Summary
The extracellular polymeric substance produced by many human pathogens during biofilm formation often contains extracellular DNA (eDNA). Strands of bacterial eDNA within the biofilm matrix can occur in a lattice-like network wherein a member of the DNABII family of DNA-binding proteins is positioned at the vertex of each crossed strand. To date, treatment of all biofilms tested with antibodies directed against one DNABII protein, Integration Host Factor (IHF), results in significant disruption. Here, using nontypeable Haemophilus influenzae as a model organism, we report that this effect was rapid, IHF-specific and mediated by binding of transiently dissociated IHF by anti-IHF even when physically separated from the biofilm by a nucleopore membrane. Further, biofilm disruption fostered killing of resident bacteria by previously ineffective antibiotics. We propose the mechanism of action to be the sequestration of IHF upon dissociation from the biofilm eDNA, forcing an equilibrium shift and ultimately, collapse of the biofilm. Further, antibodies against a peptide positioned at the DNA-binding tips of IHF were as effective as antibodies directed against the native protein. As incorporating eDNA and associated DNABII proteins is a common strategy for biofilms formed by multiple human pathogens, this novel therapeutic approach is likely to have broad utility.
Keywords: Nontypeable Haemophilus influenzae, DNABII protein, eDNA
Introduction
The chronic and recurrent nature of most bacterial infections is attributed to the ability of the causative agents to form a biofilm, communities of adherent bacteria encased in a self-produced extracellular polymeric substance (EPS) (Flemming and Wingender, 2010). The EPS serves as a semi-permeable barrier that protects resident bacteria from both immune effectors and therapeutics, such as antibiotics (Nickel et al., 1985). Moreover, bacteria resident within a biofilm demonstrate multiple unique characteristics compared to their planktonic (free living) counterparts, including a reduced metabolism and altered proteome (Romling and Balsalobre, 2012). As a result, bacteria within a biofilm are up to 1000 times more resistant to antibiotics than their planktonic counterparts (Nickel et al., 1985).
Nontypeable Haemophilus influenzae (NTHI) is the causative agent of multiple diseases of the upper and lower respiratory tracts including otitis media, sinusitis, and exacerbations of both cystic fibrosis and chronic obstructive pulmonary disease (Murphy, 2003; Starner et al., 2006). It is well-established that the ability of NTHI to form biofilms contributes significantly to disease chronicity and recurrence (Post, 2001; Swords, 2012). Clinically, NTHI biofilms have been detected on middle ear mucosal biopsies recovered from children undergoing tympanostomy tube placement for chronic or recurrent otitis media (Hall-Stoodley et al., 2006) (Wessman et al., 2014) and in bronchoalveolar lavage fluids obtained from pediatric cystic fibrosis patients (Starner et al., 2006). We, and others, have demonstrated in chinchilla models of experimental otitis media that NTHI forms robust biofilms in the middle ear and have characterized multiple molecular events that underlie the contributions of biofilms to the pathogenesis of OM (Ehrlich et al., 2002; Hong et al., 2007a; Hong et al., 2007b; Jurcisek and Bakaletz, 2007; Jurcisek et al., 2007; Leroy et al., 2007). Biofilms formed by NTHI in the chinchilla middle ear contain both host-derived and bacterial extracellular DNA (eDNA) organized into a mesh-like structure, with host eDNA largely encasing the biofilm and bacterial eDNA predominating within the inner reaches of the biofilm (Jurcisek and Bakaletz, 2007). Observed at each junction of crossed strands of bacterial eDNA is a DNABII protein that is immunolabeled by antibodies directed against Integration Host Factor (IHF), the localization of which indicates that this family of proteins may play a critical role in the structural stability of the biofilm (Goodman et al., 2011). Support for this theory is shown both in vitro by resolution of pre-formed NTHI biofilms after incubation with antiserum against IHF and in vivo wherein use of native IHF as an immunogen in chinchillas with pre-existing NTHI biofilms in their middle ears results in rapid disease resolution and eradication of mucosal biofilms (Goodman et al., 2011).
These observations also extend to biofilms formed by other important human bacterial pathogens. In vitro, biofilms formed by Staphylococcus epidermidis, uropathogenic Escherichia coli (UPEC), Pseudomonas aeruginosa, Moraxella catarrhalis, Streptococcus pneumoniae and Streptococcus mutans exposed to antiserum directed against IHF were significantly disrupted (Goodman et al., 2011). Moreover, incubation of biofilms formed in vitro by Burkholderia cenocepacia, a highly lethal and antibiotic-resistant pathogen of cystic fibrosis patients, with anti-IHF results in significant disruption and increased sensitivity to killing by traditional antibiotics (Novotny et al., 2013). We've also shown that pre-treatment of a widely-used surgical packing material with anti-IHF prevents in vitro biofilm formation by NTHI or Staphylococcus aureus, as well as disrupts pre-formed biofilms by either species (Brandstetter et al., 2013). Ex vivo, antibodies against IHF disrupt eDNA- and IHF-containing sputum solids recovered from patients with cystic fibrosis that were culture-positive for multiple microbes, including Pseudomonas aeruginosa as well as both methicillin sensitive and resistant S. aureus (Gustave et al., 2013). In a mouse model of UPEC-induced urinary tract infection, E. coli strains deficient in production of one of the two IHF subunits were attenuated in ability to colonize the mouse bladder and kidney (Justice et al., 2012). Further, encapsulation of anti-IHF within PGLA microspheres was highly effective in a rat model of Aggregatibacter acetemycetemcomitans mediated peri-implantitis (manuscript in preparation). Collectively, targeting the DNABII proteins to mediate biofilm disruption has the potential to provide a broadly effective therapeutic option to treat multiple chronic or recurrent bacterial diseases with a biofilm component within the disease course.
Whereas multiple data illustrate the benefits of targeting extra-bacterial members of the DNABII family within bacterial biofilms using specific antibody, the exact mechanisms behind these observations remain to be determined. Here, we sought to unravel the molecular mechanisms and define the kinetics of anti-IHF-mediated biofilm disruption using NTHI as a model organism. We demonstrated the utility of a biofilm-reversal approach against biofilms of up to two weeks of age, showed the onset of biofilm reduction within 6 hr after treatment and elucidated that direct physical contact between IHF-specific antibody and DNABII protein(s) within the biofilm itself was not required. Moreover, we revealed that, whereas treatment of biofilms with antibiotics was ineffective, the combined delivery of anti-IHF plus antibiotics facilitated resolution of biofilms and killing of planktonic and adherent NTHI at antimicrobial concentrations a minimum of 4-fold less than the MIC90 for planktonic NTHI. Finally, we identified an immunodominant epitope within a DNA binding tip of IHF that also mediated biofilm disruption. These data support the continued development of IHF-based therapeutics against diseases with a biofilm component.
Results
Anti-IHF induced resolution of NTHI biofilms
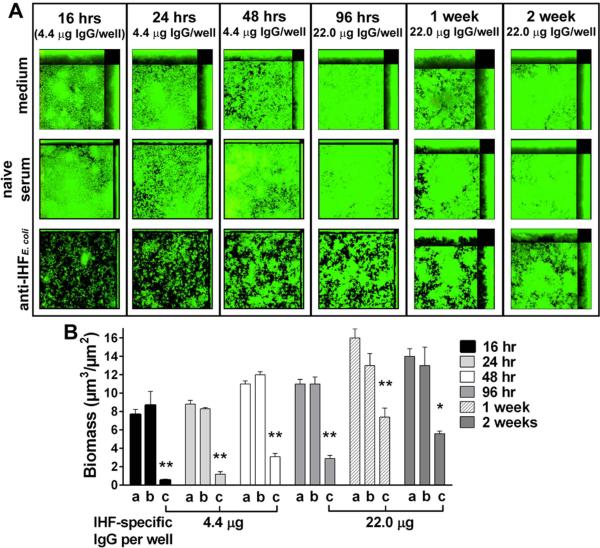
Previous work demonstrates that polyclonal rabbit antiserum against E. coli IHF (or ‘anti-IHFE. coli’) used at a 1:50 dilution, equivalent to 4.4 μg IHF-specific IgG ml-1, disrupts a 24-hr NTHI biofilm in vitro (Goodman et al., 2011). We next examined the relative effectiveness of anti-IHFE. coli on both early-forming and mature NTHI biofilms. Biofilms formed for 16, 24, 48 or 96 hr and 1 or 2 weeks prior to treatment showed a marked decrease in remaining biofilm after incubation with anti-IHFE. coli, whereas biofilms exposed to naive serum were comparable to those maintained in medium (Fig. 1A). Quantitatively, biomasses of 16-, 24- and 48-hr biofilms exposed to anti-IHFE. coli were significantly reduced 94%, 86%, and 74%, respectively, compared to naive serum (p<0.01, Fig. 1B). To achieve a similar effect against more mature biofilms, anti-IHFE. coli was diluted 1:10. Consequently, the biomasses for 96-hr or 1- or 2-week biofilms were significantly reduced by 74%, 43%, and 57%, respectively, compared to naive serum (p<0.01 or 0.05). As NTHI biofilms mature in vitro, the relative quantity of eDNA increases (Jones et al., 2013), thus it was not unexpected that a greater concentration of IHF-specific antiserum was required to achieve a similar reduction in biomass of these ‘older’ and more dense biofilms. Collectively, these data demonstrated that anti-IHFE. coli was significantly effective and capable of disrupting both early-forming and mature NTHI biofilms.
Fig. 1.
Disruption of NTHI biofilms by anti-IHFE. coli. (A) Representative images and (B) calculated mean biomass of NTHI biofilms established for the indicated times then treated for 16 hr with either a, medium; b, naive serum; or c, anti-IHFE. coli. Sera were used at a 1:50 dilution for 16, 24 and 48 hr biofilms, and at a 1:10 dilution for older biofilms. At all timepoints tested NTHI biofilms of all ages were significantly disrupted by incubation with anti-IHFE. coli as compared to naive serum or medium. Data are expressed as the mean ± SEM of three independent assays. *p<0.05; **p<0.01 compared to respective naive serum treatment, one way ANOVA.
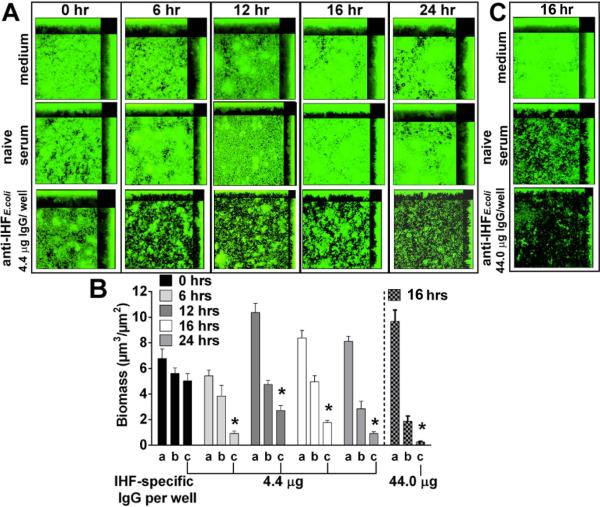
We next examined the kinetics of anti-IHF-mediated disruption. Biofilms established for 24 hrs were subsequently treated for 0, 6, 12, 16 or 24 hr with medium or either anti-IHFE. coli or naive serum at a dilution of 1:50. Application, then instantaneous removal of treatments did not induce alteration in biofilm biomass relative to medium (Fig. 2A&B). However, biofilms exposed anti-IHFE. coli for 6, 12, 16 or 24 hr demonstrated 76%, 43%, 65%, or 67% reduction in biomass compared to naive serum, respectively (p<0.01) with maximal reduction observed at the 6 hr time point. As seen here, and as we have previously reported, naive serum from non-SPF rabbits contains enough antibodies directed against outer membrane proteins of many members of the family Pasteurellaceae to mediate a modest but not significant effect in terms of reduction of biofilm biomass compared to biofilms maintained in medium (Goodman et al., 2011; Novotny et al., 2013). We next examined whether a greater concentration of anti-IHFE. coli or an extended exposure time could further reduce, or eradicate a pre-formed biofilm. Incubation of NTHI biofilms with anti-IHFE. coli diluted 1:5 resulted in a decrease in biomass by 86% compared to naive serum (p<0.01; Fig. 2B&C). This outcome appeared maximal as neither a greater concentration of anti-IHFE. coli nor an increase in treatment time to 24 hr fully eradicated all viable bacteria (data not shown). In either case, a single layer of bacteria remained after treatment, which suggested that there was no target for anti-IHFE. coli in these monolayers.
Fig. 2.
Kinetics of biofilm disruption by anti-IHFE. coli. (A) Representative images and (B) calculated mean biomass of NTHI biofilms established for 24 hr then treated for indicated times with a, medium, or a 1:50 dilution of b, naive serum; or c, anti-IHFE. coli. Reduction in biomass mediated by anti-IHFE. coli was maximal at 6 hr and sustained for 24 hr. (C) 24 hr biofilm treated for 16 hr with a, medium, or a 1:5 dilution of b, naive serum or c, anti-IHFE. coli. Treatment with this greater concentration of anti-IHFE. coli eradicated the biofilm, leaving a monolayer of bacteria. Data are expressed as the mean ± SEM of three independent assays. *p<0.01 compared to respective naive serum treatment, one way ANOVA.
Direct contact was not required for anti-IHF to disrupt NTHI biofilms
Up to this point, anti-IHF was applied directly to the NTHI biofilms. To now determine if direct contact between anti-IHFE. coli and the biofilm was required, NTHI biofilms were established in the basolateral chamber of a transwell. IgG-enriched anti-IHFE. coli covalently bound to agarose beads was placed into the apical chamber, thus physically separating the antibodies from the biofilm by the presence of a membrane (Fig. S1A). We first confirmed that anti-IHFE. coli coupled to agarose beads did not diffuse into the basolateral chamber by collection of medium within the basolateral chamber 24 hr after placement of antibody-bound beads into the apical chamber of the transwell by Western blot (Fig. S2). Compared to application of IHFE. coli directly to the biofilm (Fig. 3A), biofilm reduction in the presence of anti-IHFE. coli tethered to agarose beads within the apical chamber of the transwell was equivalent (Fig. 3C), and a significant reduction in biomass (p<0.05) was observed compared to tethered IgG from naive serum (Fig. 3B&F). Although three concentrations of antibodies coupled to agarose beads were assayed, and all were effective, dose-dependent biofilm disruption was not observed. These data suggested a saturation of available antibody binding sites at the interface between the biofilm medium and the transwell membrane. To test this theory, we first placed a layer of naked agarose beads below those to which IHFE. coli antibodies had been bound, thus sterically blocking the ability to bind ‘free’ IHF (Fig. S1B). As anticipated, no disruption of the biofilm was observed (Fig. 3D & F). However, biofilm disruption was restored upon mixing the naked beads and anti-IHF-bound antibodies (Fig. 3E). Collectively, these data demonstrated that anti-IHFE. coli did not require direct contact with an NTHI biofilm to mediate disruption, and further, that this disruption was likely mediated by a forced equilibrium shift as free IHF is captured by antibodies when this protein naturally disassociated from eDNA within the biofilm (i.e. was in an ‘off’ state).
Fig. 3.
Direct contact between anti-IHFE. coli and biofilm was not required to mediate disruption. (A) Representative images of 24 hr biofilms treated with either sterile medium or a 1:50 dilution of serum added to the basolateral chamber. (B-C) Biofilms treated by placement of indicated amount of antibody coupled to agarose beads into the apical chamber of a transwell, (D) NTHI biofilms after naked beads were layered under antibody-coupled beads in the apical chamber, (E) Biofilms after mixing of naked and antibody-coupled beads. (F) Biomass values after incubation with: a, medium; b, naive serum; c, anti-IHFE. coli; d, coupled IgG-enriched naive serum; e, coupled IgG-enriched anti-IHFE. coli; f, naked beads layered under coupled IgG-enriched naive serum; g, naked beads layered under coupled IgG-enriched anti-IHFE. coli; h, mixed naked and coupled IgG-enriched naive serum; i, mixed naked and coupled IgG-enriched anti-IHFE. coli. Data are expressed as the mean ± SEM of three independent assays. *p<0.05 compared to respective naive serum or IgG-enriched naive serum conjugated to agarose beads treatment, one way ANOVA.
To demonstrate that the observed biofilm disruption was specifically mediated by antibodies directed against IHF and not due to the influence of other components within the hyperimmune rabbit serum (as this serum was not heat-activated prior to use) aliquots of anti-IHFE. coli were adsorbed with either purified IHF, or as a negative control, an unrelated NTHI protein of comparable molecular mass called rsPilA (Novotny et al., 2009). We first established that the arbitrarily-selected 1:50 dilution of antiserum utilized in the biofilm assays equated to application of 4.4 μg IHF-specific IgG to each biofilm. Western blotting revealed reduced reactivity of IHF-specific antibody after incubation with increasing concentrations of purified IHF (Fig. S3). This result was specific, as no decrease in reactivity was noted by incubation with the unrelated recombinant NTHI protein. Further, incubation of biofilms with the IHF-adsorbed sera showed a reduced ability to effectively disrupt the pre-formed biofilms, compared to whole serum (Fig. 4A&B). These data revealed that the observed NTHI biofilm disruption was mediated by IHF-specific antibodies within the polyclonal rabbit serum.
Fig. 4.
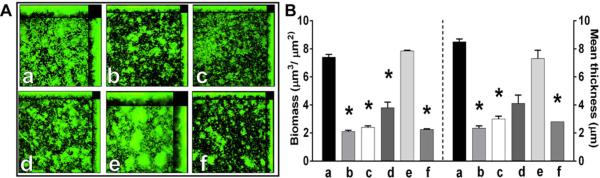
Adsorption of anti-IHF-specific antibody. (A) Representative images of 24 hr biofilms after incubation for 16 hr with a, naive serum or b, anti-IHFE. coli at a 1:50 dilution or anti-IHFE. coli (4.4 μg) adsorbed with: c, 0 μg IHF E. coli; d, 2.2 μg IHF E. coli; e, 4.4 μg IHF E. coli; or f, 4.4 μg rsPilA. (B) Changes in biofilm biomass and mean thickness.. Adsorption of IHF-specific antibody counteracted biofilm disruption capability of anti-IHFE. coli. Data are expressed as the mean ± SEM of three independent assays. *p<0.05 compared to naive serum, one way ANOVA.
Synergy of anti-IHF with antibiotics
We next examined whether biofilm disruption by anti-IHFE. coli increased susceptibility of the biofilm-resident bacteria to killing by antibiotics commonly used to treat NTHI infections. The minimum inhibitory concentration required to inhibit the growth of 90% (MIC90) of planktonically-grown NTHI was determined prior (Tristram et al., 2007) and the following concentrations were employed: ampicillin (32 μg ml-1), amoxicillin-clavulanate (1 μg ml−1 and 0.5 μg ml−1, respectively) and cefdinir (0.25 μg ml−1). We then exposed 24 hr NTHI biofilms to anti-IHFE. coli, diluted 1:50, antibiotic or a combination of anti-IHFE. coli plus antibiotic. As anticipated, anti-IHFE. coli mediated significant biofilm disruption (Fig. 5A), however incubation with any of the three antibiotics at the MIC90 for planktonic NTHI did not result in bacterial death as determined by viability stain (Fig. 5B-D) and the mean biofilm thickness and biomass was comparable between medium and antibiotic alone (Fig. 5E). Notably, treatment of an established NTHI biofilm with a combination of anti-IHFE. coli and any of the three antibiotics induced a marked alteration in biofilm architecture and a statistically significant reduction in mean biomass (p ≤ 0.05), compared to treatment with antibiotic alone. Moreover, bacterial death was noted within the biofilms treated with anti-IHFE. coli plus any of the three antibiotics (note yellow color of biofilms indicating that bacteria are dying). These data suggested that anti-IHF-mediated disruption of NTHI biofilms rendered the resident bacteria more susceptible to the actions of previously ineffective antimicrobials.
Fig. 5.
Anti-IHFE. coli acted synergistically with antibiotics. (A-D) Representative images of 24 hr biofilms treated for 16 hr with a 1:50 dilution of antiserum in the presence of medium or antibiotic at the MIC90 for planktonically grown NTHI strain 86-028NP. (E) Biomass and mean thickness of treated biofilms. a, medium; b, naive serum; c, anti-IHFE. coli. Incubation of NTHI biofilms with anti-IHFE. coli plus antibiotic markedly altered biofilm architecture, significantly reduced biofilm biomass and mean thickness and negatively impacted viability (note yellow color of biofilms which indicates that bacteria are dying). Data are expressed as the mean ± SEM of three independent assays. Bars indicate p<0.05, one way ANOVA.
Since biofilms resident within the middle ear of a child are likely to be older than 24 hours, to determine if this combinatorial approach was effective on more mature biofilms, we treated both 1 and 2 week old biofilms with anti-IHFE.coli (at a 1:10 dilution) with or without amoxicillinclavulanate at the MIC90 for NTHI strain 86-028NP (Fig. S4). As observed with 24 hr biofilms, whereas use of anti-IHFE. coli alone was significantly disruptive to the biofilm in terms of biomass (p < 0.05) , it did not induce death (notice robust green color of biofilm) (Fig. S4, Panels A&C, bottom images and Panels E&F). Use of amoxicillin-clavulanate alone also did not induce death, nor did it disrupt the biofilm (Fig. S4, Panels B&D top and middle images and Panels E&F). However, use of both anti-IHFE.coli and amoxicillin-clavulanate resulted in both disruption of the biofilm (p < 0.05) and bacterial death (as noted by the yellow color) (Fig. S4, Panels B&D, bottom image and Panels E&F). Moreover, use of amoxicillin-clavulante at a 4-fold dilution of the MIC90 in combination with anti-IHFE.coli was also capable of both significantly disrupting the biofilm as well as inducing bacterial death (Fig. S4, Panels E&F).
To extend this line of investigation beyond descriptive data of physical disruption of pre-formed biofilms, we examined whether anti-IHFE. coli exposure augmented antibiotic-mediated killing of the newly released bacteria. To do so, the three targeted antibiotics were assayed at both the MIC90 for planktonic NTHI (Biedenbach et al., 2003; Tristram et al., 2007) as well as at a 4-fold or 8-fold dilution thereof. When incubated with any of the three antibiotics at the MIC90, we observed no significant difference in adherent colony-forming units (CFU) bacteria ml−1 relative to medium (Fig. 6A-C). However, addition of anti-IHFE. coli resulted in a significant reduction in number of NTHI that remained adherent to the chamber slide compared to medium (p<0.05) (Goodman et al., 2011). Moreover, use of any antibiotic at the MIC90 in combination with anti-IHFE. coli induced an even greater significant reduction in the number of adherent bacteria (p<0.05) relative to medium alone. This reduction was maintained at an 8-fold less concentrations of ampicillin (4 μg ml−1) and amoxicillin-clavulanate (0.125 & 0.0625 μg each ml−1), as well as at a 4-fold less concentration of cefdinir (0.0625 μg ml−1). Given that planktonic bacteria represent only approximately 20-26% of the total population within a given chamber slide well, antibiotics used at the MIC90 would be expected to reduce the number of total bacteria by approximately 18-23% which is what we observed. Actual mean values for ampicillin, amoxicillin-clavulanate and cefdinir were 22%, 24% and 26%, respectively.
Fig. 6.
Bacteria newly released from the biofilm by treatment with anti-IHFE. coli showed enhanced sensitivity to antibiotics. 24 hr biofilms were incubated with ampicillin (A&D), amoxicillin-clavulanate (B&E), or cefdinir (C&F) for 16 hr in the absence or presence of a 1:50 dilution of anti-IHFE. coli or naive serum. (A-C) CFU of NTHI adherent within the biofilms. (DF) Sum of the planktonic and adherent NTHI. a, medium alone; b, naive serum; c, anti-IHFE. coli. Data are expressed as the mean ± SEM of three independent assays. Bars indicate p< 0.05, one way ANOVA.
To demonstrate that disruption of biofilms by anti-IHFE. coli mediated enhanced killing of bacteria in both the adherent and planktonic populations, we examined the total viable bacteria per well following treatment with each antibiotic delivered either with or without anti-IHFE. coli (Fig. 6D-F). For all antibiotics, we observed a significant reduction in total viable CFU NTHI/chamber slide well when the antibiotic was used at either the MIC90 or a 4-fold or 8-fold dilution thereof in combination with a 1:50 dilution of anti-IHFE. coli. In all cases, these differences were significant compared to treatment with antibiotic alone, anti-IHFE. coli alone or antibiotic plus naive serum (p< 0.05).
To determine if those NTHI newly released from the biofilm, or perhaps those still in less stringent association with the disrupted biofilm, were unusually susceptible to the action of these antibiotics we treated NTHI broth cultures with a 1:50 dilution of either anti-IHFE. coli or naive serum, delivered alone or in the presence of ampicillin, amoxicillin/clavulanate, or cefdinir. Antibiotics were used at both the MIC90 for planktonic NTHI and a 4-fold or 8-fold dilution thereof. We observed no enhancement of antibiotic-mediated killing upon exposure of planktonically grown NTHI to anti-IHFE. coli alone (Fig. S5A-C), suggesting that NTHI newly released from the biofilm by the action of anti-IHFE. coli were phenotypically unique from either their biofilm or planktonic counterparts in terms of relative sensitivity to the tested antibiotics.
Identification of immunodominant regions within IHF
Previous work showed that immunization of chinchillas with native IHFE.coli induced the formation of antibodies that rapidly resolved established NTHI biofilms within chinchilla middle ears during experimental OM; however, immunization with IHFE.coli that had been precomplexed to DNA (the form likely present in nature, during disease) did not resolve disease (Goodman et al., 2011). Collectively, our data to date imply that there is a conserved domain within DNABII proteins (IHF and HU) of many bacteria which can be targeted for effective disruption of biofilm structure and that this domain is masked or occluded when IHF/HU is in association with DNA. To determine the location of this effective/masked domain, we epitope mapped IHF from NTHI (referred to as ‘IHFNTHI’) using a series of 20-mer overlapping peptides (Table S1) designed to mimic the deduced N- to C-terminus of the α-subunit of this DNABII protein. We screened these peptides with antisera recovered from chinchillas that had been immunized with either native IHFE.coli or IHFE.coli that had been complexed to an excess of DNA (Goodman et al., 2011). Antiserum against native IHFE.coli was reactive with peptides predicted to represent the DNA-binding tip regions (Fig. 7A, note regions in fuchsia) whereas antiserum against the IHFE.coli-DNA complex yielded the greatest reactivity to peptides representing the N-terminal tails of IHFNTHI (Fig. 7B). This result was logical as the DNA-binding tip regions are likely occluded when IHFE.coli is bound to DNA as shown in Fig. 7C; therefore whereas the tail region is exposed, the tip binding regions are predicted to be immunologically inaccessible.
Fig. 7.
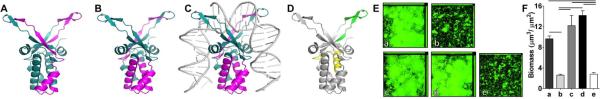
Epitope mapping IHFNTHI and design of a minimal IHFNTHI-targeted peptide. 3-D model depicting reactivity of (A) chinchilla anti-IHFE. coli and (B) chinchilla anti-IHF E. coli – complexed to DNA to synthetic peptides representing IHFNTHI. Regions with reactivity are indicated in fuchsia, nonreactive regions in blue. (C) 3-D model of DNA bound to IHF to show occlusion of tip-binding regions. (D) Localization of IhfA-3NTHI (yellow) and IhfA-5 NTHI (green) within IHF model. (E) Representative images and (F) mean biomass of 24 hr biofilms after incubation with a 1:50 dilutions of chinchilla sera for 16 hr. Data are expressed as the mean ± SEM of three independent assays. Bars indicate p< 0.01, one way ANOVA. a, naive serum; b, anti-IHFE. coli; c, anti-IHFE. coli complexed to DNA; d, anti-IhfA-3NTHI; e, anti-IhfA-5NTHI.
As our epitope mapping study revealed specific regions within the IHF molecule to which serum antibodies were reactive, we next sought to determine if antibodies directed against this targeted epitope were as effective as those directed against the native protein. To do so, we selected two peptides with which to generate immune serum in chinchillas: the peptide IhfA-5NTHI, representing the reactive DNA tip-binding region within the α-subunit of IHF (Fig. 7D, green) and as a negative control, IhfA-3 NTHI, which represents a peptide of equal size but that was unreactive by epitope mapping (Fig. 7D, yellow). Purified IHFE.coli and IHFE.coli pre-complexed to DNA served as comparative immunogens. As expected, compared to naive chinchilla serum, NTHI biofilms incubated with anti-IHFE.coli complexed to DNA or anti-IhfA-3 NTHI were not altered in biofilm morphology or biomass (Fig. 7E&F). However, similar to that observed with anti-IHFE.coli, incubation with anti-IhfA-5NTHI was equally efficacious to induce a significant reduction in biofilm biomass (p< 0.01) compared to naive serum.
Discussion
Bacterial biofilms are a significant contributor to most recurrent and chronic bacterial diseases, including those of the respiratory tract, urogenital tract and oral cavity. Biofilms are recalcitrant to action by the host immune system and antimicrobial agents, necessitating the development of novel treatment modalities for diseases with a biofilm component. eDNA is a prevalent component of the biofilm EPS of many microbes, and we previously demonstrated that members of the DNABII family of proteins play a crucial role in stabilizing the biofilm structure as exposure of biofilms to antibodies directed against IHF mediates significant disruption (Goodman et al., 2011).
As NTHI biofilm age, there is an increase in the eDNA concentration within the EPS (Jones et al., 2013), and by inference, a concordant relative increase in the concentration of associated DNABII proteins. Here we showed that older biofilms thus required a greater concentration of anti-IHFE.coli to mediate disruption. The ability of anti-IHFE.coli to disrupt an established 24 hr NTHI biofilm was rapid, with maximal effects occurring within 6 hr of exposure (76% reduction in biomass and 71% reduction in mean thickness compared to naive serum) and no further disruption occurred after an additional 24 hr incubation with a single treatment. Regardless of the relative increase in concentration of anti-IHFE.coli antibodies or exposure time, complete eradication of NTHI could not be accomplished. Instead a monolayer of viable bacteria remained, suggesting that in the absence of an EPS containing eDNA and IHF, there is no target for anti-IHF directed therapy. Thereby, a combinatorial approach would likely be ideal and result in the ability to use already existing antibiotics, or other therapeutics, to resolve these diseases.
We then sought to better define the mechanism of action. We investigated whether direct contact between the biofilm and anti-IHFE.coli antibodies was necessary for biofilm disruption as we previously hypothesized was not the case (Goodman et al., 2011) and, as expected, found that separation of antibodies tethered to agarose beads from the biofilm by a microporous membrane did not inhibit biofilm disruption, suggesting that direct contact was not needed. Instead, collectively our data indicated that antibodies directed against IHFE.coli captured free IHF as it disassociated from eDNA within the biofilm as part of the normal equilibrium between IHF with DNA. Indeed, epitope mapping experiments point to competitive inhibition of the DNA binding domain of IHF as the site of antibody action. The presence of an excess of antibodies to IHF thus shifts this equilibrium, mediating collapse or disruption of the biofilm structure.
As treatment of a biofilm with anti-IHF mediates release of bacteria into the planktonic phase (Goodman et al., 2011), here we tested whether anti-IHFE.coli could act in a combinatorial manner with traditional antibiotics to augment their killing ability. For three antibiotics traditionally used to treat recalcitrant respiratory tract infections due to NTHI, we found that this was indeed the case. Treatment of established NTHI biofilms with anti-IHF E.coli plus antibiotic rendered resident bacteria susceptible to killing as we have reported earlier for both Burkholderia cenocepacia when anti-IHFE.coli was used in combination with ciprofloxacin, imipenem and minocycline (Novotny et al., 2013) as well as for NTHI biofilms grown on the surgical packing material Nasopore® when anti-IHFE.coli was used in combination with augmentin (Brandstetter et al., 2013). Moreover, these newly released bacteria demonstrated increased susceptibility to killing that was not due to exposure to anti-IHFE.coli alone. All three antibiotics tested were able to mediate killing when used at a concentration 4- to 8-fold less than the MIC90 for planktonically growing cells, thus demonstrating true synergy. These findings also suggested the possibility of a unique phenotype for NTHI that had been newly released from biofilm growth, compared to either those resident within a biofilm or planktonically grown. Importantly, a similar observation has been made for S. pneumoniae in pioneering work by Anders Hakansson, who found that pneumococci released from biofilms, as mediated via several treatments, have a unique transcriptome and increased virulence compared to both bacteria growing as a biofilm as well as to planktonic bacteria grown in rich medium (Marks et al., 2013).
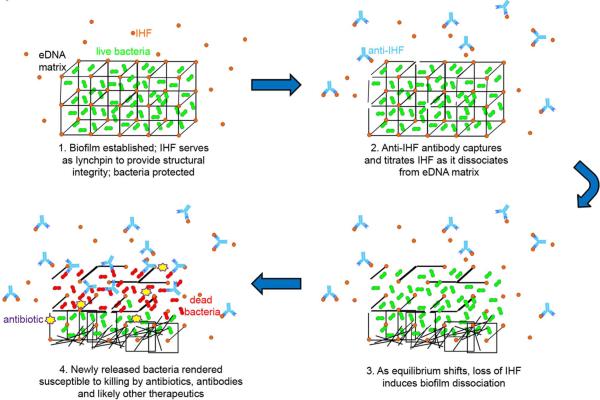
Collectively, these data support a model we propose to describe the mechanism by which we now believe that antiserum against IHF induces disruption of a established biofilm formed by multiple human pathogens (Fig. 8). We propose that exposure of biofilms to anti-IHF induces an equilibrium shift between IHF molecules bound to eDNA within a biofilm (or ‘on’) and those in an ‘off’ state. IHF molecules free in the surrounding aqueous milieu are removed, forcing bound IHF to dissociate from biofilm eDNA, thus mediating a structural collapse of the biofilm. These observations demonstrate the potential to target a molecule of critical structural importance to biofilm integrity for the treatment of multiple diseases with a biofilm component.
Fig. 8.
Model of proposed mechanism by which antibodies directed against DNABII protein(s) disrupt a bacterial biofilm. In an established biofilm, IHF is bound to bacterial eDNA, thus stabilizing the 3-dimensional structure of the biofilm matrix. As such, bacteria within the biofilm are protected. 2. Antibodies directed against IHF bind to free IHF as it transiently dissociates from the eDNA matrix. 3. As the equilibrium shifts, loss of IHF from the matrix induces structural collapse of the biofilm with release of resident bacteria. 4. Newly released bacteria are highly susceptible to therapeutics, including antibodies and antibiotics.
Experimental Procedures
Bacterial strain, biofilm formation, IHF and sera
NTHI 86-028NP is a minimally passaged clinical isolate cultured from the nasopharynx of a child undergoing tympanostomy tube insertion for chronic otitis media. Formation of NTHI biofilms in 8-well chambered coverglass slides has been described (Jurcisek et al., 2011). For all biofilm assays, duplicate wells were viewed on a Zeiss 510 Meta-laser scanning confocal microscope, images compiled with Zeiss Zen software and biomass and/or mean biofilm thickness values calculated with COMSTAT2 software (Heydorn et al., 2000; Vorregaard, 2008). All biofilm assays were repeated a minimum of three times, on separate days. Data represent mean ± SEM.
Purified E. coli IHF and rabbit antiserum against purified E. coli IHF (‘anti-IHFE. coli’) were gifts from Howard Nash (Granston and Nash, 1993; Rice et al., 1996). Naive rabbit serum was purchased from Spring Valley Laboratories.
Quantitation of IHF-specific IgG
IHF-specific IgG was purified from polyclonal serum using HiTrap Protein G HP columns (GE Healthcare). Quantitation of IHF-specific IgG in both polyclonal and IgG-enriched anti-IHFE. coli was determined by slot blot versus purified IHFE. coli. A standard curve was generated using rabbit reference serum versus purified rabbit IgG (Bethyl Laboratories, Inc.) and band intensity analyzed using AlphaView software (ProteinSimple).
Resolution of mature biofilms
Biofilms were established for 16-, 24-, 48-, and 96 hr, or 1- and 2 weeks. To maintain bacterial viability, medium (brain heart infusion broth supplemented with 2 μg ml−1 each of β-NAD and heme) was changed twice daily. Biofilms were incubated with medium, anti-IHFE. coli (a 1:50 dilution, equivalent to 4.4 μg IHF-specific IgG well−1 for biofilms of ≤48 hr or a 1:10 dilution, equivalent to 22.0 μg IHF-specific IgG well−1 for biofilms of ≥96 hr) or an equivalent volume of naive serum. After 16 hr, biofilms were stained with BacLightTM Bacterial Viability Kit (Molecular Probes), rinsed twice with sterile saline, then fixed in a solution of 1.6% paraformaldehyde, 2.5% glutaraldehyde and 4.0% acetic acid in 0.1M phosphate buffer as we have previously utilized for biofilms formed by Burkholderia cenocepacia, Legionella pneumophila and NTHI (Abu Khweek et al., 2013; Brandstetter et al., 2013; Novotny et al., 2013) and viewed as described.
Kinetics of biofilm resolution
Biofilms established for 24 hr were incubated with medium or either anti-IHFE. coli or naive serum diluted 1:50 for 0, 6, 12, 16 or 24 hr prior to viability staining and fixation as described. For the 0 hr time point, treatment was applied, and then immediately removed. To maintain bacterial viability of biofilms treated for 24 hr, treatments were replaced after 16 hr and incubated an additional 8 hr. To attempt to completely eradicate a 24 hr biofilm, a 1:5 dilution of anti-IHFE. coli or an equivalent volume of naive serum was applied.
Inhibition of direct contact between anti-IHF antibodies and NTHI biofilms
IgG-enriched anti-IHFE. coli was covalently coupled to agarose beads (>45 μm diameter) via AminoLink Plus kit (Thermo Scientific). To determine whether direct contact of anti-IHFE. coli antibodies with the biofilm was required to induce resolution, 24 hr biofilms were established in optical bottom 96-well plates, then incubated with: medium, 1:50 dilution of anti-IHFE. coli or an equivalent volume of naive serum, applied directly to the biofilm (80 μl total volume) or after insertion of a 5 μm pore size HTS Transwell (Corning) into the 96-well plate, medium, 0.5-, 5.0-or 50.0 μg IgG-enriched anti-IHFE. coli covalently bound to agarose beads, or an equal volume of IgG-enriched naive serum bound to agarose beads, were placed into the apical chamber (80 μl total volume). Biofilms were incubated for 16 hr prior to processing as described. To confirm that IHF-specific antibody did not diffuse into the basolateral chamber, supernatants from the basolateral chamber were collected and assayed by Western blotting for reactivity to purified IHFE. coli.
To determine whether biofilm resolution by anti-IHFE. coli could be blocked by steric hindrance, 80 μl of naked agarose beads were added to the apical chamber one hr prior to layering 50.0 μg IgG-enriched anti-IHFE. coli bound to beads or a comparable volume of IgG-enriched from naive serum coupled to beads. Plates were incubated an additional 16 hr, then biofilms were stained, viewed and analyzed as described.
To identify whether the ability of IHF-specific antibody to sequester free IHF was limited by relative accessibility, 50.0 μg IgG-enriched anti- IHFE. coli conjugated to beads or an equivalent volume of IgG-enriched from naive serum bound to beads was applied to the apical chamber of a transwell in which the basolateral chamber contained a 24 hr NTHI biofilm. After 6 hr, the contents of apical chamber were mixed by stirring, incubated an additional 10 hr, then the biofilms were stained, viewed and analyzed as described.
Adsorption of anti-IHFE. coli
To neutralize anti-IHFE.coli-mediated biofilm debulking, IHF-specific antibodies were adsorbed from serum by incubation with purified IHFE. coli. Aliquots of anti-IHFE. coli (4.4 μg IHF-specific IgG) were incubated with 2.2- or 4.4 μg purified IHFE. coli, saline diluent, or a recombinant protein of equivalent molecular mass called ‘rsPilA’ (Novotny et al., 2009), for 1 hr. Western blot was performed to confirm adsorption of anti-IHFE. coli. To assess the functional consequence of adsorption of anti-IHFE. coli, the adsorbed sera were then applied to 24 hr NTHI biofilms per standard treatment and processing protocol.
Synergy of anti-IHFE. coli and antibiotics against NTHI biofilms
To visualize changes in viability of NTHI biofilms upon exposure to antibiotics typically used to treat NTHI infections, 24 hr biofilms were established and incubated with 1:50 dilution of anti-IHFE. coli or naive serum, ampicillin (32.0 μg ml−1), cefdinir (0.25 μg ml−1) or amoxicillin (1.0 μg ml−1) plus clavulanate-lithium (0.5 μg ml−1) for 16 hr. Each antibiotic was used at the MIC90 for planktonic NTHI as determined via standard broth microdilution method (Biedenbach and Jones, 2003; Tristram et al., 2007). For synergy studies with older (1- and 2-week biofilms), anti-IHFE. coli was used at a 1:10 dilution either with or without amoxicillin-clavulanate at the MIC90 for planktonic NTHI.
To quantitate NTHI adherent within the biofilm and bacteria newly released into the planktonic form after treatment, 24 hr biofilms were incubated with each antibiotic at the MIC90 or a 4- or 8-fold dilution thereof, with or without antiserum. To culture newly released NTHI, supernatants were collected by aspiration, the biofilm gently washed twice with sterile saline to remove loosely adherent bacteria, and NTHI within the biofilm were recovered by repeated forceful pipetting. Planktonic and adherent bacteria were plated separately to determine the CFU NTHI ml−1 and these values were combined to demonstrate total CFU bacteria as shown. Data represent mean ± SEM of three independent assays.
Synergy of anti-IHFE. coli and antibiotics against planktonic NTHI
NTHI were prepared as described (Jurcisek et al., 2011) and 106 CFU NTHI inoculated into wells of a 96-well plate prior to incubation with antibiotic at the MIC90 or a 4- or 8-fold dilution thereof, with or without 1:50 dilution of anti-IHFE. coli or an equivalent volume of naive serum. After 16 hr, cultures were serially diluted and plated on to chocolate agar to semi-quantitate CFU NTHI/ well. Data represent mean ± SEM of three independent assays.
Epitope mapping NTHI IHF
To identify immunodominant regions within IHF, a series of twelve 20-mer synthetic peptides with 5-residue overlaps were synthesized to mimic the N- to C-terminus of the α-subunit of IHF predicted to be expressed by NTHI strain 86-028NP (‘IHFNTHI’). Synthesis, purification and sequence confirmation of all synthetic peptides was performed by Ohio Peptide, LLC. Archived samples of polyclonal sera recovered from chinchillas that had been immunized with either native IHFE. coli or IHFE. coli pre-bound to an excess of double stranded DNA (Goodman et al., 2011) were used to map immunodominant epitopes of IHF. Analysis of interaction between IHFNTHI synthetic peptides and antibodies present in chinchilla sera was determined using a Biacore 3000 (GE Healthcare) as described (Novotny et al., 2000; Novotny et al., 2009). Reactivity of chinchilla sera to IHFNTHI peptides was rendered using PyMol software (Schrödinger) to generate 3D model images.
Assessment of IHFNTHI epitope-specific antisera to disrupt NTHI biofilms
Based on results obtained from the epitope mapping study, two regions within the IHFNTHI α-subunit were selected to generate polyclonal chinchilla antiserum: IhfA-3NTHI (a non-reactive region) and IhfA-5NTHI (reactive by antibodies against IHFE. coli but nonreactive by anti-IHFE. coli prebound to DNA). NTHI biofilms established for 24 hr were treated with 1:50 dilution of the following chinchilla sera: anti-IHFE. coli, anti-IHFE. coli pre-bound to DNA, naive serum, anti-IhfA-3NTHI or anti-IhfA-5NTHI for 16 hr prior to staining and assessment. Animal work was performed following the NIH Guide for the Care and Use of Laboratory Animals and under a protocol approved by the Nationwide Children's Hospital Institutional Animal Care and Use Committee.
Statistical analyses
Statistical analyses were performed using GraphPad Prism software. Comparisons of biofilm biomass and thickness were determined using one-way analysis of variance (ANOVA) followed by Tukey's multiple comparisons test set at 5%. Significant differences in CFU NTHI following treatment were determined by one-way ANOVA followed by the Holm-Sidak test for multiple comparisons; a p-value ≤ 0.05 was considered significant.
Supplementary Material
Acknowledgments
We thank Elizabeth Norton (Tulane University) for slot blot protocol and Jennifer Neelans for manuscript preparation. This study was funded by grant R01 DC011818 to LOB and SDG from the NIH/NIDCD.
References
- Abu Khweek A, Fernandez Davila NS, Caution K, Akhter A, Abdulrahman BA, Tazi M, et al. Biofilm-derived Legionella pneumophila evades the innate immune response in macrophages. Front Cell Infect Microbiol. 2013;3:18. doi: 10.3389/fcimb.2013.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biedenbach DJ, Jones RN. Five-year analysis of Haemophilus influenzae isolates with reduced susceptibility to fluoroquinolones: prevalence results from the SENTRY antimicrobial surveillance program. Diagn Microbiol Infect Dis. 2003;46:55–61. doi: 10.1016/s0732-8893(03)00016-6. [DOI] [PubMed] [Google Scholar]
- Biedenbach DJ, Stephen JM, Jones RN. Antimicrobial susceptibility profile among beta-haemolytic Streptococcus spp. collected in the SENTRY Antimicrobial Surveillance Program--North America, 2001. Diagn Microbiol Infect Dis. 2003;46:291–294. doi: 10.1016/s0732-8893(03)00065-8. [DOI] [PubMed] [Google Scholar]
- Brandstetter KA, Jurcisek JA, Goodman SD, Bakaletz LO, Das S. Antibodies directed against integration host factor mediate biofilm clearance from Nasopore. Laryngoscope. 2013;123:2626–2632. doi: 10.1002/lary.24183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich GD, Veeh R, Wang X, Costerton JW, Hayes JD, Hu FZ, et al. Mucosal biofilm formation on middle-ear mucosa in the chinchilla model of otitis media. JAMA. 2002;287:1710–1715. doi: 10.1001/jama.287.13.1710. [DOI] [PubMed] [Google Scholar]
- Flemming HC, Wingender J. The biofilm matrix. Nat Rev Microbiol. 2010;8:623–633. doi: 10.1038/nrmicro2415. [DOI] [PubMed] [Google Scholar]
- Goodman SD, Obergfell KP, Jurcisek JA, Novotny LA, Downey JS, Ayala EA, et al. Biofilms can be dispersed by focusing the immune system on a common family of bacterial nucleoid-associated proteins. Mucosal Immunol. 2011;4:625–637. doi: 10.1038/mi.2011.27. [DOI] [PubMed] [Google Scholar]
- Granston AE, Nash HA. Characterization of a set of integration host factor mutants deficient for DNA binding. J Mol Biol. 1993;234:45–59. doi: 10.1006/jmbi.1993.1562. [DOI] [PubMed] [Google Scholar]
- Gustave JE, Jurcisek JA, McCoy KS, Goodman SD, Bakaletz LO. Targeting bacterial integration host factor to disrupt biofilms associated with cystic fibrosis. J Cyst Fibros. 2013;12:384–389. doi: 10.1016/j.jcf.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall-Stoodley L, Hu FZ, Gieseke A, Nistico L, Nguyen D, Hayes J, et al. Direct detection of bacterial biofilms on the middle-ear mucosa of children with chronic otitis media. JAMA. 2006;296:202–211. doi: 10.1001/jama.296.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydorn A, Nielsen AT, Hentzer M, Sternberg C, Givskov M, Ersboll BK, Molin S. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology. 2000;146(Pt 10):2395–2407. doi: 10.1099/00221287-146-10-2395. [DOI] [PubMed] [Google Scholar]
- Hong W, Mason K, Jurcisek J, Novotny L, Bakaletz LO, Swords WE. Phosphorylcholine decreases early inflammation and promotes the establishment of stable biofilm communities of nontypeable Haemophilus influenzae strain 86-028NP in a chinchilla model of otitis media. Infect Immun. 2007a;75:958–965. doi: 10.1128/IAI.01691-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong W, Pang B, West-Barnette S, Swords WE. Phosphorylcholine expression by nontypeable Haemophilus influenzae correlates with maturation of biofilm communities in vitro and in vivo. J Bacteriol. 2007b;189:8300–8307. doi: 10.1128/JB.00532-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EA, McGillivary G, Bakaletz LO. Extracellular DNA within a nontypeable Haemophilus influenzae-induced biofilm binds human beta defensin-3 and reduces its antimicrobial activity. J Innate Immun. 2013;5:24–38. doi: 10.1159/000339961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurcisek JA, Bakaletz LO. Biofilms formed by nontypeable Haemophilus influenzae in vivo contain both double-stranded DNA and type IV pilin protein. J Bacteriol. 2007;189:3868–3875. doi: 10.1128/JB.01935-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurcisek JA, Bookwalter JE, Baker BD, Fernandez S, Novotny LA, Munson RS, Jr., Bakaletz LO. The PilA protein of non-typeable Haemophilus influenzae plays a role in biofilm formation, adherence to epithelial cells and colonization of the mammalian upper respiratory tract. Mol Microbiol. 2007;65:1288–1299. doi: 10.1111/j.1365-2958.2007.05864.x. [DOI] [PubMed] [Google Scholar]
- Jurcisek JA, Dickson AC, Bruggeman ME, Bakaletz LO. In vitro biofilm formation in an 8-well chamber slide. J Vis Exp. 2011 doi: 10.3791/2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice SS, Li B, Downey JS, Dabdoub SM, Brockson ME, Probst GD, et al. Aberrant community architecture and attenuated persistence of uropathogenic Escherichia coli in the absence of individual IHF subunits. PLoS One. 2012;7:e48349. doi: 10.1371/journal.pone.0048349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy M, Cabral H, Figueira M, Bouchet V, Huot H, Ram S, et al. Multiple consecutive lavage samplings reveal greater burden of disease and provide direct access to the nontypeable Haemophilus influenzae biofilm in experimental otitis media. Infect Immun. 2007;75:4158–4172. doi: 10.1128/IAI.00318-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks LR, Davidson BA, Knight PR, Hakansson AP. Interkingdom signaling induces Streptococcus pneumoniae biofilm dispersion and transition from asymptomatic colonization to disease. MBio. 2013;4 doi: 10.1128/mBio.00438-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TF. Respiratory infections caused by non-typeable Haemophilus influenzae. Curr Opin Infect Dis. 2003;16:129–134. doi: 10.1097/00001432-200304000-00009. [DOI] [PubMed] [Google Scholar]
- Nickel JC, Ruseska I, Wright JB, Costerton JW. Tobramycin resistance of Pseudomonas aeruginosa cells growing as a biofilm on urinary catheter material. Antimicrob Agents Chemother. 1985;27:619–624. doi: 10.1128/aac.27.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotny LA, Jurcisek JA, Pichichero ME, Bakaletz LO. Epitope mapping of the outer membrane protein P5-homologous fimbrin adhesin of nontypeable Haemophilus influenzae. Infect Immun. 2000;68:2119–2128. doi: 10.1128/iai.68.4.2119-2128.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotny LA, Adams LD, Kang DR, Wiet GJ, Cai X, Sethi S, et al. Epitope mapping immunodominant regions of the PilA protein of nontypeable Haemophilus influenzae (NTHI) to facilitate the design of two novel chimeric vaccine candidates. Vaccine. 2009;28:279–289. doi: 10.1016/j.vaccine.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotny LA, Amer AO, Brockson ME, Goodman SD, Bakaletz LO. Structural stability of Burkholderia cenocepacia biofilms is reliant on eDNA structure and presence of a bacterial nucleic acid binding protein. PLoS One. 2013;8:e67629. doi: 10.1371/journal.pone.0067629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post JC. Direct evidence of bacterial biofilms in otitis media. Laryngoscope. 2001;111:2083–2094. doi: 10.1097/00005537-200112000-00001. [DOI] [PubMed] [Google Scholar]
- Rice PA, Yang S, Mizuuchi K, Nash HA. Crystal structure of an IHF-DNA complex: a protein-induced DNA U-turn. Cell. 1996;87:1295–1306. doi: 10.1016/s0092-8674(00)81824-3. [DOI] [PubMed] [Google Scholar]
- Romling U, Balsalobre C. Biofilm infections, their resilience to therapy and innovative treatment strategies. J Intern Med. 2012;272:541–561. doi: 10.1111/joim.12004. [DOI] [PubMed] [Google Scholar]
- Starner TD, Zhang N, Kim G, Apicella MA, McCray PB., Jr. Haemophilus influenzae forms biofilms on airway epithelia: implications in cystic fibrosis. Am J Respir Crit Care Med. 2006;174:213–220. doi: 10.1164/rccm.200509-1459OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swords WE. Nontypeable Haemophilus influenzae biofilms: role in chronic airway infections. Front Cell Infect Microbiol. 2012;2:97. doi: 10.3389/fcimb.2012.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tristram S, Jacobs MR, Appelbaum PC. Antimicrobial resistance in Haemophilus influenzae. Clin Microbiol Rev. 2007;20:368–389. doi: 10.1128/CMR.00040-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorregaard M. Master's thesis. Technical University of Denmark (DTU), Informatics and Mathematical Modeling. Kongens Lyngby; Denmark: 2008. COMSTAT2 - a modern 3D image analysis environment for biofilms. http://www2.imm.dtu.dk/pubdb/views/edoc_download.php/5628/pdf/imm5628.pdf. [Google Scholar]
- Wessman M, Bjarnsholt T, Eickhardt-Sorensen SR, Johansen HK, Homoe P. Mucosal biofilm detection in chronic otitis media: a study of middle ear biopsies from Greenlandic patients. Eur Arch Otorhinolaryngol. 2014 doi: 10.1007/s00405-014-2886-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.