Abstract
The aim of this study was to explore the feasibility and repeatability of amide proton transfer weighted (APTw) MRI for the head and neck on clinical MRI scanners. Six healthy volunteers and four patients with head and neck tumors underwent APTw-MRI scanning at 3T. The APTw signal was quantified by the asymmetric magnetization transfer ratio (MTRasym) at 3.5 ppm. Z-spectra of normal tissues in the head and neck (masseter muscle, parotid glands, submandibular glands and thyroid glands) were analyzed in healthy volunteers. Inter-scan repeatability of APTw-MRI was evaluated in six healthy volunteers. Z-spectra of head and neck tumor patients were produced and APTw signals in these tumors were analyzed. APTw-MRI scanning was successful for all ten subjects. The parotid glands showed the highest APTw signal (~7.6% averagely), while the APTw signals in other tissues were relatively moderate. The repeatability of APTw signals from the masseter muscle, parotid gland, submandibular gland and thyroid gland of healthy volunteers was established. Four head and neck tumors showed positive mean APTw ranging from 1.2% to 3.2%, distinguishable from surrounding normal tissues. APTw-MRI was feasible for the use in the head and neck regions at 3T. The preliminary results on patients with head and neck tumors indicated the potential of APTw-MRI for clinical applications.
Keywords: chemical exchange saturation transfer (CEST), amide proton transfer (APT), asymmetric magnetization transfer ratio, head and neck tumor, parotid gland
INTRODUCTION
Magnetic resonance imaging (MRI) plays an important role in the management of patients with head and neck tumors. This includes, but is not limited to, the initial investigation for tumor detection, characterization and staging; prediction of treatment outcome; post-treatment assessment and surveillance. Conventional anatomical MRI provides excellent anatomical detail of the tumor and the surrounding structures of the head and neck, but has limitations in the characterization of the molecular nature of tumors. Hence, more advanced MRI techniques are needed that can evaluate the microstructure and function of head and neck cancers beyond morphology. Advanced MRI functional techniques, such as diffusion weighted imaging (DWI), dynamic contrast enhanced (DCE) MRI, and magnetic resonance spectroscopy (MRS), are already undergoing clinical evaluation (1–5).
Chemical exchange saturation transfer (CEST) MRI has recently emerged as a new contrast mechanism for molecular and cellular MR imaging by exploring the chemical exchange processes between free water and mobile exchangeable agents (exogenous or endogenous) in vivo that are relevant to physiology or pathology. Despite the fact that ultra-high field strength MRI and exogenous contrast agents are heavily used in experimental and preclinical CEST studies, the use of endogenous CEST-MRI in clinical practice has been increasing in recent years. Based on different endogenous biochemical components in tissues that provide CEST effects at different off-resonant frequencies, different variants of endogenous CEST-MRI have been derived, including amide proton transfer weighted (APTw) imaging for mobile proteins and peptides (6–9), gagCEST imaging for glycosaminoglycans in cartilage and intervertebral discs (10–12), glycoCEST imaging for glycogen in liver (13), gluCEST imaging for glutamates (14), as well as glucoCEST imaging for glucoses (15, 16).
APTw-MRI is as a promising molecular MRI technique which investigates the chemical exchange processes between free water and mobile amide protons of protein/peptide backbones. APTw-MRI is sensitive to the detection of small variations in amide proton concentration associated with tumor proteins/peptide backbone and potentially could provide a novel tool of tumor detection and characterization. The feasibility and usefulness of APTw-MRI has been demonstrated in some clinical cancer studies applied to relatively stationary tissues in the brain (17–19), prostate (20) and breast (21).
To the best of our knowledge, there have been no studies applying CEST-MRI for the head and neck imaging to date. On the other hand, head and neck tumors have been probed for spectral markers such as amides (I, II, III), phosphate, nucleic acids and carbohydrates vibrational modes, using optical techniques such as Raman spectroscopy and infrared spectroscopy (22–25). In particular, Stone et al found in their Raman spectroscopy study that the relative intensity of the DNA mode and the amide III mode increases with progression of laryngeal malignancies, and could be used as a potential diagnostic algorithm for differentiation of normal tissue, dysplasia and carcinoma (25). We hypothesize that amide variations associated with the protein changes in the head and neck tumors may be detected and characterized non-invasively using APTw-MRI, thus providing a new tool for clinical evaluation of head and neck cancer. However, advanced MRI techniques can be difficult to perform in the head and neck because of large susceptibility artifacts, movement, and the wide range of different anatomical tissues that make up the normal structures of this complex region of the body. To this end, the major purpose of this study is to investigate the feasibility of performing APTw-MRI in the head and neck at the clinical field strength of 3T, to analyze the Z-spectra and APTw images from normal head and neck tissues in healthy volunteers and to establish the repeatability. We sought also to analyze the Z-spectra and APTw signal features in patients with head and neck tumors.
METHODS
Subjects
The local institutional ethics committee approved this prospective study and written consent forms were obtained from each subject. Six healthy volunteers (age: 21–25 years; mean: 23 years) were recruited to assess the feasibility and repeatability of APTw-MRI for head and neck at 3T. Four patients (age: 31–65 years; mean: 49 years) with an untreated head and neck tumor (parotid pleomorphic adenoma, nerve sheath tumor, primary nasopharyngeal carcinoma, metastatic node from squamous cell carcinoma) were also recruited for a preliminary assessment of the APTw-MRI for head and neck cancer imaging.
MR Imaging Protocol
All subjects were scanned on a Philips Achieva TX 3T scanner (Philips Healthcare, Best, The Netherlands) with a body coil for radiofrequency (RF) transmission and a 16-channel Philips neurovascular phased-array coil for reception. After the conventional MRI using T2-weighted images to localize the anatomy, APT imaging data was acquired using a single-slice turbo-spin-echo (TSE) sequence with chemical-shift selective fat suppression to reduce possible fat artifacts (26, 27). A continuous rectangular RF pulse with the B1 field strength of 2μT and the fixed time of 200 ms was used for saturation. This saturation pulse duration was relatively short but was the longest allowable continuous pulse duration on our scanner (even if the saturation field strength was reduced to 1μT) according to our testing. This short saturation pulse was probably restricted by the RF amplifier performance and protection. Localized high-order shimming was also performed for APT imaging to reduce the static magnetic field inhomogeneity ΔB0. Major imaging parameters were: field-of-view (FOV) = 230×230 mm2, voxel size = 2×2 mm2, slice thickness = 4 mm, TE/TR = 8 ms/2000 ms, echo train length (ETL) = 14, number of signal average (NSA) = 1, sensitivity encoding (SENSE) factor = 2, and partial Fourier factor = 0.7. A baseline image without applying the saturation pulse was acquired first, and then the saturated images were obtained at the offsets of (±0.25, ±0.5, ±1, ±1.5, ±2, ±2.5, ±3, ±3.5, ±4, ±4.5, ±5, ±5.5, ±6.5, ±7.5) parts-per-million (ppm). The total scan time of APTw-MRI lasted about two minutes. The saturation images at positive and negative offsets were acquired in an interleaved fashion. The offset frequency range applied here was broader than the normal brain scan protocol to address the possible large ΔB0 inhomogeneities in the head and neck region. The frequencies around the nominal water resonance and APT effect were not densely sampled, due also to the possible large ΔB0 inhomogeneities and the reduction of total scan time.
Each patient was scanned once while healthy volunteers were scanned twice with a time interval of at least 9 days to test the repeatability of APTw-MRI. For patients, only one slice was scanned for APTw-MRI which was selected at the level of the largest cross section through the tumor. For healthy volunteers, four individual representative slices through the nasopharynx, upper oropharynx, lower oropharynx and the mid neck were scanned, to include the major tissues of interest, namely masseter muscle, parotid gland, submandibular gland and thyroid gland.
Z-spectrum analysis
Data processing was performed by home-developed Matlab (MathWorks, Natick, MA, USA) programs. Z-spectrum for each voxel except for the background was first least-square fitted by a 12th-order polynomial model and the fitted curve was then interpolated to a finer resolution of 0.001 ppm. The actual water resonance was assumed to be at the frequency with the lowest intensity of the interpolated Z-spectrum, and the field inhomogeneity ΔB0 map was obtained. The interpolated Z-spectra were shifted correspondingly along the offset axis to correct for the field inhomogeneity ΔB0. It has been shown previously (28) that the Z-spectrum ΔB0 method and gradient echo ΔB0 method were highly consistent in CEST imaging corrections. As usual, the APT effect was quantified to reduce the interference of direct water saturation (DS) and traditional semisolid magnetization transfer (MT) by calculating the asymmetric magnetization transfer ratio (MTRasym) at the offset of 3.5 ppm using:
| [1] |
where S(ΔΩ) and S0 denote the signal intensities obtained with the saturation RF pulse at the offset ΔΩ and without the saturation pulse, respectively. MTRasym image at 3.5 ppm (named APTw image (29, 30) rather than pure APT map hereafter as this MTRasym(3.5ppm) includes some other contributions like nuclear Overhauser effect, NOE) (31–35) and ΔB0 map were produced by calculating the voxel-wise MTRasym and ΔB0 values. Besides, voxel-wise coefficient of determination R2 was also calculated to evaluate the goodness-of-fit of Z-spectrum fitting to the 12th-order polynomial model. To address the possible large field inhomogeneity ΔB0 and intrinsic tissue heterogeneities in the head and neck, some criteria were set to exclude the voxels associated with possible unreliable fitting results. In detail, a voxel would be excluded from APTw image if it showed either relatively poor goodness-of-fit (R2<0.99), or had extreme calculated MTRasym value (e.g. >0.3 or <−0.3), or had large ΔB0 shift (>2 ppm or <−2 ppm), or was not fully saturated due to the relatively short saturation pulse and/or incomplete fat suppression (if the ΔB0 corrected Z-spectrum bottom intensity was larger than 0.15S0).
Repeatability analysis
For each healthy volunteer, regions of interests (ROIs) were drawn on the head and neck MR images, around the masseter muscles, parotid glands, submandibular glands and thyroid gland lobes in both sides of the neck. The difference d, between the repeated scans of the mean APTw for each ROI was calculated. The Shapiro-Wilk test was performed to test the normality of d distribution. Then the significant difference between repeated scans was tested by using a Student’s t-test. To ensure the measurement error was independent of the mean, a Kendall’s tau test was performed. The following statistical measurements were computed to obtain the statistical measures of repeatability (36):
-
The mean squared difference dsd was calculated by:The dsd can then be used to compute the 95% confidence interval (CI):
The confidence interval value indicated that any change of APTw value in a group of n greater than this value would be significant at the 5% level.
- The within-subject standard deviation (wSD) was then obtained by:
The repeatability r of APTw was finally quantified as 2.77·wSD. The difference between two measurements of the same subject will be less than this figure for 95% of pairs of observations.
RESULTS
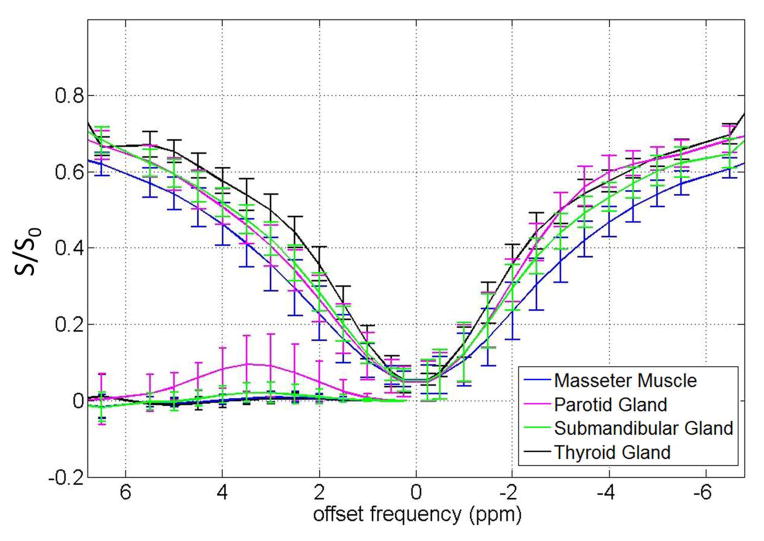
Data were successfully acquired in all 10 subjects (6 healthy volunteers and 4 patients with head and neck tumors). The ROI-averaged Z-spectra with corresponding MTRasym of the masseter muscle, parotid gland, submandibular gland and thyroid gland of the six healthy volunteers are shown in Figure 1. For the masseter muscle, its Z-spectrum was quite homogeneous in intensity (with very small standard deviation). The Z-spectrum shape was nearly symmetric within the scanned offset range, so no apparent CEST effect was observed on the whole MTRasym curve. It is found that the parotid gland, the largest of the major salivary glands, exhibited the most pronounced CEST effect on its Z-spectrum. The positive (compared to baseline) CEST MTRasym in the parotid gland ranged from less than 1 ppm to over 6 ppm with the maximum amplitude about 7.6% at around 3.5 ppm, the APT offset. In contrast, the submandibular gland (another major salivary gland) showed inconspicuous CEST MTRasym, generally smaller than 1.8%, also with the maximum amplitude at 3.5 ppm. For the thyroid gland, the MTRasym curve was very close to that of masseter muscle, without apparent CEST effect. However, the Z-spectrum of thyroid gland was higher than the Z-spectrum of masseter muscle, which indicated the more direct saturation of the masseter muscle. The APTw values of the normal masseter muscle, parotid gland, submandibular gland and thyroid gland for all six healthy volunteers are summarized in Table 1.
Figure 1.
Table 1.
APTw values in normal head and neck tissues
| Masseter Muscle (n=12) | Parotid gland (n=12) | Submandibular gland (n=12) | Thyroid gland (n=12) | |
|---|---|---|---|---|
| Mean | 0.78% | 7.62% | 1.60% | 0.59% |
| Median | 0.76% | 6.82% | 1.40% | 0.57% |
| Maximum | 6.47% | 29.91% | 18.50% | 7.10% |
| Minimum | −4.38% | −7.93% | −7.19% | −7.84% |
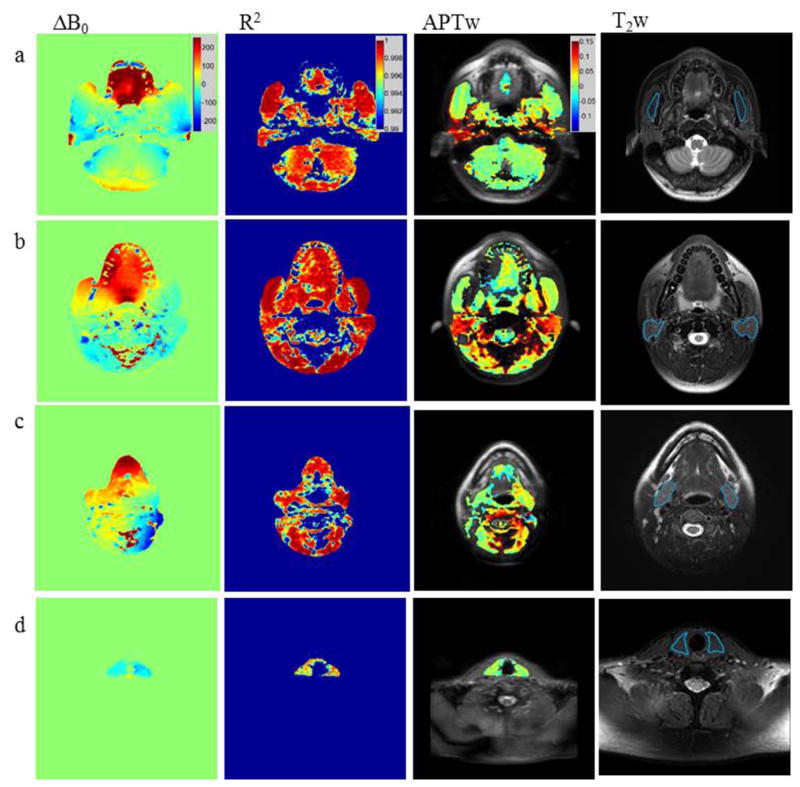
ΔB0 maps, R2 maps, APTw images (overlaid on the baseline image without saturation) and T2w anatomic images through the four representative slices at the level of the nasopharynx, upper oropharynx, lower oropharynx and the mid neck, are shown in Figures 2a–d. The ROIs of the masseter muscle, parotid gland, submandibular gland and thyroid gland are shown on the T2w anatomic images (Figure 2a–d). The most pronounced ΔB0 field inhomogeneities were found in the mouth (around the teeth and on the tongue) and near air-containing cavities such as the pharynx and paranasal sinuses. The field inhomogeneities could be much larger than 2 ppm even under high-order shimming. As such, it is no surprise that many voxels in these areas were excluded on APTw images. Even under these pronounced inhomogeneities, Z-spectra could still be well fitted to polynomial model with high R2 values, indicating the good image signal-to-noise ratio by using the optimized APTw-MRI protocol. It is clearly shown on APTw images that the parotid glands generally had much higher APTw signal compared to other tissues of interest.
Figure 2.
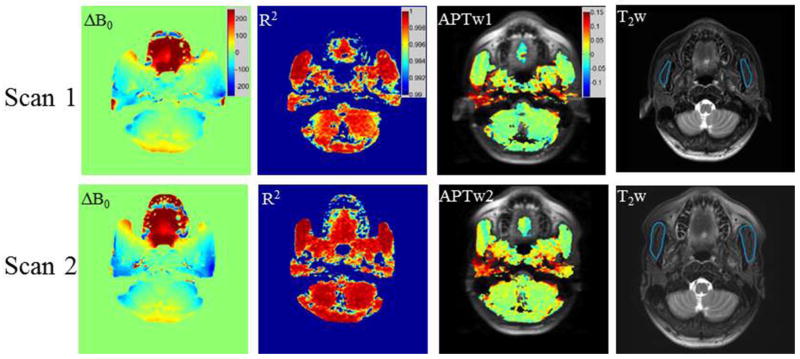
ΔB0 maps, R2 maps, APTw images and T2w anatomic images that were obtained in a healthy volunteer at the level of the nasopharynx (Figure 3 scan 1) and those obtained 9 days later in the same volunteer at the same level (Figure 3 scan 2) were illustrated in Figure 3. Although ΔB0 map exhibited differences between two scans, particularly in the region of tongue, the APTw images still showed quite good inter-scan consistency, and the coefficient of determination R2 maps showed satisfactory goodness-of-fit over 0.99.
Figure 3.
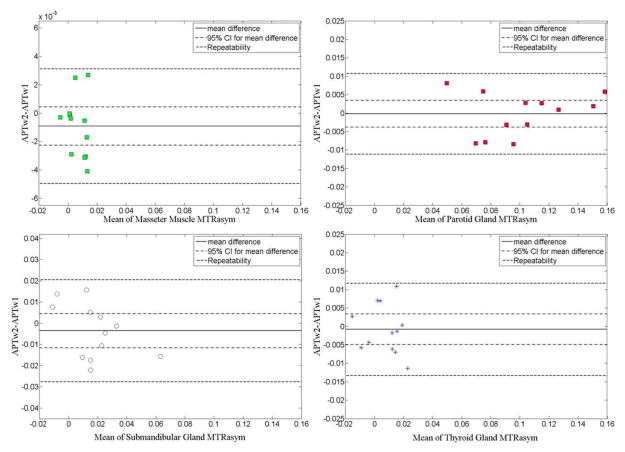
The values of the APTw difference between the two scans were plotted against the mean MTRasym of these two scans. The mean difference, the 95% confidence intervals for the mean difference and the repeatability are shown in the Bland-Altman plots (Figure 4). The calculated repeatability is the level of change that would be significant in an individual. In these four tissues of interest, the submandibular gland presented the largest repeatability value (0.0241), indicating the largest inter-scan variability. In comparison, the repeatability value for the masseter muscle was the smallest (0.0040), indicating the best consistency and the smallest variability between two scans. Overall, the inter-scan within-subject repeatability of APTw-MRI of four tissues in the head and neck was established. The statistics for the repeatability analysis of these four tissues are summarized in Table 2.
Figure 4.
Table 2.
Mean difference, 95% CI and repeatability of each region
| Parameter | Mean difference | 95%CI | Repeatability |
|---|---|---|---|
| Masseter muscle | −0.0009 | ±0.0013 | 0.0040 |
| Parotid gland | −0.0002 | ±0.0036 | 0.0109 |
| Submandibular gland | −0.0036 | ±0.0080 | 0.0241 |
| Thyroid gland | −0.0008 | ±0.0042 | 0.0125 |
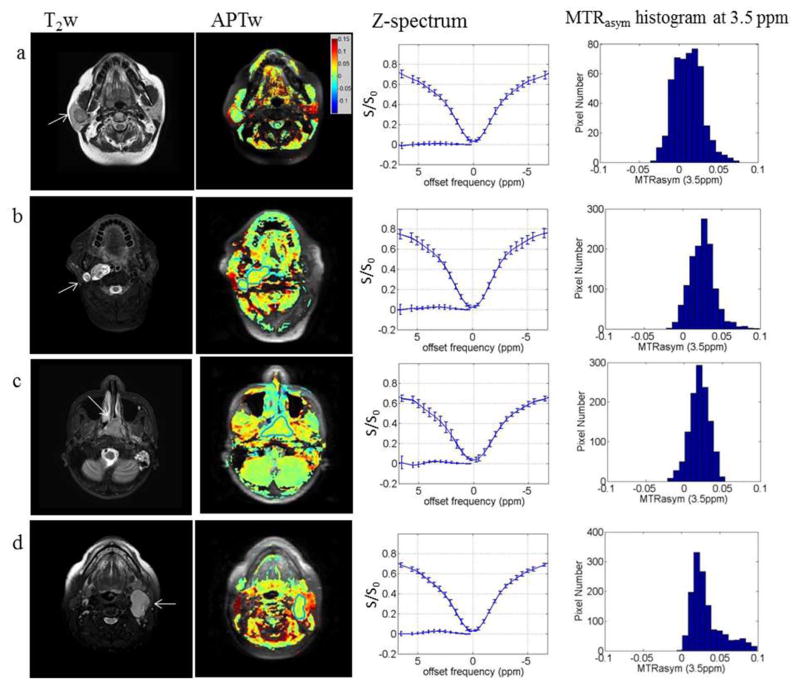
T2w anatomic images, APTw images (overlaid on the baseline image without saturation), Z spectrum and MTRasym histograms at 3.5 ppm are shown in Figure 5, for the four patients with head and neck tumors comprising of (a) pleomorphic adenoma in the right parotid gland; (b) nerve sheath tumor in the right parapharyngeal region; (c) primary carcinoma in the nasopharynx; (d) metastatic cervical node from squamous cell carcinoma. Patient details and APTw signals are summarized in Table 3. Compared with the surrounding tissues, the tumor tissues exhibited distinguishable signal differences in the APTw images. Even though there was an exception for the parotid pleomorphic adenoma, in which the APTw was just slightly higher than zero, the other three tumors showed apparently enhanced, positive APTw signals compared to adjacent tissues. In particular for the parotid pleomorphic adenoma (Fig. 5a) and the metastatic cervical node (Fig. 5d), their APTw values were significantly smaller than those of the normal parotid glands. The mean difference was over 5% so could be well delineated from the parotid glands. The APTw distribution for the nerve sheath tumor, primary nasopharyngeal carcinoma and metastatic lymph node were quite different, but most APTw values were positive and concentrated around 2.5% (the highest histogram bar). In comparison, the pleomorphic adenoma contained a fraction of negative APTw values and the APTw was more broadly distributed.
Figure 5.
Table 3.
Information and APTw values of the patients
| Gender | Age | Type | APTw Mean | APTw Median | APTw Maximum | APTw Minimum |
|---|---|---|---|---|---|---|
| Female | 53 | Pleomorphic Adenoma | 1.18% | 1.14% | 7.34% | −3.02% |
| Male | 65 | Nerve Sheath Tumor | 2.54% | 2.51% | 9.17% | −1.61% |
| Male | 31 | Nasopharyngeal Cancer | 3.24% | 2.56% | 9.90% | 0.06% |
| Female | 47 | Metastatic Node | 2.03% | 2.05% | 4.97% | −1.92% |
DISCUSSION
In this study, we for the first time explored the feasibility and repeatability of APTw-MRI in the head and neck at the clinical field of 3T. Imaging protocols were set up and optimized for head and neck. APTw signal characteristics of major tissues in the head and neck were investigated. We reported the repeatability of masseter muscle, parotid gland, submandibular gland and thyroid gland in the head and neck. The preliminary results on patients with head and neck tumors indicated the potential of APTw-MRI for clinical application.
We measured the Z-spectra and APTw signals quantified by MTRasym(3.5 ppm) in normal head and neck tissues as well as different tumor types. In normal tissues, the parotid glands were found to show the largest APTw (and other CEST) effects. Here, it is important to note that according to the Z-spectrum characteristics (Figure 1), the large APTw signals in the parotid glands and all other tissues may be attributed to the large APT effect (low z-spectrum) at 3.5ppm downfield from the water resonance, the small NOE effect (high z-spectrum) at about −3.5ppm upfield from the water resonance, or their combination (30). Furthermore, it was interesting that this high APTw signal was only exhibited in the parotid glands but not in the submandibular glands, indicating the substantial metabolism differences in these two pairs of major salivary glands. These APTw differences may be attributed to differences in the composition of the parotid glands and submandibular glands (37). Some recent studies observed very high APTw signals in blood (38) and highly vascular tumors (including hemorrhage) (39), with comparable MTRasym values to that observed in the parotid glands. In addition, previous studies also have shown a direct relationship between the composition of parotid saliva and blood (40–42). Parotid saliva has been shown to contain constituents of blood such as amino acids, pyruvate and lactate as revealed by a recent NMR study (43). We hypothesize that the high APT weighting in both the parotid gland and blood may be attributed to their similar underlying biochemical properties, but these metabolic sources should be further examined in the future.
For the four types of head and neck tumors in this study, they showed different APT signal weighting and distributions. The parotid pleomorphic adenoma showed relatively small APTw signal just slightly higher than zero, while the other three tumors showed apparently enhanced positive APTw signals. These different APTw signal properties may reflect the underlying differences in biochemical environment and property of these four different tumors, potentially providing a new mechanism for tumor characterization. The origin of the APTw signal in the tumors could be complicated and dependent on many factors such as the labile amide concentration and its exchange with water, as well as tissue acidity. Future investigation is required to evaluate the potential role of APTw-MRI for clinical head and neck cancer characterization.
It is worth noting that the APTw images of the head and neck could be much more heterogeneous than in the brain due to the different anatomical structures in this area. In the presence of this heterogeneity, delineation of tumors by the difference in APT weighting from normal tissues in the head and neck may not be as easy as that in the relatively homogeneous tissues such as brain and prostate. This potential problem could be overcome by superimposing the APTw images on the conventional anatomical images (2, 44). In relationship to tumor delineation, the very high APTw signal of the normal parotid glands may provide an excellent hypo-APTw contrast for the tumor delineation located within the parotid glands. Despite the possible difficulty in the lesion delineation, we believe that APTw-MRI would have potential clinical merits on quantitative tumor characterization in the head and neck as it provides substantially different information on tumor properties compared to diffusivity and perfusivity characterized by DWI and perfusion imaging, respectively. To this end, clinical validation based on a larger patient sample size is warranted in future studies.
In this preliminary study, we only investigated the APTw signal of head and neck tissues at the single saturation B1 field strength of 2μT adopted from clinical brain APTw-MRI. Although 2μT was proposed as an optimal saturation B1 for brain as the normal tissues in brain provides a nearly zero MTRasym background to delineate the high-APT weighting in the tumor, whether this B1 strength is also optimal for the head and neck should be further investigated. More importantly, Z-spectrum and MTRasym quantification are dependent on many factors including not only CEST effect but also conventional magnetization transfer, water DS and NOE (31–35). All these factors have different contributions to the Z-spectrum and MTRasym at different saturation powers, which could potentially affect tumor characterization (30). In particular, the Z-spectrum obtained at low B1 strengths may allow the Lorentzian analysis to individually characterize and quantify APT and NOE effect, providing additional information above MTRasym analysis (32, 45). Therefore, it is worthwhile to investigate the head and neck APTw-MRI at different saturation powers in the future.
Compared to other tissues to which APTw-MRI has been applied, like brain, breast and prostate, acquisition in the head and neck is more technically challenging. First, there are a variety of different heterogeneous tissues within this area, including muscle, glandular tissue, bone, fat, tongue and teeth, etc., and air containing structures such as the pharynx and paranasal sinuses which account for the intrinsic heterogeneities of APTw image in the head and neck even under the normal status. There are also a lot of air-tissue boundaries in this area that introduce strong susceptibility. This issue may become more severe in the presence of metallic surgical implants and dental fillings. Furthermore, head and neck is subject to a number of motions including breathing, swallowing, coughing and jaw movements during MRI scan. These motions could lead to various artifacts and displacement of APTw images and hence confound quantification.
Efforts have been taken to optimize the APTw-MRI scan protocol in the head and neck through different approaches to address these technical difficulties. Spin echo sequence was applied to mitigate the field inhomogeneity induced artifacts and localized high-order shimming was also used to further reduce the field inhomogeneity. In addition, a much wider offset frequency sweeping range was set in the head and neck than in the brain in order to reduce the possibility of APTw signal missing (at the true 3.5 ppm offset) after Z-spectrum shift for ΔB0 correction. Because MR images of the head and neck are usually associated with relatively low signal-to-noise ratio (SNR) and the accurate MTRasym quantification is SNR demanding (46), relatively large voxel size (2×2mm) was used to enhance SNR and reduce scan time. We did not densely sample the data points at the small offset frequencies (−1 ppm to 1 ppm) as which is done for brain APTw-MRI because these nominal offset frequencies might not necessarily and truly indicate the Z-spectrum bottom in the presence of severe susceptibility and B0 inhomogeneities (29). Similarly, we did not overly sample the data points around the nominal 3.5 ppm offsets either for the same reason (29). The presence of large B0 inhomogeneities also explains the reason that water saturation shift referencing (WASSR) was not used for ΔB0 mapping in this study (47). In the presence of large B0 inhomogeneity, a large offset frequency sweeping range as well as small offset frequency spacing may have to be applied for WASSR which typically uses small saturation B1 field (≤1μT). This could significantly prolong the scan time in order to accurately capture the true Z-spectrum bottom. In the aspect of Z-spectrum analysis, a very high R2 value (>0.99) criterion was set to ensure the reliable MTRasym quantification under excellent goodness-of-fit, effectively excluding the voxels associated with low SNR and/or artifacts. Voxels exhibited large ΔB0 shift (>2 ppm or <−2 ppm) were also excluded because the large Z-spectrum shift correction might affect the accuracy of MTRasym due to the loose offset frequency acquisition beyond ±5.5 ppm. Because of the relatively short saturation pulse restricted by the hardware, some tissues may not be sufficiently saturated. In addition, the chemical shift selective fat suppression may not sufficiently suppress fat in the presence of large ΔB0. As such, those voxels showing high residual signal level at the corrected Z-spectrum bottom should also be excluded to ensure the MTRasym quantification reliability.
Despite the efforts taken as describe above, the further investigation to improve APTw-MRI acquisition and quantification should be conducted to facilitate future clinical use. For example, the pulse train saturation approach rather than a single saturation pulse of 200ms may effectively ease the hardware RF pulse duration requirement in clinical practice and thus increase the APTw-MRI sensitivity (48–51). Better shimming and fast imaging sequences (52–55), as well as reconstruction techniques (56), should be further explored to yield better tradeoff between SNR, imaging volume, scan time, spatial resolution and artifacts. Better sampling strategies for head and neck APTw-MRI even in the presence of large ΔB0 are also worth to be investigated (57).
Acknowledgments
Mr. Tom Wing Cheung Yuen, Mr. Benjamin King Hong Law and Mr. Wang Lam are greatly acknowledged for their kind help in patient recruitment and data management. Hong Kong RGC grant SEG_CUHK02 was acknowledged for the MRI equipment used in this study. Hong Kong RGC grant CUHK418811 and China NSFC grant 81201076 were acknowledged for the support of pulse sequence development in this study. Dr. Jinyuan Zhou was supported by USA NIH grants R01EB009731 and R01CA166171.
Abbreviation used
- MRI
magnetic resonance imaging
- DWI
diffusion weighted imaging
- DCE
dynamic contrast enhanced
- MRS
magnetic resonance spectroscopy
- CEST
chemical exchange saturation transfer
- APT
amide proton transfer
- RF
radiofrequency
- TSE
turbo-spin-echo
- ΔB0
static magnetic field inhomogeneity
- FOV
field-of-view
- ETL
echo train length
- NSA
number of signal average
- SENSE
sensitivity encoding
- ppm
parts-per-million
- DS
direct water saturation
- MT
magnetization transfer
- MTRasym
asymmetric magnetization transfer ratio
- NOE
nuclear Overhauser effect
- R2
coefficient of determination
- ROI
region of interest
- CI
confidence interval
- SNR
signal-to-noise ratio
References
- 1.Abdel Razek AA, Poptani H. MR spectrscopy of head and neck cancer. Eur J Radiol. 2013;82:982–989. doi: 10.1016/j.ejrad.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 2.Thoeny HC, De Keyzer F, King AD. Diffusion-weighted MR Imaging in the Head and Neck. Radiology. 2012;263:19–32. doi: 10.1148/radiol.11101821. [DOI] [PubMed] [Google Scholar]
- 3.Shukla-Dave A, Lee NY, Jansen JF, Thaler HT, Stambuk HE, Fury MG, Patel SG, Moreira AL, Sherman E, Karimi S, Wang Y, Kraus D, Shah JP, Pfister DG, Koutcher JA. Dynamic contrast-enhanced magnetic resonance imaging as a predictor of outcome in head-and-neck squamous cell carcinoma patients with nodal metastases. Int J Radiat Oncol Biol Phys. 2012;82:1837–1844. doi: 10.1016/j.ijrobp.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.King AD, Mo FK, Yu KH, Yeung DK, Zhou H, Bhatia KS, Tse GM, Vlantis AC, Wong JK, Ahuja AT. Squamous cell carcinoma of the head and neck: diffusion-weighted MR imaging for prediction and monitoring of treatment response. Eur Radiol. 2010;20:2213–2220. doi: 10.1007/s00330-010-1769-8. [DOI] [PubMed] [Google Scholar]
- 5.King AD, Chow KK, Yu KH, Mo FK, Yeung DK, Yuan J, Bhatia KS, Vlantis AC, Ahuja AT. Head and Neck Squamous Cell Carcinoma: Diagnostic Performance of Diffusion-weighted MR Imaging for the Prediction of Treatment Response. Radiology. 2013;266:531–538. doi: 10.1148/radiol.12120167. [DOI] [PubMed] [Google Scholar]
- 6.Zhou J, Payen J, Wilson DA, Traystman RJ, van Zijl PCM. Using the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI. Nature Med. 2003;9:1085–1090. doi: 10.1038/nm907. [DOI] [PubMed] [Google Scholar]
- 7.Sun PZ, Zhou J, Sun W, Huang J, van Zijl PCM. Detection of the ischemic penumbra using pH-weighted MRI. J Cereb Blood Flow Metab. 2007;27:1129–1136. doi: 10.1038/sj.jcbfm.9600424. [DOI] [PubMed] [Google Scholar]
- 8.Zhou J, Lal B, Wilson DA, Laterra J, van Zijl PCM. Amide proton transfer (APT) contrast for imaging of brain tumors. Magn Reson Med. 2003;50:1120–1126. doi: 10.1002/mrm.10651. [DOI] [PubMed] [Google Scholar]
- 9.Zhou J, Tryggestad E, Wen Z, Lal B, Zhou T, Grossman R, Wang S, Yan K, Fu D-X, Ford E, Tyler B, Blakeley J, Laterra J, van Zijl PCM. Differentiation between glioma and radiation necrosis using molecular magnetic resonance imaging of endogenous proteins and peptides. Nature Med. 2011;17:130–134. doi: 10.1038/nm.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ling W, Regatte RR, Navon G, Jerschow A. Assessment of glycosaminoglycan concentration in vivo by chemical exchange-dependent saturation transfer (gagCEST) Proc Natl Acad Sci (USA) 2008;105:2266–2270. doi: 10.1073/pnas.0707666105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmitt B, Zbyn S, Stelzeneder D, Jellus V, Paul D, Lauer L, Bachert P, Trattnig S. Cartilage quality assessment by using glycosaminoglycan chemical exchange saturation transfer and Na-23 MR imaging at 7T. Radiology. 2011;260:257–264. doi: 10.1148/radiol.11101841. [DOI] [PubMed] [Google Scholar]
- 12.Singh A, Haris M, Cai K, Kassey VB, Kogan F, Reddy D, Hariharan H, Reddy R. Chemical exchange saturation transfer magnetic resonance imaging of human knee cartilage at 3 T and 7 T. Magn Reson Med. 2012;68:588–594. doi: 10.1002/mrm.23250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Zijl PC, Jones CK, Ren J, Malloy CR, Sherry AD. MRI detection of glycogen in vivo by using chemical exchange saturation transfer imaging (glycoCEST) Proc Natl Acad Sci U S A. 2007;104:4359–4364. doi: 10.1073/pnas.0700281104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cai KJ, Haris M, Singh A, Kogan F, Greenberg JH, Hariharan H, Detre JA, Reddy R. Magnetic resonance imaging of glutamate. Nature Med. 2012;18:302–306. doi: 10.1038/nm.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan KWY, McMahon MT, Kato Y, Liu GS, Bulte JWM, Bhujwalla ZM, Artemov D, van Zijl PCM. Natural D-glucose as a biodegradable MRI contrast agent for detecting cancer. Magn Reson Med. 2012;68:1764–1773. doi: 10.1002/mrm.24520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walker-Samuel S, Ramasawmy R, Torrealdea F, Rega M, Rajkumar V, Johnson SP, Richardson S, Goncalves M, Parkes HG, Arstad E, Thomas DL, Pedley RB, Lythgoe MF, Golay X. In vivo imaging of glucose uptake and metabolism in tumors. Nat Med. 2013;19:1067–1072. doi: 10.1038/nm.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou J, Zhu H, Lim M, Blair L, Quinones-Hinojosa A, Messina SA, Eberhart CG, Pomper MG, Laterra J, Barker PB, van Zijl PC, Blakeley JO. Three-dimensional amide proton transfer MR imaging of gliomas: Initial experience and comparison with gadolinium enhancement. J Magn Reson Imaging. 2013;38:1119–128. doi: 10.1002/jmri.24067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wen Z, Hu S, Huang F, Wang X, Guo L, Quan X, Wang S, Zhou J. MR imaging of high-grade brain tumors using endogenous protein and peptide-based contrast. Neuroimage. 2010;51:616–622. doi: 10.1016/j.neuroimage.2010.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones CK, Schlosser MJ, van Zijl PC, Pomper MG, Golay X, Zhou J. Amide proton transfer imaging of human brain tumors at 3T. Magn Reson Med. 2006;56:585–592. doi: 10.1002/mrm.20989. [DOI] [PubMed] [Google Scholar]
- 20.Jia G, Abaza R, Williams JD, Zynger DL, Zhou J, Shah ZK, Patel M, Sammet S, Wei L, Bahnson RR, Knopp MV. Amide proton transfer MR imaging of prostate cancer: a preliminary study. J Magn Reson Imaging. 2011;33:647–654. doi: 10.1002/jmri.22480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dula AN, Arlinghaus LR, Dortch RD, Dewey BE, Whisenant JG, Ayers GD, Yankeelov TE, Smith SA. Amide proton transfer imaging of the breast at 3 T: Establishing reproducibility and possible feasibility assessing chemotherapy response. Magn Reson Med. 2013;70:216–224. doi: 10.1002/mrm.24450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris AT, Rennie A, Waqar-Uddin H, Wheatley SR, Ghosh SK, Martin-Hirsch DP, Fisher SE, High AS, Kirkham J, Upile T. Raman spectroscopy in head and neck cancer. Head Neck Oncol. 2010;2:26. doi: 10.1186/1758-3284-2-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Conti C, Ferraris P, Garavaglia M, Giorgini E, Rubini C, Sabbatini S, Tosi G. Microimaging FTIR of Head and Neck Tumors. IV. Microsc Res Techniq. 2009;72:67–75. doi: 10.1002/jemt.20644. [DOI] [PubMed] [Google Scholar]
- 24.Lau DP, Huang Z, Lui H, Man CS, Berean K, Morrison MD, Zeng H. Raman spectroscopy for optical diagnosis in normal and cancerous tissue of the nasopharynx-preliminary findings. Lasers Surg Med. 2003;32:210–214. doi: 10.1002/lsm.10084. [DOI] [PubMed] [Google Scholar]
- 25.Stone N, Stavroulaki P, Kendall C, Birchall M, Barr H. Raman spectroscopy for early detection of laryngeal malignancy: Preliminary results. Laryngoscope. 2000;110:1756–1763. doi: 10.1097/00005537-200010000-00037. [DOI] [PubMed] [Google Scholar]
- 26.Sun PZ, Zhou J, Sun W, Huang J, van Zijl PC. Suppression of lipid artifacts in amide proton transfer (APT) imaging. Magn Reson Med. 2005;54:222–225. doi: 10.1002/mrm.20530. [DOI] [PubMed] [Google Scholar]
- 27.Lu J, Zhou J, Cai C, Cai S, Chen Z. Observation of true and pseudo NOE signals using CEST-MRI and CEST-MRS sequences with and without lipid suppression. Magn Reson Med. 2014 doi: 10.1002/mrm.25277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei W, Jia G, Flanigan D, Zhou J, Knopp MV. Chemical exchange saturation transfer MR imaging of articular cartilage glycosaminoglycans at 3 T: Accuracy of B0 Field Inhomogeneity corrections with gradient echo method. Magn Reson Imaging. 2014;32:41–47. doi: 10.1016/j.mri.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou J, Blakeley JO, Hua J, Kim M, Laterra J, Pomper MG, van Zijl PCM. Practical data acquisition method for human brain tumor amide proton transfer (APT) imaging. Magn Reson Med. 2008;60:842–849. doi: 10.1002/mrm.21712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou J, Hong X, Zhao X, Gao J-H, Yuan J. APT-weighted and NOE-weighted image contrasts in glioma with different RF saturation powers based on magnetization transfer ratio asymmetry analyses. Magn Reson Med. 2013;70:320–327. doi: 10.1002/mrm.24784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ling W, Regatte RR, Navon G, Jerschow A. Assessment of glycosaminoglycan concentration in vivo by chemical exchange-dependent saturation transfer (gagCEST) Proc Natl Acad Sci U S A. 2008;105:2266–2270. doi: 10.1073/pnas.0707666105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones CK, Huang A, Xu J, Edden RA, Schar M, Hua J, Oskolkov N, Zaca D, Zhou J, McMahon MT, Pillai JJ, van Zijl PC. Nuclear Overhauser enhancement (NOE) imaging in the human brain at 7T. Neuroimage. 2013;77:114–124. doi: 10.1016/j.neuroimage.2013.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jin T, Wang P, Zong X, Kim SG. MR imaging of the amide-proton transfer effect and the pH-insensitive nuclear overhauser effect at 9. 4 T. Magn Reson Med. 2013;69:760–770. doi: 10.1002/mrm.24315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu D, Zhou J, Xue R, Zuo Z, An J, Wang DJJ. Quantitative characterization of nuclear Overhauser enhancement and amide proton transfer effects in the human brain at 7 Tesla. Magn Reson Med. 2013;70:1070–1081. doi: 10.1002/mrm.24560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Zijl PC, Yadav NN. Chemical exchange saturation transfer (CEST): what is in a name and what isn’t? Magn Reson Med. 2011;65:927–948. doi: 10.1002/mrm.22761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Galbraith SM, Lodge MA, Taylor NJ, Rustin GJ, Bentzen S, Stirling JJ, Padhani AR. Reproducibility of dynamic contrast-enhanced MRI in human muscle and tumours: comparison of quantitative and semi-quantitative analysis. NMR Biomed. 2002;15:132–142. doi: 10.1002/nbm.731. [DOI] [PubMed] [Google Scholar]
- 37.Humphrey SP, Williamson RT. A review of saliva: normal composition, flow, and function. J Prosthet Dent. 2001;85:162–169. doi: 10.1067/mpr.2001.113778. [DOI] [PubMed] [Google Scholar]
- 38.Zheng S, van der Bom IM, Zu Z, Lin G, Zhao Y, Gounis MJ. Chemical exchange saturation transfer effect in blood. Magn Reson Med. 2013 doi: 10.1002/mrm.24770. [DOI] [PubMed] [Google Scholar]
- 39.Grossman R, Tyler B, Brem H, Eberhart CG, Wang SL, Fu DX, Wen ZB, Zhou JY. Growth properties of SF188/V+ human glioma in rats in vivo observed by magnetic resonance imaging. J Neuro-Oncol. 2012;110:315–323. doi: 10.1007/s11060-012-0974-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borg A, Birkhed D. Secretion of Glucose in Human-Parotid Saliva after Carbohydrate Intake. Scand J Dent Res. 1988;96:551–556. doi: 10.1111/j.1600-0722.1988.tb01595.x. [DOI] [PubMed] [Google Scholar]
- 41.Englander HR, Jeffay AI, Fuller JB, Chauncey HH. Glucose Concentrations in Blood Plasma and Parotid Saliva of Individuals with and without Diabetes Mellitus. J Dent Res. 1963;42:1246. doi: 10.1177/00220345630420052301. [DOI] [PubMed] [Google Scholar]
- 42.Kelsay JL, Behall KM, Holden JM, Crutchfield HC. Pyruvate and lactate in human blood and saliva in response to different carbohydrates. J Nutr. 1972;102:661–666. doi: 10.1093/jn/102.5.661. [DOI] [PubMed] [Google Scholar]
- 43.Takeda I, Stretch C, Barnaby P, Bhatnager K, Rankin K, Fu H, Weljie A, Jha N, Slupsky C. Understanding the human salivary metabolome. NMR Biomed. 2009;22:577–584. doi: 10.1002/nbm.1369. [DOI] [PubMed] [Google Scholar]
- 44.Yuan J, Yeung DK, Mok GS, Bhatia KS, Wang YX, Ahuja AT, King AD. Non-Gaussian Analysis of Diffusion Weighted Imaging in Head and Neck at 3T: A Pilot Study in Patients with Nasopharyngeal Carcinoma. PLoS One. 2014;9:e87024. doi: 10.1371/journal.pone.0087024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zaiss M, Schmitt B, Bachert P. Quantitative separation of CEST effect from magnetization transfer and spillover effects by Lorentzian-line-fit analysis of z-spectra. J Magn Reson. 2011;211:149–155. doi: 10.1016/j.jmr.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 46.Yuan J, Zhang Q, Wang Y-X, Wei J, Zhou J. Accuracy and uncertainty of asymmetric magnetization transfer ratio quantification for amide proton transfer (APT) imaging at 3T: A Monte Carlo study. Engineering in Medicine and Biology Society (EMBC); 35th Annual International Conference of the IEEE; 2013; 2013. pp. 5139–5142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim M, Gillen J, Landman BA, Zhou J, van Zijl PC. Water saturation shift referencing (WASSR) for chemical exchange saturation transfer (CEST) experiments. Magn Reson Med. 2009;61:1441–1450. doi: 10.1002/mrm.21873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun PZ, Benner T, Kumar A, Sorensen AG. Investigation of optimizing and translating pH-sensitive pulsed-chemical exchange saturation transfer (CEST) imaging to a 3T clinical scanner. Magn Reson Med. 2008;60:834–841. doi: 10.1002/mrm.21714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schmitt B, Zaiss M, Zhou J, Bachert P. Optimization of pulse train presaturation for CEST imaging in clinical scanners. Magn Reson Med. 2011;65:1620–1629. doi: 10.1002/mrm.22750. [DOI] [PubMed] [Google Scholar]
- 50.Desmond KL, Stanisz GJ. Understanding quantitative pulsed CEST in the presence of MT. Magn Reson Med. 2012;67:979–990. doi: 10.1002/mrm.23074. [DOI] [PubMed] [Google Scholar]
- 51.Jones CK, Polders D, Hua J, Zhu H, Hoogduin HJ, Zhou J, Luijten P, van Zijl P. In vivo three-dimensional whole-brain pulsed steady-state chemical exchange saturation transfer at 7 T. Magnet Reson Med. 2012;67:1579–1589. doi: 10.1002/mrm.23141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao X, Wen Z, Zhang G, Huang F, Lu S, Wang X, Hu S, Chen M, Zhou J. Three-Dimensional Turbo-Spin-Echo Amide Proton Transfer MR Imaging at 3-Tesla and Its Application to High-Grade Human Brain Tumors. Mol Imaging Biol. 2013;15:114–122. doi: 10.1007/s11307-012-0563-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Varma G, Lenkinski RE, Vinogradov E. Keyhole chemical exchange saturation transfer. Magn Reson Med. 2012;68:1228–1233. doi: 10.1002/mrm.23310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhu H, Jones CK, van Zijl PC, Barker PB, Zhou J. Fast 3D chemical exchange saturation transfer (CEST) imaging of the human brain. Magn Reson Med. 2010;64:638–644. doi: 10.1002/mrm.22546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dixon WT, Hancu I, Ratnakar SJ, Sherry AD, Lenkinski RE, Alsop DC. A multislice gradient echo pulse sequence for CEST imaging. Magn Reson Med. 2010;63:253–256. doi: 10.1002/mrm.22193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lustig M, Donoho D, Pauly JM. Sparse MRI: The application of compressed sensing for rapid MR imaging. Magn Reson Med. 2007;58:1182–1195. doi: 10.1002/mrm.21391. [DOI] [PubMed] [Google Scholar]
- 57.Tee YK, Khrapitchev AA, Sibson NR, Payne SJ, Chappell MA. Optimal sampling schedule for chemical exchange saturation transfer. Magn Reson Med. 2013;70:1251–1262. doi: 10.1002/mrm.24567. [DOI] [PubMed] [Google Scholar]