SUMMARY
Bacteria harbor both ferrous and ferric iron transporters. We now report that infection of macrophages and mice with a Salmonella enterica Typhimurium strain containing an inactivated feoB-encoded ferrous iron transporter results in increased bacterial replication, compared to infection with wild-type. Inactivation of other cation transporters, SitABCD or MntH, did not increase bacterial replication. The feoB mutant strain does not have an intrinsically faster growth rate. Instead, increased replication correlated with increased expression in macrophages of the fepB-encoded bacterial ferric iron transporter and also required siderophores, which capture ferric iron. Co-infection of mice with wild type and a feoB mutant strain yielded a different outcome: FeoB is clearly required for tissue colonization. In co-infected primary mouse macrophages, FeoB is required for S. Typhimurium replication if the macrophages were IFNγ treated and contain phagocytosed erythrocytes, a model for hemophagocytosis. Hemophagocytes are macrophages that have engulfed erythrocytes and/or leukocytes and can harbor Salmonella in mice. These observations suggest that Salmonella acquires ferrous iron from hemophagocytic macrophages.
Keywords: Salmonella, macrophages, iron, hemophagocytosis, bacterial pathogenesis, host-pathogen interaction
INTRODUCTION
Microbial pathogens encode not only genes required for virulence, but also genes that limit virulence, so-called anti-virulence factors. Genes that limit virulence reduce pathogen growth in host tissues or decrease the dose required for host death (Baek CH, 2009; Foreman-Wykert AK, 2003; Gal-Mor O, 2008). In laboratory screens, genes that reduce virulence have been identified based on hyper-virulence phenotypes in loss-of-function mutant strains upon single-infection of mice or macrophages (Ho TD, 2001). Indicators of hyper-virulence include colonization advantages, decreased survival time of the host, and reduced lethal or infectious dose (Baek CH, 2009; Foreman-Wykert AK, 2003; Gal-Mor O, 2008). While multiple examples of pathogen loci that limit virulence have been described (Baek CH, 2009; Gal-Mor O, 2008; Pilonieta MC, 2012), it remains largely unknown as to how they limit virulence and why mutations in these loci do not come to dominate the population.
The Gram-negative bacterium Salmonella enterica acts as a stealth pathogen to colonize tissues in human typhoid fever and in murine models of typhoid fever. During systemic infection, Salmonella evades humoral immunity by living within host cells (Tsolis RM, 2008). One factor that may make it difficult for the host to respond to Salmonella is that bacterial replication is limited in vivo within macrophages. In the mouse spleen and liver, individual macrophages typically harbor only four to five bacterial rods (Nix, 2007; Sheppard M, 2003). Low bacterial numbers likely reflect a combination of bacterial killing by cellular innate immunity and limited bacterial replication. Bacterial replication is restricted by antimicrobial peptides (Rosenberger CM, 2002) and reactive nitrogen species (Vazquez-Torres A and Fang, 2001), which have the potential to cause bacterial DNA damage (Buchmeier NA, 1993; Buchmeier NA, 1995; Craig M, 2009; Rosenberger CM, 2002; Suvarnapunya AE, 2003). It is unclear why, in the face of DNA damaging agents, the bacterial genome within a host appears to be fairly stable, and hyper-virulent clones do not typically overrun an infection or epidemic (Clairmont C, 2000; Holt KE, 2008).
Another factor that may allow Salmonella to evade killing is that the bacteria can apparently reside in different kinds of host cells. In a mouse model of chronic infection of the gall bladder, S. Typhimurium resides within gall bladder epithelial cells and macrophages, a professional phagocyte that normally kills bacteria (Gonzalez-Escobedo, 2013). In mice in which bacteria persist within the mesenteric lymph nodes, spleen and liver, S. Typhimurium resides within subsets of macrophages, including hemophagocytes and alternatively activated macrophages (Eisele NA, 2013; Nix, 2007). Hemophagocytes are macrophages that accumulate during acute inflammation and engulf apparently non-senescent red and white blood cells (Fisman, 2000; McCoy MW, 2012; Silva-Herzog E, 2008). Why S. Typhimurium resides within hemophagocytes is unclear.
Here we establish that a highly conserved iron importer, FeoB, limits S. Typhimurium replication in macrophages and tissues. However, the feoB mutant strain is severely attenuated upon co-infection of mice with wild-type bacteria. We observe a requirement for feoB upon co-infection particularly of IFNγ-activated macrophages that have engulfed erythrocytes, indicating a need for FeoB iron uptake in hemophagocytes.
RESULTS
FeoB limits S. Typhimurium replication in macrophages
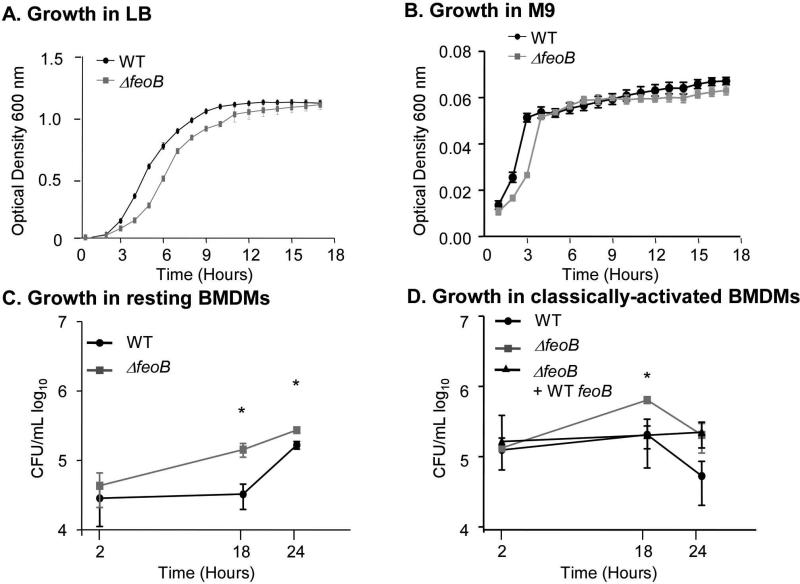
The FeoAB system transports ferrous iron from the periplasm to the cytosol (Kammler M, 1993). A feoB deletion mutant strain was constructed by replacing the feoB open reading frame with a kanamycin resistance cassette. We confirmed that there are no significant growth differences between the wild-type and ΔfeoB strains in nutrient rich media (LB) or nutrient poor media (M9) at 37°C (Figure 1 A and 1B) (Boyer E, 2002). In addition, iron chelation with 2’2 dipyridyl limited growth of the ΔfeoB strain (OD600 = 0.46 after 18 hours of growth, compared to OD600 = 0.85 without chelation), as previously reported (Tsolis RM, 1996). Macrophages are a key cell type in which S. Typhimurium resides in vivo (Monack, 2004), and yet a feoB mutant strain had no apparent survival defect in macrophage-like RAW264.7 cells (Boyer E, 2002). However, RAW264.7 cells are especially permissive for S. Typhimurium replication because they express an unstable mutant form of Nramp1/Slc11a1, a divalent cation transporter with pleiotropic effects on innate immunity (Barton CH, 1995; Canonne-Hergaux F, 1999; Govoni G, 1998). To establish whether a feoB mutant has a survival defect in Nramp1+ macrophages, bone marrow-derived macrophages (BMDMs) isolated from Sv129S6 Nramp1+/+ mice were examined. We infected resting BMDMs with wild type or a feoB mutant S. Typhimurium and performed gentamicin protection assays. Unexpectedly, strains lacking feoB replicated at a higher rate than wild type (Figure 1C). Classically (IFNγ and LPS) activated BMDMs also allowed the feoB mutant to replicate more than wild type (Figure 1D). A strain in which the deleted feoB locus was restored with a wild-type feoB gene on the chromosome replicated only as well as wild type (Figure 1D). RAW264.7 cells carrying a wild-type Nramp1G169 transgene showed similar results (Van Zandt KE, 2008) (Figure S1). A control strain in which type three secretion systems (T3SS) 1 and 2 were inactivated in a feoB mutant background (ΔfeoB, ΔinvA, ΔspiC) was unable to replicate in macrophages, indicating that, as expected, intracellular replication requires T3SS-2 (data not shown) (Cirillo, 1998; Hensel, 2000). These data collectively show that FeoB limits S. Typhimurium replication in cultured macrophages.
Figure 1. FeoB limits S. Typhimurium replication in macrophages.
Wild-type (WT) or ΔfeoB mutant strains were grown in LB (A) or M9 (B) minimal medium and optical density at 600 nm was monitored for 17 hours. Error bars are SD, n ≥ 3 experiments. Bone marrow-derived macrophages (BMDMs) isolated from Sv129S6 (Nramp1+/+) mice were resting (C) or classically activated (IFNγ and LPS) (D). Macrophages were inoculated with the strains indicated at an MOI of 10. Mean and SD of representative experiments are shown. p-values were determined as described in the methods. p ≤ 0.05 (*) vs. WT, n ≥ 3 experiments.
FeoB limits S. Typhimurium tissue colonization in the spleen and liver of mice
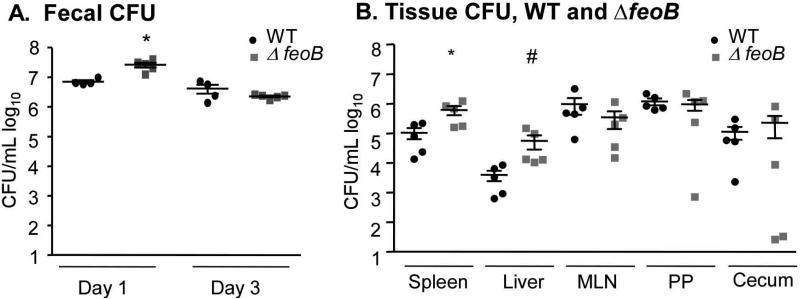
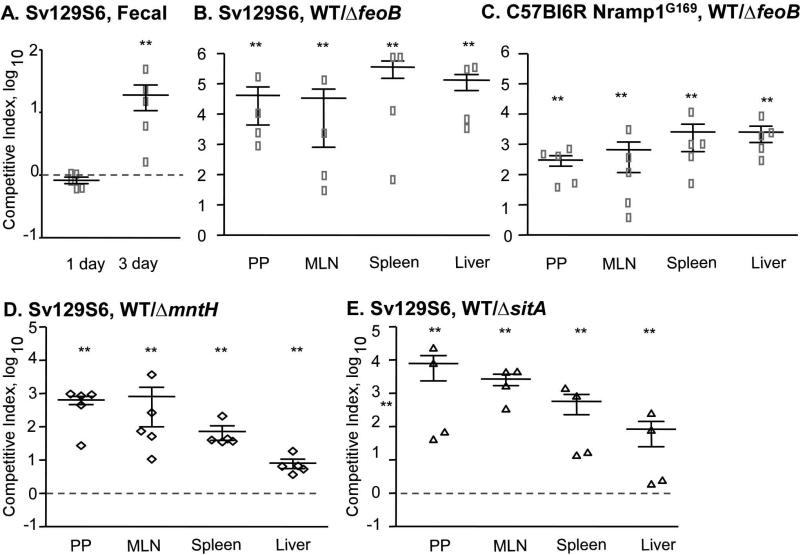
We next examined tissue colonization by the ΔfeoB mutant strain in Sv129S6 Nramp1+/+ mice orogastrically inoculated with a wild type or mutant strain. Feces were collected and plated for CFU at one and three days post-infection to determine the degree of intestinal colonization for each strain. At one-day post infection, more ΔfeoB bacteria were recovered from fecal pellets, but by three days there was no difference (Figure 2A). At two weeks post-infection, mice were sacrificed and the spleen, liver, mesenteric lymph nodes, and Peyer's patches were removed, homogenized and plated to determine bacterial tissue loads. The feoB mutant colonized the spleen and the liver to significantly higher levels than the wild-type strain (Figure 2B). These results are consistent with the increased replication of the feoB mutant in Nramp1+ macrophages and suggest that FeoB limits tissue colonization in mice.
Figure 2. FeoB limits colonization of the spleen and liver in Sv129S6 (Nramp1+/+) mice.
Mice were orogastrically inoculated with either the WT or a ΔfeoB mutant strain. Fecal pellets were harvested 1 and 3 days post-infection (A). Tissues were harvested and plated for CFU at 2 weeks post-infection (B). Mean and SEM are shown. p-values were determined as described in the methods. p < 0.05 (*) vs. WT; p < 0.01 (#) vs. WT. Each symbol represents one mouse, n = 5.
The divalent cation transporters SitABCD and MntH do not limit tissue or macrophage colonization
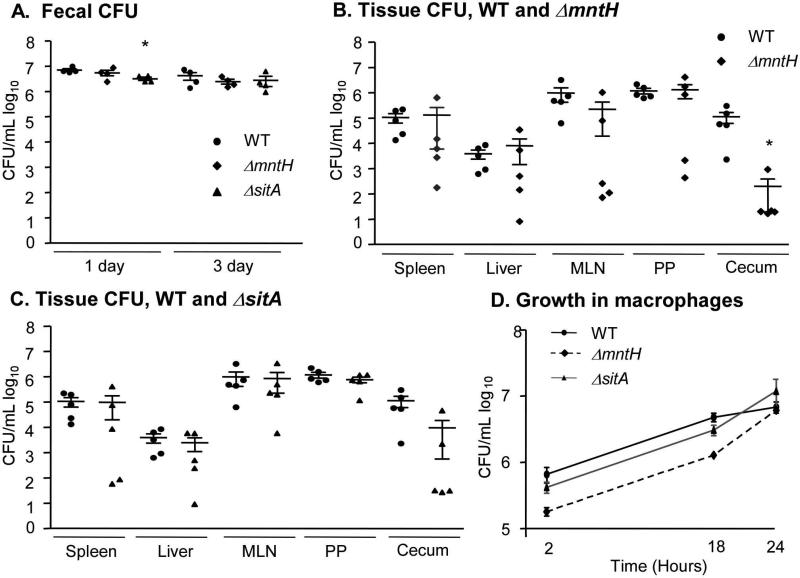
While FeoB appears to be largely dedicated to transporting ferrous iron (Fe2+), S. Typhimurium also encodes two divalent cation transporters with broader specificity (Kammler M, 1993; Zhou D, 1999). MntH and SitABCD have high affinity for manganese (Mn2+) and 10-100-fold lower affinity for Fe2+ (Kehres, 2000; Makui H, 2000). To establish whether SitABCD or MntH influence the course of infection, mice were orogastrically inoculated with individual wild-type, ΔsitA or ΔmntH strains. The sitA deletion strain was recovered from fecal pellets at slightly lower levels at one-day post-infection compared to wild-type, but within three days all strains were recovered equivalently (Figure 3A). At two weeks post-infection, colonization of both mutant strains was indistinguishable from that of wild type in the spleen, liver, mesenteric lymph nodes and Peyer's patches (Figure 3B, C). In the cecum, fewer ΔmntH mutant bacteria were recovered compared to wild type, indicating this transporter may be required specifically for intestinal colonization. In RAW264.7 Nramp1+ macrophage-like cells, ΔmntH and ΔsitA mutants replicated at similar rates as the wild-type strain (Figure 3D). These data show that neither the SitABCD nor MntH cation transporters limit bacterial replication in vivo in the Sv129S6 Nramp1+/+ orogastric infection model.
Figure 3. Strains lacking sitA or ΔmntH do not limit replication in mice or macrophages.
Mice were orogastrically inoculated with WT, ΔsitA or ΔmntH strains. Fecal pellets were harvested at 1 and 3 days post-infection (A). Tissues were harvested and plated for CFU at 2 weeks post-infection (B, C). Mean and SEM are shown. p-values were determined as described in the methods. p < 0.05 (*) vs. WT. Each symbol represents one mouse, n = 5. D) RAW264.7 Nramp1+ cells were treated with IFNγ and LPS and inoculated with the strains indicated at an MOI of 10. Mean and SD of a representative experiment are shown. n ≥ 3 experiments.
FeoB reduces expression of the Fep ferric iron transporter in macrophages
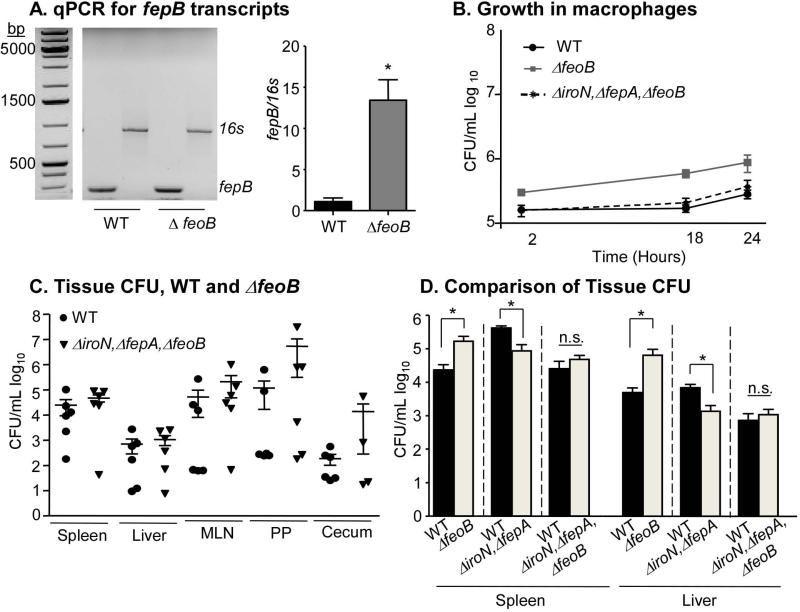
In the presence of ferrous iron, the Fur transcription factor binds DNA and represses genes encoding iron uptake proteins. Therefore, disruption of the feoB encoded ferrous iron importer may increase expression of ferric iron uptake genes (Hantke, 1981, 1987; Pecqueur L, 2006; Tsolis RM, 1995). One such Fur-regulated gene is fepB, which encodes a key periplasmic protein of a ferric iron uptake system required for S. Typhimurium survival in macrophages and mice (Crouch ML, 2008; Nagy TA, 2013). To determine whether a feoB mutant strain has increased fepB expression, total RNA was isolated from macrophages infected with wild-type or ΔfeoB strains. RTPCR revealed that fepB transcripts were approximately 8-fold more abundant in ΔfeoB mutant bacteria compared to wild-type (Figure 4A), indicating that in macrophages S. Typhimurium increases fepB expression in the absence of feoB.
Figure 4. Increased replication in the absence of feoB is dependent on ferric iron uptake.
A) RAW264.7 Nramp1+ cells were infected with WT or ΔfeoB mutant strains at an MOI of 10. At 2 hours post-infection, macrophages were lysed and isolated RNA was subjected to real-time RT-PCR. Agarose gel, left, quantification, right. fepB amplicon, 245bp and 16s amplicon, 850bp. Mean and SEM are shown. p < 0.05 (*), n = 3 experiments. B) IFNγ and LPS activated RAW264.7 Nramp1+ cells were inoculated with the strains indicated at an MOI of 10. Mean and SD of a representative experiment is shown. p < 0.05 (*), n ≥ 3 experiments. C) Mice were orogastrically inoculated with WT or ΔiroN, ΔfepA, ΔfeoB strains. Tissues were harvested and plated for CFU at 2 weeks post-infection. Mean and SEM are shown. Each symbol represents one mouse, n ≥ 5. p-values for all experiments were determined as described in the methods. D) Comparison of colonization of the spleen and liver by the indicated strains. P < 0.05 (*)
Siderophores are required for increased colonization by the feoB mutant strain
Siderophores capture ferric iron and are transported through the outer membrane for delivery to FepB. IroN and FepA are siderophore transporters required for virulence in S. Typhimurium (Crouch ML, 2008; Gorbacheva VY, 2001; Nagy TA, 2013; Rabsch W, 2003; Rabsch W, 1999; Williams PH, 2006). To establish whether increased replication of strains lacking feoB requires siderophore capture through iroN and fepA, we constructed a triple mutant ΔfeoB, ΔiroN, ΔfepA strain. The triple mutant and wild-type control strains replicated similarly in macrophages and colonized tissues to similar levels in mice (Figure 4B, C). Thus, increased replication of the ΔfeoB mutant strain depends upon the presence of siderophores. We also compared the ability of the wild type, ΔiroN, ΔfepA double mutant (Nagy TA, 2013), and ΔiroN, ΔfepA, ΔfeoB triple mutant strains to colonize the spleen and liver (Figure 4D), the two tissues in which ΔfeoB mutant strains hyper-replicate (Figure 2B). The colonization defect of the double mutant (Nagy 2013) is suppressed by the loss of feoB. One possibility is that this suppression is mediated by the induction of Fur-repressed iron acquisition genes.
In mixed-infections of mice, FeoB is required for S. Typhimurium replication
We next established whether FeoB limits bacterial replication when the mutant strain is competing with the wild-type parent strain within an animal. We inoculated Sv129S6 Nramp1+/+ mice with equivalent numbers (1 × 109 CFU) of differentially marked wild-type and mutant bacteria in PBS. At one day post-infection, wild type and the feoB mutant strain were recovered from fecal pellets at similar levels. By three days post-infection, wild type outcompeted ΔfeoB in fecal pellets by 10-fold (Figure 5A). Tissues were harvested, serially diluted and plated to enumerate CFU two weeks post-infection. Fewer than 100 wild-type and mutant colonies were recovered from the cecum of all animals (data not shown). In the Peyer's patches, mesenteric lymph nodes, spleen, and liver wild-type bacteria outcompeted the ΔfeoB mutant by 10,000-fold (Figure 5B). In a different strain background, C57Bl6 mice containing an Nramp1G169 transgene and therefore resistant to S. Typhimurium, wild-type bacteria also outcompeted the ΔfeoB mutant strain (Figure 5C), consistent with previous observations (Fritsche G, 2012). The cation transporters MntH and SitABCD were also confirmed to be important in mixed-infections of mice (Figures 5D and 5E) (Janakiraman A, 2000). Thus, the feoB transport system is strongly required for tissue colonization in competition with wild-type bacteria.
Figure 5. In mixed infections of mice, FeoB is required for S. Typhimurium replication.
Mice were orogastrically inoculated with a 1:1 mixture of WT and ΔfeoB strains (A-C), WT and ΔmntH strains (D), or WT and ΔsitA strains (E). Fecal pellets were harvested 1 and 3 days post-infection (A). Tissues from SV129S6 (B) or C57BL6 Nramp1G169 (C-E) mice were harvested and plated for CFU at 2 weeks post-infection. Mean and SEM of competitive index are shown; dashed lines indicate equivalent competition. p-values were determined as described in the methods. p < 0.03 (**) vs. the null hypothesis. Each symbol represents one mouse, n = 4-5.
FeoB limits S. Typhimurium replication in macrophages upon mixed-infection
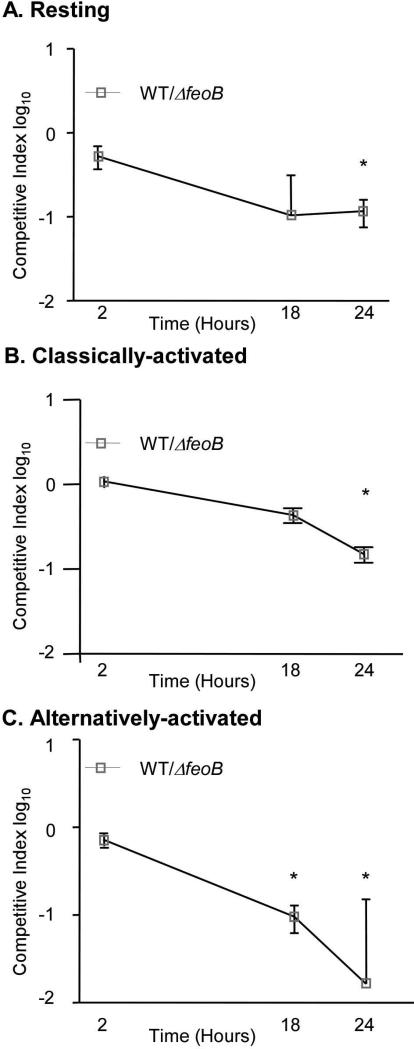
The difference in tissue colonization of the ΔfeoB strain compared to wild type in single (+10-fold) versus mixed (-10,000-fold) infection of mice was striking. To establish whether this difference may reflect macrophage responses to infection, we examined bacterial replication upon co-infection of macrophages. RAW264.7 Nramp1+ macrophages that were resting, classically activated (treated with IFNγ and LPS), or alternatively activated (treated with IL-4), were inoculated with equivalent numbers of both strains. As in single-infections, the feoB mutant outcompeted wildtype (Figure 6A-C), indicating that co-infection of RAW264.7 Nramp1+ macrophages does not model co-infection in Nramp1+ mice with regard to FeoB.
Figure 6. In mixed-infections of macrophages, FeoB limits S. Typhimurium replication.
RAW264.7 Nramp1+ cells were resting (A) or treated with IFNγ and LPS (B) or IL-4 (C) and were inoculated with a 1:1 mixture of WT and ΔfeoB strains. Mean and SD of representative experiments are shown. p-values were determined as described in the methods. p < 0.05 (*) vs. the null hypothesis, n ≥ 3 experiments.
Since RAW264.7 Nramp1+ macrophages allow for S. Typhimurium replication, whereas BMDMs kill the bacteria (Figure S2), we next examined replication of the feoB mutant strain in competition with wild type in BMDMs from Sv129S6 (Nramp1+/+) mice. BMDMs remained resting, or were treated with IFNγ, both LPS and IFNγ, or IL-4. Under these conditions, the ΔfeoB mutant strain had a small but insignificant advantage over the wild-type strain within 24 hours of infection in resting and IL-4 treated macrophages (Figure 7A). As expected, treatment with IL-4 increased the percentage of BMDMs that express CD301, a marker of alternative activation (Figure 7C). These data indicate that mixed-infection of BMDMs does not recapitulate the strong requirement for feoB observed in mixed-infections of mice.
Figure 7. FeoB is required for replication upon mixed infection in BMDMs incubated with erythrocytes.
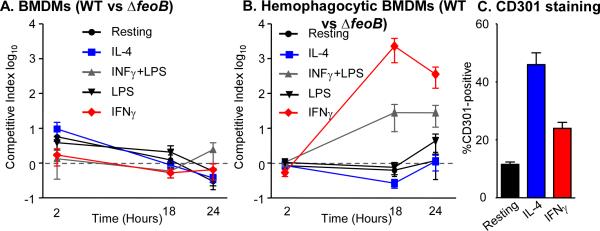
A) BMDMs were resting or treated with IL-4, IFNγ, or IFNγ and LPS and then inoculated with a 1:1 mixture of WT and ΔfeoB strains. Dashed line indicates equivalent competition. n ≥ 3 experiments. B) BMDMs were treated as in (A) and then incubated with erythrocytes. After one hour, cells were inoculated with a 1:1 mixture of WT and ΔfeoB strains. Mean and SD of representative experiments are shown. Dashed line indicates equal competition. n ≥ 3 experiments. C) BMDMs were resting or treated with IL-4 or IFNγ for 18 hours. Cells were fixed and stained with anti-CD301 and analyzed by flow cytometry. Mean and SD of the percentage of BMDMs positive for CD301 from a representative experiment are shown. p-values were determined as described in the methods. p < 0.005 (*), n ≥ 3 experiments.
FeoB is required for replication in hemophagocytes upon mixed-infection
Since S. Typhimurium can reside within hemophagocytes in mice (Nix, 2007), to model hemophagocytosis we inoculated BMDMs with freshly isolated erythrocytes. Prior to adding erythrocytes, the BMDMs were resting or activated. IFNγ treated or IFNγ and LPS treated BMDMs allowed wild-type bacteria to outcompete the ΔfeoB mutant (Figure 7B), similar to results obtained in mice (Figure 5B,C). These data suggest that macrophages incubated with erythrocytes more accurately model the mouse response to infection with respect to feoB.
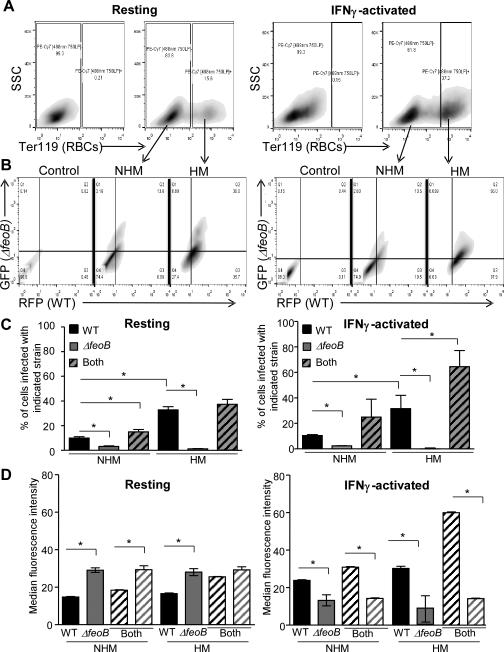
We next determined whether macrophage engulfment of erythrocytes contributed to the ability of the wild-type strain to outcompete the feoB mutant strain in individual BMDMs. Hemophagocytes (HMs) and non-hemophagocytes (NHMs) were distinguished by flow cytometry; extracellular erythrocytes were removed by lysis and macrophages containing erythrocytes (HMs) were identified with an antibody to an erythrocyte epitope, TER-119. IFNγ activation resulted in the accumulation of more HMs than did treatment with media alone (Figure 8A). HMs and NHMs were examined for the presence of wild-type (RFP signal) and/or ΔfeoB (GFP signal) bacteria (Figure 8B). Few macrophages under any conditions supported the feoB mutant strain (gray bars) within 18 hour of infection. In contrast, more HMs than NHMs, whether resting or activated, harbored the wild-type strain (black bars) (Figure 8C). These data indicate that macrophages containing erythrocytes provide wild type but not feoB mutant bacteria with a survival niche.
Figure 8. FeoB is required for replication upon mixed-infection in hemophagocytes.
BMDMs were resting (A-D, left panels) or activated with IFNγ (A-D, right panels), incubated with erythrocytes for one hour, and then inoculated with a 1:1 mixture of WT-RFP and ΔfeoB-GFP strains. At 18 hours post-infection, cells were fixed and stained with anti-Ter-119 and analyzed by flow cytometry. A) Gating scheme for non-hemophagocytic (NHM) and hemophagocytic (HM) populations. B) Gating scheme to identify RFP- and GFP-positive BMDMs. C) Percentage of BMDMs that were positive for WT (RFP, black), ΔfeoB (GFP, gray) or both (striped). D) Median fluorescence intensity of WT and ΔfeoB of singly and co-infected BMDMs from (C). Mean and SD of a representative experiment is shown. p-values were determined as described in the methods. p < 0.05 (*) vs. WT, n ≥ 3.
Median fluorescence intensity (MFI) can serve as a proxy for the relative number of bacteria in a given cell population (Figure S3). In resting macrophages (Figure 8D, left) the feoB mutant strain outcompeted the wild-type strain, consistent with expression of Fur-repressed genes in the feoB mutant in macrophages (Figure 4). In activated macrophages, the feoB mutant replicated poorly under all conditions compared to wild type, including in macrophages in which the feoB mutant strain was the only bacterial strain detected (Figure 8D, right). This result appears to contradict previous observations of increased feoB mutant replication in activated primary macrophages (Figure 1D). However, the activated NHMs in the current experiment were exposed to erythrocytes and therefore hemoglobin and heme, for which macrophages express receptors (Fabriek BO, 2005; Hvidberg V, 2005). In activated NHMs incubated with erythrocytes, the feoB mutant strain may obtain just enough iron to maintain Fur-mediated repression of iron acquisition genes, but not enough iron to replicate. In activated HMs containing both bacterial strains, the wild-type strain replicated to the highest levels (Figure 8D, right). These results support the observation that IFNγ stimulates macrophage export of iron, thereby reducing pathogen access to iron within the macrophage (Nairz M, 2008). The presence of erythrocytes in IFNγ-activated macrophages appears to compensate for iron export by increasing bacterial access to ferrous iron and thus replication. Without the feoB gene, S. Typhimurium cannot take full advantage of erythrocytes in macrophages, suggesting that wild-type S. Typhimurium requires FeoB to access ferrous iron within hemophagocytes.
DISCUSSION
Our results show that feoB is required for S. Typhimurium replication during co-infection of hemophagocytic macrophages. However, S. Typhimurium lacking feoB indeed has enhanced virulence compared to wild type upon single-infection of Sv129S6 (Nramp1+/+) mice and macrophages. This observation may reflect increased expression of iron acquisition genes in the ΔfeoB mutant strain upon macrophage infection. Under low iron conditions, induction of genes repressed by Fur in the presence of iron, including fepB, iroN and fepA, occurs in E. coli and S. enterica (Bäumler AJ, 1998; Brickman TJ, 1990; Tsolis RM, 1995).
Enhanced ferric iron uptake in response to ferrous iron limitation appears to be a common pathogen strategy. The alpha-proteobacterium Brucella abortus also lives within macrophage vesicles and causes chronic infection. B. abortus requires a ferrous iron uptake system for virulence in mice (Elhassanny AE, 2013) but also captures heme as an iron source (Paulley JT, 2007) and encodes at least one hemeoxygenase, BhuQ (Ojeda JF, 2012). Under low iron conditions, B. abortus increases expression of a siderophore-encoding gene, dhbC, in a bhuQ mutant strain (Ojeda JF, 2012). Similarly, in Nramp1+/+ mice and macrophages, S. enterica feoB mutant strains may compensate for iron starvation by inducing Fur-dependent iron acquisition genes to support enhanced bacterial replication.
Mixed-infection experiments of mice with the feoB mutant yielded very different results from single-infection experiments. This difference may reflect that the host has a more vigorous immune response to S. Typhimurium that expresses FeoB. In Yersinia pseudotuberculosis, another gamma-proteobacterium, YopE and YopH are virulence determinants secreted into host cells (Trosky JE, 2008). Infection of mice with either a yopE or yopH mutant strain yields tissue colonization comparable to infection with the parent strain, but both mutant strains are outcompeted by the wild-type strain in mixed-infections. A series of elegant experiments demonstrated that wild-type Y. pseudotuberculosis induces more robust inflammatory responses than yopE or yopH mutant strains, and that growth of a yopE mutant strain is inhibited by inflammation (Logsdon LK, 2006). With regard to S. Typhimurium, the presence of a wild-type strain expressing FeoB may stimulate in mice and in IFNγ-activated hemophagocytes an immune response that that prevents replication of a co-infected strain lacking FeoB.
A second explanation for the discrepancy of the feoB mutant phenotype in mixed and single infections is based on the iron transport role of FeoB. Erythrocytes are degraded within macrophage vesicles, followed by heme transport into the cytosol for catalysis by hemeoxygenase-1 and release of ferrous iron (Delaby C, 2012). Perhaps during mixed-infection wild-type S. Typhimurium captures heme-derived iron with FeoB and thereby outcompetes the feoB mutant strain before the latter can induce ferric iron acquisition genes. Upon single-infection, the feoB mutant appears to be sufficiently iron starved and has enough time to reverse Fur-mediated repression of ferric iron uptake. Macrophage treatment with IFNγ results in ferrous iron export from the cell and thereby restricts S. Typhimurium replication (Nairz M, 2008). However, hemophagocytosis appears to counter the effect of IFNγ, probably based on ferrous iron release from heme followed by FeoB-dependent capture of ferrous iron by S. Typhimurium. In contrast, in macrophages without erythrocytes S. Typhimurium must import ferric iron via siderophores and FepB (Nagy TA, 2013). Altogether, the data suggest that hemophagocytes more faithfully model the host environment than other kinds of macrophages by providing S. Typhimurium with iron.
In conclusion, despite appearing to be an anti-virulence determinant in single-infection experiments, FeoB is clearly required for virulence during mixed infection. Mixed infection is the more realistic context with respect to pathogen evolution in the host because genetic bacterial variants that arise in vivo must compete with much larger numbers of the wild-type parent strain. If a spontaneous loss-of-function mutation in feoB were to arise during infection, it would not become fixed within the population because it could not survive in the presence of wild-type bacteria. Our study also indicates that FeoB and thus ferrous iron transport is important particularly in hemophagocytes, indicating that S. Typhimurium exploits these macrophages for access to iron.
EXPERIMENTAL PROCEDURES
Construction of bacterial strains and growth conditions
Salmonella enterica serovar Typhimurium wild-type strain SL1344 (Merritt FF, 1984) and mutant derivatives were grown overnight at 37°C with aeration prior to infections. Antibiotics were used at the following concentrations: streptomycin, 30 μg/mL and kanamycin, 30 μg/mL.
Strains with deletions of iron transporters were made following the Wanner insertion method. Briefly, strains with marked deletions of mntH, sitA or feoB were constructed in the wild-type background by recombination of the PCR product derived from primers specific for the region flanking the corresponding gene and the kanamycin resistance marker on plasmid pKD4 (Datsenko KA and Wanner, 2000). The following primers were used: mntH fwd: 5’-ATGAAACATAGCAAAGGCTATGTTTTTGAGGCAAAAGgtgtaggctggagctgctt-3’ mntH rev: 5’-ACGCCCACGCATCGGGCCTGCTATCTTTCTATCTcatatgaatatcctccttag-3’; feoB fwd: 5’-GTAAAAAGGATTTGGCGTTAATAGAAGTGGAAGCGGgtgtaggctggagctgcttc-3’ and feoB rev: GAACCTGTATCAATGAAGCCATTTTTTACATCCCcatatgaatatcctccttag- 3’; sitA fwd: 5’-TCGATGATTAATTAACCACATTGTTGCGAGGGATACTgtgtaggctggagctgcttc-3’ and sitA rev: 5’-ACCGTGACTTGATCAACGGTAATCGCAGATTGACcatatgaatatcctccttag-3’. Lower case letters indicate the primers designed previously (Datsenko KA and Wanner, 2000). Kanamycin insertions were confirmed by PCR. The ΔfeoB, ΔinvA, ΔspiC mutant strain was created by P22 phage transduction of ΔfeoB-kanamycin resistance into to an unmarked ΔinvA, ΔspiC double mutant strain (Silva-Herzog, 2010).
Growth curves
Overnight cultures were diluted to an OD600 of 0.01 in 200 μL of Luria-Bertani (LB) medium or M9 minimal medium (M9). Bacteria were grown in 96-well plates shaking in a Synergy2 plate reader (BioTek) at 37°C for 17 hours. The OD600 was recorded at 20-minute intervals.
Mouse infections
Research protocols were approved by the University of Colorado Institutional Committees for Biosafety and for Animal Care and Use. For mixed-infection studies, 7-week-old male and female 129SvEvTac (Nramp1G169/G169) mice (Taconic Laboratories) bred in-house were fasted for two hours prior to orogastric inoculation with 1 × 109 wild-type and 1 × 109 mutant bacteria, as verified by plating for CFU on selective LB agar, in 100uL of PBS. C57/BL6 Nramp1G169D mice carrying an Nramp1G169 (SLC11a1) transgene (Govoni G, 1998; Kuhn DE, 2001) were obtained from Dr. F. Fang (Brown DE, 2013).
For single-infection experiments, mice were fasted for two hours to allow gastric contents to clear. Mice were inoculated orogastrically with 1 × 109 bacteria as verified by plating for CFU on selective LB agar. At one and three days post-infection, at least two fecal pellets were collected from each mouse in 1 mL of PBS, vortexed until homogeneous, diluted and plated on selective media. Two weeks after inoculation, tissues (spleen, liver, mesenteric lymph nodes, Peyer's patches, and cecum) were collected in 1 mL PBS, homogenized with a TissueMiser (Fisher Scientific) and diluted in PBS for plating on selective LB agar plates. CFU were enumerated and the competitive indexes (CIs) were calculated as follows: (CFUwild-type/CFUmutant) output / (CFUwild-type/CFUmutant) input. If fewer than 100 bacteria total or from each strain (for mixed-infections) were recovered, then CIs were not calculated.
Cell culture gentamicin protection assays
Primary macrophages were isolated as previously described (Nix, 2007). Briefly, marrow was flushed from the femurs and tibias of 2- to 4-month-old 129SvEvTac mice (Taconic Laboratories). Cells were resuspended in Dulbecco modified Eagle medium (DMEM) (Sigma-Aldrich, St. Louis, MO) supplemented with fetal bovine serum (10%), l-glutamine (2 mM), sodium pyruvate (1 mM), beta-mercaptoethanol (50 μM), HEPES (10 mM), and penicillin-streptomycin (50 IU/mL penicillin and 50 μg/mL streptomycin). Cells were overlaid onto an equal volume of Histopaque-1083 (Sigma-Aldrich, St. Louis, MO) and centrifuged at 1500rpm for 10 minutes. Monocytes at the interface were harvested and incubated for six days at 37°C in 5% CO2 in supplemented DMEM that also contained 30% m-CSF conditioned medium to promote monocyte differentiation into macrophages.
Bone marrow-derived macrophages and RAW264.7 Nramp1+/+ cells were seeded at 1.5 × 105 cells per well in poly-L-lysine-coated 24-well tissue culture plates. Cells were classically activated with 20 ng/mL lipopolysaccharide (S. enterica Typhimurium LPS; Sigma-Aldrich) and 20 U/mL IFNγ (PeproTech) for 18 hours and alternatively M2 activated with 20 ng/mL murine IL-4 (PeproTech) for 24 hours. Where indicated, freshly isolated murine erythrocytes were added at a 10:1 ratio for 1 hour. Bacteria were added to macrophages at a multiplicity of infection of 10. After 30 minutes, cells were washed and incubated for 1.5 hours at 37°C in fresh media supplemented with gentamicin (100 μg/mL) to kill extracellular bacteria. Media was then exchanged for media supplemented with gentamicin (10 μg/mL) to prevent extracellular bacterial growth. At 2, 18 and 24 hours, wells were washed twice with pre-warmed PBS, incubated with 1% Triton X-100 for 5 minutes, lysed, and serial dilutions plated for colony-forming units.
Real-time reverse transcriptase PCR
1.5 × 105 macrophages were infected with wild-type Salmonella or ΔfeoB mutants or medium alone for two hours as described in Cell culture gentamicin protection assays. Macrophages were scraped and collected into 1.5 mL eppendorf tubes and spun at 16,000rpm for 10 minutes to lyse macrophages. The supernatant from lysed macrophages was removed and RNA was prepared from bacterial pellets using RNAeasy kit (Qiagen) following the manufacturer's instructions after treatment with lysozyme. Reverse transcriptase-PCR was performed using High Capacity cDNA Reverse Transcription kit (Applied Biosystems), which was followed by quantitative PCR using SYBR green (Applied Biosystems) and the Eppendorf Mastercycler ep realplex2 S (Eppendorf) qPCR machine. Relative differences between bacteria were calculated based on values for the fepB (fwd primer: 5’ggcactaaggcttgcatctc3’; rev primer: 5’tcaacgtcacgctggtagag3’) gene normalized to values of the 16s (universal fwd primer: 5’gtgccagcmgccgcggtaa3’; rev primer: 5’gacgggcggtgtgtrca3’) gene.
Flow cytometry
Resting or IFNγ-activated macrophages seeded in a 6-well plate were incubated with freshly isolated murine erythrocytes at a 10:1 ratio for one hour prior to co-infection with wild-type SL1344 containing a plasmid expressing dsRed and the ΔfeoB strain containing a plasmid expressing eGFP. At 18 hours post-infection, cells were washed with cold PBS and erythrocytes were lysed with potassium bicarbonate ACK lysis buffer (Bossuyt X, 1997) and harvested by gentle scraping. Cells from each condition were equally distributed into 96-well plates and resuspended in FACS staining buffer (PBS plus 1% fetal bovine serum (FBS), 0.02% azide) containing anti-mouse CD16/32 (eBioscience) to block Fc receptors. Cells were then fixed on ice in 1% paraformaldehyde–1% sucrose, permeabilized in staining buffer with 0.1% saponin, and then incubated in staining buffer containing 0.5 μg/sample anti-mouse Ter-119-phycoerythrin (PE)-Cy7 (eBioscience). Fluorescently labeled cells were quantified with a CyAn ADP flow cytometer (Beckman Coulter, Brea, CA) and analyzed with appropriate compensation using FlowJo software (Tree Star, Inc.). Any remaining intact extracellular erythrocytes were removed by gating, as erythrocytes have less SSC and FSC relative to macrophages.
Statistics
p values were calculated with GraphPad Prism 5 (GraphPad Software Inc.) and considered significant if p < 0.05. For nonparametric data, Wilcoxon signed-rank or Mann-Whitney tests were used. Otherwise, student's t-test or ANOVA were used.
Supplementary Material
Acknowledgements
We thank M. Carolina Pilonieta for development of the hemophagocytic macrophage flow cytometry technique (manuscript in preparation) and all the members of the Detweiler lab for helpful discussions and technical help over the course of this project.
This work was supported by NIH grants 1F32AI094766 (T.A.N.) and AI072492 and AI095395 (C.S.D.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest: All of the authors declare that there are no conflicts of interest
REFERENCES
- Baek CH, Wang S, Roland KL, Curtiss R. Leucine-responsive regulatory protein (Lrp) acts as a virulence repressor in Salmonella enterica serovar Typhimurium. J Bacteriol. 2009;191:1278–1292. doi: 10.1128/JB.01142-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton CH, Whitehead SH, Blackwell JM. Nramp transfection transfers Ity/Lsh/Bcg-related pleiotropic effects on macrophage activation: influence on oxidative burst and nitric oxide pathways. Mol Med. 1995;1:267–279. [PMC free article] [PubMed] [Google Scholar]
- Bäumler AJ, Norris TL, Lasco T, Voight W, Reissbrodt R, Rabsch W, Heffron F. IroN, a novel outer membrane siderophore receptor characteristic of Salmonella enterica. J Bacteriol. 1998;180:1446–1453. doi: 10.1128/jb.180.6.1446-1453.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer E, Bergevin I, Malo D, Gros P, Cellier MF. Acquisition of Mn(II) in addition to Fe(II) is required for full virulence of Salmonella enterica serovar Typhimurium. Infect Immun. 2002;70:6032–6042. doi: 10.1128/IAI.70.11.6032-6042.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman TJ, Ozenberger BA, McIntosh MA. Regulation of divergent transcription from the iron-responsive fepB-entC promoter-operator regions in Escherichia coli. J Mol Biol. 1990;212:669–682. doi: 10.1016/0022-2836(90)90229-F. [DOI] [PubMed] [Google Scholar]
- Brown DE, Libby SJ, Moreland SM, McCoy MW, Brabb T, Stepanek A, Fang FC, Detweiler CS. Salmonella enterica Causes More Severe Inflammatory Disease in C57/BL6 Nramp1G169 Mice Than Sv129S6 Mice. Vet Pathol. 2013;50:867–76. doi: 10.1177/0300985813478213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmeier NA, Lipps CJ, So MY, Heffron F. Recombination-deficient mutants of Salmonella typhimurium are avirulent and sensitive to the oxidative burst of macrophages. Mol Microbiol. 1993;7:933–936. doi: 10.1111/j.1365-2958.1993.tb01184.x. [DOI] [PubMed] [Google Scholar]
- Buchmeier NA, Libby SJ, Xu Y, Loewen PC, Switala J, Guiney DG, Fang FC. DNA repair is more important than catalase for Salmonella virulence in mice. J Clin Invest. 1995;95:1047–1053. doi: 10.1172/JCI117750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canonne-Hergaux F, Gruenheid S, Govoni G, Gros P. The Nramp1 protein and its role in resistance to infection and macrophage function. Proc Assoc Am Physicians. 1999;111:283–289. doi: 10.1046/j.1525-1381.1999.99236.x. [DOI] [PubMed] [Google Scholar]
- Cirillo DM, Valdivia RH, Monack DM, Falkow S. Macrophage-dependent induction of the Salmonella pathogenicity island 2 type III secretion system and its role in intracellular survival. Mol. Microbiol. 1998;30:175–188. doi: 10.1046/j.1365-2958.1998.01048.x. [DOI] [PubMed] [Google Scholar]
- Clairmont C, Lee KC, Pike J, Ittensohn M, Low KB, Pawelek J, Bermudes D, Brecher SM, Margitich D, Turnier J, Li Z, Luo X, King I, Zheng LM. Biodistribution and genetic stability of the novel antitumor agent VNP20009, a genetically modified strain of Salmonella typhimurium. J Infect Dis. 2000;181:1996–2002. doi: 10.1086/315497. [DOI] [PubMed] [Google Scholar]
- Craig M, Slauch JM. Phagocytic superoxide specifically damages an extracytoplasmic target to inhibit or kill Salmonella. PLoS One. 2009;4:e4975. doi: 10.1371/journal.pone.0004975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouch ML, Castor M, Karlinsey JE, Kalhorn T, Fang FC. Biosynthesis and IroC-dependent export of the siderophore salmochelin are essential for virulence of Salmonella enterica serovar Typhimurium. Mol Microbiol. 2008;67:971–983. doi: 10.1111/j.1365-2958.2007.06089.x. [DOI] [PubMed] [Google Scholar]
- Datsenko KA, Wanner B. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaby C, Rondeau C, Pouzet C, Willemetz A, Pilard N, Desjardins M, Canonne-Hergaux F. Subcellular localization of iron and heme metabolism related proteins at early stages of erythrophagocytosis. PLoS One. 2012;7:e42199. doi: 10.1371/journal.pone.0042199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisele NA, Ruby T, Jacobson A, Manzanillo PS, Cox JS, Lam L, Mukundan L, Chawla A, Monack DM. Salmonella require the fatty acid regulator PPARδ for the establishment of a metabolic environment essential for long-term persistence. Cell Host Microbe. 2013;14:171–178. doi: 10.1016/j.chom.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhassanny AE, Anderson ES, Menscher EA, Roop RM., 2nd The ferrous iron transporter FtrABCD is required for the virulence of Brucella abortus 2308 in mice. Mol Microbiol. 2013;88:1070–1082. doi: 10.1111/mmi.12242. [DOI] [PubMed] [Google Scholar]
- Fabriek BO, Dijkstra CD, van den Berg TK. The macrophage scavenger receptor CD163. Immunobiology. 2005;210:153–160. doi: 10.1016/j.imbio.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Fisman DN. Hemophagocytic syndromes and infection. Emerg. Infect. Dis. 2000;6:601–608. doi: 10.3201/eid0606.000608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foreman-Wykert AK, Miller JF. Hypervirulence and pathogen fitness. Trends Microbiol. 2003;11:105–108. doi: 10.1016/s0966-842x(03)00007-6. [DOI] [PubMed] [Google Scholar]
- Fritsche G, Nairz M, Libby SJ, Fang FC, Weiss G. Slc11a1 (Nramp1) impairs growth of Salmonella enterica serovar typhimurium in macrophages via stimulation of lipocalin-2 expression. J Leukoc Biol. 2012;92:353–359. doi: 10.1189/jlb.1111554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gal-Mor O, Gibson DL, Baluta D, Vallance BA, Finlay BB. A novel secretion pathway of Salmonella enterica acts as an antivirulence modulator during salmonellosis. PLoS Pathogens. 2008;4:e1000036. doi: 10.1371/journal.ppat.1000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Escobedo G, Gunn JS. Gallbladder epithelium as a niche for chronic Salmonella carriage. Infect Immun. 2013;81:2920–2930. doi: 10.1128/IAI.00258-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbacheva VY, Faundez G, Godfrey HP, Cabello FC. Restricted growth of ent(-) and tonB mutants of Salmonella enterica serovar Typhi in human Mono Mac 6 monocytic cells. FEMS Microbiol Lett. 2001;196:7–11. doi: 10.1111/j.1574-6968.2001.tb10532.x. [DOI] [PubMed] [Google Scholar]
- Govoni G, Gros P. Macrophage NRAMP1 and its role in resistance to microbial infections. Inflamm Res. 1998;47:277–284. doi: 10.1007/s000110050330. [DOI] [PubMed] [Google Scholar]
- Hantke K. Regulation of ferric iron transport in Escherichia coli K12: isolation of a constitutive mutant. Mol Gen Genet. 1981;182:288–292. doi: 10.1007/BF00269672. [DOI] [PubMed] [Google Scholar]
- Hantke K. Selection procedure for deregulated iron transport mutants (fur) in Escherichia coli K 12: fur not only affects iron metabolism. Mol Gen Genet. 1987;210:135–139. doi: 10.1007/BF00337769. [DOI] [PubMed] [Google Scholar]
- Hensel M. Salmonella pathogenicity island 2. Mol. Microbiol. 2000;36:1015–1023. doi: 10.1046/j.1365-2958.2000.01935.x. [DOI] [PubMed] [Google Scholar]
- Ho TD, Slauch JM. Characterization of grvA, an antivirulence gene on the gifsy-2 phage in Salmonella enterica serovar typhimurium. J Bacteriol. 2001;183:611–620. doi: 10.1128/JB.183.2.611-620.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt KE, Parkhill J, Mazzoni CJ, Roumagnac P, Weill FX, Goodhead I, Rance R, Baker S, Maskell DJ, Wain J, Dolecek C, Achtman M, Dougan G. High-throughput sequencing provides insights into genome variation and evolution in Salmonella Typhi. Nat Genet. 2008;40:987–993. doi: 10.1038/ng.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hvidberg V, Maniecki MB, Jacobsen C, Højrup P, Møller HJ, Moestrup SK. Identification of the receptor scavenging hemopexin-heme complexes. Blood. 2005;106:2572–2579. doi: 10.1182/blood-2005-03-1185. [DOI] [PubMed] [Google Scholar]
- Janakiraman A, Slauch JM. The putative iron transport system SitABCD encoded on SPI1 is required for full virulence of Salmonella typhimurium. Mol Microbiol. 2000;35:1146–1155. doi: 10.1046/j.1365-2958.2000.01783.x. [DOI] [PubMed] [Google Scholar]
- Kammler M, Schon C, Hantke K. Characterization of the ferrous iron uptake system of Escherichia coli. J Bacteriol. 1993;175:6212–6219. doi: 10.1128/jb.175.19.6212-6219.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehres DG, Zaharik ML, Finlay BB, Maguire ME. The NRAMP proteins of Salmonella typhimurium and Escherichia coli are selective manganese transporters involved in the response to reactive oxygen. Mol. Microbiol. 2000;36:1085–1100. doi: 10.1046/j.1365-2958.2000.01922.x. [DOI] [PubMed] [Google Scholar]
- Kuhn DE, Lafuse WP, Zwilling BS. Iron transport into mycobacterium avium-containing phagosomes from an Nramp1(Gly169)-transfected RAW264.7 macrophage cell line. J Leukoc Biol. 2001;69:43–49. [PubMed] [Google Scholar]
- Logsdon LK, Mescas J. The proinflammatory response induced by wild-type Yersinia pseudotuberculosis infection inhibits survival of yop mutants in the gastrointestinal tract and Peyer's patches. Infect Immun. 2006;74:1516–1527. doi: 10.1128/IAI.74.3.1516-1527.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makui H, Roig E, Cole ST, Helmann JD, Gros P, Cellier MF. Identification of the Escherichia coli K-12 Nramp orthologue (mntH) as a selective divalent metal ion transporter. Mol Microbiol. 2000;35:1065–1078. doi: 10.1046/j.1365-2958.2000.01774.x. [DOI] [PubMed] [Google Scholar]
- McCoy MW, Moreland SM, Detweiler CS. Hemophagocytic Macrophages in Murine Typhoid Fever Have an Anti-inflammatory Phenotype. Infect Immun. 2012;80:3642–9. doi: 10.1128/IAI.00656-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt FF, Smith BP, Reina-Guerra M, Habasha F, Johnson E. Relationship of cutaneous delayed hypersensitivity to protection from challenge exposure with Salmonella typhimurium in calves. Am J Vet Res. 1984;45:1081–1085. [PubMed] [Google Scholar]
- Monack DM, Bouley DM, Falkow S. Salmonella typhimurium persists within macrophages in the mesenteric lymph nodes of chronically infected Nramp1+/+ mice and can be reactivated by IFNgamma neutralization. J. Exp. Med. 2004;199:231–241. doi: 10.1084/jem.20031319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy TA, Moreland SM, Andrews-Polymenis H, Detweiler CS. The ferric enterobactin transporter Fep is required for persistent Salmonella enterica serovar typhimurium infection. Infect Immun. 2013;81:4063–4070. doi: 10.1128/IAI.00412-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nairz M, Fritsche G, Brunner P, Talasz H, Hantke K, Weiss G. Interferon-gamma limits the availability of iron for intramacrophage Salmonella typhimurium. Eur J Immunol. 2008;38:1923–1936. doi: 10.1002/eji.200738056. [DOI] [PubMed] [Google Scholar]
- Nix RN, Altschuler SE, Henson PM, Detweiler CS. Hemophagocytic macrophages harbor Salmonella enterica during persistent infection. PLoS Pathog. 2007;3:e193. doi: 10.1371/journal.ppat.0030193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojeda JF, Martinson DA, Menscher EA, Roop RM., 2nd The bhuQ Gene Encodes a Heme Oxygenase That Contributes to the Ability of Brucella abortus 2308 To Use Heme as an Iron Source and Is Regulated by Irr. J Bacteriol. 2012;194:4052–4058. doi: 10.1128/JB.00367-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulley JT, Anderson ES, Roop RM., 2nd Brucella abortus requires the heme transporter BhuA for maintenance of chronic infection in BALB/c mice. Infect Immun. 2007;75:5248–5254. doi: 10.1128/IAI.00460-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecqueur L, D'Autreaux B, Dupuy J, Nicolet Y, Jacquamet L, Brutscher B, Michaud-Soret I, Bersch B. Structural changes of Escherichia coli ferric uptake regulator during metal-dependent dimerization and activation explored by NMR and X-ray crystallography. J Biol Chem. 2006;281:21286–21295. doi: 10.1074/jbc.M601278200. [DOI] [PubMed] [Google Scholar]
- Pilonieta MC, Nagy TA, Jorgensen DR, Detweiler CS. A glycine betaine importer limits Salmonella stress resistance and tissue colonization by reducing trehalose production. Mol Microbiol. 2012;84:296–309. doi: 10.1111/j.1365-2958.2012.08022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabsch W, Methner U, Voigt W, Tschäpe H, Reissbrodt R, Williams PH. Role of receptor proteins for enterobactin and 2,3-dihydroxybenzoylserine in virulence of Salmonella enterica. Infect Immun. 71. 2003:6953–6961. doi: 10.1128/IAI.71.12.6953-6961.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabsch W, Voigt W, Reissbrodt R, Tsolis RM, Bäumler AJ. Salmonella typhimurium IroN and FepA proteins mediate uptake of enterobactin but differ in their specificity for other siderophores. J Bacteriol. 1999;181:3610–3612. doi: 10.1128/jb.181.11.3610-3612.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberger CM, Finlay BB. Macrophages inhibit Salmonella typhimurium replication through MEK/ERK kinase and phagocyte NADPH oxidase activities. J Biol Chem. 2002;277:18753–18762. doi: 10.1074/jbc.M110649200. [DOI] [PubMed] [Google Scholar]
- Sheppard M, Webb C, Heath F, Mallows V, Emilianus R, Maskell D, Mastroeni P. Dynamics of bacterial growth and distribution within the liver during Salmonella infection. Cell Microbiol. 2003;5:593–600. doi: 10.1046/j.1462-5822.2003.00296.x. [DOI] [PubMed] [Google Scholar]
- Silva-Herzog E, Detweiler CS. Intracellular microbes and haemophagocytosis. Cell Microbiol. 2008;10:2151–2158. doi: 10.1111/j.1462-5822.2008.01192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva-Herzog E, Detweiler CS. Salmonella enterica Replication in Hemophagocytic Macrophages Requires Two Type Three Secretion Systems. Infect. Immun. 2010;78:3369–3377. doi: 10.1128/IAI.00292-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suvarnapunya AE, Lagasse HA, Stein MA. The role of DNA base excision repair in the pathogenesis of Salmonella enterica serovar Typhimurium. Mol Microbiol. 2003;48:549–559. doi: 10.1046/j.1365-2958.2003.03460.x. [DOI] [PubMed] [Google Scholar]
- Trosky JE, Liverman AD, Orth K. Yersinia outer proteins: Yops. Cell Microbiol. 2008;10:557–565. doi: 10.1111/j.1462-5822.2007.01109.x. [DOI] [PubMed] [Google Scholar]
- Tsolis RM, Baumler AJ, Heffron F, Stojiljkovic I. Contribution of TonB- and Feo-mediated iron uptake to growth of Salmonella typhimurium in the mouse. Infect Immun. 1996;64:4549–4556. doi: 10.1128/iai.64.11.4549-4556.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsolis RM, Baumler AJ, Stojiljkovic I, Heffron F. Fur regulon of Salmonella typhimurium: identification of new iron-regulated genes. J Bacteriol. 1995;177:4628–4637. doi: 10.1128/jb.177.16.4628-4637.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsolis RM, Young GM, Solnick JV, Bäumler AJ. From bench to bedside: stealth of enteroinvasive pathogens. Nat Rev Microbiol. 2008;6:883–892. doi: 10.1038/nrmicro2012. [DOI] [PubMed] [Google Scholar]
- Van Zandt KE, Sow FB, Florence WC, Zwilling BS, Satoskar AR, Schlesinger LS, Lafuse WP. The iron export protein ferroportin 1 is differentially expressed in mouse macrophage populations and is present in the mycobacterial-containing phagosome. J Leukoc Biol. 2008;84:689–700. doi: 10.1189/jlb.1107781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Torres A, Fang F. Oxygen-dependent anti-Salmonella activity of macrophages. Trends Microbiol. 2001;9:29–33. doi: 10.1016/s0966-842x(00)01897-7. [DOI] [PubMed] [Google Scholar]
- Williams PH, Rabsch W, Methner U, Voigt W, Tschäpe H, Reissbrodt R. Catecholate receptor proteins in Salmonella enterica: role in virulence and implications for vaccine development. Vaccine. 2006;24:3840–3844. doi: 10.1016/j.vaccine.2005.07.020. [DOI] [PubMed] [Google Scholar]
- Zhou D, Hardt WD, Galán JE. Salmonella typhimurium encodes a putative iron transport system within the centisome 63 pathogenicity island. Infect Immun. 1999;67:1974–1981. doi: 10.1128/iai.67.4.1974-1981.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.