Abstract
Objectives
Soy isoflavones are commonly used to alleviate menopause-related symptoms. Menopausal women are at an increased risk for hypothyroidism and there are concerns that isoflavones may be detrimental to thyroid health. The aim of this study was to examine the effects of soy protein and isoflavones on thyroid function and the relationship between thyroid and ovarian function.
Methods
Adult female cynomolgus monkeys (Macaca fascicularis) were randomized to consume two diets differing only in protein source: casein-lactalbumin (n = 44) or soy protein with isoflavones (n = 41). After 34 months all animals were ovariectomized via laparotomy. Half of the monkeys from each diet treatment group continued to consume their Pre-Ovariectomy treatment phase diet (either SOY [n = 19] or CL [n = 21]) for an additional 34 months. The remaining animals did not continue their diets, and thus were not considered further. Circulating progesterone, triiodothyronine, thyroxine, and thyroid stimulating hormone were measured at baseline. Thyroid hormones were remeasured during each treatment phase.
Results
Dietary soy increased triiodothyronine in pre-ovariectomized monkeys and prevented a decline in thyroxine following surgical menopause (both p’s < 0.05). Mean progesterone concentrations were positively correlated with triiodothyronine at baseline in pre-ovariectomized monkeys (p < 0.01).
Conclusions
Progesterone levels and triiodothyronine are positively correlated in macaques. Dietary soy increases triiodothyronine in pre-ovariectomized monkeys and prevents a decline in thyroxine following surgical menopause. The outcomes observed in this study suggest soy protein and isoflavone consumption does not adversely affect and may even preserve thyroid function in postmenopausal women.
Keywords: Thyroid, monkey, macaque, soy, isoflavone, menopause
INTRODUCTION
The menopausal transition is often accompanied by life-disrupting symptoms such as hot flashes and vaginal dryness, as well as increased risk of osteoporosis, cardiovascular disease, and cognitive decline. While estrogen replacement through hormone therapy (HT) is the most effective method for treating menopausal symptoms,1 results of the Women’s Health Initiative2 and Heart and Estrogen/Progestin Replacement Study3,4 suggest that the health risks associated with HT may outweigh the benefits. These observations have stimulated the use of alternatives to HT, including soy isoflavones, a plant-derived compound with estrogen-like activity. In humans and animals isoflavones bind to estrogen receptors (ER). They have a greater affinity of ERβ than ERα and can exert both estrogen-agonist and estrogen-antagonist properties. Supplements commonly contain soy protein which is a rich source of isoflavones; specifically genistein, daidzein, and glycitein.5
The popularity of soy isoflavones in menopausal women results from an apparent absence of adverse effects. However soy isoflavones may negatively affect thyroid function.6 Thyroid function relies on the release of thyroid stimulating hormone (TSH) from anterior pituitary in response to circulating triiodothyronine (T3) and thyroxine (T4) levels. TSH regulates thyroid peroxidase (TPO) activity and iodine uptake by the thyroid. TPO couples iodine to thyroglobulin in order to yield T3 or T4. In vitro, soy isoflavones inhibit TPO, but this inhibition can be prevented by iodine supplementation.7 The thyroid predominantly releases T4 which is then converted to the more active T3 by peripheral deiodinases (type 1 and 2).
Soy was originally thought to be goitrogenic and cause hypothyroidism.7 In humans, rats, and monkeys hypothyroidism has been associated with menstrual irregularities and infertility. 8-11 In post-menopausal women hypothyroidism prevalence reaches 10%12 and may exacerbate disorders associated with menopause such as osteoporosis, cardiovascular disease, and neuropsychiatric disease.13
While mechanisms have been identified through which soy or soy isoflavones could interfere with thyroid function, reported effects are inconsistent. The majority of human studies, including both men and women’s studies, have found no significant effect of soy isoflavones on adult thyroid function.6 These studies were all short-term, cross-sectional, and used variable doses of isolated soy protein (ISP) supplementation. Persky et al14 found no significant effect of ISP on thyroid hormone concentrations after 3 and 6 months in women aged 49-83 years. This did not control for individual differences in age, ethnicity,15 BMI,16 smoking status,17 diet,18 and underlying disease19 which may have confounded the results. However, Duncan et al20 studied a far more select and homogeneous sample and also found no thyroidal effects in post-menopausal women after 3 months of ISP treatment. Neither study controlled for the effects of caloric intake, circadian rhythm, and seasonality all of which may affect thyroid function.21,22
Studies using animal models can control for many of the environmental variables thought to influence thyroid function. Controlled experiments with rodents demonstrate that isoflavones induce increases in serum T4 concentration23,24 However, rodent thyroid function and regulation differs from that of primates.25 The main thyroid hormone carrier protein in primates, thyroid binding globulin (TBG), is not present in rodents. Isoflavone metabolism also differs markedly between rodents and humans.26 Accordingly, we used non-human primates (NHPs) in a long-term, controlled randomized study to determine the effects of dietary soy on thyroid function, and the relationship between thyroid and ovarian function.
METHODS
Animals
Eighty-two adult (based on radiographic evidence of complete epiphysial closure at the distal radius, ulna, and proximal tibia) female cynomolgus monkeys (Macaca fascicularis) were imported from Indonesia, and housed in social groups (5-6 animals each), indoors, on a 12h:12h light:dark cycle. All animal manipulations were performed according to the guidelines of state and federal laws, the US Department of Health and Human Services, and the Animal Care and Use Committee, Wake Forest University School of Medicine. Wake Forest University is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care.
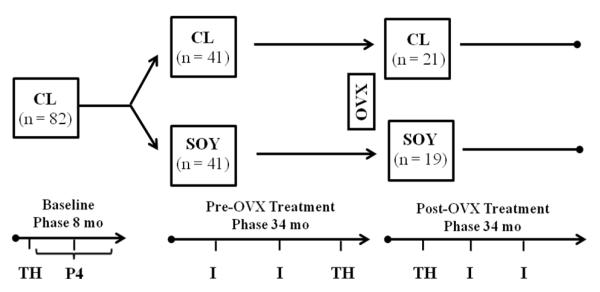
Study Design (Figure 1)
Figure 1.
Experimental Protocol. Morning blood samples were obtained for thyroid hormone (thyroxine, triiodothyronine, thyroid stimulating hormone; TH) determination 3 weeks after the onset of the study (Baseline phase), 30 months after onset of treatment (Pre-OVX treatment phase), and 9 months after ovariectomy (Post-OVX treatment phase). During the last 6 months of the Baseline phase awake blood samples were obtained for peak luteal phase progesterone (P4) concentration determination in a subset of n = 55 animals. Circulating isoflavone (I) concentrations were sampled at 7 and 19 months after the Pre-OVX treatment phase began and were repeated at 12 and 24 months following initiation of the Post-OVX treatment phase. CL, casein-lactalbumin diet; SOY, isolated soy protein diet; OVX, ovariectomy
Baseline Phase
During an 8 month Baseline phase, monkeys were fed a casein-lactalbumin (CL) diet (19% of calories from protein; 35% of calories from fat; 46% of calories from carbohydrate; 0.28 mg cholesterol/kcal which was reduced to 0.20 mg/kcal later in the study). This diet was designed to have the composition of a typical American diet, which is thought to increase the risk of chronic disease.27,28
Pre-Ovariectomy (Pre-OVX) Treatment Phase
Social groups of monkeys were assigned to one of two treatment conditions for 34 months. Since individual differences in plasma cholesterol responses to dietary fat and cholesterol could influence outcomes, the two treatment groups were matched on total serum cholesterol and high-density lipoprotein cholesterol responses to the baseline diet. The two treatment groups differed only in the main protein source: SOY (n=41), derived from isolated soy protein (SUPRO ©, Solae Corporation, St. Louis, MO, USA); or CL (n=41), derived from casein-lactalbumin (the same diet consumed by all animals during the Pre-Treatment Phase). In addition, approximately 20% of protein fed in each diet was derived from wheat flour.
Post-Ovariectomy (Post-OVX) Treatment Phase
After 34 months surgical menopause was induced in all animals by ovariectomy via laparotomy. Half of the monkeys from each diet treatment group continued to consume their Pre-treatment phase diet (either SOY [n = 19] or CL [n = 21]) for an additional 34 months. The remainder of the animals did not continue their diets, and thus were not considered further.
Diet
The SOY diet contained 1.88 mg total isoflavone/g protein and was fed at a concentration designed to provide the equivalent of a women’s daily consumption of approximately 140 mg aglycone units of isoflavone, assuming a daily intake of about 1800 kcal (i.e. 0.0775 mg isoflavone/kcal). Monkeys received 120 kcal of diet/kg body weight or approximately 9.3 mg isoflavone/kg body weight. Caloric adjustment of dose reflects the higher metabolic rate in monkeys as compared to humans. The diets were matched in macronutrients (19% of calories from protein; 35% of calories from fat; 46% of calories from carbohydrate), cholesterol (0.20 mg/kcal), and vitamin/mineral content.29
Ovarian Function
The methods used to collect data describing ovarian function has been previously described.30 Briefly, monkeys were trained to present for daily blood sampling without anesthesia during the last 6 months of the Baseline phase. Due to the extensive training required for awake blood sampling, ovarian function was assessed in a subset of n = 55 animals (29 of which were later assigned to the SOY treatment group, and 26 of which were later assigned to the CL treatment group). Blood samples were assessed for progesterone concentrations and the two highest progesterone concentrations from the luteal phase of each cycle were averaged (mean peak progesterone for each cycle). These values were then averaged over all cycles to provide a mean progesterone value for each animal.
Isoflavone Concentration
Seven and 19 months after the Pre-OVX treatment phase began blood samples were collected from all animals after ketamine sedation (10-15 mg/kg) to determine circulating isoflavone levels. 30 Animals were fed in the morning and sedated for blood collection 4 h post-feeding. Blood was immediately processed, frozen, and protected from light until analysis. Serum isoflavones from soy protein (genistein, daidzein, and glycitein) and a daidzein metabolite (equol) were analyzed by liquid chromatography photo-diode array tandem mass spectrometry. Isoflavone assessments were repeated at 12 and 24 months following initiation of the Post-OVX treatment phase.
Thyroid Hormones
Blood samples were taken between 08:00 and 10:30 h, following ketamine sedation (10-15 mg/kg), 3 weeks after the onset of the study (Baseline phase), 30 months after onset of treatment (Pre-OVX treatment phase), and 9 months after ovariectomy (Post-OVX treatment phase). After clotting all samples were centrifuged, serum was drawn off, aliquoted, and frozen at −70©C until samples from all study phases were ready for analysis.
Serum levels of total T3, free T4, and TSH were determined by radioimmunoassay (T3, T4) and immunoradiometric assay (TSH) kits (Diagnostic Product Corp; Los Angeles, CA, USA) at the Yerkes Biomarker Laboratory (Atlanta, GA, USA). The inter-assay variations for total T3 were 8.96% at 78.41 ng/dl, 11.17% at 150.91 ng/dl, and 4.57% at 291.51 ng/dl. Intra-assay variation for total T3 was 2.06%. Inter-assay variations for free T4 were 2.0% at 0.40 ng/dl, 5.6% at 1.21 ng/dl, and 6.9% at 2.08 ng/dl. Intra-assay variation for free T4 was 12.78% at 1.82 ng/dl. Inter-assay variations were 5.9% at 0.33uIU/ml, 3.4% at 3.50uIU/ml, and 5.5% at 27.0 uIU/ml. Intra-assay variations for TSH were 5.8% at 0.36uIU/ml, 2.5% at 3.83uIU/ml, and 2.3% at 29.20uIU/ml.
Statistical Analysis
Baseline characteristics of the population (body weight, T3, T4, TSH, and progesterone) were compared between treatment groups using the Student’s t-test. The effect of treatment on thyroid hormones was analyzed by 2 (treatment phase) -by- 2 (SOY, CL) mixed model analysis of variance (ANOVA) and by analysis of covariance (ANCOVA) using Baseline or Pre-OVX treatment phase values as covariates. Baseline thyroid function measurements were not obtained for three animals later assigned to the CL condition. Correlations between T3, T4, and TSH and progesterone were assessed by Pearson’s r. Statistical significance was set at p < 0.05 (two-tailed), and all results were expressed as mean ± standard error of the mean (SEM). Statistical analysis was performed using STATISTICA 10.0 for Windows, StatSoft Inc. (Tulsa, OK).
RESULTS
Baseline Characteristics of the Study Population
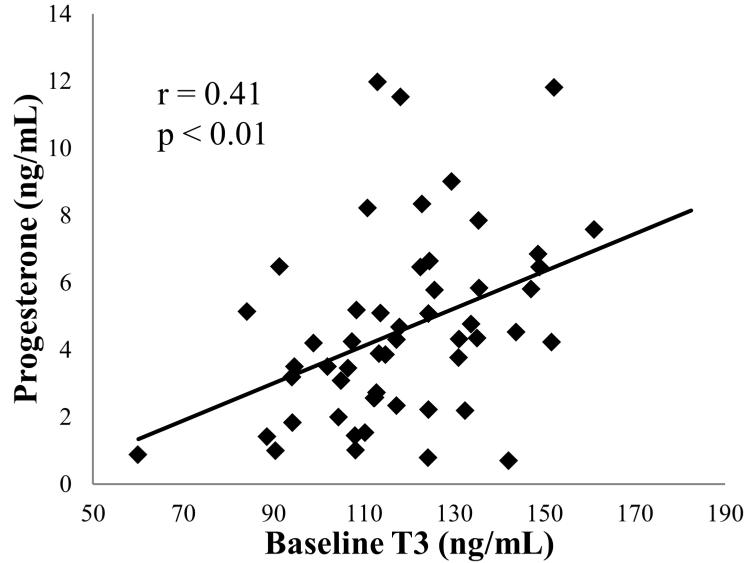
During the Baseline phase there were no significant differences in body weight, T3, T4, and progesterone among animals later assigned to either CL or SOY; however TSH was higher in animals later assigned to CL as compared to the SOY condition (Table 1). Baseline phase measures obtained from all animals demonstrated that mean progesterone concentrations correlated with T3 (r = +0.41, p < 0.01; Figure 2) but not T4 (r = +0.11, p = 0.41) or TSH (r = +0.01, p = 0.94).
Table 1.
Baseline Characteristics
| CLa | SOYa | T | P | |
|---|---|---|---|---|
| n | 41 | 41 | ||
| Body Weight (kg) | 2.9 ± 0.09 | 2.9 ± 0.06 | −0.56 | 0.57 |
| T3 (ng/dL) | 123.3 ± 3.1 | 119.2 ± 3.2 | −0.92 | 0.36 |
| T4 (ng/dL) | 2.3 ± 0.05 | 1.2 ± 0.06 | −1.0 | 0.32 |
| TSH (uIU/mL) | 1.0 ± 0.1 | 0.7 ± 0.06 | −2.26 | 0.03 |
| P4 (ng/mL) b | 4.5 ± 0.04 | |||
Values expressed as mean ± SEM.
CL, casein and lactalbumin diet; SOY, isolated soy protein diet; T3, triiodothyronine; T4, thyroxine; TSH, thyroid stimulating hormone; P4, progesterone; T, T-value; P, P-value
Refers to Pre-Ovariectomy/ Post-Ovariectomy treatment conditions; all animals were on the CL diet during baseline measures
n = 55
Figure 2.
Association between T3 and mean luteal phase progesterone from across the Pre-Treatment Phase. T3, triiodothyronine. n = 55 (29 of which were later assigned to the SOY treatment group, and 26 of which were later assigned to the CL treatment group)
Isoflavones (Table 2)
Table 2.
Isoflavone concentrations
| Baselinea | Treatment | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-Ovariectomy | Post-Ovariectomy | |||||||||||
| CLb | SOYb | T | P | CL | SOY | T | P | CL | SOY | T | P | |
| n | 44 | 41 | 44 | 41 | 21 | 19 | ||||||
|
Total Isoflavones
(nmol/L) c |
15.0 ± 2.5 | 16.6 ± 3.7 | 0.37 | 0.71 | 6.2 ± 1.5 | 405 ± 39 | 17 | <0.01 | 3.7 ± 2.8 | 354 ± 46 | 7.3 | <0.01 |
| Daidzein (nmol/L) | 12.5 ± 2.1 | 12.1 ± 2.6 | −0.10 | 0.92 | 4.6 ± 1.1 | 212 ± 21 | 10 | <0.01 | 2.1 ± 1.5 | 196 ± 21 | 9.6 | <0.01 |
| Genistein (nmol/L) | 2.5 ± 0.5 | 4.5 ± 1.2 | 1.6 | 0.12 | 1.4 ± 0.6 | 164 ± 17 | 10 | <0.01 | 1.6 ± 1.3 | 159 ± 22 | 7.5 | <0.01 |
| Equol (nmol/L) | 259 ± 29 | 237 ± 21 | −0.60 | 0.55 | 3.3 ± 1.5 | 624 ± 39 | 17 | <0.01 | 6.7 ± 5.6 | 498 ± 104 | 5.0 | <0.01 |
Values expressed as mean ± SEM.
CL, casein and lactalbumin diet; SOY, isolated soy protein diet; T, T-value; P, P-value
All animals were on the CL diet at this timepoint
Pre-Ovariectomy/ Post-Ovariectomy dietary treatment condition
Total serum isoflavones include daidzein, genistein, and glycitein
Total serum isoflavones were present in only trace amounts during the Baseline phase and did not differ significantly between treatment groups.30 Total serum isoflavones, genistein, daidzein, and equol were significantly elevated in the SOY group during both Treatment Phases (Table 2).
Diet Effects on Thyroid Function in Reproductively Intact Monkeys
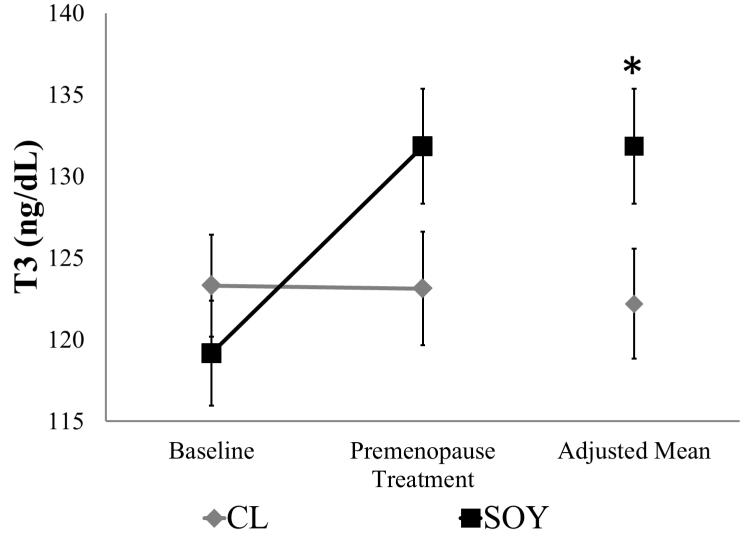
The means (±SEMs) of thyroid hormones and body weight during the Treatment Phases are given in Table 3. Repeated measures ANOVA comparing Baseline and Pre–OVX treatment phase indicated no significant differences in BW. Repeated measures ANOVA comparing Baseline and Pre-OVX treatment phase values indicated a significant time-by-diet interaction for T3 (F1,80 = 7.01, p = 0.01; Figure 3), but not T4 (F1,80 = 2.73, p = 0.10; data not shown). Although nonsignificant, differences in Baseline means for animals later assigned to CL and SOY suggested the use of ANCOVA to adjust for data collected during Baseline. Covariance of the Baseline values revealed a significant increase in T3 but not T4 following SOY treatment (T3: F1,79 = 6.23, p = 0.01; Figure 3; T4: F1,79 = 1.71, p = 0.19).
Table 3.
Treatment Phase Characteristics
| Pre-Ovariectomy | Post-Ovariectomy | |||
|---|---|---|---|---|
| CL | SOY | CL | SOY | |
| n | 44 | 41 | 21 | 19 |
| Body Weight | 3.5 ± 0.09 | 3.5 ± 0.12 | 3.4 ± 0.19 | 3.4 ± 0.16 |
| T3 (ng/dL) | 123 ± 3.5 | 132 ± 3.5 | 117 ± 7.4 | 131 ± 4.9 |
| T4 (ng/dL) | 1.3 ± 0.05 | 1.4 ± 0.05 | 1.2 ± 0.07 | 1.4 ± 0.09 |
| TSH (uIU/mL) | 1.3 ± 0.13 | 1.3 ± 0.11 | 1.07 ± 0.35 | 0.64 ± 0.09 |
Values expressed as mean ± SEM.
CL, casein and lactalbumin diet; SOY, isolated soy protein diet; T3, triiodothyronine; T4, thyroxine; TSH, thyroid stimulating hormone
Figure 3.
T3 concentration during the Pre-Treatment Phase and the Pre-OVX Treatment Phase (30 months after dietary intervention). Values presented as mean ± SEM; n = 41 per group. *p < 0.05 versus CL. T3, triiodothyronine; OVX, ovariectomy; CL, casein- lactalbumin diet; SOY isolated soy protein diet.
A repeated measures ANOVA comparing Baseline and Pre-OVX treatment phase values indicated that TSH increased significantly over time (F1,80 = 44.58, p < 0.01), but was not affected by diet (F1,80 = 1.80, p = 0.18; data not shown). Moreover, the time-by-diet interaction was not signficant (F1,80 = 2.76, p = 0.20). Because TSH assessed during Baseline differed between animals later assigned to the CL and SOY conditions, an ANCOVA using Baseline phase values as the covariate was again performed; here there were no significant differences between dietary conditions (F1,79 = 1.69, p = 0.20).
Diet Effects on Thyroid Function in Ovariectomized Monkeys
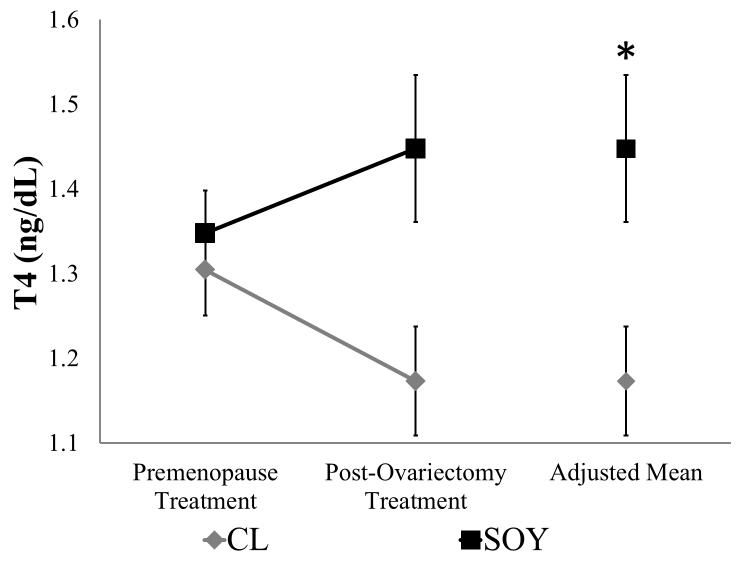
Repeated measures ANOVA comparing Pre-OVX and Post-OVX treatment phase values revealed a significant ovariectomy-by-diet interaction for T4 (F1,38 = 4.64, p = 0.04; Figure 4), but not T3 ( F1,38 = 0.11, p = 0.74; data not shown). A subsequent ANCOVA adjusting for Pre-OVX treatment phase values demonstrated the presence of a significant effect of diet on T4 (F1,38 = 7.25, p < 0.01; Figure 4) but not T3 (F1,38 = 1.70, p = 0.20). There were no significant effects of ovariectomy, diet, or their interaction on TSH (Ovariectomy: F1,37 = 1.80, p = 0.19; Diet: F1,37 = 0.76, p = 0.39; Ovariectomy-x-Diet:F1,37 = 1.38, p = 0.25; data not shown).
Figure 4.
T4 concentrations during the Pre-OVX Treatment Phase and the Post-OVX Treatment Phase (9 – 12 months post ovariectomy). Values are presented as mean ± SEM; CL group, n=21; SOY group, n=19. *p < 0.05 versus CL. T4, thyroxine; OVX, ovariectomy; CL, casein-lactalbumin diet; SOY, isolated soy protein diet.
Isoflavones and Thyroid Function
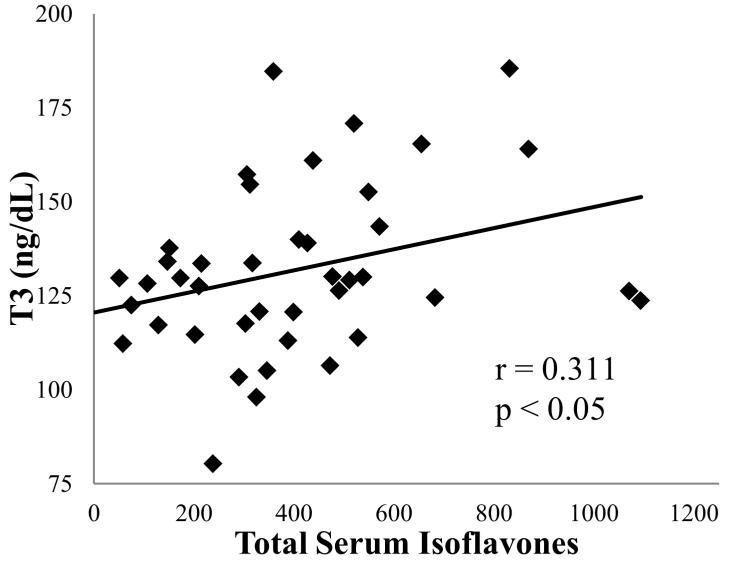
Correlational analyses between individual and total serum isoflavones and T3, T4, and TSH concentrations in the SOY condition revealed a significant positive association between T3 and genistein (r = 0.33, p = 0.03), and between T3 and total serum isoflavones (r =0.31, p < 0.05; Figure 5) during the Pre-OVX treatment phase. No significant associations were observed in the data collected during the Post-OVX treatment phase. There were no significant associations between thyroid hormones and equol.
Figure 5.
Association between mean total serum isoflavones and T3 in SOY fed monkeys during the Pre-OVX Treatment Phase. n = 41. OVX, ovariectomy; T3, triiodothyronine; SOY, isolated soy protein diet.
DISCUSSION
Reported here are the results of the first pre-clinical experiment evaluating the effects of dietary soy on thyroid function in a single NHP cohort treated both before and after surgical menopause. Notably, all animals consumed a typical North American diet that differed only in the source of protein (high isoflavone soy vs. casein-lactalbumin) The major findings were that dietary soy increased T3 in intact adult female monkeys and prevented a decline in T4 following surgical menopause. Additionally, ovarian function (progesterone concentration) was correlated with thyroid function (T3), demonstrating for the first time a relationship between these physiologic pathways in female NHPs.
This study is unique and these observations are compelling due to the use of NHPs, a human-like diet, and the multi-year experimental design that studied the same animals before and after surgical menopause. The translation to human beings of data from rodent studies is limited due to species differences in thyroid function and isoflavone metabolism. The available data from human studies derive largely from cross-sectional studies and are thus correlational, and the interpretation of the data are further limited by lack of control over environmental variables known to influence thyroid function, e.g. diet, caloric intake, health status, social environment, or circadian and seasonal rhythms. In addition to the use of an appropriate model and experimental design, a number of variables known to influence thyroid function were controlled including diet, physical and social characteristics of housing, light:dark cycle, and sampling timing.
In intact animals, SOY increased T3 but not T4 or TSH. These results differ from rodent studies that showed an increase in T423,24 and one small, short-term premenopausal women’s study which showed a decrease in T331. Changes in only T3 suggest that soy affects T4 conversion to T3 by peripheral deiodinases. Isoflavones have estrogenic activity and estradiol has been shown to increase type 1 deiodinase activity in rodent liver.32 The caveat is that T3 is produced at the tissue level and plasma levels do not represent total metabolic T3 production.33 Additionally a modest amount of T3 is released directly from the thyroid gland. TSH has been shown to trigger preferential release of T3 from the thyroid gland in man.34 Although TSH was not significantly different between the diet treatment conditions, preferential T3 secretion represents a possible mechanism by which isoflavones increase plasma T3 concentration.
SOY prevented the dramatic decrease in T4 observed in the CL condition following ovariectomy. A post-ovariectomy drop in T4 has also been described in rodents, a response that is reversed by exogenous estradiol administration.35,36 Although there are no studies in humans directly addressing the effects of surgical menopause on thyroid function, a dietary isoflavone supplementation study in oophorectomized women found alterations in free T3 concentrations across time (increased at 6 weeks, decreased at 12 weeks).37 Isoflavone studies done in naturally menopausal women have not shown significant changes in thyroid hormones.14,20,38-40 In general, theses were short term studies that were not fully controlled and individual variation may have obscured the results. Alterations in thyroid function are dependent on a subject’s normal set point.41 In the current study baseline measurements allowed the demonstration of a change in function in thyroid function in response to dietary isoflavones.
The observed alterations in T4, but not T3, in the SOY condition may have occured at the thyroid or peripheral deiodinase level. In ovariectomized rats, T3 and T4 decreases are accompanied by reduced thyroid size and histologic evidence of thyroid hypoactivity. All are reversed by physiologic doses of estradiol.35,36 Isoflavones may act similarly, possibly via thyroidal ER binding. In ovariectomized sheep an isoflavone rich diet results in greater ERα immunoactivity, higher concentrations of T3 and T4, and morphologic changes to the thyroid follicles.42 In the periphery isoflavones may boost free T4 levels by decreasing TBG. Greater than 99% of plasma T4 is protein bound, TBG being the predominant binding protein. Reduction of TBG following isoflavone administration31 may have increased free T4 in SOY fed animals. Unfortunately, while TBG has been isolated in NHP species, assays have not been validated in macaques.
To our knowledge this is the first study to demonstrate a relationship between thyroid and ovarian function in macaques. Records from a rhesus monkey breeding colony suggest an association between hypothyroxinemia (decreased plasma T4) and diminished reproductive performance.10 In this study T3, but not T4, concentrations were associated with progesterone. This may reflect the fact that T3 possesses greater biological activity compared to T4. Thyroid hormones can influence reproductive function through their effects on gonadotropin-releasing hormone pulse generation, prolactin release, sex hormone-binding globulin turnover, and by binding to thyroid hormone receptors (THRs) in the reproductive tract.7 The human ovary43 and the uterus of cynomolgus macaques44 express THRs. It is possible that THRs are also present in the macaque ovary and function as they do in humans. In humans, T3 and T4 are present in follicular fluid and addition of these hormones to granulosa cells has been shown to enhance progesterone production in vitro.45 However, the relationship between thyroid and ovarian function is complex and bidirectional, and the effect of isoflavones on thyroid function depends on ovarian status (intact versus surgical menopause).
There were some limitations to this present study. First, this study did not account for menstrual cycle related fluctuations in thyroid function. Kaplan et al30 previously published no differences in reproductive measures (ovarian hormone profile or menstrual cyclicity) between the two diet treatment conditions in this cohort; however, thyroid samples were not collected in relation to menstrual cycle phase. This may have affected outcomes as both estradiol and progesterone affect thyroid hormone concentrations.46 A related study limitation was that we were unable to measure TBG which may have been affected by isoflavone administration and reflected in the diet treatment group differences in thyroid hormone concentration. The main translational limitation of this study was that women consume a variety of protein sources; our treatment diets consisted of only wheat protein and either soy or casein-lactalbumin derived protein. This and other dietary components may have affected dietary soy-thyroid relationships. However, the effects of dietary soy in this NHP model do reflect many of those observed in premenopausal women including reduced atherogenic plaque formation,29 reduced ovarian aging,47 and the absence of effects on bone mineral content.48
Future studies might benefit from assessment of thyroid gland histological and morphometric changes. Additionally, it is essential to analyze deiodinase activity, including type 3 deiodinase which is involved in T3 and T4 degradation, to better understand the effects of soy isoflavones on thyroid hormone production and metabolism.
CONCLUSIONS
These observations suggest that dietary soy increases T3 in intact female monkeys, and prevents a decline in T4 following surgical menopause in nonhuman primates. Further there was a positive correlation between ovarian-derived progesterone levels and T3 secretion. The outcomes observed in this study suggest soy protein and isoflavone consumption does not adversely affect and may even preserve thyroid function in postmenopausal women.
Acknowledgments
This work was supported in part by the National Center for Research Resources S10 RR020890, the National Heart, Lung, and Blood Institute P01 HL45666 (JRK), R01HL79421 (JRK), 2 R01 HL 087103 (CAS), and the National Institute of Health T32 OD 010957 (MS).
Footnotes
Disclosures: Drs. Silverstein, Kaplan, Appt, Register and Shively reported no biomedical financial interests or potential conflicts of interest.
Contributor Information
Marnie G. Silverstein, Department of Pathology, Section on Comparative Medicine Integrative Physiology and Pharmacology Graduate Program Wake Forest University School of Medicine, Winston-Salem, NC.
Jay R. Kaplan, Department of Pathology, Section on Comparative Medicine Wake Forest University School of Medicine, Winston-Salem, NC.
Susan E. Appt, Department of Pathology, Section on Comparative Medicine Wake Forest University School of Medicine, Winston-Salem, NC.
Thomas C. Register, Department of Pathology, Section on Comparative Medicine Wake Forest University School of Medicine, Winston-Salem, NC.
Carol A. Shively, Department of Pathology, Section on Comparative Medicine Wake Forest University School of Medicine, Winston-Salem, NC.
REFERENCES
- 1.Bedell S, Nachtigall M, Naftolin F. [Accessed June 21, 2013];The pros and cons of plant estrogens for menopause [published online ahead of print December 25 2012] J Steroid Biochem Mol Biol. 2012 doi: 10.1016/j.jsbmb.2012.12.004. doi: 10.1016/j.jsbmb.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 2.Lawton B, Rose S, McLeod D, Dowell A. Changes in use of hormone replacement therapy after the report from the Women’s Health Initiative: cross sectional survey of users. BMJ. 2003;327:845–6. doi: 10.1136/bmj.327.7419.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grady D, Herrington D, Bittner V, Blumenthal R, Davidson M, Hlatky M, et al. HERS Research Group. Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II) JAMA. 2002;288:49–57. doi: 10.1001/jama.288.1.49. [DOI] [PubMed] [Google Scholar]
- 4.Hulley S, Furberg C, Barrett-Connor E, Cauley J, Grady D, Haskell W, et al. HERS Research Group. Noncardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II) JAMA. 2002;288:58–66. doi: 10.1001/jama.288.1.58. [DOI] [PubMed] [Google Scholar]
- 5.Clarkson TB, Utian WH, Allmen TI, et al. The role of soy isoflavones in menopausal health: report of The North American Menopause Society/Wulf H. Utian Translational Science Symposium in Chicago, IL (October 2010) Menopause. 2011;18:732–53. doi: 10.1097/gme.0b013e31821fc8e0. [DOI] [PubMed] [Google Scholar]
- 6.Marini H, Polito F, Adamo EB, Bitto A, Squadrito F, Benvenga S. Update on genistein and thyroid: an overall message of safety. Front Endocrinol (Lausanne) 2012;3:94. doi: 10.3389/fendo.2012.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Divi RL, Chang HC, Doerge DR. Anti-thyroid isoflavones from soybean: isolation, characterization, and mechanisms of action. Biochem Pharmacol. 1997;54:1087–96. doi: 10.1016/s0006-2952(97)00301-8. [DOI] [PubMed] [Google Scholar]
- 8.Poppe K, Velkeniers B. Female infertility and the thyroid. Best Pract Res Cl En. 2004;18:153–165. doi: 10.1016/j.beem.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Hatsuta M, Abe K, Tamura K, Ryuno T, Watanabe G, Taya K, Kogo H. Effects of hypothyroidism on the estrous cycle and reproductive hormones in mature female rat. Eur J Pharmacol. 2004;486:343–8. doi: 10.1016/j.ejphar.2003.12.035. [DOI] [PubMed] [Google Scholar]
- 10.Parrot MW, Johnston ME, Durbin PW. The effects of thyroid and parathyroid deficiency on reproduction in the rat. Endocrinology. 1960;67:467–483. doi: 10.1210/endo-67-4-467. [DOI] [PubMed] [Google Scholar]
- 11.Ozpinar A, Golub MS, Poppenga RH, Blount BC, Gillespie JR. Thyroid status of female rhesus monkeys and preliminary information on impact of perchlorate administration. Lab Anim. 2011;45:209–214. doi: 10.1258/la.2011.010047. [DOI] [PubMed] [Google Scholar]
- 12.Mazer NA. Interaction of estrogen therapy and thyroid hormone replacement in postmenopausal women. Thyroid. 2004;14:S27–S34. doi: 10.1089/105072504323024561. [DOI] [PubMed] [Google Scholar]
- 13.Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III) J Clin Endocrinol Metab. 2002;87:489–499. doi: 10.1210/jcem.87.2.8182. [DOI] [PubMed] [Google Scholar]
- 14.Persky VW, Turyk ME, Wang L, et al. Effect of soy protein on endogenous hormones in postmenopausal women. Am J Clin Nutr. 2002;75:145–53. doi: 10.1093/ajcn/75.1.145. [DOI] [PubMed] [Google Scholar]
- 15.Surks MI, Boucai L. Age- and race-based serum thyrotropin reference limits. J Clin Endocrinol Metab. 2010;95:496–502. doi: 10.1210/jc.2009-1845. [DOI] [PubMed] [Google Scholar]
- 16.Knudsen N, Laurberg P, Rasmussen LB, et al. Small differences in thyroid function may be important for body mass index and the occurrence of obesity in the population. J Clin Endocrinol Metab. 2005;90:4019–4024. doi: 10.1210/jc.2004-2225. [DOI] [PubMed] [Google Scholar]
- 17.Asvold BO, Bjoro T, Nilsen TI, Vatten LJ. Tobacco smoking and thyroid function: a population-based study. Arch Intern Med. 2007;167:1428–1432. doi: 10.1001/archinte.167.13.1428. [DOI] [PubMed] [Google Scholar]
- 18.Franco JG, Fernandes TP, Rocha CP, et al. [Accessed July 8, 2013];Maternal high-fat diet induces obesity and adrenal and thyroid dysfunction in male rat offspring at weaning [published online ahead of print November 1 2012] J Physiol. 2012 doi: 10.1113/jphysiol.2012.240655. doi: 10.1113/jphysiol.2012.240655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chopra IJ, Hershman JM, Pardridge WM, Nicoloff JT. Thyroid function in nonthyroidal illnesses. Ann Intern Med. 1983;98:946–57. doi: 10.7326/0003-4819-98-6-946. [DOI] [PubMed] [Google Scholar]
- 20.Duncan AM, Underhill KE, Xu X, Lavalleur J, Phipps WR, Kurzer MS. Modest hormonal effects of soy isoflavones in postmenopausal women. J Clin Endocrinol Metab. 1999;84:3479–84. doi: 10.1210/jcem.84.10.6067. [DOI] [PubMed] [Google Scholar]
- 21.Fisher DA. Physiological variations in thyroid hormones: physiological and pathophysiological considerations. Clin Chem. 1996;42:135–9. [PubMed] [Google Scholar]
- 22.Kim TH, Kim KW, Ahn HY, et al. [Accessed July 8, 2013];Effect of seasonal changes on the transition between subclinical hypothyroid and euthyroid status [published online ahead of print Jun 14 2013] J Clin Endocrinol Metab. 2013 doi: 10.1210/jc.2013-1607. doi: 10.1210/jc.2013-1607. [DOI] [PubMed] [Google Scholar]
- 23.Balmir F, Staack R, Jeffrey E, Jimenez MD, Wang L, Potter SM. An extract of soy flour influences serum cholesterol and thyroid hormones in rats and hamsters. J Nutr. 1996;126:3046–53. doi: 10.1093/jn/126.12.3046. [DOI] [PubMed] [Google Scholar]
- 24.Xiao CW, L’Abbe MR, Gilani GS, Cooke GM, Curran IH, Papademetriou SA. Dietary soy protein isolate and isoflavones modulate hepatic thyroid hormone receptors in rats. J Nutr. 2004;134:743–9. doi: 10.1093/jn/134.4.743. [DOI] [PubMed] [Google Scholar]
- 25.National Research Council . Health implications of perchlorate ingestion. The National Academies Press; Washington, DC: [Accessed July 8, 2013]. 2005. Available at: http://www.nap.edu/openbook. [Google Scholar]
- 26.Wu KM, Farrelly JG. Preclinical development of new drugs that enhance thyroid hormone metabolism and clearance: inadequacy of using rats as an animal model for predicting human risks in an IND and NDA. Am J Ther. 2006;13:141–4. doi: 10.1097/01.mjt.0000209673.01885.b0. [DOI] [PubMed] [Google Scholar]
- 27.Craig WJ, Mangels AR. Position of the American Dietetic Association: vegetarian diets; American Dietetic Association. J Am Diet Assoc. 2009;109:1266–1282. doi: 10.1016/j.jada.2009.05.027. [DOI] [PubMed] [Google Scholar]
- 28.Dall TM, Fulgoni VL, 3rd, Zhang Y, Reimers KJ, Packard PT, Astwood JD. Potential health benefits and medical cost savings from calorie, sodium, and saturated fat reductions in the American diet. Am J Health Promot. 2009;23:412–422. doi: 10.4278/ajhp.080930-QUAN-226. [DOI] [PubMed] [Google Scholar]
- 29.Walker SE, Register TC, Appt SE, et al. Plasma lipid-dependent and -independent effects of dietary soy protein and social status on atherogenesis in premenopausal monkeys: implications for postmenopausal atherosclerosis burden. Menopause. 2008;15:950–957. doi: 10.1097/gme.0b013e3181612cef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaplan JR, Chen H, Appt SE, et al. Impairment of ovarian function and associated health-related abnormalities are attributable to low social status in premenopausal monkeys and not mitigated by a high-isoflavone soy diet. Hum Reprod. 2010;25:3083–94. doi: 10.1093/humrep/deq288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duncan AM, Merz BE, Xu X, Nagel TC, Phipps WR, Kurzer MS. Soy isoflavones exert modest hormonal effects in premenopausal women. J Clin Endocrinol Metab. 1999 Jan;84:192–7. doi: 10.1210/jcem.84.1.5387. [DOI] [PubMed] [Google Scholar]
- 32.Lisbôa PC, Curty FH, Moreira RM, Oliveira KJ, Pazos-Moura CC. Sex steroids modulate rat anterior pituitary and liver iodothyronine deiodinase activities. Horm Metab Res. 2001;33:532–5. doi: 10.1055/s-2001-17211. [DOI] [PubMed] [Google Scholar]
- 33.Sapin R, Schlienger JL. Thyroxine (T4) and tri-iodothyronine (T3) determinations: techniques and value in the assessment of thyroid function. Ann Biol Clin (Paris) 2003;61:411–20. [PubMed] [Google Scholar]
- 34.Carpi A, Bianchi R, Zucchelli GC, et al. Effect of endogenous thyroid stimulating hormone levels on the secretion of thyroid hormones in man. Acta Endocrinol (Copenh) 1979;92:73–84. doi: 10.1530/acta.0.0920073. [DOI] [PubMed] [Google Scholar]
- 35.Lima LP, Barros IA, Lisbôa PC, et al. Estrogen effects on thyroid iodine uptake and thyroperoxidase activity in normal and ovariectomized rats. Steriods. 2006;71:653–659. doi: 10.1016/j.steroids.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 36.Abdel-Dayem MM, Elgendy MS. Effects of chronic estradiol treatment on the thyroid gland structure and function of ovariectomized rats. BMC Res Notes. 2009;2:173. doi: 10.1186/1756-0500-2-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mittal N, Hota D, Dutta P, et al. Evaluation of effect of isoflavone on thyroid economy & autoimmunity in oophorectomised women: a randomised, double-blind, placebo-controlled trial. Indian J Med Res. 2011;133:633–40. [PMC free article] [PubMed] [Google Scholar]
- 38.Bruce B, Messina M, Spiller GA. Isoflavone supplements do not affect thyroid function in iodine-replete postmenopausal women. J Med Food. 2003;6:309–316. doi: 10.1089/109662003772519859. [DOI] [PubMed] [Google Scholar]
- 39.Ryan-Borchers T, Chew B, Park JS, McGuire M, Fournier L, Beerman K. Effects of dietary and supplemental forms of isoflavones on thyroid function in healthy post-menopausal women. Top Clin Nutr. 2008;23:13–22. [Google Scholar]
- 40.Bitto A, Polito F, Atteritano M, et al. Genistein aglycone does not affect thyroid function: results from a three-year, randomized, double-blind, placebo-controlled trial. J Clin Endocrinol Metab. 2010;95:3067–3072. doi: 10.1210/jc.2009-2779. [DOI] [PubMed] [Google Scholar]
- 41.Andersen S, Pedersen KM, Bruun NH, Laurberg P. Narrow individual variations in serum T(4) and T(3) in normal subjects: a clue to the understanding of subclinical thyroid disease. J Clin Endocrinol Metab. 2002;87:1068–72. doi: 10.1210/jcem.87.3.8165. [DOI] [PubMed] [Google Scholar]
- 42.Madej A, Person E, Lundh T, Ridderstrale Y. Thyroid gland function in ovariectomized ewes exposed to phytoestrogens. J Chromatogr B. 2002;77:281–7. doi: 10.1016/s1570-0232(02)00082-x. [DOI] [PubMed] [Google Scholar]
- 43.Aghajanova L, Lindeberg M, Carlsson IB, Stavreus-Evers A, Zhang P, Scott JE, Hovatta O, Skjöldebrand-Sparre L. Receptors for thyroid-stimulating hormone and thyroid hormones in human ovarian tissue. Reprod Biomed Online. 2009;18:337–347. doi: 10.1016/s1472-6483(10)60091-0. [DOI] [PubMed] [Google Scholar]
- 44.Hulchiy M, Hua Z, Cline JM, Hirschberg AL, Sahlin L. Receptors for thyrotropin-releasing hormone, thyroid-stimulating hormone, and thyroid hormones in the macaque uterus: effects of long-term sex hormone treatment. Menopause. 2012;19:1253–59. doi: 10.1097/gme.0b013e318252e450. [DOI] [PubMed] [Google Scholar]
- 45.Wakim AN, Polizotto SL, Burholt DR. Augmentation by thyroxine of human granulosa cell gonadotrophin-induced steroidogenesis. Hum Reprod. 1995;10:2845–8. doi: 10.1093/oxfordjournals.humrep.a135805. [DOI] [PubMed] [Google Scholar]
- 46.Sathi P, Kalyan S, Hitchcock CL, Pudek M, Prior JC. [Accessed July 8, 2013];Progesterone Therapy increases Free Thyroxine Levels-data from a randomized placebo-controlled 12-week hot flush trial [published online ahead of print Dec 17 2012] Clin Endocrinol (Oxf) 2012 doi: 10.1111/cen.12128. doi: 10.1111/cen.12128. [DOI] [PubMed] [Google Scholar]
- 47.Appt SE, Chen H, Goode AK, Hoyer PB, Clarkson TB, Adams MR, Wilson ME, Franke AA, Kaplan JR. The effect of diet and cardiovascular risk on ovarian aging in cynomolgus monkeys. (Macaca fascicularis) Menopause. 2010;17:741–8. doi: 10.1097/gme.0b013e3181d20cd2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lees CJ, Kaplan JR, Chen H, Jerome CP, Register TC, Franke AA. Bone mass and soy isoflavones in socially housed, premenopausal Macaques. Am J Clin Nutr. 2007;86:245–50. doi: 10.1093/ajcn/86.1.245. [DOI] [PubMed] [Google Scholar]