Abstract
Two major issues in total joint arthroplasty are loosening of implants and osteolysis caused by wear particle-induced inflammation. Wear particles stimulate the release of pro-inflammatory cytokines, chemokines and other inflammatory mediators from macrophages and other cells. Although the biological response of macrophages to wear debris is well established, the role of other cell types such as natural killer T lymphocytes (NKT) and dendritic cells (DCs) is limited. Here we show that ultra-high molecular weight polyethylene (UHMWPE) particles stimulate NKT cells to secrete Interferon-γ (IFN-γ); co-culture with DCs further enhanced IFN-γ secretion. Furthermore, UHMWPE particles did not stimulate NKT cells to secrete IL-4, while the NKT cell natural ligand α-Galactosylceramide (α-GalCer) treatment in the co-culture system significantly enhanced both IFN-γ and IL-4 expression by NKT cells. Comparatively, NKT cells and/or DCs exposed to polymethylmethacrylate particles did not stimulate Interferon-γ or IL-4 expression. Mouse bone marrow derived macrophage polarization by lipopolysaccharide and conditioned medium from NKT cells and/or DCs exposed to UHMWPE particles increased TNF-α, but reduced arginase-1 expression in macrophages. The current findings indicate that UHMWPE particles stimulate NKT cells/DCs to produce pro-inflammatory cytokines; this pathway is a novel therapeutic target to mitigate wear particle induced peri-prosthetic osteolysis.
Keywords: UHMWPE, wear particles, Natural killer T lymphocytes, dendritic cells, periprosthetic osteolysis
Introduction
Total joint replacement is a cost-effective surgical procedure for end-stage arthritis. However, wear of the implant bearing surfaces with use of the joint replacement produces wear debris and other byproducts. Wear particles generated from implants produce periprosthetic osteolysis, which is a major issue related to long-term outcome. 1,2 Natural killer T (NKT) cells are a sub-population of T lymphocytes that can recognize self and foreign glycolipid antigens in the presence of antigen-presenting cells including dendritic cells (DCs)3,4. Upon activation, NKT cells are known to modulate the immune system by rapidly secreting either pro-inflammatory cytokines such as Interferon-γ (IFN-γ), or anti-inflammatory mediators such as IL-43,4. This ability to further activate or suppress the inflammatory response makes these cells unique. The purpose of current study is to evaluate cytokines released by NKT cells in response to phagocytosable polymer particles with/without DCs. This information may suggest new avenues to mitigate wear particle induced chronic inflammation and periprosthetic osteolysis.
Materials and methods
Isolation of NKT cells, DCs, and macrophages
Institutional guidelines for the care and use of laboratory animals were observed in all aspects of this project. C57BL6/J male mice 10 to 12 weeks of age (Jackson Laboratory) were euthanized with carbon dioxide (CO2) gas, and sterilized by 70% ethanol before surgery. Splenocytes were collected while maintaining sterile technique. Red blood cells were first depleted by using RBC lysis buffer (Sigma-Aldrich St. Louis, MO). After washing the cells, NKT cells were isolated by NK1.1 iNKT cell isolation kit (Miltenyi Biotec, Auburn, CA), and DCs were isolated by CD11c magnetic microbeads (Miltenyi Biotec. Auburn, CA). The instructions for cell isolation system were followed carefully. After isolation, the cells were re-suspended in the RPMI medium (Invitrogen, Cat.No.11875-093) containing 1mM sodium pyruvate (Invitrogen, Cat.No.11360070), 10% heat inactivated fetal bovine serum (FBS, Invitrogen, Cat.No.10082147), 55μM 2-mercaptoethanol (Invitrogen, Cat.No.21985023), antibiotic/antimycotic solution (100 units of penicillin, 100μg of streptomycin, and 0.25 μg of Amphotericin B per ml; Hyclone, Thermo Scientific), 1x non-essential amino acid solution(Invitrogen, Cat.No.11140-050), and 1x MEM vitamin solution (Invitrogen, Cat.No.11120-052)5. The cells were used immediately for the co-culture system.
Bone marrow derived macrophages (BMDMs) were collected from the femora of the same mice. The femora were surgically removed while maintaining sterile technique. Using a syringe and 25-gauge needle, the bone marrow was flushed by injecting 4 mL of culture medium (RPMI1640 medium supplemented with 10% heat inactivated FBS, and the antibiotic/antimycotic solution) through the marrow, passed through a 70μm strainer, spun down (400g/10mins), washed 3 times with culture medium, re-suspended in the culture medium containing 30% of L929 cells conditioned medium and 10 ng/ml mouse macrophage colony stimulation factor (M-CSF, R&D, Cat.No.416-ML-50/CF), and re-plated in T-175 culture flasks (BD, Cat.No.353112) at a concentration of 4×107 cells per flask. Cells were allowed to expand for 5–7 days, with a medium change at the second day to remove non-adherent cells. The BMDMs were used after 7 days in culture.
Ultra-high molecular weight polyethylene (UHMWPE) and polymethylmethacrylate (PMMA) particles
Conventional UHMWPE particles were a gift from Dr. Timothy Wright (Hospital for Special Surgery, New York) and obtained from knee joint simulator tests and isolated according to an established protocol6. Frozen aliquots of the particles containing serum were lyophilized for 4–7 days. The dried material was digested in 5 M sodium hydroxide at 60 °C for 1h, and ultrasonicated for 10 min. The digested particle suspension was centrifuged through a 5% sucrose gradient at 40 K rpm at 10 °C for 3 h. The collected particles at the surface of the sucrose solution were incubated at 80 °C for 1 h and centrifuged again through an isopropanol gradient (0.96 and 0.90 g/cm3) at 40K rpm at 10 °C for 1 h. The purified particles at the interface between the two layers of isopropanol were harvested and the isopropanol was evaporated from the particle mixture then lyophilized until dry. Particles were then re-suspended in 95% ethanol which was evaporated completely. The particles tested negative for endotoxin using a Limulus Amebocyte Lysate Kit (Lonza, Cat.No.50-647U). The mean diameter of the particles was 1.0 ± 0.1 mm (mean ± SE) measured by electron microscopy.
PMMA particles (Polysciences, Warrington, PA, USA) 1–10 μm in diameter, were washed with 70% ethanol and incubated overnight with shaking at 4 °C. The particles were then washed extensively with Dulbecco’s phosphate-buffered saline (DPBS, Invitrogen, Cat.No.14190250) and re-suspended to obtain a concentrated 5% v/v stock solution. The particles were shown to be free of endotoxin according to the Limulus Amebocyte Lysate Assay.
NKT/DC Co-culture system
The isolated NKT (1 × 105/well in 24 wells plate) cells were co-cultured with/without DCs (1 × 104/well), and exposed to two different particle types, UHMWPE and PMMA for 24 hours. UHMWPE was coated on the bottom of culture plate by adding 200μl/well of 1.5mg/ml UHMWPE, and air-dried in a culture hood overnight. PMMA was re-suspended in DPBS and added directly to the culture medium. The expression profiles of IFN-γ and IL-4 were analyzed at both mRNA and protein levels using qPCR and ELISA respectively. The NKT activation ligand α-galactosylceramide (α-GalCer, 100ng/ml) was used as a positive control. All the experiments were done in duplicate.
Macrophage polarization
Isolated primary BMDMs (1 × 105/well in 24 wells plate) were plated and exposed to the conditioned medium collected from the NKT cells/DCs co-culture system for 24 hours. The BMDMs were then treated with 100 ng/ml Lipopolysaccharide (LPS, purchased from Sigma-Aldrich St. Louis, MO) for additional 24 hours. Cells were then harvested and the RNAs were extracted for quantitative PCR analysis. All the experiments were done in duplicate.
Enzyme-linked immunosorbent assay (ELISA)
The supernatants from the co-cultured cells were analyzed for their cytokine expression levels. The concentration of IFN-γ (R&D, Minneapolis, MN) and IL-4 (eBioscience, San Diego, CA) were determined by ELISA. The user instructions were followed carefully.
RNA extraction and quantitative PCR
Cellular RNAs were extracted by using RNeasy RNA purification kit (Qiagen, Valencia, CA). RNAs were reverse transcribed into complementary DNA (cDNA) using a high-capacity cDNA archive kit (Applied Biosystems, Foster City, CA). Probes for 18s rRNA, IFN-γ, IL-4, TNF-α, iNOS, arginase 1, and CD206 (mannose receptor) were purchased from Applied Biosystems. Reverse-transcriptase polymerase chain reaction (RT-PCR) was performed in an ABI 7900HT Sequencing Detection System (Applied Biosystems), using the 18s rRNA as the internal control. The −ΔΔCt relative quantitation method was used to evaluate gene expression level.
Statistical analysis
Unpaired t-test and two-way ANOVA were conducted using Prism 5 (GraphPad Software, San Diego, CA). Data were reported as mean ± standard error. P<0.05 was chosen as the threshold of significance.
Results
Primary isolated mouse NKT cells/DCs exposed to UHMWPE particles induced IFN-γ but not IL-4 expression
NKT cells/DCs with/without UHMWPE particles were cultured for 24 hours. NKT cells or DCs only was set as the basal level control, and NKT cells/DCs treated with 100ng/ml α-Galcer was set as positive control for NKT cell activation.
NKT cells/DCs and NKT cells alone exposed to UHMWPE particles induced IFN-γ expression at the mRNA level (Fig. 1a). IFN-γ expression in the cells without particles was very low or un-detectable, similar results were found when DCs alone were exposed to UHMWPE. The IFN-γ protein level in culture supernatants showed a similar expression pattern, while NKT cells/DCs exposed to UHMWPE particles had a higher expression compared to the NKT cells alone with UHMWPE particles (Fig. 1b). On the other hand, IL-4 expression was not induced at the mRNA or protein levels in the particle treated cells (Fig. 1c, d). NKT cells/DCs treated with α-Galcer induced both IFN-γ and IL-4 at both the mRNA and protein levels (Fig. 1), indicating that NKT cells can be activated with its natural ligand in the co-culture system.
Figure 1. Expression of IFN-γ (a, b) and IL-4 (c, d) by NKT cells and DCs was determined by quantitative PCR (a, c) and ELISA (b, d).
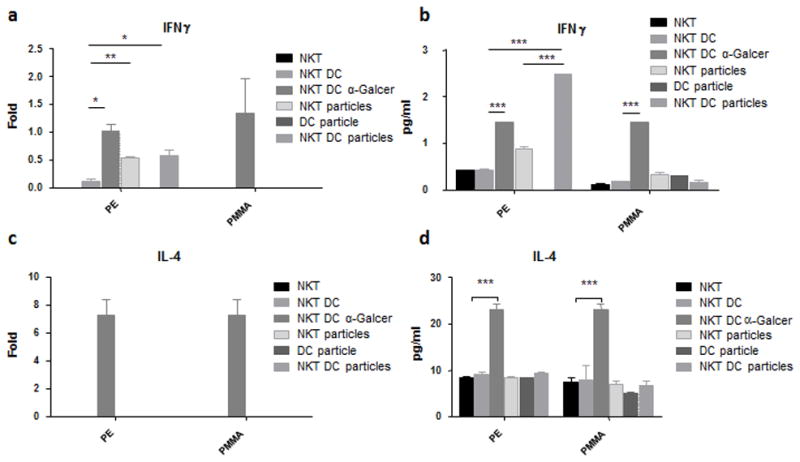
NKT cells (1×105) were exposed to UHMWPE (PE, 0.03mg) or PMMA (0.05%) only or different treatment combinations including DCs (1×104) and α-GalCer (100ng/ml) for 24 hrs. *p<0.05; **p<0.01; ***p<0.005.
NKT cells/DCs was then exposed to PMMA particles for 24 hours. The expression of IFN-γ and IL-4 were determined as described above. No induction of IFN-γ or IL-4 was observed in the cells exposed to PMMA particles at both mRNA and protein levels.
Enhanced TNF-α expression in polarized mouse macrophages by conditioned medium from NKT/DC co-cultures exposed to UHMWPE particles
Previous reports indicated that IFN-γ triggers M1 type macrophage polarization that results in the secretion of pro-inflammatory cytokines including TNF-α. We hypothesized that NKT/DC co-cultures exposed to UHMWPE would enhance macrophage-mediated inflammatory responses. To address this question, primary mouse BMDMs were treated with conditioned medium from the co-cultured system and polarized by 100ng/ml of LPS, and the mRNA expression of M1/M2 macrophage markers (M1: TNF-α and iNOS; M2: arginase-1 and mannose receptor) was determined by quantitative PCR. The polarized macrophages with no conditioned medium showed increased TNF-α (6.3 ± 0.42 fold), iNOS (8999.5 ± 344.68 fold), and arginase-1 (1.88 ± 0.35 fold) expression. Mannose receptor (M2 type marker) was down-regulated (75 ± 0.19 %) by LPS treatment (Fig. 2a). The polarized macrophages with conditioned medium from NKT/DC, NKT cells, and DCs exposed to UHMWPE particles increased TNF-α (1.73 ± 0.09, 2.50 ± 0.11, and 3.93 ± 0.26 fold, respectively), but decreased arginase-1 (59 ± 3.3% and 29 ± 9.3%, respectively). There was no significant differences in results from the NKT/DC with UHMWPE group when compared to NKT/DC controls without treatment. Comparably, the polarized macrophages with conditioned medium form NKT/DC with α-Galcer decreased TNF-α (41 ± 2.1%), but increased arginase-1 (10.13 ± 0.37 fold) expression compared to NKT/DC controls.
Figure 2. Expression of M1/M2 markers in mouse primary macrophages determined by quantitative PCR.
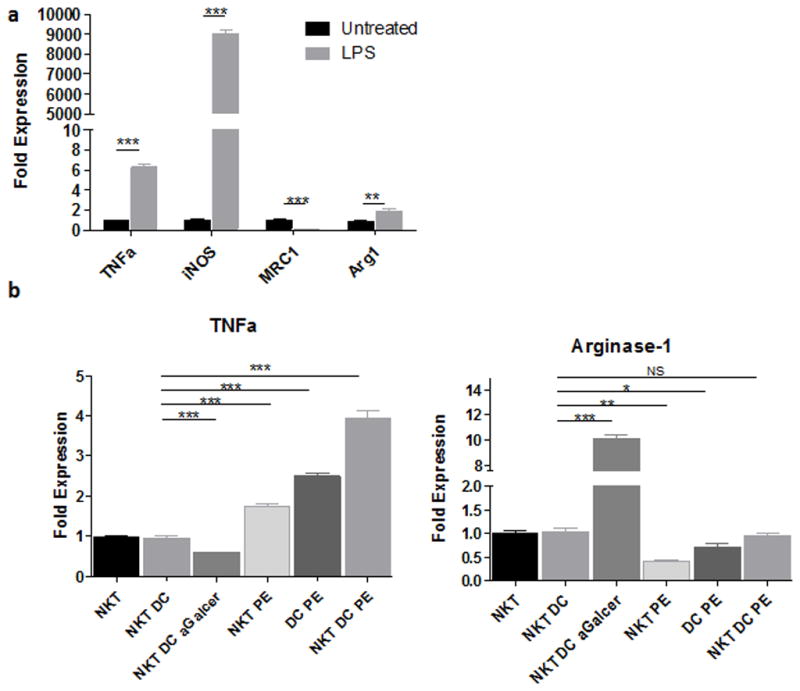
Macrophages (1×105) were exposed to control media without exposed to the cells (a) or conditioned media from NKT/DC co-cultured with/without UHMWPE or α-Galcer (b) for 24 hours, and then polarized by 100ng/ml LPS for additional 24 hrs. TNF-α and iNOS represent M1 type macrophage markers, while arginase-1 and Mannose receptor represent M2 type macrophage markers. PE=UHMWPE.
Discussion
Our results demonstrate that activation of NKT cells by UHMWPE particles modulates the pro-inflammatory response through secretion of IFN-γ in the presence of antigen presenting dendritic cells. It is well accepted that M1 macrophages (which are induced by IFN-γ) can enhance the pro-inflammatory response7, whereas M2 macrophages (which are induced by IL-4) mitigate this response7–9. Induction of IFN-γ but not IL-4 in NKT cells will increase M1 macrophage activity and enhance the wear particle related adverse tissue responses. In addition, macrophage polarization with exposure to conditioned medium showed that DCs exposed to UHMWPE particles increase TNF-α expression, suggesting that UHMWPE particles induce DCs to secrete other cytokines which modulate macrophage polarization.
It is also well accepted that NKT cell activation with glycolipid antigen presented by DCs induces both IFN-γ and IL-4 expression3,4. Recent reports have also found that DCs can recognize exogenous antigens (such as microbial) via toll-like receptors, and present endogenous antigens to activate NKT cells10,11. By secreting IL-12, DCs can modulate NKT cells to secrete IFN-γ but not IL-410,11. Since it has been reported that wear particles can be recognized by toll-like receptors and activate immune response12,13, it is likely that DCs can recognize wear particles through toll-like receptors and modulate NKT cell functions. We found that NKT cells alone exposed to UHMWPE particles can also enhance IFN-γ secretion at a lower level (Fig. 1c), suggesting that NKT cells may directly recognize these particles.
The specific roles of NKT cells in arthritis are distinct depending on the disease model. Significant correlation between NKT cell deficiency in rheumatoid arthritis patients has been reported in clinical studies14,15. Furthermore, NKT cells can promote an inflammatory response in antibody or collagen induced arthritis mouse models16,17. Here, our findings suggest that NKT cells may also contribute to the wear particle induced inflammatory response.
Depending on the specific roles in different diseases, targeting NKT cells in immune-related disorders can be achieved by preventing macrophage polarization with CD1d receptor antibody to block NKT cell function18, or by administration α-Galcer to activate NKT cell function, which may elicit anti-tumor immune responses19 or convert macrophages into an M2 type20. The potential of targeting NKT cells in wear particle induced osteolysis could be evaluated in established murine models such as the calvaria model21 or femoral particle infusion model22.
In conclusion, the cytokine expression profile of NKT cells/DCs exposed to UHMWPE particles could potentially increase M1 macrophage pro-inflammatory activity. Our current findings suggest that NKT cells/DCs exposed to UHMWPE wear particles may enhance tissue damage and promote periprosthetic osteolysis.
Acknowledgments
This work was supported by NIH grants 2R01AR055650, 1R01AR063717 and the Ellenburg Chair in Surgery at Stanford University.
References
- 1.Tsao A, Jones L, Lewallen D Implant Wear Symposium Clinical Work G. What patient and surgical factors contribute to implant wear and osteolysis in total joint arthroplasty? The Journal of the American Academy of Orthopaedic Surgeons. 2008;16 (Suppl 1):13. doi: 10.5435/00124635-200800001-00004. [DOI] [PubMed] [Google Scholar]
- 2.Purdue P, Koulouvaris P, Nestor B, Sculco T. The central role of wear debris in periprosthetic osteolysis. HSS journal : the musculoskeletal journal of Hospital for Special Surgery. 2006;2(2):102–113. doi: 10.1007/s11420-006-9003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Kaer L. NKT cells: T lymphocytes with innate effector functions. Current opinion in immunology. 2007;19(3):354–364. doi: 10.1016/j.coi.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Wu L, Gabriel C, Parekh V, Van Kaer L. Invariant natural killer T cells: innate-like T cells with potent immunomodulatory activities. Tissue antigens. 2009;73(6):535–545. doi: 10.1111/j.1399-0039.2009.01256.x. [DOI] [PubMed] [Google Scholar]
- 5.Zeissig S, Olszak T, Melum E, Blumberg R. Analyzing antigen recognition by Natural Killer T cells. Methods in molecular biology (Clifton, NJ) 2013;960:557–572. doi: 10.1007/978-1-62703-218-6_41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campbell P, Ma S, Yeom B, McKellop H, Schmalzried T, Amstutz H. Isolation of predominantly submicron-sized UHMWPE wear particles from periprosthetic tissues. Journal of biomedical materials research. 1995;29(1):127–131. doi: 10.1002/jbm.820290118. [DOI] [PubMed] [Google Scholar]
- 7.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends in immunology. 2002;23(11):549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 8.Rao A, Nich C, Dhulipala L, Gibon E, Valladares R, Zwingenberger S, Smith R, Goodman S. Local effect of IL-4 delivery on polyethylene particle induced osteolysis in the murine calvarium. Journal of biomedical materials research. Part A. 101(7):1926–1934. doi: 10.1002/jbm.a.34486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antonios J, Yao Z, Li C, Rao A, Goodman S. Macrophage polarization in response to wear particles in vitro. Cellular & molecular immunology. 10(6):471–482. doi: 10.1038/cmi.2013.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brigl M, Bry L, Kent S, Gumperz J, Brenner M. Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nature immunology. 2003;4(12):1230–1237. doi: 10.1038/ni1002. [DOI] [PubMed] [Google Scholar]
- 11.Kulkarni R, Villanueva A, Elawadli I, Jayanth P, Read L, Haeryfar SM, Sharif S. Costimulatory activation of murine invariant natural killer T cells by toll-like receptor agonists. Cellular immunology. 2012;277(1–2):33–43. doi: 10.1016/j.cellimm.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 12.Pearl JI, Ma T, Irani AR, Huang ZN, Robinson WH, Smith RL, Goodman SB. Role of the Toll-like receptor pathway in the recognition of orthopedic implant wear-debris particles. Biomaterials. 2011;32(24):5535–5542. doi: 10.1016/j.biomaterials.2011.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Potnis PA, Dutta DK, Wood SC. Toll-like receptor 4 signaling pathway mediates proinflammatory immune response to cobalt-alloy particles. Cell Immunol. 2013;282(1):53–65. doi: 10.1016/j.cellimm.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 14.Tudhope S, von Delwig A, Falconer J, Pratt A, Woolridge T, Wilson G, Isaacs J, Ng W-F. Profound invariant natural killer T-cell deficiency in inflammatory arthritis. Annals of the rheumatic diseases. 2009;69(10):1873–1879. doi: 10.1136/ard.2009.125849. [DOI] [PubMed] [Google Scholar]
- 15.van der Vliet H, von Blomberg B, Nishi N, Reijm M, Voskuyl A, van Bodegraven A, Polman C, Rustemeyer T, Lips P, van den Eertwegh A, et al. Circulating V(alpha24+) Vbeta11+ NKT cell numbers are decreased in a wide variety of diseases that are characterized by autoreactive tissue damage. Clinical immunology (Orlando, Fla) 2001;100(2):144–148. doi: 10.1006/clim.2001.5060. [DOI] [PubMed] [Google Scholar]
- 16.Miellot-Gafsou A, Biton J, Bourgeois E, Herbelin A, Boissier M-C, Bessis N. Early activation of invariant natural killer T cells in a rheumatoid arthritis model and application to disease treatment. Immunology. 2009;130(2):296–306. doi: 10.1111/j.1365-2567.2009.03235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim H, Kim H, Min H, Kim S, Park W, Park S, Chung D. NKT cells promote antibody-induced joint inflammation by suppressing transforming growth factor beta1 production. The Journal of experimental medicine. 2005;201(1):41–47. doi: 10.1084/jem.20041400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scheuplein F, Thariath A, Macdonald S, Truneh A, Mashal R, Schaub R. A humanized monoclonal antibody specific for invariant Natural Killer T (iNKT) cells for in vivo depletion. PloS one. 8(9) doi: 10.1371/journal.pone.0076692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hong S, Lee H, Jung K, Lee S, Lee S-J, Jun H, Kim Y, Song H, Bogen B, Choi I. Tumor cells loaded with α-galactosylceramide promote therapeutic NKT-dependent anti-tumor immunity in multiple myeloma. Immunology letters. 156(1–2):132–139. doi: 10.1016/j.imlet.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Horikoshi M, Goto D, Segawa S, Yoshiga Y, Iwanami K, Inoue A, Tanaka Y, Matsumoto I, Sumida T. Activation of Invariant NKT cells with glycolipid ligand α-galactosylceramide ameliorates glucose-6-phosphate isomerase peptide-induced arthritis. PloS one. 7(12) doi: 10.1371/journal.pone.0051215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rao A, Zwingenberger S, Valladares R, Li C, Lane Smith R, Goodman S, Nich C. Direct subcutaneous injection of polyethylene particles over the murine calvaria results in dramatic osteolysis. International orthopaedics. 37(7):1393–1398. doi: 10.1007/s00264-013-1887-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma T, Huang Z, Ren P-G, McCally R, Lindsey D, Smith R, Goodman S. An in vivo murine model of continuous intramedullary infusion of polyethylene particles. Biomaterials. 2008;29(27):3738–3742. doi: 10.1016/j.biomaterials.2008.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]


