Abstract
The new innovation of combining bazedoxifene plus conjugated estrogen provides a new opportunity for women’s health. The finding by the Women’s Health Initiative that the administration of conjugated equine estrogen alone to women in their 60s who have had a hysterectomy results in a decrease in the incidence of breast cancer and a drop in mortality was unanticipated but can now be exploited for another gain in women’s health. The issue to be considered is how postmenopausal women can improve their lifestyle to take advantage of conjugated equine estrogen alone therapy? The Food and Drug Administration approval of a combination of bazedoxifene /conjugated estrogen now provides an opportunity for postmenopausal women to reduce hot flashes and potentially selectively sensitize occult breast cancer cells to the apoptotic actions of estrogen. Clinical trials are proposed to advance women’s health and reduce the incidence of breast cancer.
Keywords: Breast cancer, apoptosis, hrt, chemoprevention
There are two recent clinical advances that are good news for women’s health. The first is the Food and Drug Administration (FDA) approval of a combination of conjugated equine estrogen (CEE) with the selective estrogen receptor modulator (SERM) bazedoxifene for the treatment of moderate to severe vasomotor symptoms (hot flashes) associated with the menopause, and the prevention of osteoporosis. The combination of CEE/bazedoxifene is a new innovation. The second advance is the report that CEE alone administered to hysterectomized women in their 60s, as one of the clinical trials in the Women’s Health Initiative (WHI), actually causes a decrease in the incidence of breast cancer1, a decrease in mortality from breast cancer and an overall decrease in mortality2,3. Women were treated for about six years but benefits remained for the six years after CEE was stopped3. It is proposed that CEE triggers estrogen induced apoptosis in the vulnerable estrogen-deprived ER positive breast cancer cells that are present in the breast ducts 5-10 years after menopause4. The question is whether we can build on these separate clinical advances and deploy translational research in the new biology of estrogen-induced apoptosis to conceive another paradigm to prevent breast cancer in postmenopausal women.
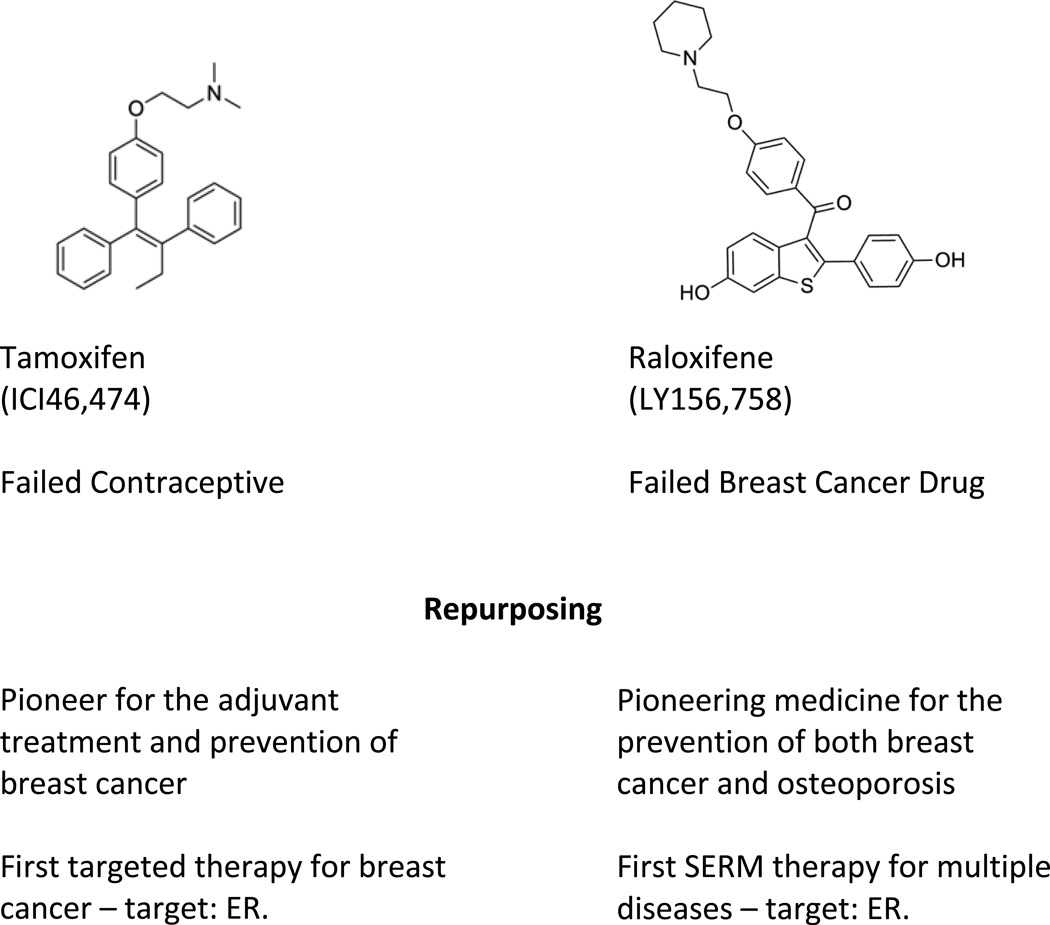
There are already two FDA approved options for the chemoprevention of breast cancer. These are the SERMs tamoxifen and raloxifene5,6 (Fig. 1) but side effects and compliance have proved to be major issues for healthy women who have an exacerbation of menopausal symptoms; compliance is reduced There is a low risk of breast cancer (6 per 1,000 women per year) even in high risk groups, so the benefit for recurrence is offset by menopausal side effects for the many. Now we must get smart if compliance is the problem that needs to be solved to maintain long term SERM therapy. Menopausal side effects must be controlled especially in women in their 50’s when quality of life is an issue. A SERM will block estrogen stimulated breast cancer cell growth at menopause but also exacerbate menopause symptoms. A SERM plus CEE, could be an answer.
Figure 1.
The early origins and repurposing of the two pioneering Selective Estrogen Receptor Modulators (SERMs) tamoxifen and raloxifene8.
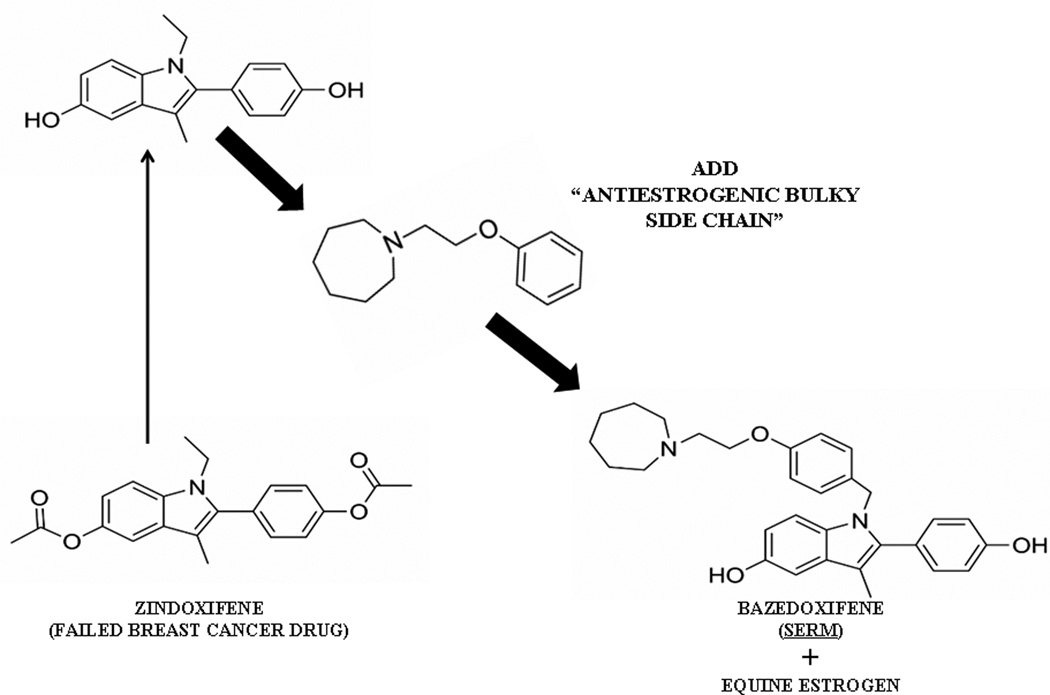
Bazedoxifene (Fig. 2) is a new SERM7,8, derived from an estrogenic derivative of the failed breast cancer drug zindoxifene9. The addition of a strategically placed bulky side chain makes bazedoxifene selectively estrogenic or antiestrogenic at target sites around a woman’s body10. Bazedoxifene has a pharmacology more like raloxifene than tamoxifen as bazedoxifene has both breast and uterine safety11. Indeed, bazedoxifene (Fig. 2) looks more like raloxifene than tamoxifen (Fig 1), but the addition of CEE to bazedoxifene ameliorates hot flashes by 85%, enhances building bone but the SERM blocks estrogen action in the uterus. Thus it is a subtle pharmacological balance between target sites for the SERM, the CEE, or in fact a combined estrogenic effect of both. Additive estrogenic effects on bone were first seen with both tamoxifen and raloxifene in the long term ovarictomized rat used to demonstrate that the combination of the SERM adds to the estrogens effect for building bone but blocks proliferation in the uterus12. Exactly what bazedoxifene/CEE does in women with the added benefits of preventing hot flashes.
Figure 2.
The building of bazedoxifene as a Selective Estrogen Receptor Modulator (SERM) from the failed breast cancer drug zindoxifene and combination with conjugated equine estrogen to create bazedoxifen/CEE8.
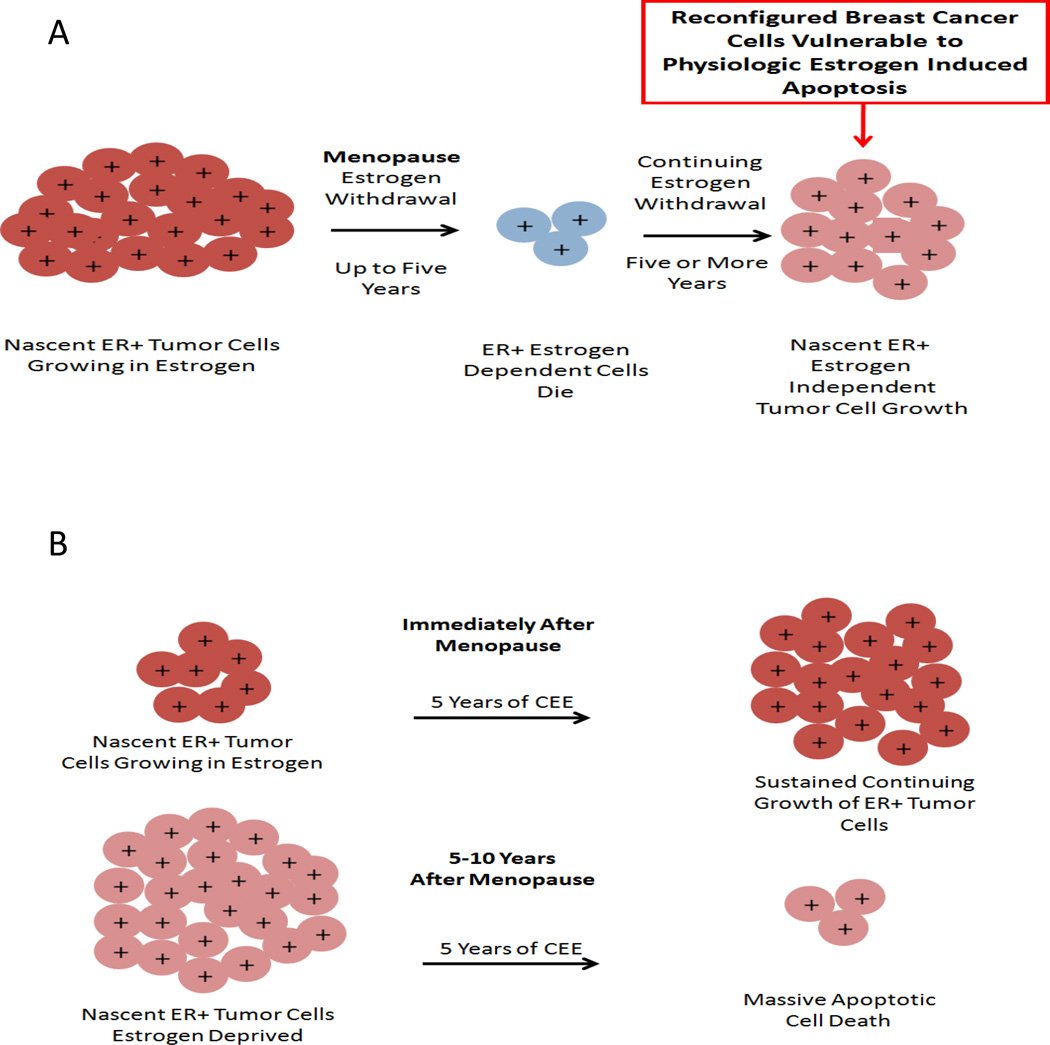
The value of CEE treatment to reduce the incidence of breast cancer appears to be dependent on a 5 year gap past the menopause when the ovary stops producing hormones and releasing eggs4,13,14,15. Estrogen deprivation is necessary to prepare the occult breast cancer cells, through clonal selection, to become vulnerable to estrogen induced apoptosis (Fig. 3). Breast cancer cells survive and grow based upon the availability of the growth signal estrogen. The dramatic decrease in estrogen at menopause causes the majority of ER positive cells that depend on estrogen to replicate, now to die. It is a simple Darwinian principle that reduced resources for survival (estrogen) results in decreased populations of cells. However, at a certain point in time the biological necessity of cancer cell survival prevails and adapted cells start to slowly repopulate in an estrogen-depleted environment. The process can be replicated in the laboratory, but the repopulating cells now respond to physiologic estrogen by triggering estrogen-induced apoptosis16,17. Surprisingly, even the long term exposure of estrogen-deprived breast cancer cells to months of physiologic estrogen that initially causes immediate and catastrophic cell death only slowly permits the surviving cells to recover18. The cancer cells that now survive in estrogen do not expand dramatically as a population18. The resulting cancer cell population does not appear to respond to estrogen with growth alone but there is now a balance of replication and apoptosis to maintain a stable population18. Thus it is not implausible that this novel biological mechanism of estrogen induced apoptosis in estrogen deprived cells results in microscopic tumors being destroyed in patients during CEE therapy.
Figure 3.
The success of estrogen replacement therapy is dependent on the menopausal status of a woman. (A) Estrogen withdrawal in postmenopausal women causes ER positive dependent cells to die but some cells continue to grow independent of estrogen. (B) Treatment of women immediately after menopause with CEE results in sustained growth of nascent ER positive tumors, whereas treatment 5 years after menopause causes apoptotic cell death (Obiorah I and Jordan VC 2013 Menopause 20:372–382 reproduced with permission)
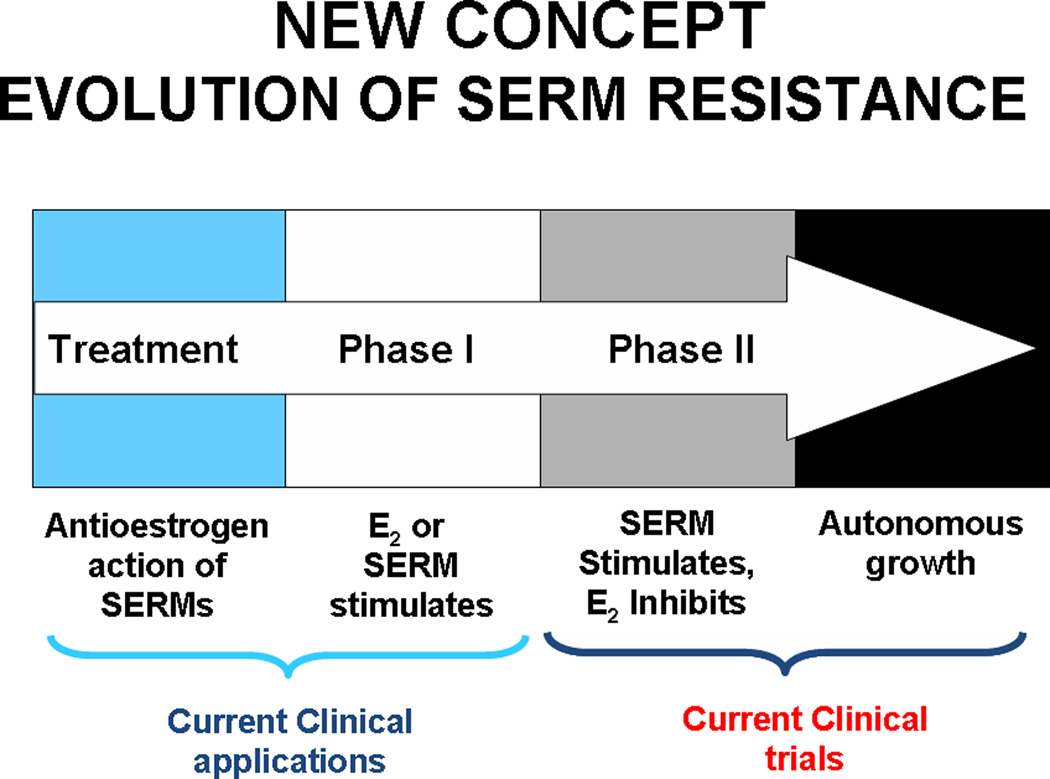
In athymic mice, the process of drug resistance to tamoxifen (antiestrogen) evolves through two distinct phases of acquired resistance over a five year period19,20 (Fig. 4). The initial phase (Phase I) of drug resistance results in tamoxifen stimulated cancer cell growth similar to estrogen . This process of cancer cell selection takes 1–2 years. In Phase II of tamoxifen resistance, tamoxifen by itself promotes tumor growth and estrogen alone causes tumor regression through apoptosis. Raloxifene can result in the same acquired resistance21 but it takes longer. So, the clinically available SERMs in an estrogen-deprived environment eventually distil a cancer cell population that will be vulnerable to estrogen induced apoptosis. The small tumors disappear with estrogen therapy but the larger tumors, with greater population plasticity to select a surviving cell, will repopulate towards the estrogenic growth signal. An estrogen stimulated tumor results as a recurrence.
Figure 4.
Evolution of acquired SERM resistance. After long-term treatment with SERMs (1–2 years in vivo), initially responsive ER-positive tumors become resistant to treatment and are stimulated by SERMs (Phase I resistance) as well as by E2. After long-term transplantation into SERM-treated animal (5+years), breast tumor growth is inhibited by E2, though still stimulated by SERMs (Phase II of resistance). This process with SERMs in vivo is replicated with estrogen deprivation with MCF-7 breast cancer cells in vitro; cells initially start to grow spontaneously but estrogen still induces growth (hypersensitivity). This is Phase I. Long-term estrogen deprivation causes spontaneous growth in culture but apoptosis with physiologic estrogens both in vitro and in vivo (Phase II) (Jordan VC 2004 Cancer Cell 5:207–213 reproduced with permission ).
The proposition to be considered here is to employ a 5 year course of bazedoxifene/CEE to avoid menopausal symptoms, and to build bone with uterine and breast safety. The antiestrogenic effects of bazedoxifene would be anticipated to drive selection of estrogen deprived ER + malignant cell population in the breast ducts. The long course of bazedoxifene (perhaps 5 years) would then be stopped and a course of a few weeks of CEE alone would be used to “purge” vulnerable cells in the ducts by inducing nascent tumor eradication by triggering apoptosis. Continuous estrogen for years may not be necessary to achieve prevention of breast cancer in a vulnerable cell population primed to die quickly.
It is therefore reasonable to advance the aforementioned propositions with clinical trials validation because there is a concern that the dominance of bazedoxifene may be insufficient in this context to drive cell selection to estrogen vulnerable population in the breast. As a polyhydroxylated molecule, bazedoxifene (Fig. 2) is very similar to the structure of raloxifene (Fig. 1). The Study of Tamoxifen and Raloxifene (STAR) trial teaches us important lessons about the effectiveness of 60mg daily of raloxifene on breast chemoprevention during 5 years of treatment5 (and after treatment stops6). While tamoxifen (20 mg daily) clearly drives cell selection during 5 years of treatment to estrogen vulnerable cancer cells like the laboratory model19, raloxifene does not6. The sustained antitumor effect does not persist in patients when raloxifene stops6, so it must be given longer to prevent breast cancer - but how long? Laboratory studies demonstrate it is possible to produce the expected selection to the estrogen vulnerable cancer cell population with raloxifene eventually21. Unfortunately the principle of maintained antitumor effects has only been demonstrated clinically with tamoxifen, ie, chemoprevention is maintained after long term tamoxifen in STAR but chemoprevention fails after raloxifene is stopped6. Tamoxifen has produced Phase II resistance in microscopic tumors after 5 years of treatment so a woman’s own estrogen now kills the vulnerable microscopic breast cancer cells. By contrast, raloxifene, it appears, has only caused the selection of Phase I resistance so a woman’s own estrogen enhances tumor growth after raloxifene stops. Be that as it may, there is a final complication when comparing bazedoxifene with raloxifene. Bazedoxifene/CEE only uses 20 mg daily of the SERM, ie only a third of the dose of raloxifene (60mg daily) in STAR. Also the combination of estrogen with bazedoxifene, a competitive inhibitor of estrogen action at the ER creates a situation where they must compete so bazedoxifen can successfully select a vulnerable populations of cells. Although we do not know whether bazedoxifene will be dominant, the good news is that compliance with bazedoxifene/CEE may be superior. Compliance is key to maintain SERM selection pressure. Raloxifene has poor compliance at higher doses because of the significant side effects. Rapid excretion of raloxifene combined with poor compliance creates a weak environment for breast cancer cells to be selected to vulnerable populations. It must be stressed, however, that laboratory studies demonstrate that constant raloxifene in an estrogen free environment does select cells that will be triggered to die when estrogen is re-introduced.22
Only clinical trials will determine the dose and timing of bazedoxifene and estrogen, but the medical advance could be profound. Resolution of menopausal symptoms with a reduction in breast cancer would be a major advance in public health.
Acknowledgements
I wish to thank Russell McDaniel for his assistance and help with the preparation of this manuscript. I am particularly grateful to Dr. Margery Gass for her contributions and counsel during the preparation of this paper. This work (VCJ) was supported by the Department of Defense Breast Program under Award number W81XWH-06-1-0590 Center of Excellence; the ‘Susan G. Komen For The Cure Foundation’ under Award number SAC100009 and the Lombardi Comprehensive Cancer Center Support Grant (CCSG) Core Grant NIH P30 CA051008. The views and opinions of the author(s) do not reflect those of the US Army or the Department of Defense.
Footnotes
No conflicts of interest are declared.
References
- 1.Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 2.LaCroix AZ, Chlebowski RT, Manson JE, et al. Health outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy: a randomized controlled trial. JAMA. 2011;305:1305–1314. doi: 10.1001/jama.2011.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson GL, Chlebowski RT, Aragaki AK, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women's Health Initiative randomised placebo-controlled trial. Lancet Oncol. 2012;13:476–486. doi: 10.1016/S1470-2045(12)70075-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Obiorah I, Jordan VC. 2012 NAMS/PFIZER- Wulf H. Utian endowed lecture. The scientific rationale for a delay after menopause in the use of conjugated equine estrogens in postmenopausal women that causes a reduction in breast cancer incidence and mortality. Menopause. 2013;20:372–382. doi: 10.1097/GME.0b013e31828865a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vogel VG, Costantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727–2741. doi: 10.1001/jama.295.23.joc60074. [DOI] [PubMed] [Google Scholar]
- 6.Vogel VG, Costantino JP, Wickerham DL, et al. Update of the National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene (STAR) P-2 Trial: Preventing breast cancer. Cancer Prev Res (Phila) 2010;3:696–706. doi: 10.1158/1940-6207.CAPR-10-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maximov PY, Lee TM, Jordan VC. The discovery and development of selective estrogen receptor modulators (SERMs) for clinical practice. Curr Clin Pharmacol. 2013;8:135–155. doi: 10.2174/1574884711308020006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McDaniel RE, Maximov PY, Jordan VC. Estrogen-mediated mechanisms to control the growth and apoptosis of breast cancer cells: a translational research success story. In: Litwack G, editor. Hormones and Breast Cancer. London: Academic Press; 2013. pp. 1–49. [DOI] [PubMed] [Google Scholar]
- 9.Robinson SP, Koch R, Jordan VC. In vitro estrogenic actions in rat and human cells of hydroxylated derivatives of D16726 (zindoxifene), an agent with known antimammary cancer activity in vivo. Cancer Res. 1988;48:784–787. [PubMed] [Google Scholar]
- 10.Jordan VC. Chemoprevention of breast cancer with selective oestrogen-receptor modulators. Nat Rev Cancer. 2007;7:46–53. doi: 10.1038/nrc2048. [DOI] [PubMed] [Google Scholar]
- 11.Pinkerton JV, Archer DF, Utian WH, et al. Bazedoxifene effects on the reproductive tract in postmenopausal women at risk for osteoporosis. Menopause. 2009;16:1102–1108. doi: 10.1097/gme.0b013e3181a816be. [DOI] [PubMed] [Google Scholar]
- 12.Jordan VC, Phelps E, Lindgren JU. Effects of anti-estrogens on bone in castrated and intact female rats. Breast Cancer Res Treat. 1987;10:31–35. doi: 10.1007/BF01806132. [DOI] [PubMed] [Google Scholar]
- 13.Prentice RL, Chlebowski RT, Stefanick ML, et al. Conjugated equine estrogens and breast cancer risk in the Women's Health Initiative clinical trial and observational study. Am J Epidemiol. 2008;167:1407–1415. doi: 10.1093/aje/kwn090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prentice RL, Chlebowski RT, Stefanick ML, et al. Estrogen plus progestin therapy and breast cancer in recently postmenopausal women. Am J Epidemiol. 2008;167:1207–1216. doi: 10.1093/aje/kwn044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beral V, Reeves G, Bull D, Green J. Breast cancer risk in relation to the interval between menopause and starting hormone therapy. J Natl Cancer Inst. 2011;103:296–305. doi: 10.1093/jnci/djq527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewis JS, Osipo C, Meeke K, Jordan VC. Estrogen-induced apoptosis in a breast cancer model resistant to long-term estrogen withdrawal. J Steroid Biochem Mol Biol. 2005;94:131–141. doi: 10.1016/j.jsbmb.2004.12.032. [DOI] [PubMed] [Google Scholar]
- 17.Lewis JS, Meeke K, Osipo C, et al. Intrinsic mechanism of estradiol-induced apoptosis in breast cancer cells resistant to estrogen deprivation. J Natl Cancer Inst. 2005;97:1746–1759. doi: 10.1093/jnci/dji400. [DOI] [PubMed] [Google Scholar]
- 18.Fan P, Agboke F, McDaniel R, et al. Inhibition of c-Src blocks oestrogen-induced apoptosis and restores oestrogen-stimulated growth in long-term oestrogen-deprived breast cancer cells. Eur J Cancer. 2013 doi: 10.1016/j.ejca.2013.10.001. E pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yao K, Lee ES, Bentrem DJ, et al. Antitumor action of physiological estradiol on tamoxifen-stimulated breast tumors grown in athymic mice. Clin Cancer Res. 2000;6:2028–2036. [PubMed] [Google Scholar]
- 20.Jordan VC. The 38th David A. Karnofsky lecture: the paradoxical actions of estrogen in breast cancer--survival or death? J Clin Oncol. 2008;26:3073–3082. doi: 10.1200/JCO.2008.17.5190. [DOI] [PubMed] [Google Scholar]
- 21.Balaburski GM, Dardes RC, Johnson M, et al. Raloxifene-stimulated experimental breast cancer with the paradoxical actions of estrogen to promote or prevent tumor growth: a unifying concept in anti-hormone resistance. Int J Oncol. 2010;37:387–398. doi: 10.3892/ijo_00000687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu H, Lee ES, Gajdos C, et al. Apoptotic action of 17beta-estradiol in raloxifene-resistant MCF-7 cells in vitro and in vivo. J Natl Cancer Inst. 2003;95:1586–1597. doi: 10.1093/jnci/djg080. [DOI] [PubMed] [Google Scholar]