivity can be enhanced through the use of a proper directing group. We prepared a panel of new palladacycles carrying the various directing groups (Table 1). The pivaloylanilide palladacycle 3,20 the phenylurea palladacycle 4,21, 22 the phenol ester palladacycle 5,23 and the O-phenylcarbamate palladacycle 624 were prepared according to the published procedures. On the other hand, the N-phenylcarbamate palladacycles 7–20 were prepared by treating the corresponding carbamate with Pd(OAc)2 and triflic acid in dichloroethane or dioxane at room temperature (see Table S1 in the Supplemental Information for yields).
Table 1.
Reactivity of Palladacycles Carrying the Various Directing Groups toward Ub-Hpga
| |||
|---|---|---|---|
| entry | palladacycle | conversion (%)b
|
|
| 10 s | 3 min | ||
| 1 |
 1 |
7 | 83 (3)c |
| 2 |
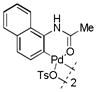 2 |
48 | >99 |
| 3 |
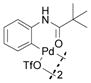 3 |
9 | 65 (25)c |
| 4 |
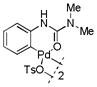 4 |
10 | 85 (10)c |
| 5 |
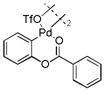 5 |
56 | 94 (6)c |
| 6 |
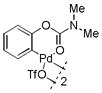 6 |
40 | 87 (11)c |
| 7 |
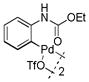 7 |
73 | >99 |
| 8 |
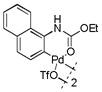 8 |
64 | >99 |
Reaction was carried out by incubating 2.5 μM Ub-Hpg with 10 μM palladacycle at room temperature for the indicated time (see detail procedure in SI, general procedures for the reaction of palladacycles with Ub-Hpg).
Conversion was determined based on LC-MS analysis: conversion % = Iproduct/(IUb-Hpg + Iproduct + Idimer side product × 2), where IUb-Hpg, Iproduct and Idimer side product represent the ion counts of Ub-Hpg, product and the protein dimer side product, respectively.
Value in parenthesis denotes the conversion of the dimer side product. The mass of the dimer side product is consistent with the structure:
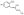 ; see ref. 25 for a possible mechanism.
; see ref. 25 for a possible mechanism.
With these palladacycles in hand, we examined their reactivity in the bioconjugation reaction with Ub-Hpg (Table 1). Compared to acetanilide palladacycle 1 (entry 1),17 pivaloylanilide palladacycle 3 (entry 3) and N-phenylurea palladacycle 4 (entry 4) gave similar conversions while the ester and carbamate palladacycles 5–8 (entries 5–8) gave significantly higher conversions at 30 sec (Table 1). Extending the reaction time to 3 min led to quantitative conversion for N-phenyl-carbamate palladacycles 7–8 (entries 7, 8), comparable to the most reactive acetanilide palladacycle 2 (entry 2) reported previously.17 Interestingly, N-phenylcarbamate palladacycle 7 showed higher reactivity than the isomeric O-phenylcarbamate palladacycle 6, indicating a subtle effect of the directing group. Notably, for the most reactive palladacycles 7 and 8, the ubiquitin dimer side products from consecutive alkyne insertion reactions (see Table 1 footnote for a possible structure) were not observed,25 suggesting that the vinyl palladium intermediates containing the N-phenylcarbamate directing group are stable before reductive depalladation to generate the styrenyl products.17 Among the palladacycles, the N-phenylcarbamate palladacycles clearly offer the fastest and clean reactions with the alkyne-containing protein substrate in PBS buffer.
Next, we evaluated the stability of the N-phenylcarbamate palladacycles. To our satisfaction, all palladacycles showed excellent stability in the deuterated PBS buffer containing 5% DMSO-d6 after incubation at room temperature for 24 hours as monitored by 1H NMR (Table S2 and Figure S1).
Then, we synthesized a series of N-phenylcarbamate palladacycle derivatives and evaluated their reactivities in the bioconjugation reaction with the alkyne-encoded protein, Ub-Hpg (Table 2). To our delight, essentially all palladacycles afforded the desired cross-coupling products in quantitative conversion after 3 min (11 entries out of 12 in Table 1). The styrenyl conjugate structure26 from palladacycle 9 was assigned based on the trypsin digestion/LC-MS analysis of the product (Figure S2). Variation of the O-alkyl group at the carbamate moiety minimally affected the reactivity (compare entries 1–3 in Table 2 to entry 7 in Table 1). For N-phenyl ring, substitution with the strong electron-donating methoxy group decreased the reaction rate as reflected by the reduced conversion at 10 sec (compare entries 4–5 in Table 2 to entry 7 in Table 1; entry 6 to 1 in Table 2), while substitution of the methyl group (entries 7–8) and the fluorine at the para-position (entry 9) had a minimum effect. However, substitution of fluorine at the meta-position (entry 10) and chlorine at the para-position (entry 11) slowed down the reactions. Interestingly, palladacycles 20 containing a BODIPY moiety at the para-position slowed down the reaction together with a significantly reduced conversion at 3 min (entry 12), suggesting that the large BODIPY group hinders the cross-coupling reaction.
Table 2.
Reactivity of Substituted N-Phenylcarbamate Palladacycles toward Ub- Hpga
| |||
|---|---|---|---|
| entry | palladacycles | conversion (%)b
|
|
| 10 s | 3 min | ||
| 1 |
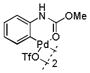 9 |
83 | >99 |
| 2 |
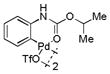 10 |
71 | >99 |
| 3 |
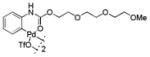 11 |
79 | 97 |
| 4 |
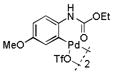 12 |
42 | >99 |
| 5 |
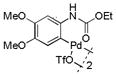 13 |
40 | >99 |
| 6 |
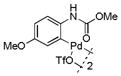 14 |
49 | >99 |
| 7 |
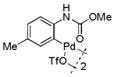 15 |
74 | >99 |
| 8 |
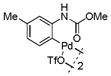 16 |
80 | >99 |
| 9 |
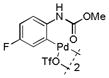 17 |
74 | >99 |
| 10 |
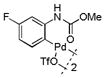 18 |
35 | >99 |
| 11 |
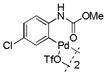 19 |
41 | >99 |
| 12 |
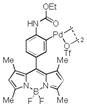 20 |
41 | 53 |
Reaction was carried out by incubating 2.5 μM Ub-Hpg with 10 μM palladacycle at room temperature for the indicated time (see detail procedure in SI, general procedures for the reaction of palladacycles with Ub-Hpg).
Conversion was determined based on LC-MS analysis: conversion % = Iproduct/(IUb-Hpg + Iproduct), where IUb-Hpg and Iproduct represent the ion counts of Ub-Hpg and the product, respectively.
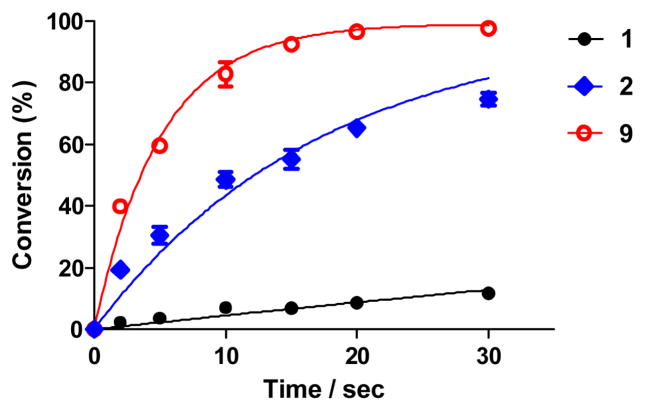
Since the difference in reactivity appears mainly to be a kinetic effect (Tables 1 and 2), we determined the second-order rate constant, k2, of N-phenylcarbamate palladacycle 9 in the cross-coupling reaction and compared it with acetanilide palladacycles 1 and 2 we synthesized previously17 (Figure 1). Palladacycle 9 afforded a k2 value of 19 770 ± 930 M−1 s−1, more than three times faster than N-naphthylacetanilide 2 (k2 = 5 764 ± 318 M−1 s−1) and 35 times faster than acetanilide 1 (k2 = 559 ± 33 M−1 s−1) (Figure 1). Since the palladacycles were used in excess in the reaction, the accelerated rate for the carbamate palladacycle 9 can be attributed to more efficient alkyne insertion into the aryl-palladium bond, leading to accelerated formation of the vinyl palladium intermediate.17
Figure 1.
Kinetics characterization of palladacycles 1, 2 and 9 in the bioconjugation reaction with Ub-Hpg. The reactions were set up by incubating 2.5 μM Ub-Hpg with 10 μM palladacycle in PBS at room temperature, and the conversions were determined by LC-MS. The plots were fitted with the one-phase decay equation in GraphPad Prism to derive the pseudo-first-order rate constant, k1. The second-order rate constant was calculated using the equation: k2 = k1/[palladacycle].
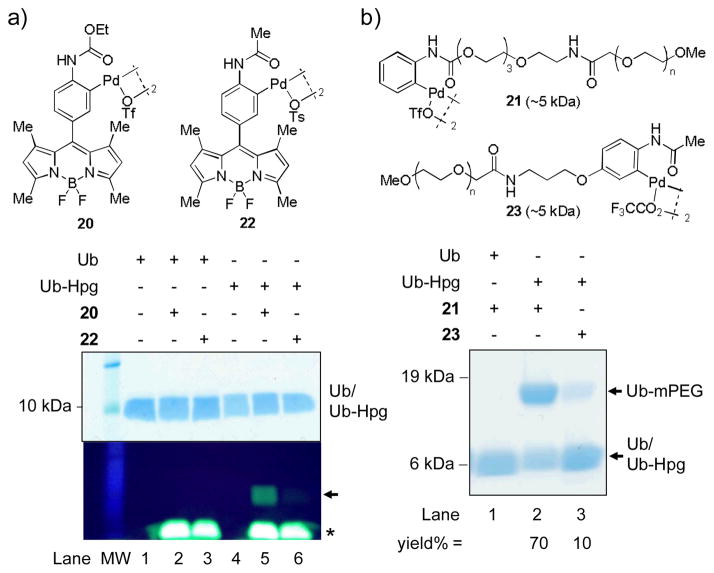
To verify the high reactivity and selectivity of the N-phenyl-carbamate palladacycles in the bioconjugation reaction with Ub-Hpg, we performed the SDS-PAGE/in-gel fluorescence analyses of the reactions involving the BODIPY-modified N-phenylcarbamate palladacycle 20 and acetanilide palladacycle 22.17 As shown in Figure 2a, palladacycle 20 showed a visible fluorescent adduct with Ub-Hpg (lane 5), but not wild-type Ub (lane 2), whereas palladacycle 22 did not show any adduct formation (lane 6) under the identical conditions, indicating a higher reactivity and selectivity for palladacycle 20. Similarly, the reaction of an mPEG-containing palladacycle 21 with Ub-Hpg gave the PEGylated product in 70% conversion after 1 min based on densitometry, significantly higher than that of a mPEG-containing acetanilide palladacycle 2317 (10% conversion; Figure 2b). No PEGylated adduct was observed for the wild-type Ub under the same condition (lane 1), indicating the palladacycle-mediated conjugation is highly selective toward the terminal alkyne group.
Figure 2.
Reactivity comparison of the N-phenylcarbamate palladacycles vs. the acetanilide palladacycles in the bioconjugation reaction with Ub-Hpg. (a) Coomassie blue stained SDS-PAGE (top) and in-gel fluorescence analysis (bottom) of the product mixture after incubating 2.5 μM Ub-Hpg with 10 μM palladacycle 20 or 22 in PBS for 1 min:λex = 365 nm. The fluorescent labeled product band is indicated with an arrow. The front migrating excess palladacycles are indicated with an asterisk. 15% SDS-PAGE gel was used. (b) Coomassie blue stained SDS-PAGE gel of the PEGylation product mixtures after incubating 2.5 μM Ub-Hpg with 10 μM palladacycle 21 or 23 in PBS for 1 min. The PEGylated product band is indicated with an arrow. Bio-Rad Any kD Mini-PROTEAN TGX Gel was used.
In summary, we have synthesized a new class of storable palladacycles from the N-phenylcarbamates. These palladacycles showed excellent stability in phosphate buffered saline and superior reactivity in selectively functionalizing the terminal alkyne-encoded protein via the Heck-type cross-coupling reaction. The kinetic measurement revealed an extremely fast bioconjugation reaction, with the second-order rate constant approaching 19 770 M−1 s−1. This rate constant is significantly greater than that of the copper-catalysed azide-alkyne cycloaddition (10–200 M−1 s−1 in the presence of 10–500 μM of CuI),27 on par with the fastest photoclick chemistry (k2 = 10 420 M−1 s−1),28 but slower than the fastest tetrazine ligation (k2 = 2.8 × 106 M−1 s−1).29 We also demonstrated the suitability of the N-phenylcarbamate palladacycles in selectively functionalizing the alkyne-encoded proteins with a fluorophore and a PEG in biological buffer. Since the preparation of the palladacycles requires the use of stoicmetric amount of triflic acid or tosylic acid, the acid-sensitive functional groups are not tolerated in the palladacycle structure. The continuous investigation of the scope of the palladacycles containing other types of biophysical and biochemical probes such as biotin30 and the unprotected carbohydrates as well as the compatibility of this class of organometallic reagents with living systems are currently underway.
Supplementary Material
Acknowledgments
We gratefully acknowledge the National Institutes of Health (GM 085092) and the National Science Foundation (CHE-1305826) for financial support. The FT-ICR mass spectrometer used in this study was supported through a grant from the NIH National Center for Research Resources (S10RR029517)
Footnotes
Electronic Supplementary Information (ESI) available. See DOI: 10.1039/c000000x/
Notes and references
- 1.Ramil CP, Lin Q. Chem Commun. 2013;49:11007–11022. doi: 10.1039/c3cc44272a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ojida A, Tsutsumi H, Kasagi N, Hamachi I. Tetrahedron Lett. 2005;46:3301–3305. [Google Scholar]
- 3.(a) Kodama K, Fukuzawa S, Nakayama H, Kigawa T, Sakamoto K, Yabuki T, Matsuda N, Shirouzu M, Takio K, Tachibana K, Yokoyama S. ChemBioChem. 2006;7:134–139. doi: 10.1002/cbic.200500290. [DOI] [PubMed] [Google Scholar]; (b) Kodama K, Fukuzawa S, Nakayama H, Sakamoto K, Kigawa T, Yabuki T, Matsuda N, Shirouzu M, Takio K, Yokoyama S, Tachibana K. ChemBioChem. 2007;8:232–238. doi: 10.1002/cbic.200600432. [DOI] [PubMed] [Google Scholar]; (c) Tilley SD, Francis MB. J Am Chem Soc. 2006;128:1080–1081. doi: 10.1021/ja057106k. [DOI] [PubMed] [Google Scholar]; (d) Brustad E, Bushey ML, Lee JW, Groff D, Liu W, Schultz PG. Angew Chem Int Ed. 2008;47:8220–8223. doi: 10.1002/anie.200803240. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Ourailidou ME, van der Meer JY, Baas BJ, Jeronimus-Stratingh M, Gottumukkala AL, Poelarends GJ, Minnaard AJ, Dekker FJ. ChemBioChem. 2014;15:209–212. doi: 10.1002/cbic.201300714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(a) Yusop RM, Unciti-Broceta A, Johansson EM, Sanchez-Martin RM, Bradley M. Nat Chem. 2011;3:239–243. doi: 10.1038/nchem.981. [DOI] [PubMed] [Google Scholar]; (b) Li J, Yu J, Zhao J, Wang J, Zheng S, Lin S, Chen L, Yang M, Jia S, Zhang X, Chen PR. Nat Chem. 2014;6:352–361. doi: 10.1038/nchem.1887. [DOI] [PubMed] [Google Scholar]
- 5.(a) Chalker JM, Wood CSC, Davis BG. J Am Chem Soc. 2009;131:16346–16347. doi: 10.1021/ja907150m. [DOI] [PubMed] [Google Scholar]; (b) Spicer CD, Davis BG. Chem Commun. 2011;47:1698–1700. doi: 10.1039/c0cc04970k. [DOI] [PubMed] [Google Scholar]; (c) Spicer CD, Triemer T, Davis BG. J Am Chem Soc. 2012;134:800–803. doi: 10.1021/ja209352s. [DOI] [PubMed] [Google Scholar]; (d) Spicer CD, Davis BG. Chem Commun. 2013;49:2747–2749. doi: 10.1039/c3cc38824g. [DOI] [PubMed] [Google Scholar]; (e) Dumas A, Spicer CD, Gao Z, Takehana T, Lin YA, Yasukohchi T, Davis BG. Angew Chem Int Ed. 2013;52:3916–3921. doi: 10.1002/anie.201208626. [DOI] [PubMed] [Google Scholar]; (f) Lercher L, McGouran JF, Kessler BM, Schofield CJ, Davis BG. Angew Chem Int Ed. 2013;52:10553–10558. doi: 10.1002/anie.201304038. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Gao Z, Gouverneur V, Davis BG. J Am Chem Soc. 2013;135:13612–13615. doi: 10.1021/ja4049114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li N, Lim RK, Edwardraja S, Lin Q. J Am Chem Soc. 2011;133:15316–15319. doi: 10.1021/ja2066913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li J, Lin S, Wang J, Jia S, Yang M, Hao Z, Zhang X, Chen PR. J Am Chem Soc. 2013;135:7330–7338. doi: 10.1021/ja402424j. [DOI] [PubMed] [Google Scholar]
- 8.Simmons RL, Yu RT, Myers AG. J Am Chem Soc. 2011;133:15870–15873. doi: 10.1021/ja206339s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dyker G. Chem Ber. 1997;130:1567–1578. [Google Scholar]
- 10.Engle KM, Mei TS, Wasa M, Yu JQ. Acc Chem Res. 2012;45:788–802. doi: 10.1021/ar200185g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lyons TW, Sanford MS. Chem Rev. 2010;110:1147–1169. doi: 10.1021/cr900184e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dupont J, Consorti CS, Spencer J. Chem Rev. 2005;105:2527–2571. doi: 10.1021/cr030681r. [DOI] [PubMed] [Google Scholar]
- 13.Yang Y, Oldenhuis NJ, Buchwald SL. Angew Chem Int Ed. 2013;52:615–619. doi: 10.1002/anie.201207750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herrmann WA, Brossmer C, Öfele K, Reisinger CP, Priermeier T, Beller M, Fischer H. Angew Chem In Ed. 1995;34:1844–1848. [Google Scholar]
- 15.Michel BW, Lippert AR, Chang CJ. J Am Chem Soc. 2012;134:15668–15671. doi: 10.1021/ja307017b. [DOI] [PubMed] [Google Scholar]
- 16.Dupont J, Pfeffer M. Palladacycles-Synthesis, Characterization and Application. 1. Wiley-VCH Verlag GmbH & Co; Weinheim, Germany: 2008. [Google Scholar]
- 17.Cheng G, Lim RK, Li N, Lin Q. Chem commun. 2013;49:6809–6811. doi: 10.1039/c3cc43479f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bertozzi CR. Acc Chem Res. 2011;44:651–653. doi: 10.1021/ar200193f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sletten EM, Bertozzi CR. Angew Chem Int Ed. 2009;48:6974–6998. doi: 10.1002/anie.200900942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yeung CS, Zhao X, Borduas N, Dong VM. Chem Sci. 2010;1:331–336. [Google Scholar]
- 21.Houlden CE, Hutchby M, Bailey CD, Ford JG, Tyler SN, Gagne MR, Lloyd-Jones GC, Booker-Milburn KI. Angew Chem Int Ed. 2009;48:1830–1833. doi: 10.1002/anie.200805842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rauf W, Thompson AL, Brown JM. Chem Commun. 2009:3874–3876. doi: 10.1039/b905717j. [DOI] [PubMed] [Google Scholar]
- 23.Xiao B, Fu Y, Xu J, Gong TJ, Dai JJ, Yi J, Liu L. J Am Chem Soc. 2010;132:468–469. doi: 10.1021/ja909818n. [DOI] [PubMed] [Google Scholar]
- 24.John A, Nicholas KM. J Org Chem. 2012;77:5600–5605. doi: 10.1021/jo300713h. [DOI] [PubMed] [Google Scholar]
- 25.Bahsoun A, Dehand J, Pfeffer M, Zinsius M, Bouaoud S-E, Le Borgne G. J Chem Soc, Dalton Trans. 1979:547–556. [Google Scholar]
- 26.The trans-alkene structure was assigned based on the consideration of the sterics during the alkyne insertion step; see: Larock RC, Yum EK, Refvik MD. J Org Chem. 1998;63:7652–7662.
- 27.Lang K, Chin JW. ACS Chem Biol. 2014;9:16–20. doi: 10.1021/cb4009292. [DOI] [PubMed] [Google Scholar]
- 28.Yu Z, Lin Q. J Am Chem Soc. 2014;136:4153–4156. doi: 10.1021/ja5012542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rossin R, van den Bosch SM, Ten Hoeve W, Carvelli M, Versteegen RM, Lub J, Robillard MS. Bioconjug Chem. 2013;24:1210–1217. doi: 10.1021/bc400153y. [DOI] [PubMed] [Google Scholar]
- 30.In our preliminary studies, a biotin-containing palladacycle can be readily prepared; see Figure S3 in the ESI for details.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.