Abstract
Background
Assembly of fibronectin matrices is associated with integrin receptor turnover and is an important determinant of tissue remodeling. While it is well established that fibronectin is the primary ligand for α5β1 receptor, the relationship between fibronectin matrix assembly and the fate of internalized α5 integrin remains poorly characterized.
Materials and Methods
To evaluate the effect of fibronectin matrix on the fate of internalized α5 integrin, fibronectin-null CHO and MEF cells were used to track the fate of α5 following exposure to exogenous fibronectin.
Results
In the absence of matrix-capable fibronectin dimer, levels of internalized α5 decreased rapidly over time. This correlated with a decline in total cellular α5 and was associated with the ubiquitination of α5 integrin. In contrast, internalized and total cellular α5 protein levels were maintained when matrix-capable fibronectin was present in the extracellular space. Further, we show that ubiquitination and degradation of internalized α5 integrin in the absence of fibronectin requires the presence of two specific lysine residues in the α5 cytoplasmic tail.
Conclusions
Our data demonstrate that α5 integrin turnover is dependent on fibronectin matrix assembly, where the absence of matrix-capable fibronectin in the extracellular space targets the internalized receptor for rapid degradation. These findings have important implications for understanding tissue-remodeling processes found in wound repair and tumor invasion.
Keywords: α5 integrin, fibronectin, extracellular matrix, tissue remodeling, endocytosis, matrix assembly
INTRODUCTION
Tissue remodeling is a critical component of normal physiology as well as many pathological states, and it depends on the cell-mediated assembly and degradation of extracellular matrix (ECM) proteins [1]. Fibronectin is an indispensable ECM protein (~220 kDa in monomeric form) required for tissue repair and remodeling [2, 3]. In the wound, cells assemble newly synthesized FN into a three-dimensional (3D) fibrillar matrix (fibrillogenesis) that regulates a variety of cell behaviors including contractility. As the generation of tractional force is required for wound closure, an analysis of the mechanics of FN matrix assembly has the potential to significantly impact our understanding of this essential biological process. Further, the fibrillar matrix formed by fibronectin is thought to serve as the template for the subsequent collagen matrix that is required for wound strength [4,5]. Therefore, factors that perturb fibronectin matrix assembly, either positively or negatively can impact scar strength or lead to excessive fibrosis.
Cells secrete fibronectin as a disulfide-bonded dimer that binds to the α5β1 integrin receptor via the cell-binding domain located in the tenth type III repeat of fibronectin. Recombinant fibronectin monomers lacking the carboxyl-terminal dimerization region are able to interact with integrins but are not capable of being incorporated into a matrix [4]. Integrin interactions with native fibronectin dimers allow unfolding of the soluble protein and its assembly into a detergent-insoluble fibrillar matrix that serves as the template for the deposition and assembly of other fibrillar proteins such as collagen. Fibronectin matrices are also continuously remodeled, and fibronectin turnover has been shown to be regulated by α5β1-mediated endocytosis with intracellular degradation [5-7].
Integrin internalization and subsequent recycling in the context of cell migration have been reported in several studies and may play an important role in driving tumor cell invasion as well [8-10]. Remodeling of fibronectin matrices requires that the α5β1– fibronectin complex is internalized by receptor-mediated endocytosis. Following internalization, the receptors are targeted to the early endosomes where receptor fate is determined. From there, external stimulations can mediate recycling of α5β1 back to the plasma membrane via a process that is incompletely understood and cell-type dependent [11]. Receptors that are not recycled are sent to the late endosomes and the lysosomes where they are targeted for degradation. More recent data have demonstrated that proteasomal degradation of integrin receptors may also occur [12] and may be regulated by the ubiquitin-proteasome pathway [13, 14]. The sorting of receptors from the early endosome to the recycling compartments is regulated by cell adhesion. Early studies of the life cycle of the α5β1 integrin, for example, showed that when cells are adherent, recycling of the receptor to the cell membrane following internalization is readily detected [15, 16]. In contrast, when cells are maintained in suspension, the surface expression of the integrin is lost as the receptor is re-directed from the early endosome to the lysosome for degradation. Nonetheless, the factors that regulate α5β1 cell fate following internalization remain incompletely understood.
In this report, we demonstrate that, in the presence of a fibronectin monomer that cannot undergo fibrillar matrix assembly, internalized α5 integrin is rapidly degraded via the ubiquitin-proteasome pathway. We also show that this rapid degradation depends on the presence of two lysine residues in the α5 integrin cytoplasmic tail. Loss of these lysine residues in the α5 integrin cytoplasmic tail leads to diminished α5 integrin internalization and also diminished fibronectin matrix assembly. Taken together, our findings suggest that fibronectin matrix assembly and the fate of internalized α5 integrin are interdependent processes.
RESULTS
α5 integrin internalization and degradation is regulated by “matrix-capable” fibronectin
To evaluate the effect of fibronectin matrix on the fate of internalized α5β1 receptor, the levels of internalized α5 integrin were monitored in the presence or absence of exogenous fibronectin. Chinese Hamster Ovary (CHO) cells stably expressing a functional human α5 integrin were used for the initial experiments since they are well-characterized and do not express detectable levels of endogenous fibronectin. Using an established biotin-avidin detection system [17], cells that were grown in the presence or absence of exogenous fibronectin were surface-labeled with biotin at 4°C, a temperature that inhibits endocytosis. Cells were then transferred to 37°C to allow internalization of the labeled receptor. At indicated times, the remaining surface label was stripped from the cells to allow monitoring of internalized labeled integrin. In the presence of fibronectin, the levels of internalized, labeled α5 integrin were maintained, increasing slightly over time. In contrast, the levels of internalized α5 integrin were significantly diminished in the absence of fibronectin (p<0.0001; 2-way ANOVA) and decreased rapidly over time so that they were nearly undetectable by two hours (Figure 1A; see supplemental Figure 1). Similar results were obtained when the experiments were conducted using the fibronectin-null mouse embryo fibroblast (MEF) cells that do not express endogenous fibronectin protein (Data not shown). Immunofluorescence microscopy of cells cultured in the presence or absence of fibronectin supports the idea that the α5β1 integrin is targeted to different locations within the cell depending on whether fibronectin is present (See Supplemental Figure 2). Taken together, these data suggest that the presence of fibronectin in the extracellular space determines the fate of internalized α5 integrin.
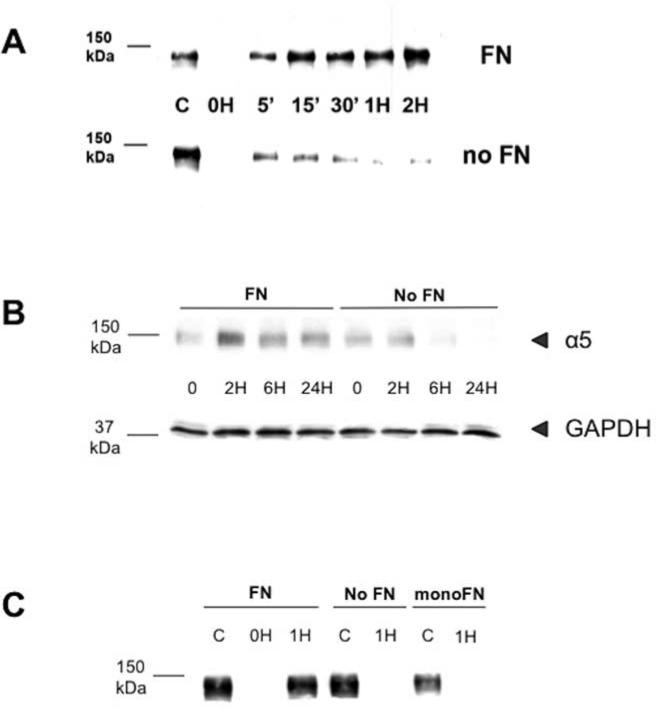
FIGURE 1. α5 integrin levels decrease in the absence of fibronectin and stabilization of internalized α5 requires ‘matrix-capable’ fibronectin.
(A): CHO-α5 cells and MEF cells, which are both FN null, were cultured overnight in the presence or absence of FN and then labeled with NHS-SS-Biotin for 30 minutes at 4°C. In some cases, cells were lysed (lane C) or stripped of surface biotin using MESNa (0H). Alternately, cells were warmed to 37°C to allow receptor internalization. At the indicated times, cells were returned to ice and the remaining surface biotin was stripped as described above. Cells were then lysed and internalized integrin identified by precipitation with Neutravidin-agarose beads followed by immunoblotting with anti-α5 antibody. In the absence of exogenous FN, detected levels of internalized α5 integrin in both CHO-α5 cells (panel A) and MEF cells (not shown) rapidly decreases over time while the presence of exogenous FN results in persistence of internalized receptor. (B): FN-null MEF cells were cultured overnight in the presence or absence of FN. Cells were then pretreated with cycloheximide for one hour to inhibit new protein synthesis and lysed at the indicated time points. Equal amounts of total cell lysates were analyzed by immunoblotting using anti-α5 antibody (upper row) or anti-GAPDH antibody as a control (lower row). (C): FN-null MEF cells were cultured overnight in the absence of fibronectin (No FN) or in the presence of either full-length native FN (FN) or a recombinant FN monomer that binds α5 integrin but cannot be incorporated into matrix (monoFN). Cells were then labeled with NHS-SS-Biotin and internalized integrin identified as described above. “C” in these experiments were unstripped whole cell lysates at 0H. After one hour, internalized α5 integrin is retained in cells cultured in the presence of FN but is barely detectable at 1H in cells cultured under No FN or monoFN conditions.
There are two possible explanations for the apparent rapid decline in detectable levels of internalized α5 receptor in the absence of exogenous fibronectin. One possibility is that the receptor is rapidly recycled back to the cell surface, where the label is stripped off, rendering the receptor undetectable. The other is that the receptor is targeted for degradation. To determine whether degradation of α5 integrin is responsible for our findings, total cellular α5 protein levels were monitored in cells grown in the presence or absence of exogenous fibronectin. When new protein synthesis is blocked by pretreatment with cycloheximide, total cellular α5 integrin levels persisted in the presence of exogenous fibronectin over a 24-hour period (Figure 1B). In contrast, in the absence of fibronectin, total α5 protein levels declined visibly by 6 hours. Taken together, these data suggest that once α5β1 receptor is internalized in the absence of exogenous fibronectin, the α5 subunit is rapidly targeted for degradation.
Cells secrete fibronectin as a disulfide-bonded dimer. Integrin-fibronectin interactions allow unfolding of the soluble, dimeric protein and its assembly into a detergent-insoluble fibrillar matrix. To determine whether fibronectin matrix assembly influences the fate of internalized α5 integrin, we conducted internalization experiments in which full-length native fibronectin was replaced by a monomeric fibronectin (monoFN). The monoFN is identical to the native fibronectin with one exception, the absence of the carboxyl-terminal dimerization region. The absence of this region renders the protein incapable of undergoing matrix assembly while still capable of binding to the α5β1 integrin receptor [4]. Surprisingly, addition of monoFN did not prevent the degradation of internalized α5 (Figure 1C). This finding suggests that ligand binding of fibronectin to the α5β1 integrin alone is not sufficient to protect the receptor once it is internalized, and instead, persistence of the receptor requires binding with fibronectin ligand that is “matrix-capable”.
α5 integrin degradation is mediated by proteasomes and α5 integrin is associated with the ubiquitination pathway in the absence of fibronectin
Our data indicate that internalized α5 integrin in the absence of fibronectin is targeted for degradation. Other investigators have found that internalized α5 integrin is sorted to multivesicular endosomes and then to the lysosomes where it is degraded [6, 18]. However, proteasomal degradation of β1 integrin receptor has also been recently reported [12]. To determine the mechanism for α5 integrin degradation in the absence of fibronectin, we incubated cells with inhibitors of either lysosomal or proteasomal degradation. Incubation of cells with the lysosomal inhibitors bafilomycin A1 and chloroquine did not prevent degradation of internalized α5 (Figure 2A). Rather, the proteasome inhibitor MG-132 resulted in the persistence of internalized α5 integrin even in the absence of fibronectin. These findings suggest that degradation of α5 integrin in the absence of matrix-capable fibronectin may occur via a proteasome-dependent pathway.
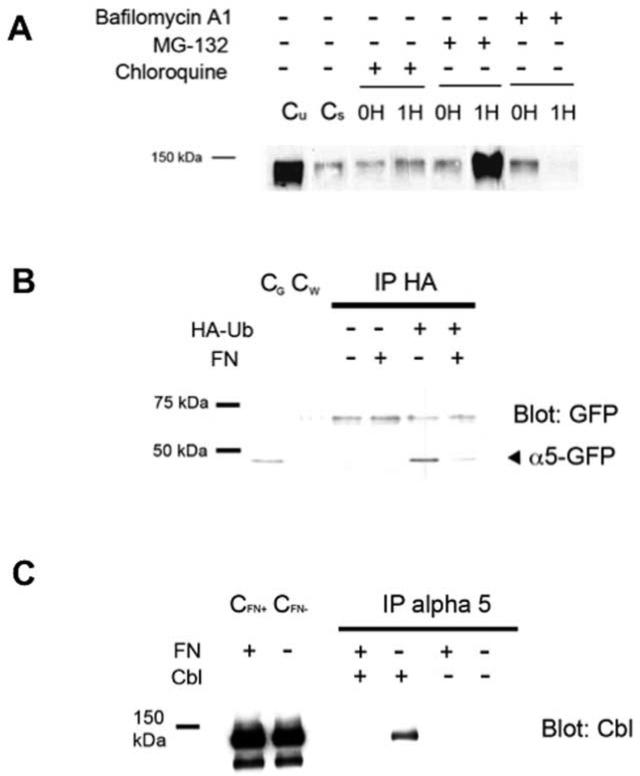
FIGURE 2. Degradation of internalized α5 integrin is MG-132 sensitive and in the absence of fibronectin, α5 integrin cytoplasmic tail is ubiquitinated and CBL associates with α5 integrin.
(A): CHO-α5 cells were cultured overnight without FN and then pretreated for one hour with either lysosomal inhibitors (Bafilomycin or Chloroquine) or the proteosomal inhibitor, MG-132. Cells were labeled with NHS-SSBiotin at 4°C and either stripped of surface biotin (0H) or transferred to 37°C. After one hour, the remaining surface label was stripped from the cells followed by cell lysis and analysis of whole cell lysates for labeled α5 to allow monitoring of internalized labeled α5 integrin as described in Fig. 1. (Untreated control lanes: Cu = unstripped cells at 0H; Cs = stripped cells at 0H). Pretreatment with MG-132 leads to accumulation of internalized α5 integrin which was not seen in cells pretreated with lysosomal inhibitors. (B): CHO cells stably expressing an α5 integrin with a C-terminal GFP-tag were transiently transfected with an HA-tagged ubiquitin cDNA (HA-Ub) or a control vector. Cells were then cultured overnight in the presence or absence of FN followed by pretreatment for one hour with MG-132. Whole cell lysates were analyzed by immunoprecipitation using an anti-HA monoclonal antibody (IP HA) followed by immunoblotting under reduced conditions with an anti-GFP antibody to detect the GFP-labelled α5 integrin cytoplasmic domain (~50 kDa). α5 integrin is found to be ubiquitinated only in the absence of FN. (whole cell lysate controls: CG = α5-GFP cells; CW = wild-type α5 cells) (C): Cells transiently transfected to express the E3 ubiquitin ligase CBL (Cbl) or with a control vector were cultured overnight in the presence or absence of FN and pretreated with MG-132 for one hour. Whole cell lysates underwent immunoprecipitation with anti-α5 antibody (IP alpha 5) followed by immunoblotting with an anti-CBL antibody. α5 integrin association with CBL is detected only in the absence of exogenous FN. (whole cell lysate controls: CFN+ = FN present; CFN- = FN absent)
Targeting of proteins to the proteasome requires ubiquitination of the target protein, usually at specific lysine residues. To determine whether the α5 integrin subunit may be ubiquitinated in the absence of fibronectin, we used CHO cells stably expressing α5 integrin tagged on the cytoplasmic domain with green fluorsescent protein (GFP). The GFP-tagged α5 integrin has been well characterized and previously shown to have normal integrin function [19, 20]. α5 integrin undergoes post-translational modification [21] and is comprised of an extracellular heavy chain (~120 kDa) that is linked by disulfide bonds to a cytoplasmic domain-associated light chain (~25 kDa). Tagging with GFP at the cytoplasmic domain results in an expected light chain fragment of approximately 50 kDa under reduced conditions, making it easier to distinguish on immunoblot. As GFP-tagged α5 integrin (α5-GFP) is also degraded in the absence of fibronectin (data not shown), we utilized these cells to investigate whether ubiquitination of internalized α5 integrin cytoplasmic tail could be detected. Cells were transiently transfected with hemaglutinin (HA)-tagged ubiquitin, and then grown overnight in the presence or absence of exogenous fibronectin. As demonstrated in Figure 2B, ubiquitination of the α5 integrin cytoplasmic domain was detected only when cells were cultured in the absence of fibronectin. These data indicate strongly that α5 can undergo ubiquitination in the absence of fibronectin and support the idea that this may be the mechanism for rapid degradation of the receptor.
Previous work has demonstrated that ubiquitination of α5 integrin results in an association between α5 integrin cytoplasmic tail and the E3 ubiquitin ligase Casitas B-lineage lymphoma (CBL; [22]). CBL has been shown to control the degradation of receptor tyrosine kinases for growth factors such as platelet-derived growth factor (PDGF) and fibroblast growth factor (FGF) by mediating polyubiquitination of receptors [10], and CBL has been reported to mediate FGF receptor-activated degradation of α5 integrin via the proteasome [22]. To determine whether this association between CBL and the α5 integrin cytoplasmic tail exists in our model system, cells transiently expressing CBL were cultured in the presence or absence of fibronectin. Cells were then lysed, followed by immunoprecipitation with an anti-α5 antibody. We found that association of α5 integrin and CBL occurred only in the absence of extracellular fibronectin (Figure 2C). Our findings therefore corroborate work by others and support a role for CBL in determining the fate of α5 integrin.
α5 integrin internalization and degradation depends on two lysine residues in the cytoplasmic tail region
Ubiquitination of target proteins typically occurs on select lysine residues. The α5 integrin cytoplasmic tail has four lysine residues. The first is proximal to the highly conserved amino acid sequence GFFKR (K1021; Figure 3A). The GFFKR amino acid sequence is present in almost all α integrin subunits and is thought to be important for heterodimer stability [23]. The second (K1027) is contained in the highly conserved GFFKR region, while the third (K1038) and fourth (K1042) are in the short cytoplasmic tail distal to this region. To determine which lysines were important to the targeted rapid degradation of α5, we utilized CHO cells which stably expressed a chimeric integrin construct in which the α5 cytoplasmic tail carboxy-terminal to the GFFKR amino acid sequence was replaced with the cytoplasmic tail of the αV integrin (X5CV cells). These cells can assemble a fibronectin matrix similar to cells that express the wild-type receptor (data not shown). When X5CV cells were examined, the levels of internalized α5 integrin were not significantly different in the presence or absence of fibronectin (Figure 3B; p=0.92 2-way ANOVA), in contrast to cells expressing the wild-type α5 integrin construct (X5C5). These data indicate that the ability of fibronectin to influence the degradation of internalized α5 integrin is likely regulated by lysines 1038 and/or 1042.
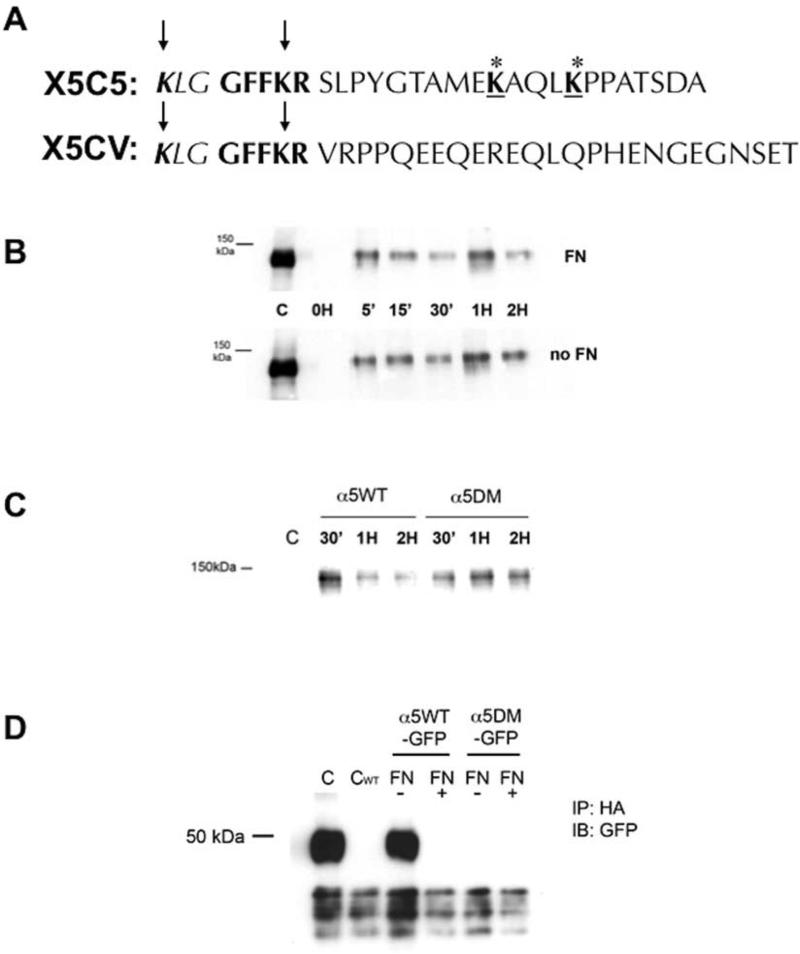
FIGURE 3. α5 integrin degradation in the absence of fibronectin requires the cytoplasmic tail of α5 integrin distal to the conserved GFFKR region and containing two lysine residues that are ubiquitinated.
(A): A chimeric integrin construct was constructed where the α5 cytoplasmic tail of the α5 integrin distal to the GFFKR amino acid sequence was replaced with the αV cytoplasmic tail (X5CV). CHO cells were bulk transfected to stably express the chimeric α5 integrin (X5CV cells) at expression levels comparable to wild-type cells (X5C5 cells). (B): Retention of internalized α5 integrin in X5CV cells was analyzed at indicated times as described in Fig. 1. Internalized X5CV receptor was not degraded in the absence of FN (Panel B) as had been observed with the wild-type receptor (Fig. 1A). (C): Site-directed mutagenesis was performed to obtain a double mutant construct (α5DM) in which the lysines at positions 1038 and 1042 in the cytoplasmic tail of α5 integrin, distal to the conserved GFFKR region, were replaced with alanine residues. α5-null CHO cells were bulk transfected to stably express the α5DM integrin at expression levels comparable to wild-type cells (α5WT cells). Cells (α5WT and α5DM) were then cultured overnight in the absence of FN. Retention of internalized α5WT and α5DM integrin was analyzed at indicated times as described in Fig. 1. α5DM integrin was readily detected at two hours, in contrast to the α5WT integrin which was significantly reduced. (D): CHO cells stably expressing α5WT or α5DM integrin with a C-terminal GFP-tag (α5WT-GFP and α5DM-GFP, respectively) were transiently transfected with an HA-tagged ubiquitin cDNA and cultured overnight in the presence or absence of FN, followed by pretreatment with MG-132 for one hour. Cell lysates were immunoprecipitated with an anti-HA monoclonal antibody followed by immunoblotting with an anti-GFP antibody to detect GFP-labeled α5 integrin cytoplasmic domain (~50 kDa; see also Figure 4 and text). Whole cell lysates of α5WTGFP (C) or α5WT (CWT) served as control. Loss of the two carboxyl-terminal lysine residues in the cytoplasmic tail of α5 integrin resulted in loss of the ability of that region to be ubiquitinated in the absence of FN.
To confirm that the carboxy-terminal lysines are important for α5 integrin degradation in the absence of fibronectin, site-directed mutagenesis was performed to obtain a double mutant construct (α5DM) in which the lysines at positions 1038 and 1042 were replaced with alanine residues. Cells expressing α5DM were bulk-sorted to obtain a cohort that expressed the mutant receptor at levels similar to cells expressing the wild-type α5 receptor (data not shown). We found that in the absence of fibronectin, the α5DM receptor escapes degradation when compared to the wild-type X5C5 receptor (Figure 3C). Furthermore, when α5DM cells were examined in the absence of fibronectin, no ubiquitination of the α5DM receptor was detected (Figure 3D). These data reinforce the conclusion that the ability of fibronectin to regulate the degradation of internalized α5 integrin depends on the ubiquitination of lysines in the α5 cytoplasmic tail distal to the conserved GFFKR sequence.
Diminished α5 integrin internalization leads to diminished fibronectin matrix assembly
Morphology of cells expressing α5DM was similar to cells expressing wild-type α5 integrin (data not shown). To see if there were any functional differences in the mutant receptor, α5DM cells were analyzed for their ability to assemble a fibronectin matrix. Despite their morphological similarities, α5DM cells had a diminished ability to internalize cell-surface α5 integrin even in the presence of fibronectin (Figure 4A). This correlated with a reduction in fibronectin matrix assembly. Cells expressing α5DM did not assemble fibronectin matrix as rapidly as cells expressing wild-type α5 integrin (Figure 4B), while overall fibronectin matrix assembly was also diminished in α5DM cells compared to wild-type cells (Figure 4C).
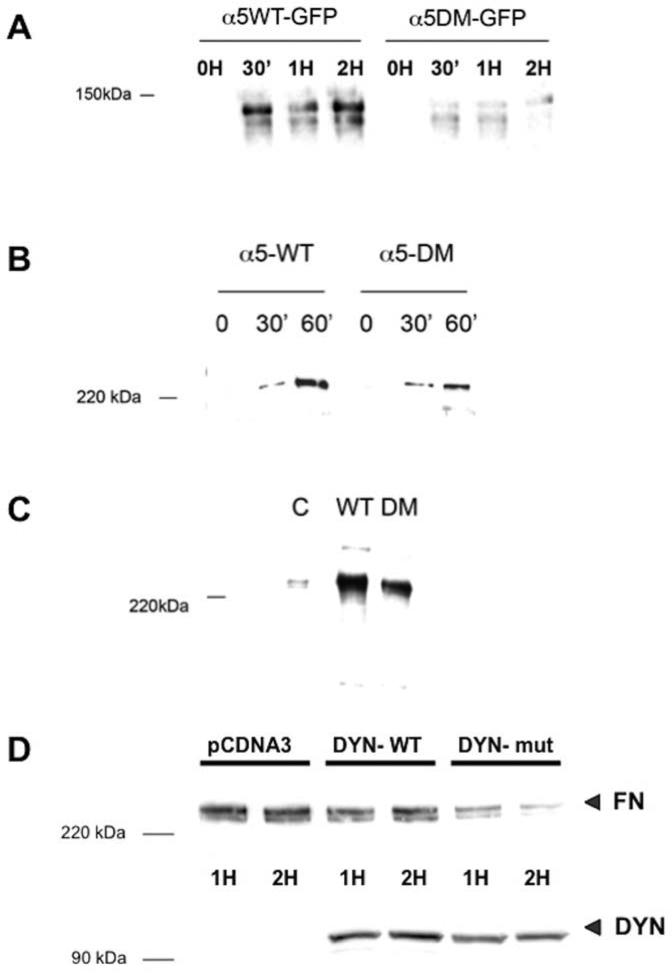
FIGURE 4. Loss of two carboxyl-terminal lysine residues results in a diminished rate of α5 integrin internalization and fibronectin matrix assembly, which is regulated by endocytosis.
(A): α5WT-GFP or α5DM-GFP cells were assayed for their ability to internalize α5 integrin in the presence of FN as described in Fig. 1. Loss of the two carboxyl-terminal lysine residues in the cytoplasmic tail of α5 integrin (α5DM) resulted in a diminished rate of internalization of cell-surface α5 integrin even when FN is present. (B and C): α5WT-GFP or α5DM-GFP cells were assessed for their ability to assemble a FN matrix using a DOC differential solubilization assay. Loss of the two carboxyl-terminal lysine residues in the cytoplasmic tail of α5 integrin (α5-DM) resulted in diminished rate of matrix assembly (panel B) as well as diminished total FN matrix assembly after 24 hours in culture (panel C) compared to cells expressing wild-type α5 integrin. (D): CHO cells stably expressing α5 integrin were transfected to express either wild-type human dynamin (DYN-WT) or a dominant-negative form (DYN-mut) prior to stimulation with soluble FN to initiate FN matrix assembly. Cells were lysed at the indicated time and matrix assembly was assessed by the DOC differential solubilization assay. Presence of non-functional dynamin (DYN) results in diminished ability to assemble a FN matrix (FN).
One possible explanation for our findings is that fibronectin matrix assembly depends on a cell's ability to internalize α5 integrin. To further corroborate this possibility, CHO cells expressing wild-type α5 integrin were transfected with a dominant-negative form of dynamin, a GTPase required for several endocytic pathways including those mediated by clathrin or caveolin [24-26] Compared to cells expressing the functional wild-type protein, cells expressing the non-functional dynamin were unable to assemble a fibronectin matrix (Figure 4D). Taken together with the findings from experiments using the α5DM cells, these data support the idea that internalization of α5 integrin is required for fibronectin matrix assembly.
DISCUSSION
Endocytosis and recycling of α5β1 integrin play a key role in extracellular matrix turnover, which is especially important for tissue remodeling and fibrotic processes associated with wound repair and tumor invasion. In this study, we have demonstrated that internalized α5 integrin is rapidly degraded via the ubiquitin-proteasome pathway in the absence of matrix-capable fibronectin in the extracellular space. Furthermore, we have identified two lysine residues in the cytoplasmic tail of the α5 integrin, distal to the highly conserved amino acid sequence GFFKR, whose ubiquitination targets the unligated receptor for rapid degradation. To our knowledge, this is the first study to present data demonstrating that extracellular matrix composition can regulate the fate of internalized integrin receptors. As inactive receptors and other unneeded cellular proteins are commonly targeted for proteasomal degradation, our findings are consistent with this principle.
In this study, fibronectin monomer that binds to α5β1 integrin does not prevent rapid turnover of the internalized receptor. These data indicate that simple binding of the receptor to ligand alone is not sufficient to prevent targeting of α5 for degradation, but rather that there are additional cues provided by the fibrillar matrix that affect receptor fate (Figure 5). The reasons for this are not clear, however, integrin-mediated mechanotransduction is an important determinant of focal adhesion complex assembly and recruitment of structural and signaling molecules to the site of cell-ECM interaction [27, 28]. This in turn triggers multiple mechanosensitive signaling pathways influencing protein and cellular responses in ways that are still not fully understood [29]. Our data suggest that the absence of tension can also mediate signaling events that trigger the rapid degradation of α5 integrin, presumably via ubiquitination of the receptor on key cytoplasmic lysine residues. This supports recent work in embryoid bodies derived from talin-1 null embryonic stem cells, which fail to form integrin-mediated adhesion and signaling complexes [12]. In these studies, a diminished level of β1 integrin was observed and found to be associated with the morphologic changes evident in the talin-null embryoid bodies. The decreased β1 protein levels reflected degradation of the integrin via a similar MG-132 sensitive proteosomal mechanism, suggesting a similar fate for unoccupied integrins exists in different cell types.
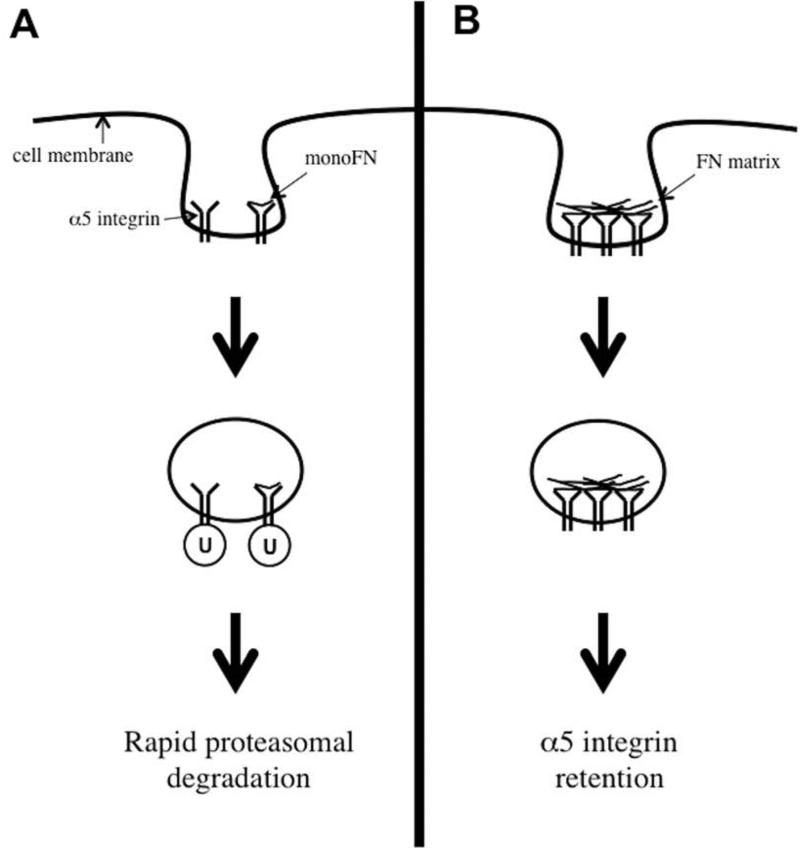
FIGURE 5. Fibronectin's impact on regulation of cell-surface α5 integrin.
This schematic diagram summarizes our findings regarding how FN affects cell-surface α5 integrin. (A): When FN is absent, or present only as a monomer (monoFN) or fragment containing the cell-binding sequence that can bind to α5 integrin but cannot be assembled into a matrix, α5 integrin is ubiquitinated (U) and targeted for proteasomal degradation. This degradation is rapid and requires two cytoplasmic tail lysine residues distal to the conserved GFFKR region. (B): In contrast, the presence of native matrix-capable FN that can undergo fibrillogenesis allows α5 integrin to avoid ubiquitination and degradation. This may occur through integrin activation and clustering or activation of co-receptors.
Alternately, the status of extracellular fibronectin itself could modulate α5 integrin fate via its impact on the binding and activation of other cell-surface receptors. Fibronectin in its matrix form is able to engage in multidomain interactions with other cell surface proteins, either directly or in concert with other extracellular components including growth factors [30]. Our finding that internalization and degradation of cell-surface α5 integrin depends on the presence of two specific lysine residues in the α5 cytoplasmic tail suggests there may be much room for combinatorial differences in how signals from these multidomain interactions are integrated within the cell and how cell-surface integrin receptors are sorted once they have been internalized based on external microenvironmental conditions.
We found that inhibitors of lysosomal degradation did not prevent rapid α5 integrin degradation in the absence of matrix fibronectin. This corroborates other recent findings showing that the β1 integrin can be degraded via a proteasomal mechanism [12]. However, α5 integrin has also been shown to undergo lysosomal degradation [6, 18]. For example, Lobert et al. have demonstrated that in migrating cells, a fraction of the endocytosed α5β1 is sorted into multivesicular endosomes, together with fibronectin, and degraded in lysosomes. The sorting of α5β1 to the lysosomes requires both fibronectin and polyubiquitination of the α5 subunit. This contrasts with our data, which suggests that ubiquitination can also occur in the absence of matrix fibronectin. One possible explanation for why α5 integrin may undergo degradation by different pathways is that alternative mechanisms for integrin sorting and degradation may exist depending on environmental factors. For example, cells grown under serum-starved conditions do not assemble fibronectin matrix as readily, even when exogenous fibronectin is provided [31]. In our study, cells were grown under conditions where full serum that had been stripped of fibronectin was provided. Thus, serum factors such as lysophosphatidic acid are available to support fibronectin matrix assembly and impact the fate of α5 and other integrins after internalization. Over the course of 24 hours internalized receptors may undergo recycling back to the cell surface or eventual lysosomal degradation. Our findings support the idea that another pathway for more rapid proteasomal degradation may exist for integrins as has been found for other cell-surface receptors [13].
Finally, we show that fibronectin matrix assembly is diminished when endocytic internalization of α5 integrin is hindered, whether by loss of the two cytoplasmic tail lysines of α5 integrin or non-functional dynamin. Although the relationship of fibronectin matrix degradation and α5 integrin endocytosis has been described previously [5-7], this is the first study to our knowledge demonstrating that assembly of fibronectin matrix depends on the ability of cells to internalize α5 integrin. Furthermore, protection of α5 integrin from rapid degradation depends on the presence of matrix-capable fibronectin that can be assembled and undergo fibrillogenesis. Taken together, our data support the concept that fibronectin matrix assembly and the fate of internalized α5β1 integrin are interdependent processes. The formation of a fibronectin matrix precedes and subsequently regulates the remodeling of the type I collagen matrix, and influences the remodeling, of the mature scar [5, 32, 33]. Therefore, determining how integrin trafficking requirements and the cell-mediated process of fibronectin fibril and matrix formation are related and could be manipulated would have important implications for our ability to understand, and in turn mitigate, fibrosis-related complications that arise as a consequence of physiological processes such as wound repair or pathological ones such as tumor invasion.
MATERIALS AND METHODS
Cell Culture
CHO-α5 cells (gift from Dr. Jean Schwarzbauer, Princeton University, NJ) are a Chinese Hamster Ovary cell line transfected with human α5 integrin and selected with G418 (250 g/ml; Invitrogen, Carlsbad, CA) that expresses functional α5β1 integrin receptor but does not secrete detectable levels of endogenous fibronectin. A stably transfected CHO-α5-GFP cell line was generated in the laboratory by co-transfecting α5-pEGFP-N3 and pCMV-Bsd (Invitrogen) constructs into fibronectin-null CHO cells that do not express human α5, using Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer's instructions and selected with blasticidin (1 μg/ml; Invivogen, San Diego, CA). Mouse embryonic fibroblast (MEF) cells lacking fibronectin expression were obtained as a gift from Dr. Jane Sottile, University of Rochester, NY. All cell lines were propagated in Dulbecco's Modified Eagle Medium (DMEM; Invitrogen) supplemented with 10% Fetal Bovine Serum (FBS; Hyclone Laboratories, Logan, UT), 2 mM L-glutamine, 1mM sodium pyruvate, 0.1 mM non-essential amino acids, and 1% Antibiotics-Antimycotic solution (Invitrogen). Normocin (Invivogen) was added to the medium at a concentration of 100 μg/ml to prevent mycoplasma contamination. Fibronectin-depleted serum was prepared by binding FBS to gelatin sepharose 4B beads (GE Healthcare, Piscataway, NJ) for one hour at room temperature, repeated twice, followed by centrifugation at 900 rpm for 10 minutes and then filter sterilized [4]. Fibronectin depletion in the serum was verified by SDS-PAGE.
Transfection
EpS15 mutant constructs (pEGFP-C2-EH29, pEGFP-C2-DIII and pEGFP-C2-D3▲) and pCDNA3-GFP-Cbl construct were obtained as a gift from Dr. Mayumi Naramura (University of Nebraska Medical Center, Omaha, NE). pRK5-HA-Ubquitin-WT construct was purchased from Addgene (Cambridge, MA). Transfections were performed by Nucleofection (Amaxa Biosystems, Inc., Gaithersburg, MD). 1 x 106 to 5 x 106 cells were washed with PBS once and resuspended in 100 μl of Nucleofector Solution V and mixed with 2-5 μg of plasmid DNA. The cell/DNA mixture was then transferred to an Amaxa-certified cuvette and subjected to nucleofection using a preexisting program (U-024) on the nucleofector device. Immediately after nucleofection, 500 μl of medium was added to the cuvette and transfected cells were transferred to a culture dish containing pre-warmed complete growth medium using an Amaxa-certified Pasteur pipette and incubated in a humidified CO2 incubator at 37°C. Analysis of gene expression was done by FN matrix assembly, immunoblot analysis, and immunofluorescence using an inverted microscope (Nikon Eclipse TE 300, Melville NY). Transfection efficiency of the cells was analyzed by FACS Calibur (Becton & Dickenson, San Jose, CA). Supplier-provided pMax-GFP DNA, non-transfected cells, and/or empty vector were used as controls.
α5 Internalization by Immunoblot Analysis
Detection of α5 integrin internalization was detected using previously published methodology [17]. Briefly, cells cultured in the presence or absence of fibronectin were labeled with 10 mM solution of EZ-Link Sulfo-NHS-SS-Biotin (Pierce Biotechnology Inc., Rockford, IL) for 30 minutes at 4°C and then warmed to 37°C for different intervals of time with or without the following proteasome/lysosome inhibitors: MG132 (1 μM; EMD Chemicals, Gibbstown, NJ), Bafilomycin (1 μM; LC Laboratories, Woburn, MA), Chloroquine (100 μM; Sigma-Aldrich, St. Louis, MO) or Recombinant Human Fibronectin-1, Fragment 3 (3.6 μg/ml; R&D Systems, Minneapolis, MN). At the indicated times, the medium was aspirated and the dishes were rapidly transferred to ice and washed twice with ice cold HBSS (Hanks Balanced Salt Solution; Invitrogen). Biotin was removed from proteins remaining at the cell surface by incubation with a solution containing 20 mM MESNa (sodium 2-mercaptoethane sulfate) in 50 mM Tris (pH 8.6) and 100 mM NaCl for 10 minutes at 4°C and quenched by the addition of 20 mM iodoacetamide (IAA) for 10 minutes. Cells were then lysed in RIPA buffer (150 mM NaCl, 50 mM Tris pH 7.5, 1% Nonidet P-40 and 0.25% Deoxycholate) supplemented with protease inhibitor cocktail (EMD Biosciences, San Diego, CA). Lysates were quantitated and equivalent protein concentrations were immobilized using neutravidin agarose beads (Pierce), solubilized in Laemmli sample buffer, analysed by 7% SDS-PAGE and transferred to a nitrocellulose membrane. The membrane was blocked in 5% non-fat dry milk, 0.1% Tween 20 and TBS followed by immunoblotting with anti-α5 integrin primary antibody (Millipore, Billerica, MA) and peroxidaseconjugated secondary antibody (Pierce). Experiments were performed in triplicate. For each experiment, the corresponding bands were detected using Supersignal enhanced chemiluminescence (ECL) detection kit (Pierce). Signal intensity was quantified by densitometry using NIH ImageJ softwareS tatistical analysis performed using a 2-way ANOVA with a Tukey correction for multiple comparisons (GraphPad; Prism TM software).
Visualization of α5 Internalization by Immunofluorescence
CHO-α5 cells were plated onto glass coverslips in 12-well culture dishes and grown to 50%-70% confluency in medium containing regular serum or FN-depleted serum. The following day cells were labeled with anti-α5 mouse monoclonal antibody (Santa Cruz Biotechnology) for one hour at 4°C. Cells were then washed and treated with pre-warmed growth medium and transferred to 37°C for 30 minutes to allow internalization. Remaining labeled antibody on the cell surface was removed by treatment with 0.5M NaCl, 2M acetic acid for 5 minutes at 4°C, following which cells were fixed with 3.7% formaldehyde for 15 minutes at 4°C, semi-permeabilized with PBS containing 1% triton X-100 for 15 minutes at room temperature, and blocked with 6% BSA in PBS for one hour. Cells were later stained with goat anti-mouse-Alexa Fluor 555 (Invitrogen) for 30 minutes at room temperature, washed three times with PBS and stained with 1:1000 dilution of 2.5 mg/ml stock solution of DAPI (4',6-diamidino-2-phenylindole; Invitrogen) for 10 minutes, washed and mounted on slides using DABCO mounting medium (Sigma-Aldrich). Internalized antibody was visualized using an inverted fluorescent microscope (Nikon Instruments, Melville, NY).
Immunoprecipitation
Cells lysates were clarified by centrifugation using a Qiashredder spin column (Invitrogen) at 14,000 RPM at 4°C. Equal aliquots of protein (300 μg to 1 mg) of protein lysates were immunoprecipitated using 2-4 μg of one of the following specific antibodies: anti-α5, anti-CBL (Santa Cruz Biotechnology), anti-HA-Tag (Cell Signaling Technology, Beverly, MA), before being incubated overnight at 4°C in a rotator. The following day 20 μg of protein A/G agarose beads (EMD Chemicals) were added and incubated for one hour at 4°C. Immunoprecipitates were then collected by centrifugation at 14000 RPM for three minutes, the pellets were washed three times with RIPA buffer, and solubilized in 25 μl of Laemmli sample buffer containing β-mercaptoethanol. Aliquots were then subjected to SDS-PAGE electrophoresis and immunoblot membranes were reacted with corresponding anti-CBL or anti-GFP (Invitrogen) antibodies. Immunoblots were probed with peroxidase-conjugated secondary antibodies and visualized by ECL detection.
α5 Integrin Degradation Assay
Fibronectin-null mouse embryonic fibroblast (MEF; gift of Dr. Jane Sottile, University of Rochester, NY) cells plated in the presence or absence of fibronectin overnight were treated with cyclohexamide (100 μg/ml; Sigma-Aldrich) for one hour to block new protein synthesis. Cells lysates were then collected after various time intervals and quantitated for total protein using standard techniques. Equal amounts of protein (40 μg) were loaded on SDS-PAGE gel, electrophoresed and analyzed by immunoblotting with anti-α5 integrin or anti-GAPDH (Sigma-Aldrich) antibodies.
Fibronectin Matrix Assembly
The assembly of fibronectin matrix was assessed using a deoxycholate (DOC) insolubility assay and immunoblot analysis as described previously [34]. Cell monolayers were rinsed once with PBS and lysed in DOC lysis buffer (2% sodium deoxycholate, 20 mM Tris-HCl pH 8.8, 2 mM PMSF, 2 mM EDTA, 2 mM iodoacetic acid and 2 mM N-ethylmaleimide) and passed five times through a 26-gauge needle to break up viscosity. The DOC-insoluble material was then collected by centrifugation at 14000 RPM for 20 minutes at 4°C and solubilized using SDS solubilization buffer (1% SDS, 20 mM Tris-HCl pH 8.8, 2 mM PMSF, 2 mM EDTA, 2 mM iodoacetic acid and 2 mM N-ethylmaleimide). The insoluble lysates were resolved by SDS-PAGE, transferred to nitrocellulose membrane and analyzed by immunoblotting using a biotinylated anti-fibronectin antibody (Abcam, Cambridge, MA) as the primary antibody and streptavidin-HRP conjugated secondary antibody (Pierce), followed by ECL detection.
Site-Directed Mutagenesis of α5 Integrin Ubiquitination Sites
Site-directed mutagenesis was performed on two lysine (K) residues at positions 15 and 19 of the α5 integrin cytoplasmic domain (GFFKRSLPYGTAMEKAQLKPPATSDA; positions 1038 and 1042 on the full-length protein) that were predicted to be ubiquitinated with high confidence using the QuikChange Lightning Site-Directed Mutagenesis Kit (Stratagene, Santa Clara, CA) according to manufacturer's protocol. Mutagenic primers (α5K15A primers, sense: 5’- CAT ATG GCA CCG CCA TGG AAG CAG CTC AGC TCA AGC C -3’; anti-sense: 5’- GGC TTG AGC TGA GCT GCT TCC ATG GCG GTG CCA TAT G -3’ and α5K19A primers- sense: 5’- GGA AAA AGC TCA GCT CGC GCC TCC AGC CAC CTC T -3’; anti-sense: 5’- AGA GGT GGC TGG AGG CGC GAG CTG AGC TTT TTC C -3’) containing the desired mutations were designed using Stratagene's QuikChange Primer Design Program based on the α5 integrin wild-type nucleotide sequence and synthesized through our DNA core facility. Both lysine (K) residues on the wild-type α5 integrin DNA (α5-pEGFP-N3) distal to the conserved GFFKR sequence were mutated to alanine (A) simultaneously, generating a double-mutant DNA construct (α5-DM).
Site-Directed Mutagenesis of Human Dynamin 2
Human dynamin 2 (DNM2) plasmid clone was purchased from OriGene (Rockville, MD). A highly conserved amino acid lysine (K) within the GTPase domain was mutated to alanine (A) by site-directed mutagenesis using QuikChange II XL Site Directed Mutagenesis Kit (Stratagene) according to the manufacturer's protocol. Both the dynamin wild type (DYN-WT) and mutant (DYN-mut) constructs were then transfected into CHO cells stably expressing α5 integrin using Lipofectamine 2000 (Invitrogen). Cells were plated to obtain 90-95% confluency. The following day, 8 μg of DNA from either the wild type or mutant DNM2 was mixed with 30 μl of lipofectamine transfection reagent in a tube and incubated at room temperature for 20 minutes. The DNA/lipofectamine mixture was later added to cells in serum-free Opti-MEM medium (Invitrogen) and incubated at 37°C in a CO2 incubator overnight to express either the wild-type dynamin (DYN-WT) or the dominant-negative form (DYN-mut). Transfection efficiency was analyzed by flow cytometer. Both the DYN-WT and DYN-mut transfected cells were treated with 10 μg/ml soluble fibronectin for one to two hour intervals to initiate fibronectin matrix assembly. Cells were lysed at the indicated time and matrix assembly was assessed by the DOC differential solubilization assay (see above). pCDNA3 empty vector was used as a negative control for transfection. Dynamin protein detected in the lysates by immunoblot analysis using human anti-dynamin primary antibody (Santa Cruz Biotechnology, Santa Cruz, CA) served as a positive control for transfection.
Supplementary Material
SUPPLEMENTAL FIGURE 1. The labels of internalized α5 integrin decline when fibronectin is absent. Experiments were conducted in triplicate as described in Fig.1. For each experiment, the corresponding bands were detected using Supersignal enhanced chemiluminescence and signal intensity was quantified by densitometry. Statistical analysis was performed using a 2-way ANOVA with a Tukey correction for multiple comparisons. (GraphPad; Prism TM software). The presence of internalized a5 integrin is significantly higher in the presence vs. absence of fibronectin (p<0.0001).
SUPPLEMENTAL FIGURE 2. Internalized α5 integrin localizes to a distinct perinuclear compartment. CHO cells cultured in the absence or presence of FN were incubated with α5 integrin antibody for one hour at 4°C before being washed and warmed to 37°C for 30 minutes to allow internalization. Cells were then fixed and the internalized antibody detected with a FITC-labeled goat-antimouse IgG. In the absence of FN, α5 integrin localizes to a distinct intracellular compartment adjacent to the cell nucleus (A). In the presence of FN, internalized α5 integrin appears to be localized to an area overlying the nucleus, although its presence within the nucleus cannot be excluded (B).
ACKNOWLEDGMENTS
Funding for this research was provided by the National Institutes of Health (R01-GM61847 to S.A.C and K08-GM072546 to H.C.H) and Feldstein Medical Foundation (H.C.H).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
(Author contributions: Conception and Design: S.A.C. Analysis and Interpretation: S.A.C., H.C.H., M.R.N. Data collection: M.R.N. Writing and critical revisions: H.C.H, S.A.C.)
A portion of this data was presented at the Academic Surgical Congress, Las Vegas, NV in February 2011.
FINANCIAL DISCLOSURES:
None of the authors have any commercial associations or financial disclosures to make with regard to this paper.
REFERENCES
- 1.Clark RAF. The molecular and cellular biology of wound repair. 2nd ed xxiii. Plenum Press; New York: 1996. p. 611. [Google Scholar]
- 2.Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5(2):146–56. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- 3.Hynes RO. Fibronectins. Springer-Verlag; New York: 1990. [Google Scholar]
- 4.Corbett SA, et al. Covalent cross-linking of fibronectin to fibrin is required for maximal cell adhesion to a fibronectin-fibrin matrix. J Biol Chem. 1997;272(40):24999–5005. doi: 10.1074/jbc.272.40.24999. [DOI] [PubMed] [Google Scholar]
- 5.Sottile J, Hocking DC. Fibronectin polymerization regulates the composition and stability of extracellular matrix fibrils and cell-matrix adhesions. Mol Biol Cell. 2002;13(10):3546–59. doi: 10.1091/mbc.E02-01-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sottile J, Chandler J. Fibronectin matrix turnover occurs through a caveolin-1-dependent process. Mol Biol Cell. 2005;16(2):757–68. doi: 10.1091/mbc.E04-08-0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi F, Sottile J. Caveolin-1-dependent beta1 integrin endocytosis is a critical regulator of fibronectin turnover. J Cell Sci. 2008;121(Pt 14):2360–71. doi: 10.1242/jcs.014977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ivaska J, et al. PKC epsilon controls the traffic of beta1 integrins in motile cells. Embo J. 2002;21(14):3608–19. doi: 10.1093/emboj/cdf371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caswell PT, Norman JC. Integrin trafficking and the control of cell migration. Traffic. 2006;7(1):14–21. doi: 10.1111/j.1600-0854.2005.00362.x. [DOI] [PubMed] [Google Scholar]
- 10.Caswell PT, Vadrevu S, Norman JC. Integrins: masters and slaves of endocytic transport. Nat Rev Mol Cell Biol. 2009;10(12):843–53. doi: 10.1038/nrm2799. [DOI] [PubMed] [Google Scholar]
- 11.Margadant C, et al. Mechanisms of integrin activation and trafficking. Current Opinion in Cell Biology. 2011;23(5):607–14. doi: 10.1016/j.ceb.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 12.Liu J, et al. Talin1 regulates integrin turnover to promote embryonic epithelial morphogenesis. Mol Cell Biol. 2011;31(16):3366–77. doi: 10.1128/MCB.01403-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hicke L, Dunn R. Regulation of membrane protein transport by ubiquitin and ubiquitin-binding proteins. Annual Review of Cell and Developmental Biology. 2003;19:141–72. doi: 10.1146/annurev.cellbio.19.110701.154617. [DOI] [PubMed] [Google Scholar]
- 14.Ciechanover A. Proteolysis: from the lysosome to ubiquitin and the proteasome. Nat Rev Mol Cell Biol. 2005;6(1):79–87. doi: 10.1038/nrm1552. [DOI] [PubMed] [Google Scholar]
- 15.Bretscher MS. Endocytosis and recycling of the fibronectin receptor in CHO cells. The EMBO journal. 1989;8(5):1341–8. doi: 10.1002/j.1460-2075.1989.tb03514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bretscher MS. Circulating integrins: alpha 5 beta 1, alpha 6 beta 4 and Mac-1, but not alpha 3 beta 1, alpha 4 beta 1 or LFA-1. The EMBO journal. 1992;11(2):405–10. doi: 10.1002/j.1460-2075.1992.tb05068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zemskov EA, et al. Cell-surface transglutaminase undergoes internalization and lysosomal degradation: an essential role for LRP1. Journal of Cell Science. 2007;120(Pt 18):3188–99. doi: 10.1242/jcs.010397. [DOI] [PubMed] [Google Scholar]
- 18.Lobert VH, et al. Ubiquitination of alpha 5 beta 1 integrin controls fibroblast migration through lysosomal degradation of fibronectin-integrin complexes. Dev Cell. 2010;19(1):148–59. doi: 10.1016/j.devcel.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 19.Laukaitis CM, et al. Differential dynamics of alpha 5 integrin, paxillin, and alpha-actinin during formation and disassembly of adhesions in migrating cells. J Cell Biol. 2001;153(7):1427–40. doi: 10.1083/jcb.153.7.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valdembri D, et al. Neuropilin-1/GIPC1 signaling regulates alpha5beta1 integrin traffic and function in endothelial cells. PLoS biology. 2009;7(1):e25. doi: 10.1371/journal.pbio.1000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 22.Kaabeche K, et al. Cbl-mediated ubiquitination of alpha5 integrin subunit mediates fibronectin-dependent osteoblast detachment and apoptosis induced by FGFR2 activation. J Cell Sci. 2005;118(Pt 6):1223–32. doi: 10.1242/jcs.01679. [DOI] [PubMed] [Google Scholar]
- 23.Pardi R, et al. Conserved regions in the cytoplasmic domains of the leukocyte integrin alpha L beta 2 are involved in endoplasmic reticulum retention, dimerization, and cytoskeletal association. Journal of immunology. 1995;155(3):1252–63. [PubMed] [Google Scholar]
- 24.van der Bliek AM, et al. Mutations in human dynamin block an intermediate stage in coated vesicle formation. J Cell Biol. 1993;122(3):553–63. doi: 10.1083/jcb.122.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henley JR, et al. Dynamin-mediated internalization of caveolae. J Cell Biol. 1998;141(1):85–99. doi: 10.1083/jcb.141.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumari S, Mg S, Mayor S. Endocytosis unplugged: multiple ways to enter the cell. Cell Res. 2010;20(3):256–75. doi: 10.1038/cr.2010.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Balaban NQ, et al. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat Cell Biol. 2001;3(5):466–72. doi: 10.1038/35074532. [DOI] [PubMed] [Google Scholar]
- 28.Bershadsky AD, Balaban NQ, Geiger B. Adhesion-dependent cell mechanosensitivity. Annual Review of Cell and Developmental Biology. 2003;19:677–95. doi: 10.1146/annurev.cellbio.19.111301.153011. [DOI] [PubMed] [Google Scholar]
- 29.Holle AW, Engler AJ. More than a feeling: discovering, understanding, and influencing mechanosensing pathways. Current opinion in biotechnology. 2011;22(5):648–54. doi: 10.1016/j.copbio.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326(5957):1216–9. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Q, Magnusson MK, Mosher DF. Lysophosphatidic acid and microtubule-destabilizing agents stimulate fibronectin matrix assembly through Rho-dependent actin stress fiber formation and cell contraction. Molecular Biology of the Cell. 1997;8(8):1415–25. doi: 10.1091/mbc.8.8.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Velling T, et al. Polymerization of type I and III collagens is dependent on fibronectin and enhanced by integrins alpha 11beta 1 and alpha 2beta 1. J Biol Chem. 2002;277(40):37377–81. doi: 10.1074/jbc.M206286200. [DOI] [PubMed] [Google Scholar]
- 33.Shi F, et al. Collagen I matrix turnover is regulated by fibronectin polymerization. American journal of physiology. Cell physiology. 2010;298(5):C1265–75. doi: 10.1152/ajpcell.00341.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Winters BS, et al. Three-dimensional culture regulates Raf-1 expression to modulate fibronectin matrix assembly. Mol Biol Cell. 2006;17(8):3386–96. doi: 10.1091/mbc.E05-09-0849. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPLEMENTAL FIGURE 1. The labels of internalized α5 integrin decline when fibronectin is absent. Experiments were conducted in triplicate as described in Fig.1. For each experiment, the corresponding bands were detected using Supersignal enhanced chemiluminescence and signal intensity was quantified by densitometry. Statistical analysis was performed using a 2-way ANOVA with a Tukey correction for multiple comparisons. (GraphPad; Prism TM software). The presence of internalized a5 integrin is significantly higher in the presence vs. absence of fibronectin (p<0.0001).
SUPPLEMENTAL FIGURE 2. Internalized α5 integrin localizes to a distinct perinuclear compartment. CHO cells cultured in the absence or presence of FN were incubated with α5 integrin antibody for one hour at 4°C before being washed and warmed to 37°C for 30 minutes to allow internalization. Cells were then fixed and the internalized antibody detected with a FITC-labeled goat-antimouse IgG. In the absence of FN, α5 integrin localizes to a distinct intracellular compartment adjacent to the cell nucleus (A). In the presence of FN, internalized α5 integrin appears to be localized to an area overlying the nucleus, although its presence within the nucleus cannot be excluded (B).