Abstract
Mechanical forces affect all the tissues of our bodies. Experiments conducted mainly on cultured cells have established that altering these forces influences cell behaviors, including migration, differentiation, apoptosis, and proliferation [1, 2]. The transcriptional co-activator YAP has been identified as a nuclear relay of mechanical signals, but the molecular mechanisms that lead to YAP activation were not identified [3]. YAP is the main transcriptional effector of the Hippo signaling pathway, a major growth regulatory pathway within metazoa [4], but at least in some instances the influence of mechanical strain on YAP were reported to be independent of Hippo signaling [5, 6]. Here we identify a molecular pathway that can promote the proliferation of cultured mammary epithelial cells in response to cyclic or static stretch. These mechanical stimuli are associated with increased activity of the transcriptional co-activator YAP, which is due at least in part to inhibition of Hippo pathway activity. Much of this influence on Hippo signaling can be accounted for by the activation of c-Jun N-terminal kinase (JNK) activity by mechanical strain, and subsequent inhibition of Hippo signaling by JNK. LATS1 is a key negative regulator of YAP within the Hippo pathway, and we further show that cyclic stretch is associated with a JNK-dependent increase in binding of a LATS inhibitor, LIMD1, to the LATS1 kinase, and that reduction of LIMD1 expression suppresses the activation of YAP by cyclic stretch. Together, these observations establish a pathway for mechanical regulation of cell proliferation via JNK-mediated inhibition of Hippo signaling.
YAP activity has been examined after varied means of altering cellular mechanics, including altering F-actin accumulation, plating cells on stiff versus soft substrates, modulating the extent of cellular attachment to the extracellular matrix (ECM), and altering cell shape through varied ECM attachments or stretching [5-10]. We examined the cellular response to a direct application of mechanical strain, achieved through a regime of cyclic stretch by attaching cells of the human mammary epithelial cell line MCF10A to a flexible substrate, and subjecting this substrate to 20% of uniaxial cyclic stretch at 1 Hz over a period of several hours. This frequency of mechanical strain is similar to that experienced by many cells in vivo, and can stimulate the proliferation of cultured cells in vitro [11, 12] (Fig 1A-B).
Figure 1. Cyclic stretch increases YAP activity through down-regulation of Hippo signaling.
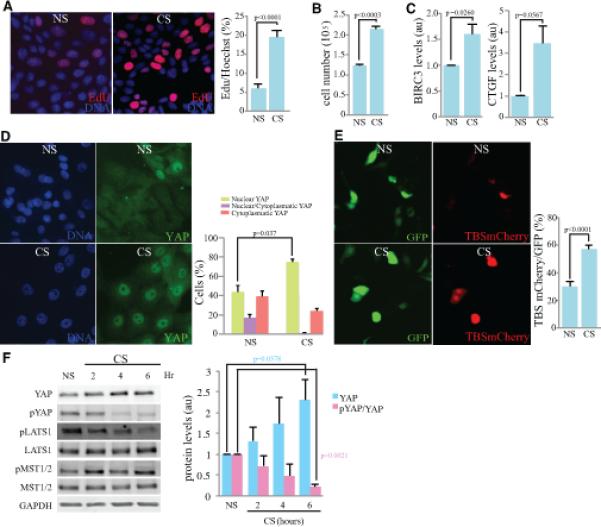
Comparisons of MCF10A cells subject to cyclic stretch (CS) and non-stretched controls (NS) are shown. A) Cell proliferation, visualized by Edu labeling (red) after 6 h CS, with nuclei labeled by Hoechst (blue). Histogram at right shows quantitation of the mean percent labeled cells, from three biological replicates. B) Quantitation of cell numbers after 6 h CS compared to NS controls, from three biological replicates. C) Histogram shows result of quantitative RT-PCR on BIRC3 and CTGF mRNA level in cells after 6 h CS compared to NS controls, from three biological replicates. The mRNA over GAPDH ratio in all samples were normalized to the ratio in NS control cells. D) Immunolocalization of YAP (green) after 6 h CS compared to NS controls. Histogram at right shows quantitation of the mean percent cells with predominantly nuclear YAP, from three biological replicates. E) Cells co-transfected with TBS-mCherry (red) and GFP (green) after 6 h CS compared to NS controls. Graph indicates the percentage of GFP-expressing cells that are positive for TBS-mCherry, from three biological replicates. F) Western blot on lysates of cells after 2h, 4h, and 6 h CS, compared to NS controls, blotted with anti-YAP, anti-pYAP, anti-pLATS, anti-LATS, anti-pMST, anti-MST and anti-GAPDH, as indicated. Histogram shows average (from three biological replicates) of relative levels of YAP normalized to GAPDH (loading control), together with ratio of pYAP/YAP. In all panels, error bars indicate standard error. See also Fig. S1.
To assess whether YAP could be activated by cyclic stretch, we first analyzed its sub-cellular localization. YAP functions as transcriptional activator within the nucleus, and Hippo signaling decreases its activity both by decreasing its stability and by promoting its cytoplasmic localization [13]. Six hours of cyclic stretch caused an increase in the nuclear localization of YAP (Fig. 1D), implying that YAP is activated by cyclic stretch. This was confirmed by using a reporter plasmid with multiple copies of a consensus binding sequence for the YAP DNA-binding partner TEAD, cloned upstream of mCherry (TBS-mCherry)[14]. Cyclic stretch increased the fraction of cells with visible TBS-mCherry expression (Fig 1E). Moreover, mRNA levels of two transcriptional targets of YAP, BIRC3 and CTGF [15], were also increased by cyclic stretch (Fig. 1C). A direct correlation between increased cell proliferation and activation of YAP induced by cyclic stretch was revealed by co-staining for YAP and, via EdU labeling, proliferating cells (Supplementary Figure S1).
To determine whether this increased activity of YAP is associated with decreased Hippo pathway activity, we examined the phosphorylation status of YAP, together with upstream kinases within the Hippo pathway. The key negative regulators of YAP within the Hippo pathway are the LATS protein kinases. YAP is phosphorylated by LATS on multiple sites, including Ser127, which promotes cytoplasmic retention of YAP, and Ser381, which promotes YAP degradation. Six hours of cyclic stretch significantly decreased phosphorylation of YAP on Ser127, while at the same time increasing total YAP levels (Fig 1F). These observations imply that cyclic stretch is associated with Hippo pathway-dependent YAP regulation, though we note that this doesn't exclude the possibility of additional, Hippo pathway-independent effects. Modulation of Hippo signaling was also indicated by examination of LATS1 phosphorylation, as LATS activation is associated with phosphorylation of Thr1079. Cyclic stretch decreased LATS1 phosphorylation at this site (Fig 1F). In some cases, LATS activation is associated with activation of the LATS kinase MST. However, we did not detect any influence of cyclic stretch on MST phosphorylation (Fig 1F), and therefore, infer that cyclic stretch decreases LATS activity without altering MST activity.
Stretch-induced actin stress, including that induced by cyclic stretch, can lead to activation of c-jun N-terminal kinase (JNK)[16, 17]. JNK is a stress-activated kinase, which promotes varied responses including cytoskeletal modulation, apoptosis or cell proliferation [18-20]. JNK is also activated by tissue damage, and was found in the context of regenerative responses to damage in Drosophila to be associated with activation of the YAP homologue Yorkie [21-25]. Moreover, JNK was found to be required for activation of CTGF expression by cyclic stretch in osteoblasts [26]. Using antibodies that recognizes the activated (phosphorylated) form of JNK, and its substrate cJun, we found that JNK is activated under our cyclical stretching regime (Fig. 2A). These observations led us to hypothesize that the activation of YAP under cyclical strain might be caused by JNK activation. This was evaluated by using a well-characterized JNK-specific inhibitor, SP600125 [27]. SP600125 treatment was effective since it was able to prevent the phosphorylation of c-Jun (Fig. 2B). This inhibition of JNK suppressed the influence of cyclic stretch on Hippo signaling, as evidenced by the reversal of the decreased YAP Ser127 phosphorylation by cyclic stretch in the presence of SP600125 (Fig. 2B). Moreover, inhibition of JNK also reduced the fraction of cells exhibiting nuclear YAP (Fig. 2C), reduced YAP activity as assayed by TBS-mCherry and BIRC3 expression (Fig. 2E,F), and decreased cell proliferation (Fig. 2D). To further confirm the role of JNK, we also transfected an shRNA against JNK1 and 2 (shJNK1/2)[28] and observed a decrease in nuclear YAP and increase in cytoplasmic YAP among transfected cells (Supplementary Figure S2). Thus, JNK contributes to YAP activation and cell proliferation induced by cyclic stretch.
Figure 2. Activation of YAP by cyclic stretch is JNK-dependent.
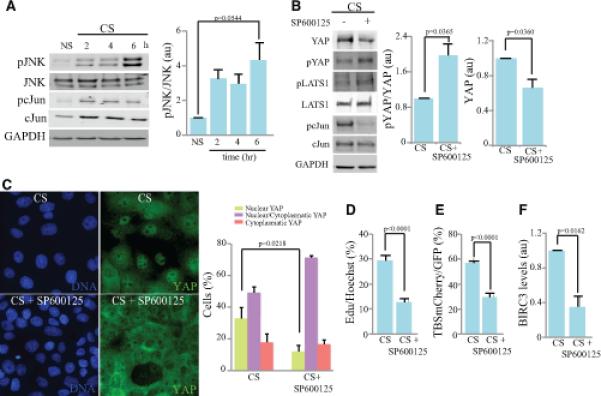
Influence of JNK inhibition on the response of MCF10A cells to cyclic stretch (CS). A) Western blot on lysates of cells after 2h, 4h, and 6 h CS, compared to non-stretched (NS) controls, blotted with anti-JNK, anti-pJNK, anti-pcJun, anti-cJun and anti-GAPDH. Histogram shows average ratio of pJNK/JNK from three biological replicates. B) Western Blot on lysates of cells treated with 50 μM SP600125 (JNK inhibitor) and subject to CS for 6 hours, blotted with anti- YAP, pYAP, LATS1, pLATS1, pcJun, cJun and GAPDH. Histogram shows average ratio of pYAP/YAP, and total YAP normalized to GAPDH from four biological replicates. C) Immunolocalization of YAP (green) and nuclei (DNA, blue) in cells treated with 50 μM SP600125 and subject to 6 h CS. Histogram at right shows quantitation of the mean percent cells (from three biological replicates) of cells with predominantly nuclear YAP (green), similar cytoplasm and nuclear staining (purple) and predominantly cytoplasmatic YAP (pink). D) Cell proliferation, visualized by Edu labeling of cells treated with 50 μM SP600125 or vehicle control, and then subject to 6 h CS. Histogram shows quantitation of the mean percent labeled cells, from three biological replicates. E) Mean percentage of transfected (GFP-expressing) cells that are positive for TBS-mCherry, treated with 50 μM SP600125 or vehicle control, and then subject to 6 h CS, from three biological replicates. F) Histogram shows result of quantitative RTPCR on BIRC3 mRNA level in cells after 6 h CS compared to NS controls three biological replicates. The BIRC3 over GAPDH ratio in all samples were normalized to the ratio in CS non-treated cells. In all panels, error bars indicate standard error of the mean. See also Fig. S2.
To investigate whether JNK activation might contribute to other modes of mechanical regulation of YAP and TAZ, we examined MCF10A cells cultured on substrates of different stiffness. Cells adjust their cytoskeletal tension to match their mechanical environment, and consequently cells grown on stiff substrates experience higher tension than those cultured on soft substrates [1, 29]. Cells grown on stiff substrates adopt a flat, spread morphology and have higher proliferation rates, whereas cells grown on soft substrates have a round morphology and lower proliferation rates [6, 29, 30]. Culturing on stiff substrates has been reported to increase YAP nuclear localization through an as yet unidentified, but Hippo pathway-independent, mechanism [6]. In accordance with these previous studies, we found that in MCF10A cells grown on stiff substrates, cell proliferation was increased (Supplementary Figure S3A). Moreover, in cells grown on soft substrates YAP was predominantly cytoplasmic, whereas in cells cultured on stiff substrates YAP was predominantly nuclear (Fig 3A), YAP protein levels were elevated, and YAP transcriptional activity was higher (Figs 3A,B, S3B,C). However, we also observed a reduction in the levels of YAP phosphorylation on Ser127 in cells cultured on stiff substrates (Fig. 3B). This observation suggests that part of the influence of substrate stiffness on YAP activity could be mediated through an effect on the Hippo pathway. This does not contradict evidence for Hippo-independent effects of substrate stiffness [6], as there could be multiple biomechanical pathways that impinge on YAP activity. Indeed, other studies have reported that substrate attachment and cell shape can influence LATS activity in some contexts [7, 8].
Figure 3. Hippo and JNK signaling contribute to YAP activation by ECM stiffness.
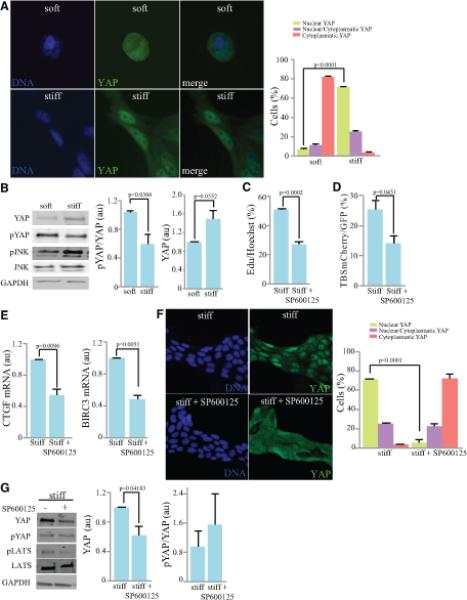
Comparisons of MCF10A cells plated on soft (0.2 kPa) versus stiff (35 kPa) substrates. A) Immunolocalization of YAP (green) and nuclei (Hoechst, blue). Histogram at right shows quantitation of the mean percent cells (from three biological replicates) with predominantly nuclear YAP (green), similar cytoplasm and nuclear staining (purple) and predominantly cytoplasmic YAP (pink). B) Western Blot on lysates of cells plated on soft and stiff substrates. Cells were lysed 24 h after of plating and then blotted with anti-YAP, pYAP, pJNK, JNK and GAPDH. Histogram shows normalized ratios of pYAP/YAP and YAP/GAPDH, from three biological replicates. C) Cell proliferation, visualized by Edu labeling of cells grown on stiff substrates and treated with 50 μM SP600125 or vehicle control for 4 h. Histogram shows quantitation of the mean percent labeled cells, from three biological replicates. D) Mean percentage of transfected (GFP-positive) cells that are positive for TBS-mCherry, among cells grown on stiff substrates and treated with 50 μM SP600125 or vehicle control for 4 h, from three biological replicates. E) Quantitation of BIRC3 and CTGF mRNA levels by RT-PCR in cells plated on stiff substrates treated with 50 μM SP600125 or vehicle control, from three biological replicates. The mRNA over GAPDH ratio in all samples was normalized to the ratio in stiff vehicle-treated cells F) Immunolocalization of YAP (green) and nuclei (Hoechst, blue) in cells plated on stiff substrates and treated with 50 μM SP600125 or vehicle control for 4 h. Histogram at right shows quantitation of the mean percent cells (from three biological replicates) of cells with predominantly nuclear YAP (green), similar cytoplasmic and nuclear staining (purple) and predominantly cytoplasmic YAP (pink). G) Western Blot on lysates of cells plated on stiff substrates and treated with 50 μM SP600125 or vehicle control for 4 h, blotted with anti- YAP, pYAP, pLATS, LATS and GAPDH. Histogram shows mean YAP levels (normalized to GAPDH) and ratio of pYAP/YAP, from three biological replicates. In all panels, error bars indicate standard error. See also Fig. S3.
As we had observed that the influence of cyclic stretch on Hippo signaling is dependent upon JNK signaling, we investigated whether JNK activation contributes to YAP activation promoted by substrate stiffness. Indeed, JNK activity is higher on stiff substrates (Fig. 3B) and treatment of cells on stiff substrates with the JNK inhibitor SP600125 shifted YAP from a predominantly nuclear to predominantly cytoplasmic localization (Fig. 3F), and consistent with this shift, reduced both YAP transcriptional activity (Fig. 3D,E) and cell proliferation (Fig 3C). However, while we detected a consistent decrease in total YAP levels upon JNK inhibitor treatment, the increase in YAP phosphorylation was more variable and was not statistically significant (Fig. 3G). These observations identify JNK activation as a process that contributes in YAP activation induced by substrate stiffness. However, the lack of consistent effects on YAP phosphorylation suggests that the mechanism by which JNK influences YAP activity on stiff substrates might be distinct from its influence on cells subject to cyclic stretch. Alternatively, it could be that the activation of JNK, and its consequent effects on Hippo signaling are simply more robust under cyclic stretch than under increased substrate stiffness.
To elucidate the mechanism of YAP activation by reduced Hippo signaling under cyclic stretch, we took advantage of recent observations on Ajuba family proteins. Ajuba family proteins (mammalian members include Ajuba, LIMD1, and WTIP) are characterized by three C-terminal LIM domains, can exhibit cytoskeletal-associated localization, and have been implicated in diverse forms of gene regulation [31, 32]. Studies in Drosophila, which have only a single Ajuba family member (Jub), have established that genetically they act as negative regulators of Hippo signaling [31, 33], and experiments in cultured cells have established that each of the three mammalian Ajuba family proteins can bind to LATS proteins, and that they inhibit LATS activity [31]. Moreover, phosphorylation of WTIP or LIMD1 by ERK or JNK can increase their binding to LATS and consequent inhibition of LATS activity [22, 34]. We observed that under conditions of cyclic stretch, binding between endogenous LIMD1 and LATS1 proteins was increased, as detected by co-immunoprecipitation experiments (Fig. 4A). Moreover, pharmacological inhibition of JNK activity decreased LATS1-LIMD1 binding under conditions of cyclic stretch (Fig. 4B). These observations suggest that cyclic stretch increases YAP activity through the ability of JNK to phosphorylate and thereby promote binding of the LATS-inhibitor LIMD1 to LATS. Consistent with this, Phos-tag gels revealed that cyclic stretch stimulated phosphorylation of LIMD1, and this effect was reversed when cells were treated with a JNK inhibitor (Fig. 4C). We also examined the requirement for LIMD1 in activation of YAP under cyclic stretch. Co-transfection of an shRNA against LIMD1 eliminated the elevated YAP activity (observed using the TBS-mCherry reporter) normally induced by cyclic stretch (Fig. 4D), implying that the activation of YAP caused by this mechanical strain requires LIMD1.
Figure 4. Cyclic Stretch increases LIMD1-LATS1 binding to activate YAP.
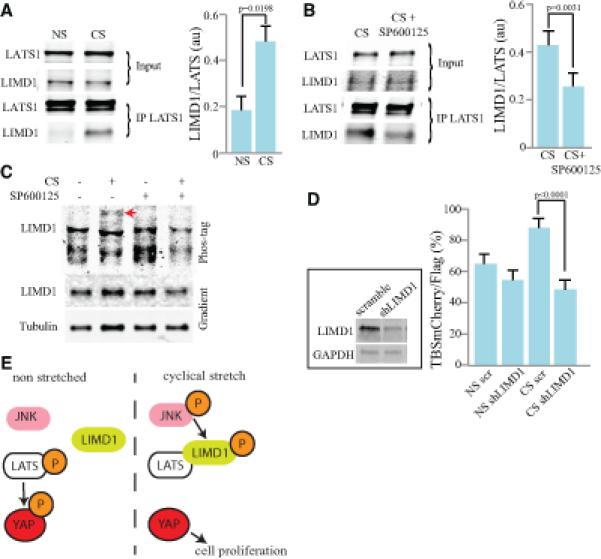
Comparisons of MCF10A cells subject to 6h cyclic stretch (CS) and non-stretched controls (NS) or cells treated with SP600125 or shRNAs, as indicated. A) Western blots showing the results of co-immunoprecipitation experiments on cells subject to CS or NS controls. Upper two blots (input) show relative amounts of endogenous LATS1 and LIMD1 in cell lysates, lower two panels show relative amounts immunoprecipitated using anti-LATS1 sera. Histogram shows average ratio of LIMD1/LATS1 from four biological replicates. B) Western blots showing the results of co-immunoprecipitation experiments on cells subject to CS and treated with SP600125 or vehicle control. Upper two blots (input) show relative amounts of endogenous LATS1 and LIMD1 in cell lysates, lower two panels show relative amounts immunoprecipitated using anti-LATS1 sera. Histogram shows average ratio of LIMD1/LATS1 from four biological replicates, normalized to levels in lysates. C) Western blots on lysates of MCF10A cells treated with DMSO or SP600125, and with or without CS, as indicated. The lower two blots show a standard 4-15% gradient gel, and the upper blot shows a Phos-tag gel. The arrow indicates a band of slow mobility (highly phosphorylated) LIMD1 induced by CS, and suppressed by SP600125. D) YAP activity, visualized by mean percentage of transfected (Flag-expressing) cells that are positive for TBS-mCherry, among cells subject to 6h CS or NS controls, and treated either with a shRNA specific for LIMD1 or a negative control shRNA (scramble), from three biological replicates. Cells expressing shRNAs are marked by expression of a FLAG-epitope from the same plasmid. Inset (upper left), western blot showing the effectiveness of shLIMD1. Lysate of total levels of HEK cells transfected with shLIMD1 for 24 hours and blotted for LIMD1 and GAPDH. E) Model illustrating the proposed mechanism in which cyclical stretch promotes the binding of LIMD1 to LATS1 by activating JNK. This inhibits LATS, and therefore increases YAP activity, which increases cell proliferation. In all panels, error bars indicate standard error. See also Fig. S4.
In conclusion, our observations have provided new insights into mechanisms that cells use to respond to their mechanical environment by defining a molecular pathway for mechanical regulation of cell proliferation, in which cyclic stretch promotes LIMD1-LATS1 binding through activation of JNK, leading to reduced LATS activity and consequently elevated YAP activity (Fig. 4E). We have also identified JNK activation as a contributor to a biomechanical pathway in which YAP is activated by increased substrate stiffness. Beyond the insights gained into the mechanism of biomechanical signal transduction, this identification of JNK and LIMD1 as key players in biomechanical signaling establishes them as key targets of future investigation for further studies of how cells respond to their mechanical environment.
Supplementary Material
Acknowledgements
We thank F. Camargo, S. Andreadis and D. Fowler for reagents. This research was supported by Human Frontiers Science Program grant RGP0016/2010 and the Howard Hughes Medical Institute.
References
- 1.Jaalouk DE, Lammerding J. Mechanotransduction gone awry. Nature reviews. Molecular cell biology. 2009;10:63–73. doi: 10.1038/nrm2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wozniak MA, Chen CS. Mechanotransduction in development: a growing role for contractility. Nature reviews. Molecular cell biology. 2009;10:34–43. doi: 10.1038/nrm2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Halder G, Dupont S, Piccolo S. Transduction of mechanical and cytoskeletal cues by YAP and TAZ. Nature Reviews Molecular Cell Biology. 2012;13:591–600. doi: 10.1038/nrm3416. [DOI] [PubMed] [Google Scholar]
- 4.Yu FX, Guan KL. The Hippo pathway: regulators and regulations. Genes & development. 2013;27:355–371. doi: 10.1101/gad.210773.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aragona M, Panciera T, Manfrin A, Giulitti S, Michielin F, Elvassore N, Dupont S, Piccolo S. A Mechanical Checkpoint Controls Multicellular Growth through YAP/TAZ Regulation by Actin-Processing Factors. Cell. 2013;154:1047–1059. doi: 10.1016/j.cell.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 6.Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 7.Zhao B, Li L, Wang L, Wang C-Y, Yu J, Guan K-L. Cell detachment activates the Hippo pathway via cytoskeleton reorganization to induce anoikis. Genes & Development. 2012;26:54–68. doi: 10.1101/gad.173435.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wada K-I, Itoga K, Okano T, Yonemura S, Sasaki H. Hippo pathway regulation by cell morphology and stress fibers. Development (Cambridge, England) 2011;138:3907–3914. doi: 10.1242/dev.070987. [DOI] [PubMed] [Google Scholar]
- 9.Sansores-Garcia L, Bossuyt W, Wada K-I, Yonemura S, Tao C, Sasaki H, Halder G. Modulating F-actin organization induces organ growth by affecting the Hippo pathway. The EMBO Journal. 2011;30:2325–2335. doi: 10.1038/emboj.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernández BG, Gaspar P, Brás-Pereira C, Jezowska B, Rebelo SR, Janody F. Actin-Capping Protein and the Hippo pathway regulate F-actin and tissue growth in Drosophila. Development (Cambridge, England) 2011;138:2337–2346. doi: 10.1242/dev.063545. [DOI] [PubMed] [Google Scholar]
- 11.Fujita H, Hida M, Kanemoto K, Fukuda K, Nagata M, Awazu M. Cyclic stretch induces proliferation and TGF-beta1-mediated apoptosis via p38 and ERK in ureteric bud cells. American journal of physiology. Renal physiology. 2010;299:F648–655. doi: 10.1152/ajprenal.00402.2009. [DOI] [PubMed] [Google Scholar]
- 12.Wang JG, Miyazu M, Xiang P, Li SN, Sokabe M, Naruse K. Stretch-induced cell proliferation is mediated by FAK-MAPK pathway. Life sciences. 2005;76:2817–2825. doi: 10.1016/j.lfs.2004.10.050. [DOI] [PubMed] [Google Scholar]
- 13.Oh H, Irvine KD. Yorkie: the final destination of Hippo signaling. Trends in cell biology. 2010;20:410–417. doi: 10.1016/j.tcb.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schlegelmilch K, Mohseni M, Kirak O, Pruszak J, Rodriguez JR, Zhou D, Kreger BT, Vasioukhin V, Avruch J, Brummelkamp TR, et al. Yap1 acts downstream of alpha-catenin to control epidermal proliferation. Cell. 2011;144:782–795. doi: 10.1016/j.cell.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao B, Ye X, Yu J, Li L, Li W, Li S, Lin JD, Wang CY, Chinnaiyan AM, Lai ZC, et al. TEAD mediates YAP-dependent gene induction and growth control. Genes & development. 2008;22:1962–1971. doi: 10.1101/gad.1664408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaunas R, Usami S, Chien S. Regulation of stretch-induced JNK activation by stress fiber orientation. Cellular signalling. 2006;18:1924–1931. doi: 10.1016/j.cellsig.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 17.Kito H, Chen EL, Wang X, Ikeda M, Azuma N, Nakajima N, Gahtan V, Sumpio BE. Role of mitogen-activated protein kinases in pulmonary endothelial cells exposed to cyclic strain. Journal of applied physiology. 2000;89:2391–2400. doi: 10.1152/jappl.2000.89.6.2391. [DOI] [PubMed] [Google Scholar]
- 18.Dhanasekaran DN, Reddy EP. JNK signaling in apoptosis. Oncogene. 2008;27:6245–6251. doi: 10.1038/onc.2008.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bogoyevitch MA, Kobe B. Uses for JNK: the many and varied substrates of the c-Jun N-terminal kinases. Microbiology and molecular biology reviews : MMBR. 2006;70:1061–1095. doi: 10.1128/MMBR.00025-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen F. JNK-induced apoptosis, compensatory growth, and cancer stem cells. Cancer Research. 2012;72:379–386. doi: 10.1158/0008-5472.CAN-11-1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun G, Irvine KD. Regulation of Hippo signaling by Jun kinase signaling during compensatory cell proliferation and regeneration, and in neoplastic tumors. Developmental biology. 2011;350:139–151. doi: 10.1016/j.ydbio.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun G, Irvine KD. Ajuba family proteins link JNK to Hippo signaling. Science signaling. 2013;6:ra81. doi: 10.1126/scisignal.2004324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grusche FA, Degoutin JL, Richardson HE, Harvey KF. The Salvador/Warts/Hippo pathway controls regenerative tissue growth in Drosophila melanogaster. Developmental biology. 2011;350:255–266. doi: 10.1016/j.ydbio.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 24.Staley BK, Irvine KD. Warts and Yorkie mediate intestinal regeneration by influencing stem cell proliferation. Current biology : CB. 2010;20:1580–1587. doi: 10.1016/j.cub.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shaw RL, Kohlmaier A, Polesello C, Veelken C, Edgar BA, Tapon N. The Hippo pathway regulates intestinal stem cell proliferation during Drosophila adult midgut regeneration. Development. 2010;137:4147–4158. doi: 10.1242/dev.052506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiao L-W, Yang M, Dong J, Xie H, Sui G-L, He Y-L, Lei J-X, Liao E-Y, Yuan X. Stretch-inducible expression of connective tissue growth factor (CTGF) in human osteoblasts-like cells is mediated by PI3K-JNK pathway. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology. 2011;28:297–304. doi: 10.1159/000331743. [DOI] [PubMed] [Google Scholar]
- 27.Bennett BL, Sasaki DT, Murray BW, O'Leary EC, Sakata ST, Xu W, Leisten JC, Motiwala A, Pierce S, Satoh Y, et al. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:13681–13686. doi: 10.1073/pnas.251194298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee MH, Koria P, Qu J, Andreadis ST. JNK phosphorylates beta-catenin and regulates adherens junctions. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2009;23:3874–3883. doi: 10.1096/fj.08-117804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwartz MA. Integrins and extracellular matrix in mechanotransduction. Cold Spring Harbor perspectives in biology. 2010;2:a005066. doi: 10.1101/cshperspect.a005066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y, Wang G, Luo X, Qiu J, Tang C. Substrate stiffness regulates the proliferation, migration, and differentiation of epidermal cells. Burns : journal of the International Society for Burn Injuries. 2012;38:414–420. doi: 10.1016/j.burns.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 31.Das Thakur M, Feng Y, Jagannathan R, Seppa MJ, Skeath JB, Longmore GD. Ajuba LIM proteins are negative regulators of the Hippo signaling pathway. Current biology : CB. 2010;20:657–662. doi: 10.1016/j.cub.2010.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Langer EM, Feng Y, Zhaoyuan H, Rauscher FJ, Kroll KL, Longmore GD. Ajuba LIM proteins are snail/slug corepressors required for neural crest development in Xenopus. Developmental cell. 2008;14:424–436. doi: 10.1016/j.devcel.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rauskolb C, Pan G, Reddy BV, Oh H, Irvine KD. Zyxin links fat signaling to the hippo pathway. PLoS biology. 2011;9:e1000624. doi: 10.1371/journal.pbio.1000624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reddy BV, Irvine KD. Regulation of Hippo signaling by EGFR-MAPK signaling through Ajuba family proteins. Developmental cell. 2013;24:459–471. doi: 10.1016/j.devcel.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


