Abstract
Background
Symptomatic neuroma occurs in 13% to 32% of amputees, causing pain and limiting or preventing the use of prosthetic devices. Targeted nerve implantation (TNI) is a procedure that seeks to prevent or treat neuroma-related pain in amputees by implanting the proximal amputated nerve stump onto a surgically denervated portion of a nearby muscle at a secondary motor point so that regenerating axons might arborize into the intramuscular motor nerve branches rather than form a neuroma. However, the efficacy of this approach has not been demonstrated.
Questions/purposes
We asked: Does TNI (1) prevent primary neuroma-related pain in the setting of acute traumatic amputation and (2) reduce established neuroma pain in upper- and lower-extremity amputees?
Methods
We retrospectively reviewed two groups of patients treated by one surgeon: (1) 12 patients who underwent primary TNI for neuroma prevention at the time of acute amputation and (2) 23 patients with established neuromas who underwent neuroma excision with secondary TNI. The primary outcome was the presence or absence of palpation-induced neuroma pain at last followup, based on a review of medical records. The patients presented here represent 71% of those who underwent primary TNI (12 of 17) and 79% of those who underwent neuroma excision with secondary TNI (23 of 29 patients) during the period in question; the others were lost to followup. Minimum followup was 8 months (mean, 22 months; range, 8–60 months) for the primary TNI group and 4 months (mean, 22 months; range, 4–72 months) for the secondary TNI group.
Results
At last followup, 11 of 12 patients (92%) after primary TNI and 20 of 23 patients (87%) after secondary TNI were free of palpation-induced neuroma pain.
Conclusions
TNI performed either primarily at the time of acute amputation or secondarily for the treatment of established symptomatic neuroma is associated with a low frequency of neuroma-related pain. By providing a distal target for regenerating axons, TNI may offer an effective strategy for the prevention and treatment of neuroma pain in amputees.
Level of Evidence
Level IV, therapeutic study. See Instructions for Authors for a complete description of levels of evidence.
Introduction
In 2005, there were approximately 1.6 million amputees in the United States, a number that is projected to more than double by 2050 [49]. Painful neuroma occurs in 13% to 32% of amputees [12, 13, 15, 36, 42], causing pain that is often exacerbated even by a well-fitted prosthesis. Neuromas can cause significant disability, as patients often limit or discontinue the use of their prosthetic device due to neuroma-related pain.
Many nonsurgical treatments for this frustrating problem have been suggested, including neuropathic medications, topical or injectable anesthetics [6], chemical axonotmesis [19], and radiofrequency ablation [39, 44]. These techniques are inconsistently successful in the setting of discrete symptomatic neuroma. A similar array of surgical treatments has been advocated, including traction neurectomy, nerve capping, end-to-side or centrocentral coaptation [1, 4, 17, 35, 40, 41, 43, 46], and nerve transposition into healthy bone, vein, or muscle [16, 20, 21, 30, 33, 34]. Given the large number of potentially effective treatments and the conflicting evidence, there is no consensus regarding the best practice for neuroma treatment. A common approach to treating painful neuromas is to bury the stump in healthy muscle. While this technique can position the nerve stump and likely subsequent neuroma in a protected location and potentially reduce pain, symptoms sometimes recur [3, 9].
After a neurotmetic injury, proximal nerve stump axons sprout into the extracellular milieu. In the case of simple transection, these axons will be guided by neurotrophic factors to the nearby distal stump [8, 38]. After entering this distal stump, the regenerating axons can proceed down the existing column of Schwann cells and basal lamina to reinnervate the denervated target tissue [24]. However, in the case of limb amputation, the transected proximal stump is deprived of a distal nerve and receptive tissue target. The result is a directionless proliferation of axons, fibroblasts, Schwann cells, and blood vessels, which manifests clinically as a painful neuroma.
To address the underlying pathophysiology of neuromas, we devised a technique wherein we provide these idle axons with a target for organized reinnervation. Targeted nerve implantation (TNI) can be applied as a primary treatment for neuroma prevention at the time of amputation or secondarily for treatment of an established neuroma. In this procedure, a secondary motor nerve branch to an expendable portion of healthy muscle is isolated and divided, and then the proximal major nerve stump is implanted atop the carefully identified secondary motor point within the surgically denervated muscle. The theoretical mechanism justifying trial of this technique is that, rather than forming (or reforming) a neuroma, the transected axons might enter this secondary motor point and arborize along the intramuscular motor branches in an organized fashion [24] such that the resulting nerve ending is less painful to palpation than if it had formed a neuroma. However, the efficacy of this approach has not been demonstrated.
In this study, we therefore determined whether TNI (1) prevents primary neuroma-related pain in the setting of acute traumatic amputation and (2) reduces established neuroma pain in upper- and lower-extremity amputees.
Patients and Methods
A retrospective cohort of eligible patients was created and clinical data for each patient were abstracted from the electronic medical record by a single investigator (MAP). Inclusion required a history of major upper- or lower-extremity amputation treated with TNI and clinical followup exceeding 4 months postoperative with documented examination by the surgeon and/or physiatrist. This minimum amount of followup required is consistent with the best available evidence for the timescale of neuroma formation in humans and animals [7, 15, 45, 48]. In the primary TNI cohort, TNI was performed in the setting of acute traumatic amputation for primary neuroma prevention. Nontraumatic mechanisms of amputation were excluded from this primary TNI cohort. In the secondary TNI cohort, TNI was performed with neuroma resection in patients with established painful neuroma at the site of previous amputation to prevent secondary neuroma recurrence. All remote mechanisms of amputation were included in this secondary TNI cohort.
Between 2006 and 2012, we performed 47 surgical procedures (132 nerves) for neuroma prevention after acute amputation. Seventeen of these procedures (36%, 48 nerves) were primary TNI, and adequate clinical followup was available in 12 of these patients (71%, 34 nerves), who comprise our primary TNI study cohort. In the same time period, we performed 101 surgical procedures (174 nerves) for the treatment of established painful neuromas in amputees. Twenty-nine of these procedures (29%, 50 nerves) were secondary TNI, and adequate clinical followup was available for 23 of these patients (79%, 42 nerves), who comprise our secondary TNI study cohort.
Since we began using TNI in 2006, we have employed this technique for all acute upper-extremity amputations at or above the level of the elbow, with the exception of three patients early in the study period. We have utilized TNI in a single patient with acute lower-extremity amputation but still generally employ simple traction neurectomy most of the time. For secondary treatment of upper-extremity neuromas, our practice slowly shifted over the course of the study period from our previous strategy of neuroma excision and nerve transection within an area protected by healthy muscle to almost exclusively TNI. For secondary treatment of lower-extremity neuromas, we still frequently use neuroma excision and nerve transection within a protected area and employ TNI selectively, often in patients with recurrent disease.
Both cohorts were predominantly young male patients. The primary TNI cohort consisted of mostly upper-extremity amputees, while the secondary TNI cohort consisted of mostly lower-extremity amputees (Table 1). Operative and clinical details are given for individual patients in the primary TNI group (Table 2) and secondary TNI group (Table 3). Minimum followup was 8 months (mean, 22 months; range, 8–60 months) for the primary TNI group and 4 months (mean, 22 months; range, 24–72 months) for the secondary TNI group. Our institutional review board committee approved this study.
Table 1.
Description of the primary and secondary TNI groups
| Variable | Primary TNI group | Secondary TNI group |
|---|---|---|
| Number of patients screened | 17 | 29 |
| Number of patients included | 12 (34 nerves) | 23 (42 nerves) |
| Age (years)* | 34 (14–59) | 44 (20–80) |
| Number of male patients | 10 (83%) | 15 (65%) |
| Upper extremity treated (number of patients) | 11 (92%) | 8 (35%) |
| Lower extremity treated (number of patients) | 1 (8%) | 15 (65%) |
| Duration from amputation to TNI* | 4 (2–10) days | 80 (8–361) months |
| Clinical followup (months)* | 22 (8–60) | 22 (4–72) |
* Values are expressed as mean, with range in parentheses; TNI = targeted nerve implantation.
Table 2.
Primary TNI in the setting of acute traumatic amputation
| Patient | Acute mechanism | Formal amputation level | Specific TNI (nerve to muscle motor point) | Number of nerve transfers | Followup (months) | Neuroma pain after TNI | Phantom pain after TNI | Comments |
|---|---|---|---|---|---|---|---|---|
| 1 | All-terrain vehicle rollover | Shoulder disarticulation | MeN to pectoralis major, RN to teres major, UN to serratus | 3 | 11 | No | Yes | Palpable, painless ulnar neuroma |
| 2 | Caught in conveyor belt | Shoulder disarticulation | MeN to pectoralis major, RN to latissimus dorsi, UN to pectoralis minor | 3 | 18 | Possible | Yes | Hypersensitivity at amputation site and phantom limb pain; neuroma possible |
| 3 | Crush injury | Short transhumeral | McN to pectoralis major, MeN to biceps, RN to triceps, UN to latissimus dorsi | 4 | 57 | No | No | |
| 4 | Caught in conveyor belt | Short transhumeral | McN to medial biceps, MeN to lateral biceps, RN to triceps, UN to latissimus dorsi | 4 | 16 | No | No | |
| 5 | Caught in conveyor belt | Short transhumeral | MeN to upper pectoralis major, RN to serratus anterior, UN to lower pectoralis major | 3 | 60 | No | Yes | |
| 6 | Motorcycle crash | Short transhumeral | MeN to pectoralis major, UN to teres major, RN to latissimus dorsi | 3 | 23 | No | Yes | |
| 7 | Hit by train | Long transhumeral | MeN to medial biceps, RN to lateral triceps, UN to brachialis | 3 | 14 | No | No | |
| 8 | Motor vehicle crash | Long transhumeral | McN to lateral biceps, MeN to medial biceps, RN to lateral triceps, UN to brachialis | 4 | 9 | No | No | |
| 9 | Motor vehicle crash | Long transhumeral | MeN to biceps, RN to brachialis | 2 | 8 | No | No | Phantom sensation but no phantom pain |
| 10 | Motor vehicle crash | Elbow disarticulation | MeN to medial biceps, UN to triceps | 2 | 29 | No | Yes | |
| 11 | Caught in conveyor belt | Above elbow | MeN to pectoralis major, formal UN to TDN transfer | 1 | 21 | No | Yes | |
| 12 | Motorcycle crash | Knee disarticulation | TN to medial hamstring, PN to lateral hamstring | 2 | 12 | No | No |
All primary TNI procedures were performed within several days of injury and formal surgical amputation; TNI = targeted nerve implantation; MeN= median nerve; RN = radial nerve; UN = ulnar nerve; McN = musculocutaneous nerve; TDN = thoracodorsal nerve; TN = tibial nerve; PN = peroneal nerve.
Table 3.
Secondary TNI with excision of established painful amputation stump neuroma
| Patient | Remote mechanism | Amputation level | Neuromas excised | Specific TNI (nerve to muscle motor point) |
Number of TNI transfers | Concomitant surgical treatments* | Duration between amputation and TNI (years) | Neuroma pain before TNI | Phantom pain before TNI | Neuroma pain after TNI | Phantom pain after TNI | Followup (months) | Comments |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 13 | Crushed by machinery | Shoulder disarticulation | McN, MeN, RN, UN | McN to clavicular head of pectoralis major | 1 | Excision of HO, MeN to pectoralis minor motor branch nerve transfer, RN to LTN transfer, UN to pectoralis major motor branch nerve transfer | 1 | Yes | Yes | No | No | 5 | Only one true TNI; because of very short proximal nerve stumps, three other transfers done to distal nerve trunks, rather than to secondary motor points |
| 14 | Motor vehicle crash | Transhumeral | MeN, UN, RN | MeN to medial biceps, UN to medial triceps, RN to lateral triceps | 3 | Excision of HO | 4 | Yes | Yes | No | Yes | 6 | |
| 15 | Motor vehicle crash | Transhumeral | McN, MeN, RN, UN | McN to medial biceps, MeN to medial biceps, RN to medial triceps, UN to posterior triceps | 4 | Excision of HO | 7 | Yes | Yes | Possible | Yes | 39 | Never experienced significant pain relief; has stump pain similar to previous neuroma; ultrasound recommended to evaluate for recurrent neuroma |
| 16 | Crushed by machinery | Transhumeral | McN, RN | McN to biceps, RN to triceps | 2 | Removal of hardware | 2 | Yes | Yes | No | Yes | 57 | Also had excision of ulnar neuroma, but TNI target not available |
| 17 | Electrocution | Transradial | MeN, superficial RN, UN | MeN to biceps, RN to brachialis, UN to triceps | 2 | Excision of HO, traction neurectomy of superficial RN | 3 | Yes | No | No | No | 9 | |
| 18 | Crushed by machinery | Transradial | RN | RN to supinator or abductor pollicus longus | 1 | 2 | Yes | No | No | No | 72 | Has significant stump pain; neuroma not suspected (negative MRI, atypical description) | |
| 19 | Explosion | Transradial | UN, MeN, RN | MeN to undersurface of FDS, RN to lateral FDS, UN to medial triceps | 3 | Excision of HO | 45 | Yes | No | No | Yes | 4 | |
| 20 | Purpura fulminans | CMC joint | MeN, UN | MeN to FDP, UN to FDS | 2 | 1 | Yes | Yes | Yes | Yes | 17 | Surgery had no effect at all on stump pain or phantom pain | |
| 21 | Necrotizing fasciitis | Above knee | ScN | ScN to medial hamstring | 1 | 12 | Yes | No | No | No | 4 | ||
| 22 | Motor vehicle crash | Above knee | TN, PN | TN to medial hamstring, PN to lateral hamstring | 2 | 14 | Yes | No | No | No | 9 | ||
| 23 | Fall | Above knee | TN, PN | TN to medial hamstring, PN to lateral hamstring | 2 | 1 | Yes | No | No | No | 20 | ||
| 24 | Kicked by horse | Above knee | TN | TN to hamstring | 1 | Repair of tibiofibular bone bridge nonunion | 5 | Yes | No | No | No | 23 | |
| 25 | Necrotizing soft tissue infection | Above knee | TN, PN | TN to medial hamstring, PN to lateral hamstring | 2 | Excision of HO | 1 | Yes | No | No | No | 11 | Has new saphenous neuroma |
| 26 | Gunshot wound | Above knee | ScN | Tibial portion of ScN to medial hamstring, peroneal portion of ScN to lateral hamstring | 2 | Excision of HO | 15 | Yes | No | No | No | 49 | |
| 27 | Mangled in auger | Above knee | ScN | Tibial portion of ScN to medial hamstring, peroneal portion of ScN to lateral hamstring | 2 | Excision of HO | 1 | Yes | Yes | No | Yes | 33 | |
| 28 | Pedestrian struck by automobile | Above knee | ScN | ScN to lateral hamstring | 1 | 28 | Yes | No | Yes | No | 4 | Pain previously occurred several times per month, lasting for days; now it occurs a few times per week but lasts only moments; patient much less symptomatic postoperatively | |
| 29 | Logging accident | Below knee | TN | TN to medial hamstring | 1 | 7 | Yes | No | No | No | 5 | ||
| 30 | Burn | Below knee | PN | PN to lateral hamstring | 1 | 21 | Yes | No | No | No | 13 | Postoperative infection requiring I&D | |
| 31 | Hit by train | Below knee | Superficial and deep PN | Superficial PN to peroneus brevis | 1 | 4 | Yes | No | No | No | 48 | Deep PN not transferred because it was transected deep in well-padded muscle | |
| 32 | Motor vehicle collision | Below knee | TN, PN | TN to medial hamstring, PN to lateral hamstring | 2 | Excision of prominent portion of fibula | 14 | Yes | No | No | No | 12 | |
| 33 | Motor vehicle collision | Below knee | Superficial and deep PN | Superficial and deep PN to tibialis anterior | 2 | 11 | Yes | Yes | No | Yes | 19 | ||
| 34 | Peripheral vascular disease | Below knee | TN, PN | TN to medial hamstring, PN to lateral hamstring | 2 | Excision of HO | 3 | Yes | Yes | No | Yes | 4 | |
| 35 | Crushed by machinery | Below knee | TN, PN | TN to medial hamstring, PN to lateral hamstring | 2 | Excision of HO, tibiofibular arthrodesis | 4 | Yes | Yes | No | Yes | 41 |
* Revision of the amputation stump was also performed when indicated; TNI = targeted nerve implantation; CMC = carpometacarpal; McN = musculocutaneous nerve; MeN = median nerve; RN = radial nerve; UN = ulnar nerve; ScN = sciatic nerve; TN = tibial nerve; PN = peroneal nerve; SuN = sural nerve; FDS = flexor digitorum superficialis; FDP = flexor digitorum profundus; HO = hypertrophic ossification; LTN = long thoracic nerve; I&D = irrigation and débridement.
All TNI surgeries and clinical followups were completed at our Level I trauma center with an established amputation program. All procedures were performed at a single major academic trauma center by the same surgeon (DGS). Neuroma-related pain was defined as localizable tenderness and a reproducible Tinel’s sign within the amputation stump in the expected anatomic location of a transected nerve. Neuromas were confirmed in each patient receiving secondary TNI by the operative identification of the neuroma. Imaging was not utilized routinely for the purposes of diagnosing neuroma. A diagnosis of phantom pain was made using the subjective history relayed by the patient [23].
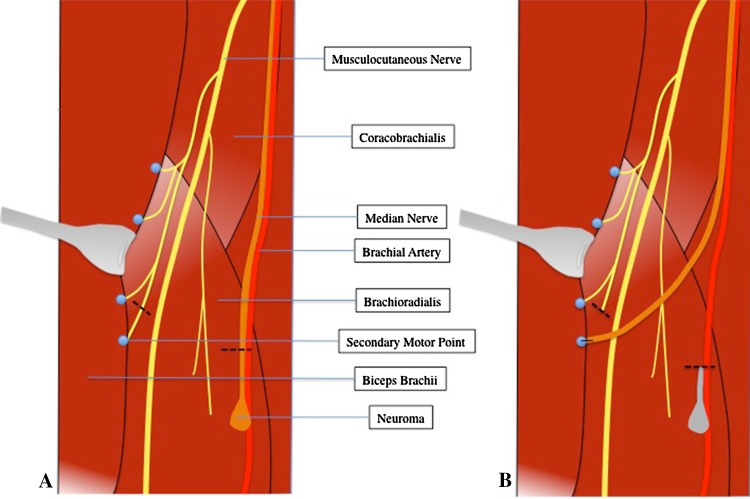
The operative approach and specific nerve implantations performed in each patient depended on the amputation level, anatomy, zone of injury, and other problems concomitantly addressed. For the TNI portion of the procedure, the proximal nerve stump was prepared by trimming it proximal to the zone of injury as observed grossly in the case of primary TNI or by resection of the end neuroma in the case of secondary TNI. A section of healthy proximal muscle was then exposed, and using monopolar cautery at a setting of five, the surface of the muscle was serially stimulated in different areas. This was done until we encountered a location where stimulation triggered a broad concentric contraction, rather than the usual local trace muscle fiber contraction. This was our indication of a motor point or secondary motor nerve branch coursing superficially within the muscle. Here, gentle blunt intramuscular dissection was undertaken until the small nerve could be isolated (generally just a few millimeters below the muscle surface) and sharply transected. The proximal major nerve stump was then trimmed back to the level of the chosen secondary motor point and then carefully sutured to the muscle such that the cut end lay directly over the distal stump of the transected intramuscular motor branch (Fig. 1). The intention of this procedure was to allow nerve ingrowth into this denervated muscle segment via the transected nerve to avoid formation (or reformation) of an end neuroma. A representative TNI procedure is illustrated (Fig. 1).
Fig. 1A–B.
Diagrams illustrate an example of secondary TNI. (A) A median neuroma in the setting of previous elbow disarticulation is shown. (B) The median neuroma has been resected and the median nerve stump has been implanted into a secondary motor point of the biceps brachii muscle. Primary TNI would be illustrated similarly, except without the component of neuroma resection.
In lower-extremity amputations, donor-recipient proximity was generally the most important consideration in planning multiple implantations. At the transfemoral level, we generally found it easiest to implant the tibial and peroneal portions of the sciatic nerve into the medial and lateral hamstring musculature, respectively. In long transhumeral amputees, this would ideally mean implantation of the median nerve into the biceps muscle, radial nerve into the triceps, and ulnar nerve into the brachialis. At the shoulder level, the pectoralis major and minor, teres major, serratus, and latissimus dorsi are potential target muscles.
The primary outcome was the presence of typical neuroma pain as assessed by clinical examination. Determination of this outcome was by chart review, performed by a single investigator (MAP) who was not involved in the care of included patients.
Results
Eleven of 12 patients (92%) treated with primary TNI at the time of traumatic amputation were free of palpation-induced neuroma pain at their last followup (Table 2). The sole patient with potential treatment failure (Patient 2) developed nonspecific hypersensitivity at his amputation stump, for which neuroma has not been ruled out as a cause. Painful phantom limb sensations developed in six of the 12 patients in this cohort.
Of the 23 previous amputees who underwent neuroma resection and secondary TNI, 20 (87%) were free of palpation-induced neuroma pain at their last followup (Table 3). Patient 20 experienced no relief of symptoms with surgery but subsequently responded completely to mirror therapy. Patient 15 had a postoperative course dominated by medical and psychiatric comorbidity, which complicated serial assessments. However, at last followup, she described persistent stump pain, for which neuroma cannot be ruled out as a contributing factor. Patient 28 had drastic improvement in his palpation-induced neuroma pain but had some infrequent and brief residual symptoms. Painful phantom limb sensation was present in eight patients before secondary TNI and in eight patients afterward. This represents persistent phantom pain in seven patients, new onset of phantom pain in one, and resolution of preoperative phantom pain in one.
Discussion
Symptomatic neuroma occurs in 13% to 32% of amputees [12, 13, 15, 36, 42], causing pain and limiting the use of prosthetic devices. In this retrospective study, we examined the clinical course of patients who have undergone TNI in an attempt to either prevent formation of neuromas after acute traumatic upper- and lower-extremity amputation or to treat painful neuromas in amputees.
There are several limitations of this study that must be noted, most of which derive from its design as a retrospective study. First is the issue of transfer bias (loss to followup); 11 of 46 (24%) eligible patients were lost to followup, and while our mean followup is nearly 2 years, late recurrence of symptomatic neuroma is still a possibility. Additionally, our conclusions are limited by the uncertainty that is inherent in attributing pre- and/or postintervention pain to neuroma. For example, nine of 23 had heterotopic ossification resected concomitantly with their TNI, which could potentially confound our results by allowing misattribution of clinical improvement. Furthermore, the use of palpation-induced pain greatly simplifies the complex pain complaints of our amputee population. Still, while our records were reasonably specific in distinguishing neuroma complaints from phantom limb pain, there remains the potential for overlapping diagnoses, especially in cases of spontaneously firing neuroma that may mimic phantom limb pain. Additionally, we treated neuroma pain generally as an all-or-none phenomenon. While this was generally the pattern we observed in our patients, it may be that more detailed questioning would reveal subtler gradation.
Selection bias cannot be ruled out, as patients were assigned to TNI (rather than alternative treatments) in a nonrandomized fashion using a decision algorithm that was not necessarily constant nor entirely based on objective findings. Furthermore, as the treating team was involved in the assessment of outcomes, assessor bias is a possibility. For these and other reasons, our preliminary findings will require further study and validation. Finally, while we theorize that TNI may prevent neuroma occurrence/recurrence by offering a viable path and destination for regenerating axons, this study relied on clinical presentation and examination as our only indicator of neuroma recurrence. In no group did we have imaging studies to confirm the absence of neuroma after surgery. It is possible that neuromas could have occurred or recurred asymptomatically and that any clinical improvement might simply reflect proximal relocation rather than nonrecurrence. By examining the histologic outcomes of TNI and comparing them to those of muscle implantation [30], one might determine whether our proposed model of axonal arborization into the denervated muscle occurs in vivo and whether this truly represents any improvement over simple muscle implantation. This type of investigation would also be necessary to exclude the possibility of iatrogenic neuroma formation due to the secondary motor branch transection required for TNI.
In this preliminary study, we found TNI to be effective in the prevention of neuroma formation at the time of amputation. Only one of 12 patients in the primary treatment group developed neuroma-related pain over a mean followup of 20 months. Given that 13% to 32% of patients with amputation develop neuroma-related pain [12, 13, 42], our rate of 8% suggests that treatment of the resected nerve with TNI at the time of amputation might offer some benefit to this population. While numerous surgical techniques for neuroma prevention have shown promise in animal models of major limb amputation [2, 7, 14, 31, 41, 43, 45], to our knowledge, the few available human studies are limited to the treatment of digital amputations. In 1984, Gorkisch et al. [18] found only a single instance of symptomatic neuroma in a series of 30 digital amputations treated with centrocentral coaptation. This finding was supported by a randomized, controlled trial done by Belcher and Pandya [5] in 2000, wherein they found that digital amputation stumps managed with centrocentral coaptation were less tender than those managed with simple nerve transection. Yüksel et al. [47] compared three methods of using epineural tissue to cover transected digital nerve ends and found that epineural grafts performed better than epineural flaps or epineural ligatures. While these studies provide some context, the different goals, mechanics, and demands on digital and major limb amputations make them inappropriate for comparison to our current series. Further study, ideally in the form or randomized, controlled trials in humans, are needed to compare available techniques for the primary prevention of amputation neuromas.
Among patients treated with TNI for established neuroma pain, the majority had resolution of their neuroma symptoms. As other authors have observed [15], we found that surgical treatment of neuromas had little effect on other types of concomitant pain, including centrally mediated phantom limb pain. In an attempt to contextualize our results, we performed a literature search seeking any case series from the past 30 years describing interventions for the treatment of established neuroma pain after major limb amputation (notable exclusions include digital amputation neuromas and reports with five patients or less) (Table 4). Among the papers reviewed, our results are most instructively compared to a similar case series in which symptomatic amputation site neuromas were managed using the popular method of resection and burial of the proximal stump within healthy muscle [10]. In that series, Ducic et al. [10] observed a decrease in palpation-induced neuroma pain without any symptomatic neuroma recurrence over 22 months. While these studies are not directly comparable due to methodologic and population differences, the results of Ducic et al. [10] do provide a counterpoint that underscores that our series cannot demonstrate a clear-cut benefit of secondary TNI over existing techniques at this time.
Table 4.
Literature review of the human data regarding techniques for the treatment of symptomatic neuroma within the stump after previous major limb amputation
| Study | Year | Technique | Number of patients | Extremity | Mean followup (months) | Number of patients lost to followup | Outcome summary | Notes |
|---|---|---|---|---|---|---|---|---|
| Martini and Fromm [32] | 1989 | Fascicular shortening and epineural sealing with tissue glue | 36 | 8 UE 14 LE 25 digital |
17 | None | Complete relief in 28 patients; improvement in 5; no change in 3 | Series included 14 patients with digital neuromas; limb subseries was not extractable from the presented data |
| Barberá and Albert-Pampló [4] | 1993 | Centrocentral anastomosis | 22 | 22 LE | 15 | None | Resolution of neuroma pain in 21 patients; recurrent painful neuroma in 1 |
|
| Koch et al. [26] | 2003 | Neuroma resection and vein implantation | 23 | 13 UE 7 LE 3 digital |
27 | None | No pain in 12 patients; mild in 8; moderate in 2; severe in 1 |
Only 7 patients had amputation stump neuromas. this subseries was not extractable from the presented data |
| Ducic et al. [10] | 2008 | Neuroma excision with stump implantation into proximal muscle | 21 | 1 UE 20 LE |
23 | None | Mean VAS pain score reduced from 8.04 to 1.07 | |
| Gruber et al. [19] | 2008 | Ultrasound-guided phenol injection | 82 | 11 UE 71 LE |
6 | 30/82 | Median VAS pain score reduced from of 10 to 3 | Patients underwent 1–3 treatment sessions |
| Kesikburun et al. [25] | 2013 | Ultrasound-guided steroid injection | 14 | 14 LE | 1.5 | None | 50% of patients had > 50% decrease in pain score | |
| Current study | 2014 | Secondary targeted nerve implantation | 23 | 7 UE 15 LE 1 shoulder |
22 | 6/29 | 20 patients free of neuroma pain |
Outcome data are simplified for concise presentation; the reader is directed to the references provided for details such as subgroup analysis and statistical calculations; small series later supplanted by larger series from the same group are excluded; UE = upper extremity; LE = lower extremity.
Targeted muscle reinnervation (TMR), as described by Kuiken and Dumanian [11, 22, 27–29, 37], is a related method of primary nerve stump management in amputees, which is based on many of the same principles as TNI. Both procedures entail transfer of a transected proximal nerve stump and rely on a surgically denervated muscle to encourage orderly reinnervation. While conceptually similar, there exist important differences that are informed by the specific goals of each procedure. TMR is distinct in its employment of much more formal and proximal nerve transfers into defined muscle segments with the aim of creating separate EMG signals usable for myoelectric prosthesis control. As a means to the end of improved myoelectric prosthesis control (which has been demonstrated in many patients), TMR seeks to provide a large number of healthy regenerating axons to a defined downstream muscle unit, which when reinnervated will provide a clean and easily detectable surface EMG. As such, large proximal recipient motor nerves are used (allowing formal neurorraphy), thereby denervating and reinnervating defined portions of muscle. With its more restricted goal of neuroma prevention, TNI conversely is unconcerned with the ultimate spatial arrangement and EMG characteristics of the nascent nerve-muscle unit, allowing more distal transfer.
As a method to control myoelectric prostheses, we believe TMR represents an advance. In situations where myoelectric prosthesis control is not at stake, the technique of TNI may be advantageous in that it avoids proximal dissection within a potentially unstable stump and does not require the surgical sacrifice of a proximal major motor nerve, as does TMR. TNI should be considered as a strategy for neuroma prevention in patients who are not candidates for immediate TMR and myoelectric prosthesis. This may include many lower-extremity amputees, distal upper-extremity amputees, and transhumeral or shoulder disarticulation amputees who are unsuited for or uninterested in a myoelectric prosthesis at the time of operation. In addition, because TNI requires the denervation of a portion of muscle (albeit small), there is some concern that primary TNI might compromise the outcomes of secondary TMR. We believe that the small portion of muscle denervated by TNI is negligible in this context, and it seems unlikely that its sacrifice would prevent a patient from being able to undergo a successful TMR procedure in the future for myoelectric prosthesis control.
The technique we used is intriguing in that it may prevent neuroma recurrence, rather than simply controlling it. While our preliminary results are encouraging, our study cannot directly demonstrate neuroma occurrence, recurrence, or the absence thereof. Patient-reported outcome measures or imaging modalities, such as MRI and ultrasound, may help quantify pre- and postoperative neuroma pain and size, respectively. In addition, histologic examination of TNI sites could help determine whether axonal arborization into target muscle occurs after TNI in vivo, especially when compared to simple muscle implantation [30]. Although TNI demonstrates potential for the prevention and treatment of neuromas, further research is necessary to confirm the mechanism and compare TNI to existing techniques.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Ashley L, Stallings JO. End-to-side nerve flap for treatment of painful neuroma: a 15-year follow-up. J Am Osteopath Assoc. 1988;88:621–624. [PubMed] [Google Scholar]
- 2.Aszmann OC, Korak KJ, Rab M, Grünbeck M, Lassmann H, Frey M. Neuroma prevention by end-to-side neurorraphy: an experimental study in rats. J Hand Surg Am. 2003;28:1022–1028. doi: 10.1016/S0363-5023(03)00379-4. [DOI] [PubMed] [Google Scholar]
- 3.Balcin H, Erba P, Wettstein R, Schaefer DJ, Pierer G, Kalbermatten DF. A comparative study of two methods of surgical treatment for painful neuroma. J Bone Joint Surg Br. 2009;91:803–808. doi: 10.1302/0301-620X.91B6.22145. [DOI] [PubMed] [Google Scholar]
- 4.Barberá J, Albert-Pampló R. Centrocentral anastomosis of the proximal nerve stump in the treatment of painful amputation neuromas of major nerves. J Neurosurg. 1993;79:331–334. doi: 10.3171/jns.1993.79.3.0331. [DOI] [PubMed] [Google Scholar]
- 5.Belcher HJ, Pandya AN. Centro-central union for the prevention of neuroma formation after finger amputation. J Hand Surg Br. 2000;25:154–159. doi: 10.1054/jhsb.2000.0372. [DOI] [PubMed] [Google Scholar]
- 6.Chabal C, Jacobson L, Russell LC, Burchiel KJ. Pain response to perineuromal injection of normal saline, epinephrine, and lidocaine in humans. Pain. 1992;49:9–12. doi: 10.1016/0304-3959(92)90181-A. [DOI] [PubMed] [Google Scholar]
- 7.Chim H, Miller E, Gliniak C, Cohen ML, Guyuron B. The role of different methods of nerve ablation in prevention of neuroma. Plast Reconstr Surg. 2013;131:1004–1012. doi: 10.1097/PRS.0b013e3182879ec2. [DOI] [PubMed] [Google Scholar]
- 8.Chiu DT, Smahel J, Chen L, Meyer V. Neurotropism revisited. Neurol Res. 2004;26:381–387. doi: 10.1179/016164104225013815. [DOI] [PubMed] [Google Scholar]
- 9.Dellon AL, Mackinnon SE. Treatment of the painful neuroma by neuroma resection and muscle implantation. Plast Reconstr Surg. 1986;77:427–438. doi: 10.1097/00006534-198603000-00016. [DOI] [PubMed] [Google Scholar]
- 10.Ducic I, Mesbahi AN, Attinger CE, Graw K. The role of peripheral nerve surgery in the treatment of chronic pain associated with amputation stumps. Plast Reconstr Surg. 2008;121:908–914; discussion 915–917. [DOI] [PubMed]
- 11.Dumanian GA, Ko JH, O’Shaughnessy KD, Kim PS, Wilson CJ, Kuiken TA. Targeted reinnervation for transhumeral amputees: current surgical technique and update on results. Plast Reconstr Surg. 2009;124:863–869. doi: 10.1097/PRS.0b013e3181b038c9. [DOI] [PubMed] [Google Scholar]
- 12.Ebrahimzadeh MH, Fattahi AS. Long-term clinical outcomes of Iranian veterans with unilateral transfemoral amputation. Disabil Rehabil. 2009;31:1873–1877. doi: 10.1080/09638280902810968. [DOI] [PubMed] [Google Scholar]
- 13.Ebrahimzadeh MH, Hariri S. Long-term outcomes of unilateral transtibial amputations. Mil Med. 2009;174:593–597. doi: 10.7205/MILMED-D-02-8907. [DOI] [PubMed] [Google Scholar]
- 14.Elwakil TF, Elkharbotly AA. Role of Nd: YAG laser for prevention of neuroma formation: an in vivo experimental study. Lasers Med Sci. 2008;23:163–168. doi: 10.1007/s10103-007-0461-y. [DOI] [PubMed] [Google Scholar]
- 15.Geraghty TJ, Jones LE. Painful neuromata following upper limb amputation. Prosthet Orthot Int. 1996;20:176–181. doi: 10.3109/03093649609164440. [DOI] [PubMed] [Google Scholar]
- 16.Goldstein SA, Sturim HS. Intraosseous nerve transposition for treatment of painful neuromas. J Hand Surg Am. 1985;10:270–274. doi: 10.1016/S0363-5023(85)80120-9. [DOI] [PubMed] [Google Scholar]
- 17.González-Darder J, Barberá J, Abellán MJ, Mora A. Centrocentral anastomosis in the prevention and treatment of painful terminal neuroma: an experimental study in the rat. J Neurosurg. 1985;63:754–758. doi: 10.3171/jns.1985.63.5.0754. [DOI] [PubMed] [Google Scholar]
- 18.Gorkisch K, Boese-Landgraf J, Vaubel E. Treatment and prevention of amputation neuromas in hand surgery. Plast Reconstr Surg. 1984;73:293–299. doi: 10.1097/00006534-198402000-00027. [DOI] [PubMed] [Google Scholar]
- 19.Gruber H, Glodny B, Bodner G, Kopf H, Bendix N, Galiano K, Strasak A, Peer S. Practical experience with sonographically guided phenol instillation of stump neuroma: predictors of effects, success, and outcome. AJR Am J Roentgenol. 2008;190:1263–1269. doi: 10.2214/AJR.07.2050. [DOI] [PubMed] [Google Scholar]
- 20.Hazari A, Elliot D. Treatment of end-neuromas, neuromas-in-continuity and scarred nerves of the digits by proximal relocation. J Hand Surg Br. 2004;29:338–350. doi: 10.1016/j.jhsb.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 21.Herbert TJ, Filan SL. Vein implantation for treatment of painful cutaneous neuromas: a preliminary report. J Hand Surg Br. 1998;23:220–224. doi: 10.1016/S0266-7681(98)80178-2. [DOI] [PubMed] [Google Scholar]
- 22.Hijjawi JB, Kuiken TA, Lipschutz RD, Miller LA, Stubblefield KA, Dumanian GA. Improved myoelectric prosthesis control accomplished using multiple nerve transfers. Plast Reconstr Surg. 2006;118:1573–1578. doi: 10.1097/01.prs.0000242487.62487.fb. [DOI] [PubMed] [Google Scholar]
- 23.Hsu E, Cohen SP. Postamputation pain: epidemiology, mechanisms, and treatment. J Pain Res. 2013;6:121–136. doi: 10.2147/JPR.S32299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ide C. Peripheral nerve regeneration. Neurosci Res. 1996;25:101–121. doi: 10.1016/0168-0102(96)01042-5. [DOI] [PubMed] [Google Scholar]
- 25.Kesikburun S, Yaşar E, Dede I, Göktepe S, Tan AK. Ultrasound-guided steroid injection in the treatment of stump neuroma: pilot study. J Back Musculoskelet Rehabil. 2013 November 27 [Epub ahead of print]. [DOI] [PubMed]
- 26.Koch H, Haas F, Hubmer M, Rappl T, Scharnagl E. Treatment of painful neuroma by resection and nerve stump transplantation into a vein. Ann Plast Surg. 2003;51:45–50. doi: 10.1097/01.SAP.0000054187.72439.57. [DOI] [PubMed] [Google Scholar]
- 27.Kuiken TA, Dumanian GA, Lipschutz RD, Miller LA, Stubblefield KA. The use of targeted muscle reinnervation for improved myoelectric prosthesis control in a bilateral shoulder disarticulation amputee. Prosthet Orthot Int. 2004;28:245–253. doi: 10.3109/03093640409167756. [DOI] [PubMed] [Google Scholar]
- 28.Kuiken TA, Li G, Lock BA, Lipschutz RD, Miller LA, Stubblefield KA, Englehart KB. Targeted muscle reinnervation for real-time myoelectric control of multifunction artificial arms. JAMA. 2009;301:619–628. doi: 10.1001/jama.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuiken TA, Miller LA, Lipschutz RD, Lock BA, Stubblefield K, Marasco PD, Zhou P, Dumanian GA. Targeted reinnervation for enhanced prosthetic arm function in a woman with a proximal amputation: a case study. Lancet. 2007;369:371–380. doi: 10.1016/S0140-6736(07)60193-7. [DOI] [PubMed] [Google Scholar]
- 30.Mackinnon SE, Dellon AL, Hudson AR, Hunter DA. Alteration of neuroma formation by manipulation of its microenvironment. Plast Reconstr Surg. 1985;76:345–353. doi: 10.1097/00006534-198509000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Marcol W, Larysz-Brysz M, Kucharska M, Niekraszewicz A, Slusarczyk W, Kotulska K, Wlaszczuk P, Wlaszczuk A, Jedrzejowska-Szypulka H, Lewin-Kowalik J. Reduction of post-traumatic neuroma and epineural scar formation in rat sciatic nerve by application of microcrystallic chitosan. Microsurgery. 2011;31:642–649. doi: 10.1002/micr.20945. [DOI] [PubMed] [Google Scholar]
- 32.Martini A, Fromm B. A new operation for the prevention and treatment of amputation neuromas. J Bone Joint Surg Br. 1989;71:379–382. doi: 10.1302/0301-620X.71B3.2722924. [DOI] [PubMed] [Google Scholar]
- 33.Mass DP, Ciano MC, Tortosa R, Newmeyer WL, Kilgore ES. Treatment of painful hand neuromas by their transfer into bone. Plast Reconstr Surg. 1984;74:182–185. doi: 10.1097/00006534-198408000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Mobbs RJ, Vonau M, Blum P. Treatment of painful peripheral neuroma by vein implantation. J Clin Neurosci. 2003;10:338–339. doi: 10.1016/S0967-5868(03)00010-9. [DOI] [PubMed] [Google Scholar]
- 35.Muehleman C, Rahimi F. Effectiveness of an epineurial barrier in reducing axonal regeneration and neuroma formation in the rat. J Foot Surg. 1990;29(260–264):32. [PubMed] [Google Scholar]
- 36.Nelson AW. The painful neuroma: the regenerating axon verus the epineural sheath. J Surg Res. 1977;23:215–221. doi: 10.1016/0022-4804(77)90024-5. [DOI] [PubMed] [Google Scholar]
- 37.O’Shaughnessy KD, Dumanian GA, Lipschutz RD, Miller LA, Stubblefield K, Kuiken TA. Targeted reinnervation to improve prosthesis control in transhumeral amputees: a report of three cases. J Bone Joint Surg Am. 2008;90:393–400. doi: 10.2106/JBJS.G.00268. [DOI] [PubMed] [Google Scholar]
- 38.Politis MJ, Ederle K, Spencer PS. Tropism in nerve regeneration in vivo: attraction of regenerating axons by diffusible factors derived from cells in distal nerve stumps of transected peripheral nerves. Brain Res. 1982;253:1–12. doi: 10.1016/0006-8993(82)90667-9. [DOI] [PubMed] [Google Scholar]
- 39.Restrepo-Garces CE, Marinov A, McHardy P, Faclier G, Avila A. Pulsed radiofrequency under ultrasound guidance for persistent stump-neuroma pain. Pain Pract. 2011;11:98–102. doi: 10.1111/j.1533-2500.2010.00398.x. [DOI] [PubMed] [Google Scholar]
- 40.Robbins TH. Nerve capping in the treatment of troublesome terminal neuromata. Br J Plast Surg. 1986;39:239–240. doi: 10.1016/0007-1226(86)90089-5. [DOI] [PubMed] [Google Scholar]
- 41.Sakai Y, Ochi M, Uchio Y, Ryoke K, Yamamoto S. Prevention and treatment of amputation neuroma by an atelocollagen tube in rat sciatic nerves. J Biomed Mater Res Part B Appl. Biomater. 2005;73:355–360. [DOI] [PubMed]
- 42.Soroush M, Modirian E, Soroush M, Masoumi M. Neuroma in bilateral upper limb amputation. Orthopedics. 2008;31. pii: orthosupersite.com/view.asp?rID=32929. [DOI] [PubMed]
- 43.Swanson AB, Boeve NR, Lumsden RM. The prevention and treatment of amputation neuromata by silicone capping. J Hand Surg Am. 1977;2:70–78. doi: 10.1016/S0363-5023(77)80013-0. [DOI] [PubMed] [Google Scholar]
- 44.Tamimi MA, McCeney MH, Krutsch J. A case series of pulsed radiofrequency treatment of myofascial trigger points and scar neuromas. Pain Med. 2009;10:1140–1143. doi: 10.1111/j.1526-4637.2009.00646.x. [DOI] [PubMed] [Google Scholar]
- 45.Tyner TR, Parks N, Faria S, Simons M, Stapp B, Curtis B, Sian K, Yamaguchi KT. Effects of collagen nerve guide on neuroma formation and neuropathic pain in a rat model. Am J Surg. 2007;193:e1–e6. doi: 10.1016/j.amjsurg.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 46.Wood VE, Mudge MK. Treatment of neuromas about a major amputation stump. J Hand Surg Am. 1987;12:302–306. doi: 10.1016/S0363-5023(87)80297-6. [DOI] [PubMed] [Google Scholar]
- 47.Yüksel F, Kişlaoğlu E, Durak N, Uçar C, Karacaoğlu E. Prevention of painful neuromas by epineural ligatures, flaps and grafts. Br J Plast Surg. 1997;50:182–185. doi: 10.1016/S0007-1226(97)91367-9. [DOI] [PubMed] [Google Scholar]
- 48.Zeltser R, Beilin B, Zaslansky R, Seltzer Z. Comparison of autotomy behavior induced in rats by various clinically-used neurectomy methods. Pain. 2000;89:19–24. doi: 10.1016/S0304-3959(00)00342-0. [DOI] [PubMed] [Google Scholar]
- 49.Ziegler-Graham K, MacKenzie EJ, Ephraim PL, Travison TG, Brookmeyer R. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch Phys Med Rehabil. 2008;89:422–429. doi: 10.1016/j.apmr.2007.11.005. [DOI] [PubMed] [Google Scholar]