Abstract
Objectives
To develop a fully-implanted, intramuscular, bipolar, myoelectric signal recording electrode (IM-MES) for functional electrical stimulation (FES), prosthetic myoelectric control, and other permanently implantable systems.
Materials and Methods
An existing fully-implanted intramuscular stimulating electrode was modified at each end to allow bipolar recording. The design change also required a modification of the implantation method. Mechanical and in vivo testing was performed on the novel components of the electrode. The first clinical application is also described.
Results
The electrode design modifications did not create any areas of excess mechanical strain on the wires at the distal end where the leads were wound into electrode surfaces. In vivo testing showed that the IM-MES electrode recorded myoelectric signals that were equivalent to an existing epimysial MES electrode. The modified implantation method was simple to implement. The IM-MES electrode was used in an upper extremity FES system in an individual with a spinal cord injury, and provided signals that were suitable for a command signal.
Conclusions
A fully-implanted, bipolar intramuscular recording electrode (IM-MES) was developed. Implantation of the IM-MES is straightforward and almost any muscle can be targeted. Testing has been performed to demonstrate the suitability of the IM-MES electrode for clinical use. Initial clinical applications were successful.
Keywords: Myoelectric recording, EMG, electrode, functional electrical stimulation (FES), implantable system
I. Introduction
Myoelectric signal (MES) recording electrodes are often used in the control of prosthetic upper limbs using surface electrodes (1, 2), and implanted MES electrodes are also used in functional electrical stimulation (FES) systems as sources of control signals. For example, we have previously used epimysial MES electrodes in our stimulation systems (3, 4) (Figure 1) that are sutured to the surface of a muscle. The epimysial recording electrodes, like epimysial electrodes for stimulation, can be difficult to implant if the target muscle is small or located deep within a limb. Epimysial electrodes also require large surgical incisions to expose the target muscles, and their implantation can be time-consuming if many electrodes are being implanted.
Figure 1.
Epimysial MES electrode, which is sutured to the surface of a muscle and connected to an implanted stimulator/telemeter.
The earlier development of an intramuscular stimulating electrode (IM-STIM) has achieved the goals of being able to access small muscles (e.g., intrinsic hand muscles) and deeper muscles (e.g., in the forearm) while minimizing incision size and implantation time (5). The IM-STIM electrode (Figure 2) consists of a pair of helically coiled insulated cables placed in silicone tubing. At one end, the electrode is terminated with a standard pin connector for connection to an implantable stimulator. At the stimulating end, the insulation is removed from both cables and the wire is wrapped around the outside of the silicone tubing to form the stimulating surface. The electrode is stabilized in the muscle tissue by a polypropylene anchor. The IM-STIM electrode has been shown to have excellent tissue response characteristics (6) and long-term durability (7).
Figure 2.
Intramuscular stimulating (IM-STIM) electrode, with a polypropylene anchor, 316LVM stainless steel stimulating surface, and a helically-wound lead in silicone tubing.
Just as the development of the intramuscular stimulating electrode provided advantages over the epimysial stimulating electrodes, we anticipated that modifying the IM-STIM design to create an intramuscular bipolar MES electrode (IM-MES) would provide similar benefits (smaller incisions, decreased implantation time, access to smaller and deeper muscles) over the epimysial MES electrode. Implantable myoelectric sensors are also being developed by other researchers (8).
II. Methods
A. Design
Lead
The lead uses the same configuration as the stimulating electrodes and the epimysial MES electrode. Two separate conductors made of fluoropolymer-coated, 7-strand type 316LVM stainless steel wire are helically wound in tandem and placed inside silicone tubing (5, 9).
Connector
Any desired connector can be used to terminate the leads at the stimulator end, depending upon the implanted recording device being used. For example, an industry-standard bipolar IS-1 connector or the CWRU Y-branch connector (7) can be used.
Anchor
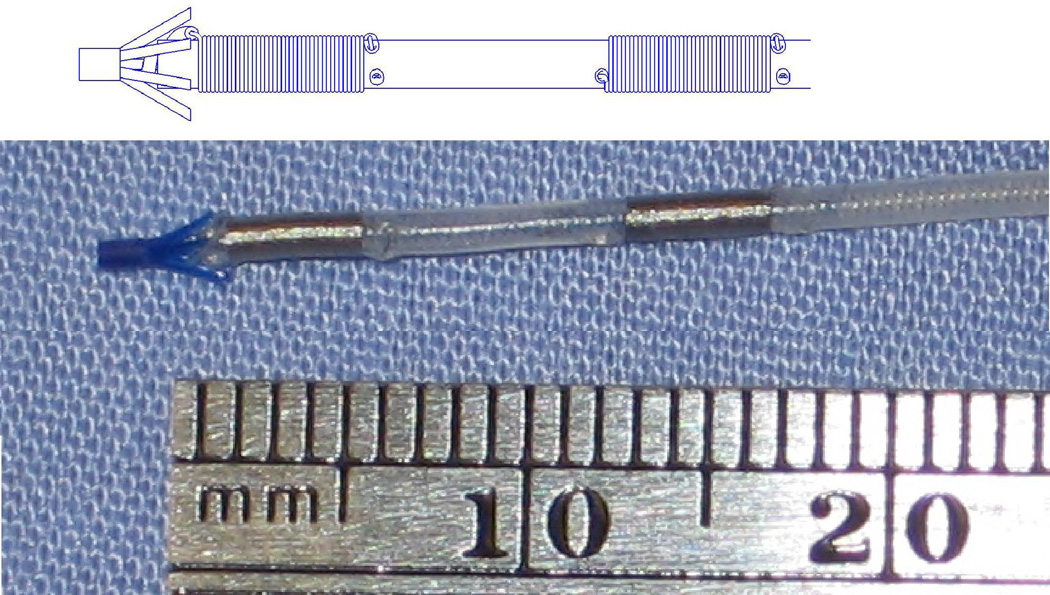
The anchor is the same as the IM-STIM electrode - a 6-barb polypropylene anchor with a central polypropylene core that is heated to melt into the coils and lock the anchor into the lead (Figure 3) (5).
Figure 3.
Schematic (upper) and photo (lower) of intramuscular MES electrode, showing the polypropylene anchor, two 316LVM stainless steel recording areas, and a helically-wound lead in silicone tubing.
Recording surface
On the recording end of the electrode, one of the two conductors is deinsulated (to provide one of the recording surfaces) where it exits the tube and is then wrapped around the outside of the tubing (Figure 3) for 4 mm. It then is inserted back into the tubing. The second conductor is fed back inside the end of the tubing in a straight segment to a small hole in the tubing 10 mm from the end. It then exits the tubing, is deinsulated (providing the second recording surface), and then is wrapped around the tubing for 4 mm and inserted back into the tubing. The insertion holes in the tubing are sealed with silicone adhesive.
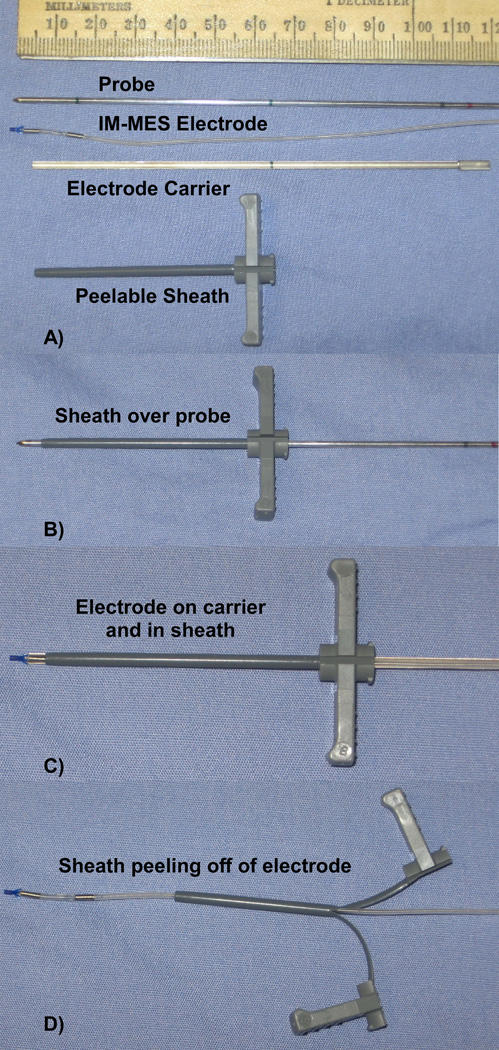
Insertion Tool
The IM-STIM insertion tools (5) were modified to accommodate the larger IS-1 connector or Y-branch connector. A commercially-available peelable sheath (EnPath Medical, 5 FR, 6 cm long) is now used in place of a metal sheath (Figure 4). To insert the electrode, an insulated metal probe is inserted to the desired location. The peelable sheath is then slid over the probe until it reaches a mark on the probe, which indicates that the tip of the sheath is aligned with the tip of the probe (Figure 4B). The probe is then removed. The IM-MES electrode is loaded onto the electrode carrier, and is loaded into the sheath until a mark on the carrier lines up with the end of the sheath, which indicates that the electrode tip has exited the sheath at the proper location (Figure 4C). The carrier is then removed, and the sheath is pulled back. The sheath is then peeled apart to remove it from the electrode (Figure 4D), leaving the electrode inserted in the desired location.
Figure 4.
A) The IM-MES insertion tool components. B) After the probe is placed in the muscle at the desired location, the sheath is placed over the probe until it lines up with the tip. C) The probe is then removed, and the electrode is placed in a carrier and inserted into the sheath until it lines up with the tip. D) The carrier is removed, and the sheath is pulled back and peeled apart to remove it from the electrode, leaving the electrode in the muscle.
B. Mechanical Testing
The section of the tip where the 2nd conductor is bent and fed back into the tubing was an area of concern from a mechanical standpoint. The bent area may have too high a strain (causing cracks or deformations). The straight section of wire that runs inside the tubing from the distal end to the second recording area may have a fatigue failure after many bends. The 7-strand type-316 stainless steel wire from an electrode was analyzed mechanically and optically (9). Tensile failures resulting from uniaxial loads were studied using a standard tensile tester (9). Fatigue failures were evaluated using cyclic strain-controlled bending around different diameter mandrels (1.15 – 12.6 mm) using a Flex tester(9). Nineteen wire samples were placed in the tester and cycled until fracture occurred, or until at least a million cycles were completed. In addition, scanning electron microscope photographs were taken of the section of one electrode where the wire areas have the sharpest bends. These photographs were evaluated for signs of cracks or deformations.
C. Animal Testing
Approval was received for this study from the Case Institutional Animal Care and Use Committee. A nerve cuff electrode (10) was placed on the peroneal nerve of an anesthetized cat. An IM-MES electrode was placed in the tibialis anterior muscle (see insertion method above), and an epimysial MES electrode was sutured to the surface of the muscle (Figure 5). The centers of the recording areas for each MES electrode were 10 mm apart. Each electrode was connected to an EMG amplifier (Model 1902, Cambridge Electronic Design, Cambridge, U.K.), and the signals were amplified (gain of 990), low pass filtered at 1 kHz, with a notch filter at 60 Hz. A trigger signal from the stimulator was connected to the amplifier to identify when a stimulus pulse was sent. The amplifier was blanked for 1.5 msec after the stimulation pulse. The MES signals were sampled at 2.4 kHz. A comparison was made of the M-wave signals recorded by the two electrode types when the nerve cuff electrode was stimulated with a balanced-charge, biphasic waveform, with a current amplitude of 1.4 mA, a pulse width of 100 usec, and a frequency of 4 Hz. Additionally, a 200 Hz sinusoidal signal was injected by a pair of needle electrodes inserted at the ends of the muscle, and the resulting waveform was recorded by the electrodes. The 200 Hz signal represented a pseudo-MES signal, since this frequency is within the typical MES power spectrum (11), and allowed a comparison of the signals recorded by the epimysial and intramuscular MES electrodes in this frequency range.
Figure 5.
Epimysial and IM-MES electrodes placed in the tibialis anterior muscle in a cat to compare the recorded myoelectric signals.
D. Clinical Usage
Amendments were made to a local IRB protocol and an existing FDA Investigational Device Exemption (IDE) for an implanted FES system to allow for usage of the IM-MES electrode in human subjects. Written informed consent was obtained from the research subjects. The initial IM-MES electrode application was as part of a high tetraplegia neuroprosthesis (12) for an individual with a high cervical level spinal cord injury. The individual received two implanted stimulator/telemeters, each of which had two MES channels for command signals (4). Two IM-MES electrodes were implanted successfully in the trapezius muscles (one in the left trapezius and one in the right trapezius), since the trapezius could still be controlled voluntarily. The MES electrodes were then connected to the implanted stimulator/telemeters. The circuitry in the implanted stimulator/telemeter can be set to amplification gains of 200 to 8000. In this subject, the gain was set to 200 for each MES channel. The MES signal was then bandwidth filtered (100 Hz – 1 kHz) full-wave rectified and bin-integrated. The integration was done in 30 msec bins. The integrated signal was then sampled and telemetered to an external control unit (4).
III. Results
A. Electrode Fabrication
The IM-STIM electrode fabrication protocols were modified to incorporate the design changes for the IM-MES electrode as described above. Existing fabrication equipment was used (no additional equipment was necessary). Sample IM-MES electrodes were fabricated that met the design specifications listed above. The electrode impedance at a 1 kHz signal with the electrode placed in a 0.9% saline bath was 400 ohms.
B. Mechanical Testing
Fatigue tests on the 7-strand type-316 stainless steel wire showed that when the wire was wrapped around mandrels with a diameter of greater than 9.8 mm, the wire did not fail after more than 1.6 million cycles (9). This indicates that fatigue failure should not be a concern, since the electrode tip is inside the muscle and is not subjected to sharp bending forces as the muscle contracts. A similar wire wrapping configuration has been used in the intramuscular stimulating electrode (5) in human subjects since 1995 without a report of fatigue failure (7).
Scanning electron microscope (SEM) views of the sharp bend in the tip showed no cracks or deformations (Figure 6) that would result from excessive strain.
Figure 6.
SEM of bend in IM-MES electrode tip. ‘B’ is an enlargement of the box in ‘A’. ‘C’ is an enlargement of the box in ‘B’. No cracks or deformations were observed.
C. Animal Testing
The IM-MES electrode was inserted easily in the desired location. The epimysial MES and IM-MES electrodes recorded similar M-wave response signals when the nerve was stimulated by the nerve cuff electrode (Figure 7). The two electrodes also had nearly identical responses to an injected 200 Hz signal (Figure 8).
Figure 7.
M-wave response of IM (solid line) and epimysial (dotted line) MES electrodes in a cat tibialis anterior muscle during nerve cuff stimulation of the peroneal nerve. The stimulus pulse occurred at t=0. A trigger signal from the stimulator to the amplifier allowed the amplifier to blank the signal for 1.5 ms after the stimulus pulse to eliminate a stimulus artifact in the signal.
Figure 8.
Recordings from epimysial (dotted line) & IM-MES (solid line) electrodes in a cat tibialis anterior muscle while a 1-volt, 200 Hz sinusoidal signal (dashed line) was injected across the muscle. The injected signal is plotted at a 1/1000 scale for comparison to the recorded signals.
D. Clinical Usage
Sample recordings of the myoelectric signals from the implanted IM-MES and epimysial MES electrodes in a human subject are shown in Figure 9. The individual was able to produce a signal on each MES electrode, and in combinations, allowing for a variety of control algorithms to be used. During the setup of a typical control algorithm, the MES signals are optimized by setting the amplifier gains to obtain the maximal signal without saturation. In addition, minimum threshold values are set to reduce the effect of any crosstalk between the MES signals from unintentional co-activation of the muscles being recorded. Subsequently, the IM-MES electrode has been used in a lower extremity FES application, in which the myoelectric signal is used to trigger stimulation patterns for walking in individuals with incomplete spinal cord injuries (13).
Figure 9.
Recorded myoelectric signals from intramuscular and epimysial MES electrodes implanted in a human subject. The electrodes are connected to an implanted stimulator/telemeter, which amplifies, filters, rectifies and integrates the MES signal (in 30 msec bins). The integrated signal is then sampled and telemetered to an external control unit. The subject was instructed to activate each muscle separately, then in pairs, as shown at the bottom of the figure.
IV. Discussion and Conclusions
The monopolar intramuscular stimulating electrode (IM-STIM) was modified into a bipolar intramuscular MES recording electrode (IM-MES). The manufacturing changes did not induce any excessive mechanical strains, thus the IM-MES electrode should have a longevity similar to the IM-STIM electrode. Replacing the solid tube insertion sheath with a plastic peelable sheath for the insertion tool did not add any additional steps to the insertion method. The animal testing and clinical usage confirmed that the insertion procedure for the IM-MES electrode is simpler and quicker than the epimysial MES electrode insertion technique (since it does not require multiple sutures at the muscle site), while at the same time requiring a smaller incision and allowing access to deeper muscles for recording.
The IM-MES electrodes have been shown to record signals that are equivalent to those recorded with epimysial MES electrodes. The slight differences between the signals recorded by the two electrode types in Figures 7 and 8 can be attributed to the difference in locations for the electrodes (one on the surface of the muscle and one inside the muscle). The design changes do not involve changes in the materials used by existing electrodes and do not decrease the expected longevity of the electrode. Since the IM-MES functions equivalently to existing MES electrodes while simplifying surgical implantation, the IM-MES electrode is an acceptable alternative to the epimysial MES electrode for use in human applications. The initial use of the IM-MES electrode in clinical FES applications has shown that the signals recorded by the electrode are suitable for use as a command signal.
Besides the applications of the IM-MES electrode in upper and lower extremity FES systems, the electrode could also be used as an implanted control source for prosthetic limbs, where myoelectric signals from deeper muscles or muscles further away from the socket could allow for control of prosthetic limbs with multiple degrees of freedom. Similarly, the electrode could prove useful in other applications that would benefit from the consistent and repeatable signals from permanently implanted EMG recordings, such as the control of robotic assistive devices or exoskeletons. Also, the IM-MES electrode could be used for monitoring motor output for implanted devices intended to treat tremor or other movement disorders.
Acknowledgments
This research was supported by NIH NINDS Grant No. N01-NS-5-2365, and in part by NIH-NIBIB Grant No.EB-001740.
Conflict of Interest statement: This research was supported by a grant from NIH that contributed to the salary of the authors. No stocks or patents were involved in this research. Two of the authors (Memberg, Stage) may potentially receive royalties since the electrode fabrication procedures were licensed to Ardiem Medical.
Footnotes
Authorship statement: Mr. William Memberg and Dr. Robert Kirsch designed and conducted the study. Mr. William Memberg and Mr. Thomas Stage designed the electrode modifications. Mr. William Memberg prepared the manuscript draft, and Dr. Robert Kirsch and Mr. Thomas Stage provided revisions to the manuscript. All authors approved the final manuscript. NIH provided funding for the study.
References
- 1.Fougner A, Stavdahl O, Kyberd PJ, Losier YG, Parker Pa. Control of upper limb prostheses: terminology and proportional myoelectric control-a review. IEEE Trans Neural Syst Rehabil Eng. 2012 Sep;20(5):663–677. doi: 10.1109/TNSRE.2012.2196711. [DOI] [PubMed] [Google Scholar]
- 2.Parker PA, Scott RN. Myoelectic Control of Prostheses. CRC Crit. Rev. Biomed. Eng. 1986 Jan;Vol 13, I(4):283–310. [PubMed] [Google Scholar]
- 3.Kilgore KL, Hoyen HA, Bryden AM, Hart RL, Keith MW, Peckham PH. An implanted upper-extremity neuroprosthesis using myoelectric control. J Hand Surg [Am] 2008;33(4):539–550. doi: 10.1016/j.jhsa.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hart RL, Bhadra N, Montague FW, Kilgore KL, Peckham PH. Design and testing of an advanced implantable neuroprosthesis with myoelectric control. IEEE Trans Neural Syst Rehabil Eng. 2011 Feb;19(1):45–53. doi: 10.1109/TNSRE.2010.2079952. [DOI] [PubMed] [Google Scholar]
- 5.Memberg WD, Peckham PH, Keith MW. A surgically-implanted intramuscular electrode for an implantable neuromuscular stimulation system. IEEE Trans Rehabil Eng. 1994 Jun;2(2):80–91. [Google Scholar]
- 6.Akers JM, Peckham PH, Keith MW, Merritt K. Tissue response to chronically stimulated implanted epimysial and intramuscular electrodes. IEEE Trans Rehabil Eng. 1997;5(2):207–220. doi: 10.1109/86.593301. [DOI] [PubMed] [Google Scholar]
- 7.Kilgore KL, Peckham PH, Keith MW, Montague FW, Hart RL, Gazdik MM, et al. Durability of implanted electrodes and leads in an upper-limb neuroprosthesis. J Rehabil Res Dev. 2003;40(6):457–468. doi: 10.1682/jrrd.2003.11.0457. [DOI] [PubMed] [Google Scholar]
- 8.Weir RF, Troyk PR, DeMichele GA, Kerns DA, Schorsch JF, Maas H. Implantable myoelectric sensors (IMESs) for intramuscular electromyogram recording. IEEE Trans. Biomed. Eng. 2009 Jan;56(1):159–171. doi: 10.1109/TBME.2008.2005942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewandowski JJ, Varadarajan R, Smith B, Tuma C, Shazly M, Vatamanu LO. Tension and fatigue behavior of 316LVM 1x7 multi-strand cables used as implantable electrodes. Mater. Sci. Eng. A. 2008 Jul 15;486(1–2):447–454. doi: 10.1016/j.msea.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naples GG, Mortimer JT, Scheiner A, Sweeney JD. A spiral nerve cuff electrode for peripheral nerve stimulation. IEEE Trans. Biomed. Eng. 1988 Nov;35(11):905–916. doi: 10.1109/10.8670. [DOI] [PubMed] [Google Scholar]
- 11.Basmajian JV, De Luca CJ. Muscles Alive. Their Funct. Reveal. by Electromyogr. 5th ed. Baltimore, Md: Williams and Wilkins; 1985. Description and Analysis of the EMG Signal; pp. 65–100. [Google Scholar]
- 12.Bryden AM, Kilgore KL, Kirsch RF, Memberg WD, Peckham PH, Keith MW. An implanted neuroprosthesis for high tetraplegia. Top. Spinal Cord Inj. Rehabil. 2005;10(3):38–52. [Google Scholar]
- 13.Dutta A, Kobetic R, Triolo RJ. Ambulation after incomplete spinal cord injury with EMG-triggered functional electrical stimulation. IEEE Trans. Biomed. Eng. 2008 Feb;55(2):791–794. doi: 10.1109/TBME.2007.902225. [DOI] [PubMed] [Google Scholar]