Abstract
Background
Innate lymphoid cells (ILCs) play important roles in innate immunity and tissue remodeling via production of various cytokines and growth factors. Group 2 ILCs (ILC2s) were recently shown to mediate the immune pathology of asthma even without adaptive immunity. However, little is known about possible interactions between ILC2s and other immune cells. We sought to investigate the capacity of ILC2s to regulate effector functions of T cells.
Methods
We isolated ILC2s from the lungs of naïve mice. We cultured CD4+ T cells with ILC2s in vitro and examined the functions of these cell types. The mechanisms were investigated by using blocking antibodies and cells isolated from cytokine-deficient mice. For the in vivo study, we adoptively transferred ILC2s and CD4+ T cells into Il7ra−/− mice and subsequently exposed the mice to ovalbumin and a cysteine protease.
Results
Lung ILC2s enhanced CD4+ T cell proliferation and promoted production of type 2 cytokines in vitro. The interaction between ILC2s and CD4+ T cells involved costimulatory molecule OX40L and cytokine IL-4, which was mainly derived from ILC2s. Adoptive transfer of both ILC2 and CD4+ T cell populations, but not each population alone, into Il7ra−/− mice resulted in induction of a robust antigen-specific type 2 cytokine response and airway inflammation.
Conclusion
Lung ILC2s function to promote adaptive immunity in addition to their established roles in innate immunity. This novel function of ILC2s needs to be taken into account when considering the pathophysiology of asthma and other allergic airway diseases.
INTRODUCTION
Innate lymphoid cells (ILCs) are emerging as important effector cells in innate immunity and tissue homeostasis (1). Type 2 ILCs (ILC2s) produce Th2 cytokines, such as IL-5 and IL-13, and play important roles in a variety of immune responses, including immunity to helminths, airway and skin inflammation, and tissue remodeling (2). However, we have limited knowledge about the ability of ILC2s to work together with other immune cells. Several prior reports provide evidence suggesting that “crosstalk” may occur between ILC2s and T cells. For example, ILC2 numbers were not maintained in Rag2−/− mice after helminth infection (3), suggesting a role for T cells in the survival of ILC2s. Conversely, ILC2s from IL-13-deficient mice restored IL-13 production, presumably from T cells, in mice infected with helminths (3). In addition, multipotent progenitor type 2 (MPPtype2) cells promoted Th2-type CD4+ T cell differentiation (4), although MPPtype2 cells may comprise a population that is distinct from ILC2s (5). In this study, to address possible interactions between ILC2s and CD4+ T cells directly, we performed a series of in vitro and in vivo experiments using isolated lung ILC2s and CD4+ T cells. Our findings indicate that synergistic interactions between innate immune and adaptive immune cell populations may generate robust type 2 immune responses.
MATERIALS AND METHODS
Mice and reagents
BALB/cJ, C57BL/6, Il4−/− and Il7ra-/- mice were from the Jackson Laboratory. Il5−/− mice were from Dr. James Lee (Mayo Clinic Arizona, AZ). Female mice ages 6-12 weeks were used in all experiments. Antibodies to CD3 (145-2C11), CD25 (PC61; 7D4), CD44 (IM7), CD14 (M5E2), CD16/CD32 (2.4G2), CD45R/B220 (RA3-6B2), ICOS (7E.17G9), CD28 (37.51), IL-4Rα (mIL4R-M1), OX40 (ACT35), and OX40L (ik-1) were from BD Biosciences. Anti-OX40L mAb (RM134L) and polyclonal anti-OX40L Ab were from eBioscience and R&D Systems, respectively. Anti-ST2 mAb (97203) was from R&D Systems. Bromelain was from Sigma-Aldrich. Endotoxin-free ovalbumin (OVA) was prepared as described previously (6).
Lung ILC2 isolation for in vitro study
ILC2s were isolated as described previously (7). Briefly, lungs were minced and digested with a cocktail of collagenases (Roche Diagnostics) and DNase I (StemCell Technologies) to obtain lung single cell suspensions. To isolate ILC2s, lineage-negative (Lin−) cells were enriched first by magnetically depleting lineage-positive (Lin+) cells with PE-conjugated antibodies to CD3, CD14, CD16/CD32 and B220 and EasySep® magnetic particles (StemCell Technologies). Lin− cell-enriched lung cells were then stained with fluorescence-labeled antibodies to CD3, CD14, CD16/CD32, B220, CD25 and CD44. ILC2s were isolated as the Lin− CD25+CD44hi cell population by sorting on a fluorescence-activated cell sorter (FACS, BD FACSAria®). Sorted ILC2s were cultured with a cocktail of IL-33 (10 ng/ml) and IL-7 (10 ng/ml) for up to 10 days. Before use, ILC2s were washed once to remove residual IL-33 and IL-7. In some experiments, purity of ILC2s was verified by staining them with anti-ST2 and FACS analysis.
CD4+ T cell isolation and culture
Splenic CD4+ T cells were isolated using the Negative Selection EasySep® CD4+ T cell enrichment kit (StemCell Technologies). CD4+ T cells were cultured with plate-bound anti-CD3 (2 μg/ml) and soluble anti-CD28 (1 μg/ml) in a 96-well plate at 2×104 cells/well with or without ILC2s at 104 cells/well unless specified otherwise. Four days later, cytokine levels in culture supernatants were analyzed by ELISA. For the Transwell® culture system (Costar, 0.4 μm pore size; Corning) experiments, CD4+ T cells (2x105 cells/well) and ILC2s (105 cells/well) were added to anti-CD3-coated lower and upper chambers, respectively. In some experiments, IL-4 or IL-12 and neutralizing antibodies to IL-4 or IFN-γ (all from R&D Systems) were added to the co-culture of ILC2s and CD4+ T cells.
Quantitative RT-PCR
ILC2s and CD4+ T cells were co-cultured in a 24-well plate at 5×105 cells/well for 20 h and then FACS sorted as CD4+CD25+ T cells and CD4−CD25+ ILC2s. RT-PCR was performed using Taqman Universal PCR Master Mix and IL-4, IL-5, IL-13 and housekeeping gene 18S primers (Applied Biosystems). Cytokine mRNA expression was normalized to the expression of 18S.
Adoptive cell transfer and antigen-induced airway inflammation
Naïve mice were injected i.p. with IL-25 and IL-33 (400 ng/mouse) daily for 4 days to expand ILC2s in vivo. Lung ILC2s were isolated by FACS sorting as the Lin-ICOS+ cell population (7). ILC2s (105 cells/mouse), CD4+ T cells (2×106 cells/mouse), or a combination of the two were adoptively transferred to recipient Il7ra−/− mice by i.v. injection. One day and 5 days after the transfer, mice were intranasally (i.n.) administered with OVA (100 μg) plus bromelain (10 μg). Nine days after the transfer, bronchoalveolar lavage (BAL) fluids were analyzed for inflammatory parameters. Lung cells were cultured at 105 cells/well with OVA antigen (100 μg/ml) for 7 days or anti-CD3/CD28 for 5 days for measurement of cytokine production in vitro.
Statistical analysis
Statistical significance was assessed with Student's t test. A p-value of < 0.05 was considered significant.
RESULTS
Co-culture of ILC2s and CD4+ T cells promotes proliferation of CD4+ T cells and type 2 cytokine production in vitro
To investigate the possible interaction between ILC2s and CD4+ T cells directly, we used an in vitro culture system. ILC2s were isolated from the lungs of naïve BALB/c mice, expanded and maintained with IL-33 plus IL-7, and washed before use. FACS analysis revealed that, similar to naïve ILC2s (7), these in vitro expanded ILC2s were lineage negative, CD44+CD25+ and ST2+ (Supplemental Fig. 1). CD4+ T cells were isolated from spleens of naïve BALB/c mice and were cultured alone or together with ILC2s. As expected, CD4+ T cells proliferated following stimulation with anti-CD3/CD28 antibodies (Fig. 1A). When lung ILC2s were added to the culture, proliferation of CD4+ T cells was clearly enhanced. Furthermore, the numbers of CD4+ T cells were 2.1±0.7-fold higher after 4 days when cultured with ILC2s as compared to when cultured without ILC2s (mean±SEM, n=4)
Figure 1. Co-culture of ILC2s and CD4+ T cells enhances proliferation of CD4+ T cells and type 2 cytokine production.
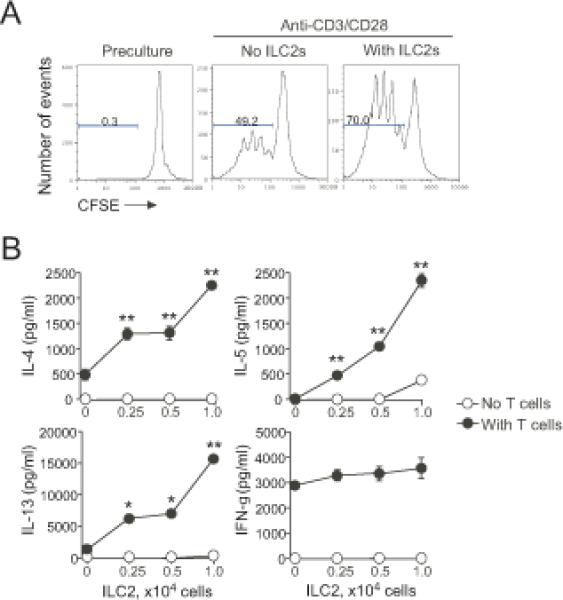
(A) CFSE-labeled CD4+ T cells (2×104 cells/well) were stimulated with anti-CD3/CD28 with or without isolated lung ILC2s (104 cells/well) for 4 days. Cells were analyzed by FACS by gating on CD4+ T cells. Data are representative of three experiments. (B) CD4+ T cells (2×104 cells/well) were stimulated with anti-CD3/CD28 with the indicated numbers of ILC2s for 4 days. Cytokine levels in the supernatants were analyzed by ELISA. *, p<0.05; **, p<0.01 as compared to no ILC2s in the culture. Data (mean±SEM, n=3) are representative of 2-3 experiments.
To investigate whether ILC2s affect cytokine production, CD4+ T cells were stimulated with anti-CD3/CD28 in the presence of increasing numbers of ILC2s. When cultured alone, CD4+ T cells produced both IL-4 and IFN-γ but negligible amounts of IL-5 and IL-13 (Fig. 1B). ILC2s alone produced small amounts of IL-5 and IL-13 but only at the highest cell number examined (1×104 cells/well); IL-4 and IFN-γ were undetectable. Notably, co-culture of CD4+ T cells with ILC2s resulted in robust increases in IL-4, IL-5, and IL-13 levels in the supernatants. In contrast, CD4+ T cell and ILC2 co-culture did not affect IFN-γ levels (Fig. 1B). Furthermore, when CD4+ T cells were cultured in Th1-skewing condition, addition of ILC2s enhanced production of IL-5 but not IFN-γ (Supplemental Fig. 2). These data suggest that co-culture of ILC2s and CD4+ T cells enhances proliferation of T cells and type 2 cytokine production.
Cytokine production is enhanced in both ILC2s and CD4+ T cells
Because both CD4+ T cells and ILC2s are capable of producing type 2 cytokines (1, 2), we sought to determine which cell type is affected by co-culture. To this end, we cultured CD4+ T cells with ILC2s and then separated the cells into the CD4+ T cell and ILC2 populations by sorting. Following ILC2/CD4+ T cell co-culture, IL-4 mRNA levels were upregulated in ILC2s (mean 13.2-fold increase) but not in CD4+ T cells (Fig. 2A). IL-5 mRNA was increased dramatically in CD4+ T cells (29.7-fold increase) and modestly in ILC2s (2.1-fold increase). A modest (2.6-fold) increase and a minimal (1.6-fold) increase in IL-13 mRNA levels were observed in CD4+ T cell and ILC2 populations, respectively.
Figure 2. The interaction between ILC2s and CD4+ T cells is likely bidirectional.

(A) CD4+ T cells (5×105 cells/well) and ILC2s (5×105 cells/well) were cultured alone or together for 20 h and then sorted by FACS. Cytokine mRNA expression in each cell population was analyzed. Data are presented as the ratio of mRNA copy numbers in co-cultured cells divided by mRNA copy numbers in cells cultured alone. Data are from three experiments. (B) CD4+ T cells derived from WT or Il5−/− mice were stimulated with anti-CD3/CD28 with or without ILC2s from WT mice for 4 days. *, p<0.05; **, p<0.01 between the groups indicated by horizontal lines. Data (mean±SEM, n=3) are representative of two experiments.
To examine whether the increased IL-5 mRNA expression in both cell populations reflected the cytokine protein levels, we utilized CD4+ T cells derived from Il5−/− mice. These T cells are unable to produce IL-5; therefore, IL-5 production should be limited to ILC2s in the co-culture, allowing us to estimate the source of the protein. ILC2s cultured alone spontaneously produced modest amounts of IL-5, while CD4+ T cells alone produced minimal IL-5 irrespective of whether the cells were derived from wild-type (WT) or Il5−/− mice (Fig. 2B). When WT CD4+ T cells were cultured with ILC2s, IL-5 levels in the supernatants increased synergistically. When IL-5-deficient CD4+ T cells were cultured with ILC2s, IL-5 levels in the supernatants were significantly decreased as compared to levels observed following co-culture of ILC2 and WT CD4+ T cells (Fig. 2B), suggesting that CD4+ T cells mostly account for the increased IL-5 levels in the co-culture. Nonetheless, IL-5 levels did not decrease to baseline levels (i.e., the levels observed in cultures of ILC2s alone), suggesting that IL-5-deficient CD4+ T cells modestly enhance IL-5 production by ILC2s. Thus, when cultured together, ILC2s and CD4+ T cells likely enhance IL-5 production mutually in each population; CD4+ T cells are affected more than ILC2s.
Cellular contact through OX40L plays a key role in the ILC2 and CD4+ T cell interaction
These observations led us to speculate that a bidirectional interaction or “crosstalk” between ILC2s and CD4+ T cells may be occurring. For Th2-type immune responses in general, IL-4 is critical for differentiation of CD4+ T cells and production of Th2-type cytokines in vitro (8). Furthermore, the OX40/OX40L interaction is critical in initiating IL-4 transcription and differentiation of naïve T cells to a Th2-type (9) and in the induction of inflammatory Th2 cells mediated by thymic stromal lymphopoietin-activated dendritic cells (10).
To investigate whether the ILC2 and CD4+ T cell interaction requires direct contact or close proximity, we used a Transwell® culture system with CD4+ T cells in the bottom chamber and ILC2s in the upper chamber separated by a culture insert. Co-culture in the Transwell® system significantly reduced IL-4, IL-5, and IL-13 levels compared to co-culture without Transwell® separation (Fig. 3A). IL-4 levels were affected less than those of IL-5 or IL-13.
Figure 3. Cellular contact through OX40L plays a key role in the ILC2 and CD4+ T cell interaction.
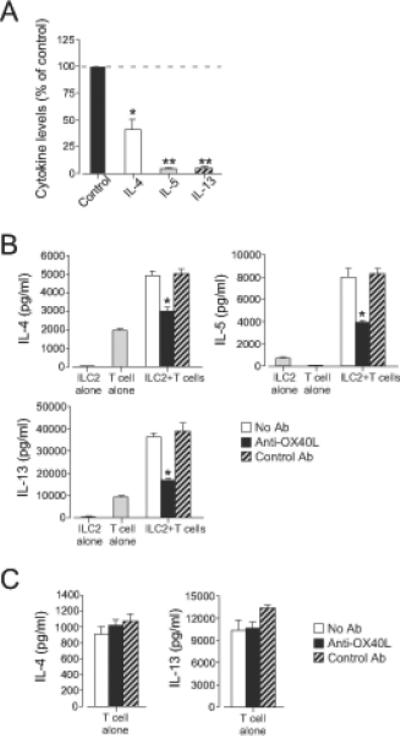
(A) ILC2s (105 cells/well) and CD4+ T cells (2×105 cells/well) were cultured in the Transwell® system for 4 days. Cytokine levels in the supernatants were analyzed by ELISA. Data were normalized to the values from co-culture without the Transwell® system as 100% (control). *, p<0.05; **, p<0.01 as compared to the control. Data (mean±SEM) are a pool of three experiments. (B) ILC2s (104 cells/well) and CD4+ T cells (2×104 cells/well) were stimulated with anti-CD3/CD28 with or without anti-OX40L (10 μg/ml) for 4 days. *, p<0.05 versus no antibody. (C) CD4+ T cells (2×104 cells/well) were stimulated with anti-CD3/CD28 with or without anti-OX40L (10 μg/ml) for 4 days. Data (mean±SEM, n=3) are representative of five experiments.
We investigated whether OX40L is involved in the interaction between ILC2s and CD4+ T cells by using blocking antibodies. Addition of polyclonal anti-OX40L antibody significantly inhibited IL-4, IL-5, and IL-13 production in the ILC2/CD4+ T cell co-culture (Fig. 3B). In contrast, anti-OX40L antibody did not significantly inhibit IL-4 and IL-13 production when T cells were stimulated with anti-CD3/CD28 without ILC2s (Fig. 3C). Similarly, a monoclonal anti-OX40L antibody also significantly inhibited IL-4, IL-5, and IL-13 production in the ILC2/CD4+ T cell co-culture (Supplemental Fig. 3A). By FACS analyses, OX40 was expressed on CD4+ T cells but not on ILC2s (Supplemental Fig. 3B); OX40L was detectable within ILC2s. These findings suggest that cellular contact through OX40L plays a key role in the interaction between CD4+ T cells and ILC2s; however, other co-stimulatory molecules may also be involved because anti-OX40L antibody only partially inhibited cytokine production in the ILC2/CD4+ T cell co-culture.
ILC2-derived IL-4 also plays a key role in the ILC2 and CD4+ T cell interaction
To investigate whether IL-4 is involved in the enhanced cytokine production detected in the ILC2/CD4+ T cell co-culture, we initially used a blocking antibody to IL-4 receptor. As described above, co-culture of CD4+ T cells and ILC2s resulted in a synergistic increase in the production of IL-5 and IL-13; anti-IL-4Rα significantly inhibited production of these cytokines (Fig. 4A). To further verify the involvement of IL-4 in the ILC2 and CD4+ T cell interaction and to identify the source of IL-4, we used a genetic approach. We isolated lung ILC2s and CD4+ T cells from Il4−/− mice and cultured these cells with CD4+ T cells or ILC2s obtained from WT mice. Co-culture of IL-4-deficient CD4+ T cells with WT ILC2s produced large amounts of IL-5 protein, similar to the levels measured in co-culture of WT CD4+ T cells and WT ILC2s (Fig. 4B). Production of IL-13 was partially inhibited when IL-4-deficient CD4+ T cells were used in place of WT CD4+ T cells in culture with WT ILC2s (Fig. 4B). Importantly, co-culture of IL-4-deficient ILC2s with WT CD4+ T cells resulted in significant decreases in both IL-5 and IL-13 levels as compared to levels from cultures of WT ILC2s with WT CD4+ T cells (Fig. 4C). Thus, ILC2-derived IL-4 likely plays a key role in type 2 cytokine upregulation in CD4+ T cell/ILC2 co-cultures.
Figure 4. ILC2-derived IL-4 plays a key role in the ILC2 and CD4+ T cell interaction.
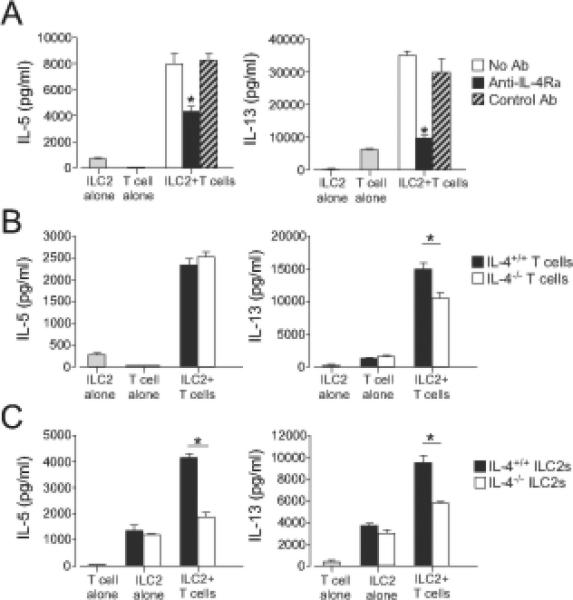
(A) ILC2s (104 cells/well) and CD4+ T cells (2x104 cells/well) were stimulated with anti-CD3/CD28 with or without anti-IL-4Rα(10 μg/ml) for 4 days. Cytokine levels in the supernatants were analyzed by ELISA. *, p<0.05 versus no antibody. (B) ILC2s from WT mice and CD4+ T cells from WT or Il4−/− mice were cultured alone or together for 4 days. *, p<0.05 between the groups indicated by horizontal bars. (C) CD4+ T cells from WT mice and ILC2s from WT or Il4−/− mice were cultured alone or together for 4 days. *, p<0.05 between the groups indicated by horizontal bars. Data (mean±SEM, n=3) are representative of three experiments.
ILC2s and CD4+ T cells synergize to mediate airway inflammation in vivo
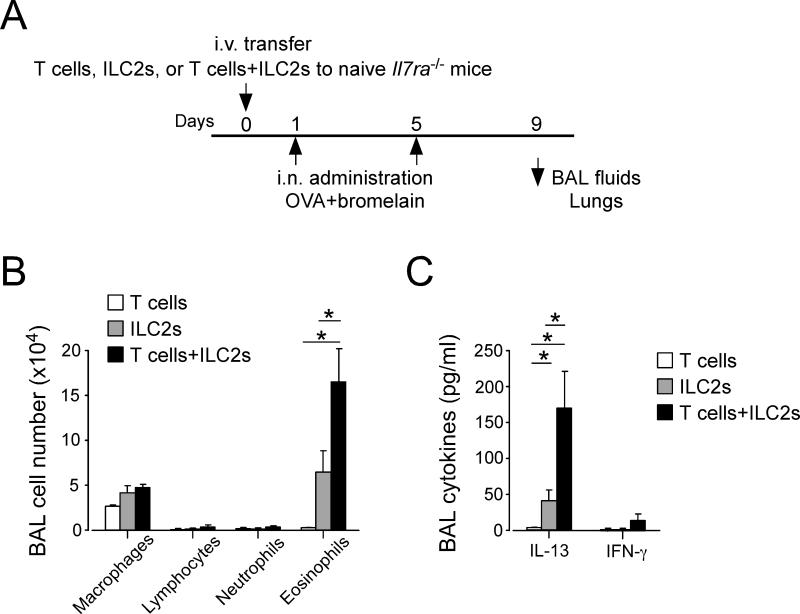
To examine whether this synergistic interaction between ILC2s and CD4+ T cell operates in vivo, we used an antigen-induced airway inflammation model in mice. Inhalation of innocuous protein is generally tolerogenic, and co-administration of adjuvants, such as proteases, is necessary to sensitize naïve animals via the airways (11, 12). We isolated lung ILC2s and CD4+ T cells from non-sensitized WT mice and immediately transferred these cells to naïve Il7ra−/− mice that are deficient in both T cells and ILC2s (7, 13). These mice were then exposed i.n. to OVA antigen plus bromelain (a cysteine protease) as an adjuvant to induce an antigen-specific immune response (Fig. 5A). Papain (another cysteine protease) was used previously as an adjuvant to induce adaptive type 2 immune responses (12); however, we found in a preliminary study that bromelain was more potent than papain (data not shown).
Figure 5. ILC2s and CD4+ T cells synergize in antigen-induced airway inflammation in vivo.
(A) Experimental protocol. Naïve Il7ra−/− mice were adoptively transferred with CD4+ T cells, ILC2s or both. Mice were then exposed i.n. to OVA plus an adjuvant protease (bromelain) on days 1 and 5 and analyzed on day 9. (B) Cell numbers and differentials in BAL fluids were analyzed and presented as mean±SEM (n =3-4). *, p<0.05 between the groups indicated by horizontal lines. Data are representative of two experiments. (C) Cytokine levels in BAL fluids were analyzed by ELISA and presented as mean±SEM (n =3-4). *, p<0.05 between the groups indicated by horizontal lines. Data are representative of two experiments.
In this model, Il7ra−/− mice reconstituted with CD4+ T cells alone showed minimal airway inflammation and no increases in cytokine levels in the BAL fluids (Fig. 5B and 5C). Il7ra−/− mice reconstituted with ILC2s alone showed a modest increase in eosinophil numbers and IL-13 levels (Fig. 5B and 5C), likely reflecting an innate ILC2 response to cysteine protease as previously described (12, 14). Importantly, Il7ra−/− mice reconstituted with both CD4+ T cells and ILC2s developed robust airway eosinophilia and displayed a marked increase in IL-13, but not IFN-γ, levels in BAL fluids. The magnitude of the response after transfer of both CD4+ T cells and ILC2s was apparently greater than the expected additive effects of CD4+ T cells alone and ILC2s alone, suggesting synergistic effects of CD4+ T cells and ILC2s.
The antigen-specific immune response in CD4+ T cells is enhanced when ILC2s are transferred together with CD4+ T cells
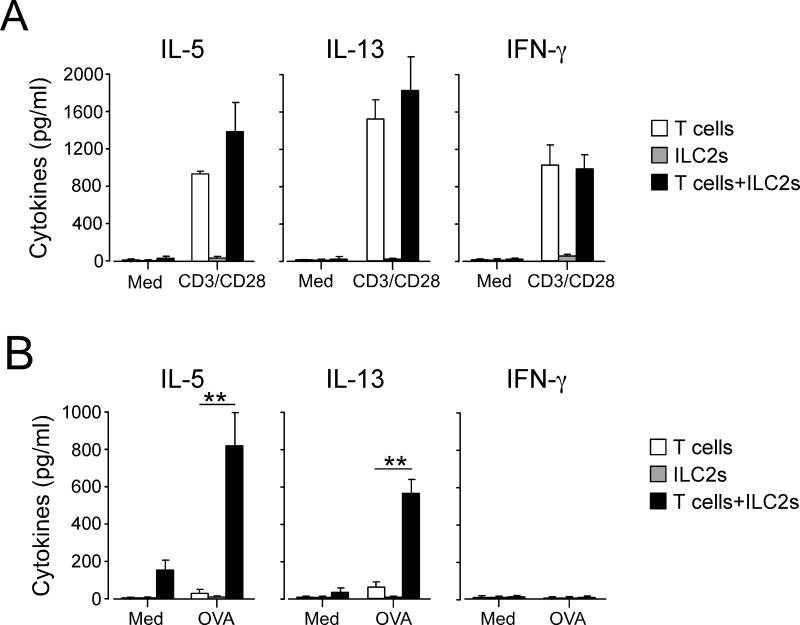
To address the mechanisms of enhanced immune response in this model, we investigated whether development of antigen-specific CD4+ T cells is affected by ILC2s. Lung cells from the Il7ra−/− mice that were reconstituted with CD4+ T cells, ILC2s or both and exposed to OVA plus bromelain were stimulated with OVA antigen in vitro. First, we stimulated lung cells with a polyclonal T cell agonist, namely anti-CD3/CD28 antibodies. Il7ra−/− mice reconstituted with CD4+ T cells alone and those reconstituted with CD4+ T cells plus ILC2s produced roughly comparable levels of Th1- and Th2-type cytokines, including IL-5, IL-13 and IFN-γ (Fig. 6A), suggesting successful reconstitution of these mice with CD4+ T cells. As expected, the Il7ra−/− mice reconstituted with ILC2s alone did not produce detectable cytokines in response to anti-CD3/CD28.
Figure 6. Antigen-specific immune response in CD4+ T cells is enhanced when ILC2s are transferred together with CD4+ T cells.
(A) Naïve Il7ra−/− mice were treated as described in Fig. 5A. Lung cells were collected on day 9 and cultured with medium alone or anti-CD3/CD28 antibodies for 5 days. Cytokine levels in the supernatants were analyzed by ELISA. (B) Lung cells were cultured with medium alone or OVA antigen (100 μg/ml) for 7 days. Cytokine levels in the supernatants were analyzed by ELISA. **, p<0.01 between the groups indicated by horizontal lines. Data are representative of two experiments.
Second, to examine the antigen-specific immune response, we stimulated the lung cells with OVA antigen. Mice reconstituted with both CD4+ T cells and ILC2s produced high levels of IL-5 and IL-13 when they were cultured with OVA in vitro (Fig. 6B). IFN-γ was undetectable, suggesting that an antigen-specific Th2-type, but not Th1-type, immune response to OVA had been established in these mice. In contrast, mice reconstituted with CD4+ T cells alone or ILC2s alone produced minimal amounts of IL-5 or IL-13 (Fig. 6B). Taken together, these results suggest that the presence of ILC2s promotes development of Th2-type antigen-specific CD4+ T cell responses in vivo in the lungs following inhaled antigen exposure.
DISCUSSION
Type 2 immune responses are orchestrated by several cell types (15). In this study, we investigated the possible interaction between two major players in type 2 immunity, namely ILC2s and CD4+ T cells. Our data demonstrate that lung ILC2s enhance effector functions of Th2-type CD4+ T cells when they are cultured together in vitro. The interaction between ILC2s and CD4+ T cells appears bidirectional and likely requires both OX40L and IL-4 and perhaps other molecules. Furthermore, when adoptively transferred into immune deficient Il7ra−/− mice, ILC2s and CD4+ T cells synergistically promoted airway inflammation and type 2 cytokine responses in vivo. Interestingly, the antigen-specific response of CD4+ T cells was enhanced when ILC2s were transferred together with CD4+ T cells. Altogether, these findings suggest that lung ILC2s and CD4+ T cells cooperate to mediate robust Th2-type immune responses in mice.
The interaction between ILCs and CD4+ T cells has been demonstrated in other experimental systems using MPPtype2 cells and group 3 ILCs (4, 16). In those studies, ILCs affected CD4+ T cell function by presenting antigens to them. In contrast, our experimental model of CD4+ T cell and ILC2 co-culture does not involve any soluble antigens (Fig. 1), suggesting that ILC2s can affect CD4+ T cells by the mechanism(s) other than antigen presentation. Instead, the model revealed the role for a co-stimulatory molecule OX40L (Fig. 3B). Previous studies have shown that engagement of OX40 on CD4+ T cells by OX40L preferentially drives Th2-type differentiation in CD4+ T cells (9, 10, 17). Thus, the interaction between ILCs and CD4+ T cells may involve antigen presentation, engagement of co-stimulatory molecules or both, depending on the experimental conditions. We detected OX40L expression intracellularly but were not able to detect it on the cell surface of ILC2s (Supplemental Fig. 3B). Perhaps, similarly to CTLA-4 (18), which is primarily localized in intracellular vesicles, OX40L in ILC2s may remain sequestered intracellularly and may be transiently expressed on the cell surface when ILC2s encounter CD4+ T cells. Alternatively, OX40L molecules may be present at a level below detection by FACS analysis. In any event, ILC2s and CD4+ T cells likely need to be in contact or in close proximity to work together because cytokine production was markedly attenuated when they were separated by a culture insert (Fig. 3A).
Our study also suggests roles for IL-4, in particular ILC2-derived IL-4. The expression of IL-4 mRNA and protein in mouse and human ILC2s has been reported previously in several other studies (4, 19-24), while a functional role for ILC2-derived IL-4 has not been demonstrated. Our data suggest that upon interaction with CD4+ T cells, ILC2s upregulate their expression of IL-4 mRNA (Fig. 2A). This IL-4 likely plays a critical role in robust production of IL-5 and IL-13 when CD4+ T cells and ILC2s are cultured together, as IL-4-deficient ILC2s were considerably less potent than WT ILC2s in mediating the cytokine response (Fig. 4C).
Although production of IL-4, IL-5 and IL-13 proteins was upregulated by ILC2 and CD4+ T cell co-culture, there was little difference in IL-13 mRNA expression (Fig. 2). In contrast, IL-5 mRNA expression by CD4+ T cells was clearly enhanced by co-culture. These findings suggest that upregulation of type 2 cytokine production in co-culture may involve several stages of cytokine production, including mRNA expression, post-transcriptional modification and secretion of the proteins. Further studies will be necessary to elucidate the molecular mechanisms involved in the enhanced cytokine production in ILC2 and CD4+ T cell co-culture.
The interaction between ILC2s and CD4+ T cells also likely occurs in vivo during allergic airway inflammation. In mouse models of allergic airway inflammation, ILC2s have been shown to be an important innate source of type 2 cytokines when mice are exposed to fungal or protease allergens even in mice deficient in T cells or B cells (7, 12, 14, 25). In this study, as shown by enhanced airway eosinophilia and IL-13 levels in BAL fluid (Fig. 5), ILC2s and CD4+ T cells likely work synergistically in vivo during allergic airway inflammation that is induced by exposure to OVA antigen plus an adjuvant. Importantly, the antigen-specific cytokine production by CD4+ T cells is upregulated when ILC2s are co-transferred with CD4+ T cells, suggesting the capacity of ILC2s to promote Th2-type antigen-specific T cell immune responses.
These findings are consistent with a recent report by others demonstrating that both innate and adaptive immunity contribute to maximum allergic airway inflammation when mice are exposed to protease allergens (12). Therefore, we propose that ILC2s possess the activities to bridge innate and adaptive immunity during allergic airway inflammation. Indeed, a recent report suggests that ILC2-derived IL-13 promotes dendritic cell (DC) function, resulting in enhanced adaptive type 2 immune response to protease allergens (26). Thus, ILC2s may promote type 2 adaptive immunity by affecting several cell types, including CD4+ T cells and DCs. One potential weakness in our study is that immune-deficient mice were used as recipients of ILC2s and CD4+ T cells. These mice may allow homeostatic proliferation of transferred immune cells and thus may exaggerate the function of particular immune cells. Therefore, additional studies to verify these results by using mice that are specifically deficient in ILC2s in the presence of otherwise intact immune components are warranted when these research tools become available.
In summary, the major observation in this study that ILC2s can work together with CD4+ T cells both in vitro and in vivo will provide a novel understanding of the underlying mechanisms of diseases mediated by type 2 immunity. Recent human studies suggest increased infiltration of ILC2s in tissues from patients with chronic type 2 inflammation, such as those with atopic dermatitis and nasal polyps (27, 28). The possible synergistic interaction between ILC2s and CD4+ T cells may explain the severity and chronicity of these diseases.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr. James Lee (Mayo Clinic Arizona) for providing Il5−/− mice, Gail Kephart for editorial assistance, and LuRaye Eischens for secretarial assistance.
This work was supported by grants from the National Institute of Health, R01AI34486, R01AI49235, and R01HL117823, and by Mayo Foundation.
Abbreviations
- BAL
bronchoalveolar lavage
- DCs
dendritic cells
- FACS
fluorescence-activated cell sorter
- ILCs
innate lymphoid cells
- ILC2s
type 2 ILCs
- i.n.
intranasal
- Lin−
lineage-negative
- Lin+
lineage-positive
- MPPtype2
multipotent progenitor type 2
- OVA
ovalbumin
- WT
wild-type
Footnotes
Conflict of interest: There is no conflict of interest.
Author contributions: L.D., K.I, and H.K. designed the studies and experiments, interpreted the data, and wrote the manuscript. L.D. and K.I. performed the experiments.
REFERENCES
- 1.Spits H, Cupedo T. Innate lymphoid cells: emerging insights in development, lineage relationships, and function. Annu. Rev. Immunol. 2012;30:647–675. doi: 10.1146/annurev-immunol-020711-075053. [DOI] [PubMed] [Google Scholar]
- 2.Walker JA, McKenzie AN. Development and function of group 2 innate lymphoid cells. Curr. Opin. Immunol. 2013;25:148–55. doi: 10.1016/j.coi.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saenz SA, Siracusa MC, Perrigoue JG, Spencer SP, Urban JF, Jr, Tocker JE, et al. IL-25 elicits a multipotent progenitor cell population that promotes Th2 cytokine responses. Nature. 2010;464:1362–1366. doi: 10.1038/nature08901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saenz SA, Siracusa MC, Monticelli LA, Ziegler CG, Kim BS, Brestoff JR, et al. IL-25 simultaneously elicits distinct populations of innate lymphoid cells and multipotent progenitor type 2 (MPPtype2) cells. J. Exp. Med. 2013;210:1823–1837. doi: 10.1084/jem.20122332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kobayashi T, Iijima K, Radhakrishnan S, Mehta V, Vassallo R, Lawrence CB, et al. Asthma-related environmental fungus, Alternaria, activates dendritic cells and produces potent Th2 adjuvant activity. J. Immunol. 2009;182:2502–2510. doi: 10.4049/jimmunol.0802773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartemes KR, Iijima K, Kobayashi T, Kephart GM, McKenzie AN, Kita H. IL-33-responsive lineage−CD25+CD44hi lymphoid cells mediate innate type 2 immunity and allergic inflammation in the lungs. J. Immunol. 2012;188:1503–1513. doi: 10.4049/jimmunol.1102832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kopf M, Le Gros G, Bachmann M, Lamers MC, Bluethmann H, Kohler G. Disruption of the murine IL-4 gene blocks Th2 cytokine responses. Nature. 1993;362:245–248. doi: 10.1038/362245a0. [DOI] [PubMed] [Google Scholar]
- 9.So T, Song J, Sugie K, Altman A, Croft M. Signals from OX40 regulate nuclear factor of activated T cells c1 and T cell helper 2 lineage commitment. Proc. Natl. Acad. Sci. USA. 2006;103:3740–3745. doi: 10.1073/pnas.0600205103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ito T, Wang YH, Duramad BO, Hori T, Delespesse GL, Watanabe N, et al. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J. Exp. Med. 2005;202:1213–23. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kheradmand F, Kiss A, Xu J, Lee SH, Kolattukudy PE, Corry DB. A protease-activated pathway underlying Th cell type 2 activation and allergic lung disease. J. Immunol. 2002;169:5904–5911. doi: 10.4049/jimmunol.169.10.5904. [DOI] [PubMed] [Google Scholar]
- 12.Kamijo S, Takeda H, Tokura T, Suzuki M, Inui K, Hara M, et al. IL-33-mediated innate response and adaptive immune cells contribute to maximum responses of protease allergen-induced allergic airway inflammation. J. Immunol. 2013;190:4489–99. doi: 10.4049/jimmunol.1201212. [DOI] [PubMed] [Google Scholar]
- 13.Peschon JJ, Morrissey PJ, Grabstein KH, Ramsdell FJ, Marakovsky E, Gliniak BC, et al. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J. Exp. Med. 1994;180:1955–60. doi: 10.1084/jem.180.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halim TY, Krauss RH, Sun AC, Takei F. Lung natural helper cells are a critical source of Th2 cell-type cytokines in protease allergen-induced airway inflammation. Immunity. 2012;36:451–463. doi: 10.1016/j.immuni.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 15.Paul WE, Zhu J. How are Th2-type immune responses initiated and amplified? Nat. Rev. Immunol. 2010;10:225–235. doi: 10.1038/nri2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hepworth MR, Monticelli LA, Fung GC, Ziegler CG, Grunberg S, et al. Innate lymphoid cells regulate CD4+ T-cell responses to intestinal commensal bacteria. Nature. 2013;498:113–7. doi: 10.1038/nature12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flynn S, Toellner KM, Raykundalia C, Goodall M, Lane P. CD4 T cell cytokine differentiation: the B cell activation molecule, OX40 ligand, instructs CD4+ T cells to express interleukin 4 and upregulates expression of the chemokine receptor Blr-1. J. Exp. Med. 1998;188:297–304. doi: 10.1084/jem.188.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Egen JG, Kuhns MS, Allison JP. CTLA-4: new insights into its biological function and use in tumor immunotherapy. Nat. Immunol. 2002;3:611–8. doi: 10.1038/ni0702-611. [DOI] [PubMed] [Google Scholar]
- 19.Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, et al. Innate production of Th2 cytokines by adipose tissue-associated c-Kit+Sca-1+ lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 20.Price AE, Liang HE, Sullivan BM, Reinhardt RL, Eisley CJ, Erle DJ, et al. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc. Natl. Acad. Sci. USA. 2010;107:11489–11494. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barlow JL, Peel S, Fox J, Panova V, Hardman CS, Camelo A, et al. IL-33 is more potent than IL-25 in provoking IL-13-producing nuocytes (type 2 innate lymphoid cells) and airway contraction. J. Allergy Clin. Immunol. 2013;132:933–941. doi: 10.1016/j.jaci.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 22.Hoyler T, Klose CS, Souabni A, Turqueti-Neves A, Pfeifer D, Rawlins EL, et al. The transcription factor GATA-3 controls cell fate and maintenance of type 2 innate lymphoid cells. Immunity. 2012;37:634–648. doi: 10.1016/j.immuni.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mjosberg J, Bernink J, Golebski K, Karrich JJ, Peters CP, Blom B, et al. The transcription factor GATA3 is essential for the function of human type 2 innate lymphoid cells. Immunity. 2012;37:649–659. doi: 10.1016/j.immuni.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 24.Doherty TA, Khorram N, Lund S, Kumar Mehta A, Croft M, Broide DH. Lung type 2 innate lymphoid cells express cysteinyl leukotriene receptor 1, which regulates Th2 cytokine production. J. Allergy Clin. Immunol. 2013;132:205–13. doi: 10.1016/j.jaci.2013.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doherty TA, Khorram N, Sugimoto K, Sheppard D, Rosenthal P, Cho JY, et al. Alternaria induces STAT6-dependent acute eosinophilia and epithelial FIZZ1 expression that promotes airway fibrosis and epithelial thickness. J. Immunol. 2012;188:2622–9. doi: 10.4049/jimmunol.1101632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Halim TY, Steer CA, Matha L, Gold MJ, Martinez-Gonzalez I, McNagny KM, et al. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity. 2014 doi: 10.1016/j.immuni.2014.01.011. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim BS, Siracusa MC, Saenz SA, Noti M, Monticelli LA, Sonnenberg GF, et al. TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Sci. Trans. Med. 2013;5:170ra116. doi: 10.1126/scitranslmed.3005374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shaw JL, Fakhri S, Citardi MJ, Porter PC, Corry DB, Kheradmand F, et al. IL-33-responsive innate lymphoid cells are an important source of IL-13 in chronic rhinosinusitis with nasal polyps. Am. J. Respir. Crit. Care Med. 2013;188:432–439. doi: 10.1164/rccm.201212-2227OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.