Abstract
Background
Restoration of biomechanics is a major goal in THA. Imageless navigation enables intraoperative control of leg length equalization and offset reconstruction. However, the effect of navigation compared with intraoperative fluoroscopy is unclear.
Questions/purposes
We asked whether intraoperative use of imageless navigation (1) improves the relative accuracy of leg length and global and femoral offset restoration; (2) increases the absolute precision of leg length and global and femoral offset equalization; and (3) reduces outliers in a reconstruction zone of ± 5 mm for leg length and global and femoral offset restoration compared with intraoperative fluoroscopy during minimally invasive (MIS) THA with the patient in a lateral decubitus position.
Methods
In this prospective study a consecutive series of 125 patients were randomized to either navigation-guided or fluoroscopy-controlled THA using sealed, opaque envelopes. All patients received the same cementless prosthetic components through an anterolateral MIS approach while they were in a lateral decubitus position. Leg length, global or total offset (representing the combination of femoral and acetabular offset), and femoral offset differences were restored using either navigation or fluoroscopy. Postoperatively, residual leg length and global and femoral offset discrepancies were analyzed on magnification-corrected radiographs of the pelvis by an independent and blinded examiner using digital planning software. Accuracy was defined as the relative postoperative difference between the surgically treated and the unaffected contralateral side for leg length and offset, respectively; precision was defined as the absolute postoperative deviation of leg length and global and femoral offset regardless of lengthening or shortening of leg length and offset throughout the THA. All analyses were performed per intention-to-treat.
Results
Analyzing the relative accuracy of leg length restoration we found a mean difference of 0.2 mm (95% CI, −1.0 to +1.4 mm; p = 0.729) between fluoroscopy and navigation, 0.2 mm (95 % CI, −0.9 to +1.3 mm; p = 0.740) for global offset and 1.7 mm (95 % CI, +0.4 to +2.9 mm; p = 0.008) for femoral offset. For the absolute precision of leg length and global and femoral offset equalization, there was a mean difference of 1.7 ± 0.3 mm (p < 0.001) between fluoroscopy and navigation. The biomechanical reconstruction with a residual leg length and global and femoral offset discrepancy less than 5 mm and less than 8 mm, respectively, succeeded in 93% and 98%, respectively, in the navigation group and in 54% and 95%, respectively, in the fluoroscopy group.
Conclusions
Intraoperative fluoroscopy and imageless navigation seem equivalent in accuracy and precision to reconstruct leg length and global and femoral offset during MIS THA with the patient in the lateral decubitus position.
Level of Evidence
Level I, therapeutic study. See the Instructions for Authors for a complete description of levels of evidence.
Introduction
The issues of leg length, global offset, and femoral offset in THA are intimately related. Leg length discrepancy after THA is associated with gait disorders, back and knee pain, aseptic loosening, early revision surgery, and litigation [7, 8, 11, 13]. Likewise, patient satisfaction as measured by the patient-reported Oxford Hip Score correlates with the reduction of leg length discrepancy [13]. Offset has been shown to correlate with hip stability, ROM, abduction strength, wear, and impingement [1, 17, 30, 32]. Minimally invasive surgical (MIS) techniques with reduced incision lengths and less-extensive exposures are safe without greater operative complication or component malrotation rates [25]. However, with these approaches it is more difficult for the orthopaedic surgeon to estimate leg length and offset changes intraoperatively [2, 35]. Intraoperative fluoroscopy is widely used to control leg length and offset restoration during THA; however, the exposure to radiation carries risks for the entire surgical team [18, 24, 31, 34]. Previous studies have shown that leg length after THA can be restored within a ± 6-mm range with intraoperative use of radiography when the patient is in a supine position [10]. With the patient in the lateral decubitus position, cup orientation can be improved with the help of intraoperative AP radiographs during the THA [9]. It was shown that use of intraoperative AP radiographs can optimize component position and leg length with the patient in a lateral decubitus position. In 25% of the cases, the radiograph led to a change in intraoperative management for leg length, and postoperatively 86% of leg length discrepancies were within ± 6 mm [6].
Imageless navigation systems without the need for preoperative or intraoperative image acquisition and exposure to radiation have been reported to increase precision in positioning the acetabular component and to assist the surgeon in achieving appropriate leg length and offset values [27, 28]. However, to our knowledge, there have been no randomized comparative trials of intraoperative fluoroscopy and navigation to evaluate the potential benefit of navigation-guided reconstruction of the biomechanics in THA.
Therefore, we asked whether intraoperative use of imageless navigation (1) improves relative accuracy; (2) increases absolute precision; and (3) reduces outliers in a reconstruction zone of ± 5 mm for leg length, global offset, and femoral offset restoration compared with intraoperative fluoroscopy during MIS THA with the patient in a lateral decubitus position.
Patients and Methods
During a registered, prospective randomized controlled trial (DRKS00000739, German Clinical Trials Register) evaluating navigation for THA, we randomized patients for with or without the use of navigation. The random allocation sequence was computer-generated in a permuted block randomization design by statisticians of the Institute of Medical Statistics and Epidemiology Munich using certificated randomization software (Rancode 3.6 Professional, IDV, Gauting, Germany). Permuted blocks of four, six, and eight participants were used to ensure a balanced allocation sequence. This sequence then was placed in sealed, consecutively numbered, opaque envelopes. These envelopes were kept in a locked filing cabinet in the office of the surgeon who opened the envelopes in order of participant recruitment on the day of surgery. This investigation was approved by the local Ethics Commission. The current study is a subgroup analysis from a larger cohort [26]. The purpose of this larger study was to assess whether the artificial joint’s ROM can be improved by a computer-assisted, functional optimization of cup position and containment. The navigated measurements of leg length and offset were independent from this cup optimization algorithm and the method and moment of the biomechanical reconstruction of leg length and offset (with the cup and the trial stem/head in place) was the same during surgery. A sovereign power calculation was performed for investigation of the three primary endpoints in this subgroup analysis: leg length equalization and global and femoral offset restoration. This relative accuracy was defined as the relative postoperative difference between the surgically treated and the unaffected contralateral side for leg length and offset, respectively. Consequently, each of the corresponding hypotheses was tested on a Bonferroni-adjusted, two-sided 5%/3 = 1.6% significance level. The relevant difference between navigation and fluoroscopy was set at 5 mm. A conservative estimate of the corresponding standard deviation after THA ie, 8 mm, was taken from literature regarding leg length [19]. Based on these considerations, a sample size of 56 in each group achieved a power of 80% using two-sample t-tests (nQuery Advisor 7.0, Statistical Solutions Ltd, Cork, Ireland). Secondary endpoints were relative precision and number of outliers. Precision was defined as the absolute postoperative deviation of leg length and global and femoral offset regardless of lengthening or shortening of leg length and offset throughout the THA. According to one study, a postoperative leg length or offset inequality greater than 5 mm was regarded as an outlier [22].
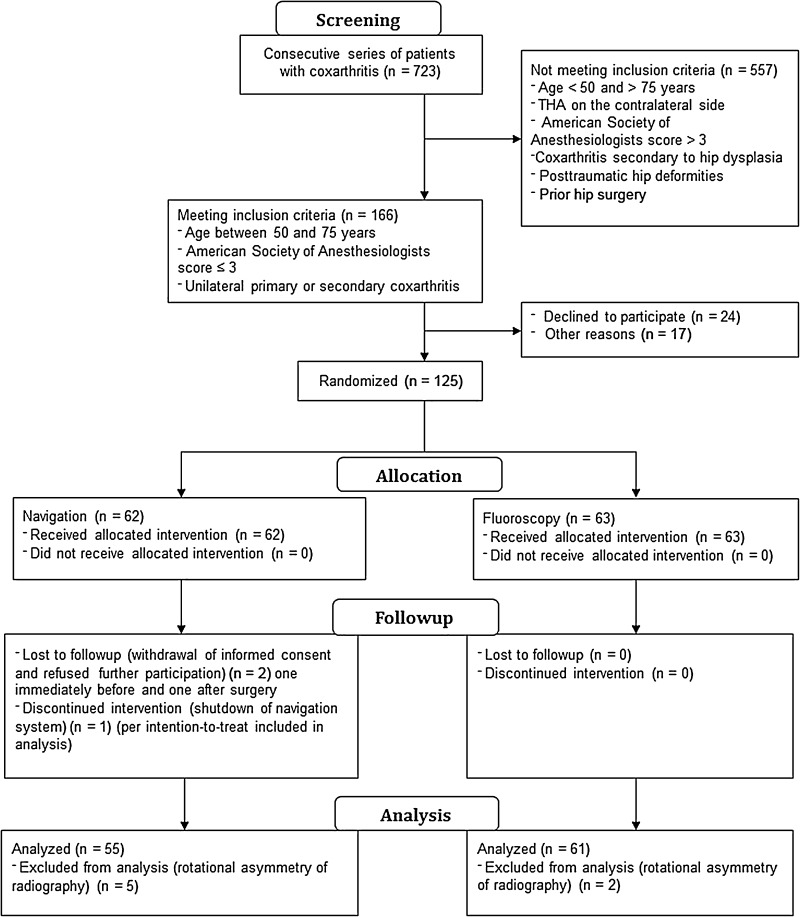
A consecutive series of 723 patients with coxarthritis was screened. Our inclusion criteria were: patients 50 to 75 years old, an American Society of Anesthesiologists (ASA) score of 3 or less, unilateral coxarthritis (up to Kellgren-Lawrence Grade 2 of the contralateral side), no prior hip surgery, and no hip dysplasia or trauma. The ASA physical status classification system assesses the fitness of patients before surgery, whereas a patient with ASA Physical Status Grade 1 is a healthy patient, ASA Physical Status Grade 2 is a patient with mild systemic disease, and ASA Physical Status Grade 3 is a patient with severe systemic disease. In total, 557 patients did not meet the inclusion criteria, 24 patients declined participation in the study, and another 17 patients could not be included for other reasons (cancellation of surgery or increased inflammatory factors during a blood examination at the day before surgery).
In total, a consecutive series of 125 patients was enrolled in this single-center study. After giving written consent, the patients were randomly allocated to either fluoroscopy or navigation-guided THA. During the study, two patients withdrew informed consent (one patient immediately before surgery and one patient after surgery) and refused further participation and use of their data. According to our informed consent and after consultation of our statistical partner in Munich, these two patients were regarded as dropouts. In one case, the navigation system shut down during the procedure and according to our intention-to-treat protocol, this patient remained in the data set. For seven patients, the postoperative radiographs were not taken according to the standardized protocol and could not be analyzed. These patients were regarded as dropouts (Fig. 1).
Fig. 1.
A flow chart of the study is shown.
In total, records of 116 patients who had THAs (61 fluoroscopies and 55 navigation-guided cases) were included for analysis. Anthropometric characteristics were comparable in both groups (Table 1). Before surgery, restoration of leg length and offset was templated on digital AP radiographs of the pelvis with the help of digital planning software (MediCAD, Hectec, Germany) for all patients. The radiographic magnification was corrected using a scaling object of known diameter. With the unaffected contralateral side serving as a reference, leg length discrepancy was equalized and global and femoral offset discrepancies restored. THAs were performed by four orthopaedic surgeons (JG, ES, MW, TR) from Regensburg University Medical Center. Each surgeon had experience with more than 200 fluoroscopy and 200 navigation-controlled THAs. All operations were performed with the patient in the lateral decubitus position through a MIS anterolateral approach to the hip after an intermuscular and interneural tissue plane between the tensor muscle and the gluteus medius muscle [21]. Press-fit components (Pinnacle®; DePuy, Warsaw, IN, USA) and cement-free hydroxyapatite-coated stems (Corail®; DePuy) were used. The tribologic pairing consisted of polyethylene liners and metal heads with a diameter of 32 or 28 mm. For patients who were randomly allocated to the navigation group, intraoperative leg length and offset changes were measured using an imageless navigation system (Hip 6.0 prototype; Brainlab, Feldkirchen, Germany). The registration process was performed as described by Renkawitz et al. [26]. As part of the navigation data entry, two connected K-wires (3.2 mm in diameter) were inserted in the ipsilateral iliac wing and the ventrolateral 1/3 of the distal femur. A dynamic reference array then was connected to these wires. A preoperative neutral reference position of the leg was defined by holding it in approximately 0° flexion, abduction, and internal and external rotation. The navigation system stored the relative orientation (transformation) between the femur and pelvis dynamic reference array according to this position. After inserting the trial and final implants and hip reduction, the initial neutral reference position was reproduced. The navigation system guided the surgeon by showing the deviation between the current and the initial neutral reference alignment (Fig. 2). After insertion of the final implants, leg length and offset change as presented on the screen were stored three times for reproducibility and the mean of these measurements was considered the true leg length and offset change. All surgeons aimed to restore leg length and offset according to the preoperative plan.
Table 1.
Anthropometric and operative characteristics of the study group
| Characteristics | Fluoroscopy | Navigated | p value |
|---|---|---|---|
| Probands (number) | 61 | 55 | |
| Age (years) | 62.5 ± 7.6 | 62.4 ± 7.6 | 0.987 |
| Sex (men/women) | 29/32 | 27/28 | 0.868 |
| BMI (kg/m2) | 27.1 ± 4.4 | 27.7 ± 4.1 | 0.459 |
| Treatment side (right/left) | 36/25 | 27/28 | 0.288 |
| ASA Class 1 | 14 | 10 | 0.763 |
| ASA Class 2 | 31 | 28 | |
| ASA Class 3 | 16 | 17 | |
| Kellgren-Lawrence score | 9 (5–10) | 8 (6–10) | 0.058 |
| Length of skin incision (cm) | 10 (8–12) | 10 (8–13) | 0.188 |
| Duration of THA (minutes) | 64 (43–115) | 77 (51–126) | < 0.001 |
ASA = American Society of Anesthesiologists.
Fig. 2.
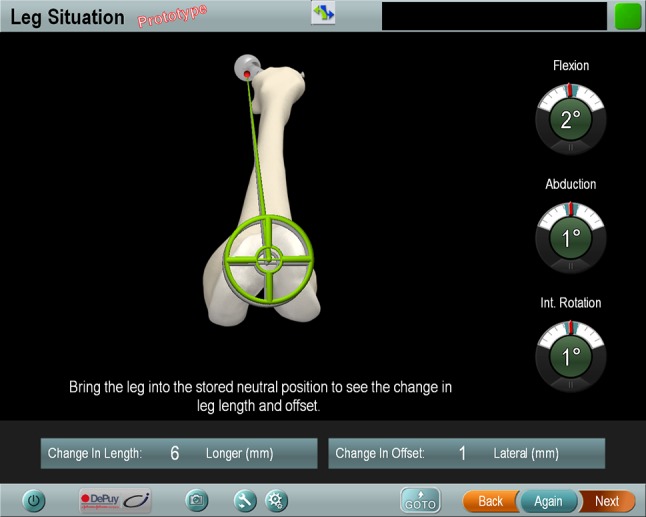
In the navigation group, intraoperative leg length and offset changes were calculated by the navigation system and shown on the screen. Int. rotation = internal rotation.
For patients who were randomly allocated to the fluoroscopy group, intraoperative leg length and offset equalization were estimated by the surgeons visually and with fluoroscopy. After insertion of the trial and final implants and hip reduction, the leg was placed in a neutral position and covered with sterile surgical cloth. Then a sterile-covered 90° rotated C-arm (Ziehm Vision; Ziehm Imaging GmbH, Nuremberg, Germany) was positioned with the detector at the back of the pelvis. After focusing the center of the beam on the symphysis, the distance from the trochanter tip to the lateral shoulder (superior edge) of the femoral stem and the distance to the center of rotation on the fluoroscopy screen were assessed by eye. This was compared with the preoperative plan as seen on a screen. In case of disagreement, biomechanical reconstruction was corrected by variation of the femoral head size, the liner, the insertion depth, or the size of the stem (Fig. 3).
Fig. 3.
In the fluoroscopy group, intraoperative fluoroscopy was performed using a sterile-covered 90° rotated C-arm with the patient in a lateral decubitus position.
Postoperatively leg length, global offset, and femoral offset discrepancies were evaluated on standardized digital AP radiographs of the pelvis using the same digital planning software as for templating. Magnification was corrected by the documented size of the metal head. Femoral length (used as a surrogate for leg length) was obtained by drawing a line through the inferior aspects of the teardrops (interteardrop line or Koehler line) and measuring the distance to the superior margin of the lower trochanter [24]. Cup offset (also known as acetabular offset) was defined as the distance from the center of rotation of the femoral head to the teardrop along the transteardrop line touching the inferior margins of the teardrop [4, 23]. Femoral offset was defined as the distance from the center of rotation of the femoral head to the central axis of the femur [14]. To maximize accuracy, the distances between the long axis and the outer contours of the femur were checked carefully on the radiographs. The axes were placed in a way that the distances between preoperative and postoperative radiographs matched in the proximal and the more distal parts of the femoral canal. All postoperative radiographic measurements were performed by a blinded observer (MW), independent from the surgical team.
The difference between the surgically treated and nonoperated sides was calculated as biomechanical discrepancy of each parameter (Fig. 4). Calculation of the study results and statistical evaluation were performed at the Institute of Medical Statistics and Epidemiology, Technische Universitaet, Munich. For statistical analysis, normally and nonnormally distributed continuous data are presented as mean ± standard deviation or median (range), respectively. Accordingly, group comparisons were performed using two-sided t-tests or Mann-Whitney U-tests. Absolute and relative frequencies were given for categorical data and compared between study groups using chi-square tests. For analysis of primary endpoints, leg length, global offset, and femoral offset values were compared between the navigation and the fluoroscopy-controlled groups using two-sided t-tests because there were no evident deviations from the normal distribution of the data. For analysis of secondary endpoints, absolute leg length, global offset, and femoral offset values were compared using a Poisson regression analysis because of their skewed distribution and the discrete scale of measurements. As an additional sensitivity analysis, baseline values were included in these models as independent factors according to the European Medicines Agency guideline [3], “Points to Consider on Adjustment for Baseline Covariates.” All secondary hypotheses were tested in an explorative manner on a two-sided 5% significance level. IBM SPSS Statistics 20 (SPSS Inc, Chicago, IL, USA) and the statistical software package R (The R Foundation for Statistical Computing, Vienna, Austria) were used for analysis.
Fig. 4.
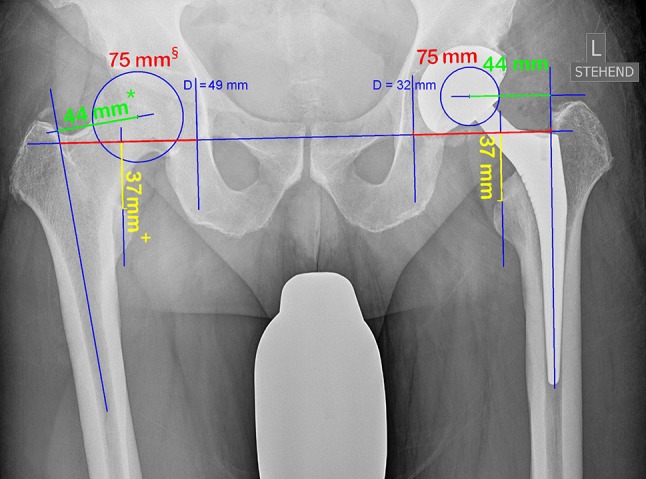
Changes of leg length and global and femoral offset were assessed on magnification-corrected postoperative AP radiographs of the pelvis. + = leg length; § = global offset; * = femoral offset.
Results
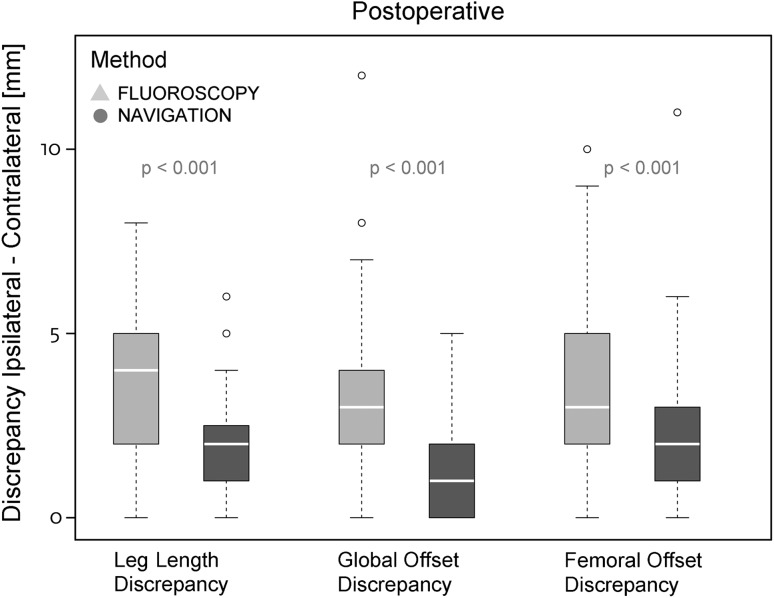
With the numbers available, there were no differences between the fluoroscopy and navigation groups in terms of accuracy of leg length difference or restoration of total offset. For femoral offset, a statistical, however not clinically relevant, effect was observed (Table 2). For leg length equalization we found a mean difference of 0.2 mm (95 % CI, −1.0 to +1.4 mm; p = 0.729) between the fluoroscopy and the navigation-based technique, 0.2 mm (95 % CI, −0.9 to +1.3 mm; p = 0.740) for global offset, and 1.7 mm (95 % CI, +0.4 to +2.9 mm; p = 0.008) for femoral offset. Preoperatively, the existing biomechanical differences were comparable with a mean leg length difference of −0.4 mm (95 % CI, −1.7 to +1.0 mm; p = 0.589) between the fluoroscopy and navigation groups, 0.2 mm (95 % CI, −1.2 to +1.6 mm; p = 0.769) for global offset, and −0.1 mm (95 % CI, −1.6 to +1.5 mm; p = 0.945) for femoral offset. Analysis of the absolute precision showed reduced deviations from the operative goal, equalization of biomechanics for leg length, global offset and femoral offset, in the navigation group compared with the fluoroscopy group (Table 3). The mean absolute leg length discrepancies were 1.8 ± 0.2 mm for navigation and 3.5 ± 0.2 mm for fluoroscopy (p < 0.001). The mean difference between fluoroscopy versus navigation was 1.7 ± 0.3 mm. Global offsets were restored with an absolute mean of 1.4 ± 0.2 mm for the navigation group and 3.1 ± 0.2 mm for the fluoroscopy group (p < 0.001), with a mean difference of 1.7 ± 0.3 mm for fluoroscopy versus navigation. Analysis for absolute femoral offset discrepancies resulted in means of 2.0 ± 0.2 mm for the navigation group and 3.6 ± 0.2 mm for the fluoroscopy group (p < 0.001), with a mean difference of 1.7 ± 0.3 mm for fluoroscopy versus navigation (Fig. 5). In addition, the assessment of differences between fluoroscopy and navigation was adjusted for baseline values because the latter were included as additional independent variables in the Poisson models. The adjusted difference of postoperative leg length discrepancy was 1.8 ± 0.3 mm between methods (= discrepancy fluoroscopy − discrepancy navigation). For global and femoral offset, this difference was 1.7 ± 0.3 mm, respectively. The p value was less than 0.001 for all comparisons.
Table 2.
Mean postoperative differences between groups
| Primary endpoints | Group | Number of patients | Mean (mm) | Standard deviation | p value |
|---|---|---|---|---|---|
| Leg length | Fluoroscopy | 61 | 0.6 | 4.1 | |
| Navigation | 55 | 0.4 | 2.2 | 0.729 | |
| Global offset | Fluoroscopy | 61 | −0.4 | 3.9 | |
| Navigation | 55 | −0.6 | 1.9 | 0.740 | |
| Femoral offset | Fluoroscopy | 61 | 2.1 | 3.9 | |
| Navigation | 55 | 0.4 | 2.7 | 0.008* |
* p < 0.016 (significance level after Bonferroni adjustment for multiple testing).
Table 3.
Absolute postoperative differences by Poisson models
| Secondary endpoints | Group | Mean (mm) | Standard error | Wald 95% CI | p value | |
|---|---|---|---|---|---|---|
| Low value | High value | |||||
| Leg length | Fluoroscopy | 3.5 | 0.2 | 3.0 | 4.0 | |
| Navigation | 1.8 | 0.2 | 1.4 | 2.1 | < 0.001* | |
| Global offset | Fluoroscopy | 3.1 | 0.2 | 2.7 | 3.6 | |
| Navigation | 1.4 | 0.2 | 1.1 | 1.8 | < 0.001* | |
| Femoral offset | Fluoroscopy | 3.6 | 0.2 | 3.2 | 4.2 | |
| Navigation | 2.0 | 0.2 | 1.6 | 2.3 | < 0.001* | |
* Significance level p < 0.05.
Fig. 5.
Absolute postoperative leg length and global and femoral offset discrepancies between the navigation and fluoroscopy controlled group were compared through Poisson models.
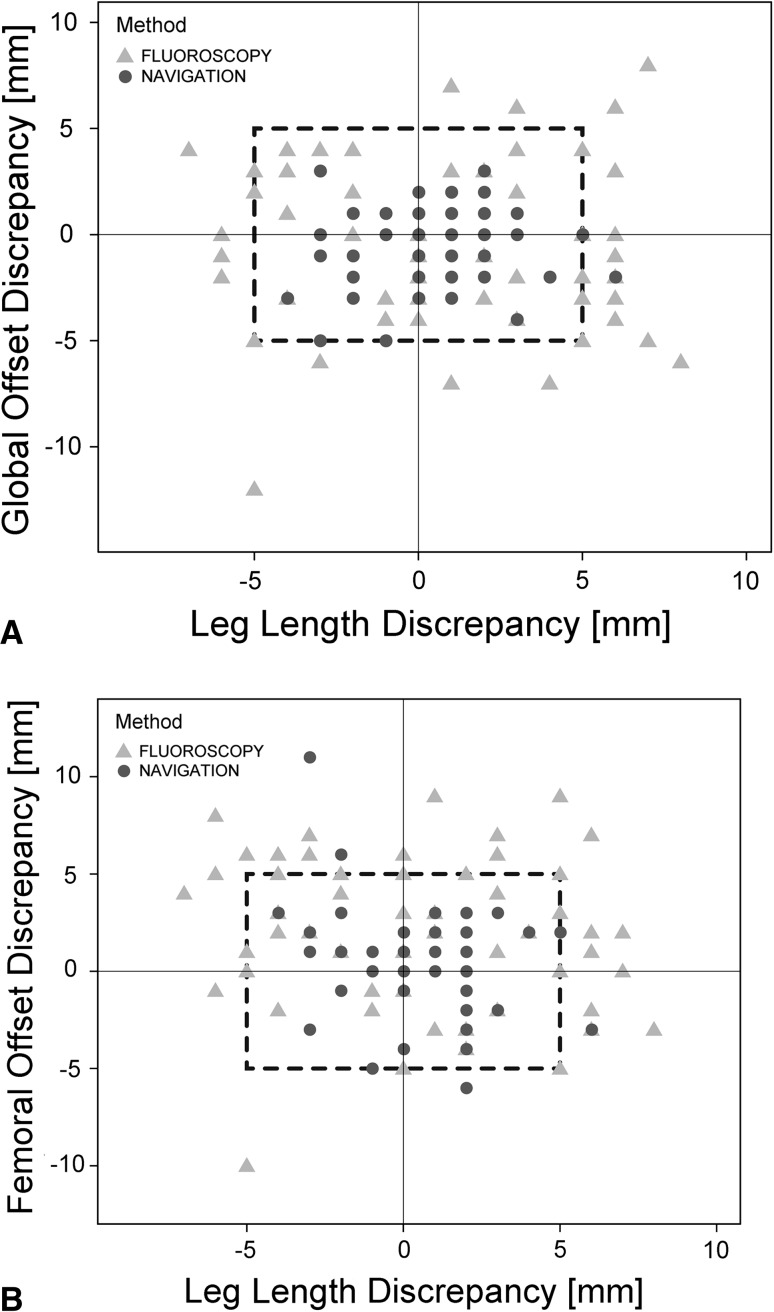
The numbers of postoperative outliers outside a reconstruction zone of 5 mm were lower in the navigation group than in the fluoroscopy group for leg length, global offset, and femoral offset (Fig. 6). Ninety-eight percent (54 of 55) of the navigation group and 77% (47 of 61) of the fluoroscopy group were inside the 5-mm tolerance limit for leg length (p < 0.001). For global offset reconstruction, 100% (55 of 55) of the navigation and 85% (52 of 61) of fluoroscopy values were within the 5-mm boundary (p = 0.003). Femoral offset was successfully restored in 95% (52 of 55) of the navigation and 80% (49 of 61) of the fluoroscopy THAs (p = 0.023). In total, 93% (51 of 55) of the navigation and 54% (33 of 61) of fluoroscopy THAs were within a benchmark of 5 mm for all three parameters (p < 0.001).
Fig. 6A–B.
The results of postoperative (A) leg length and global offset and (B) leg length and femoral offset restoration in the 5-mm reconstruction zone were compared between navigation and fluoroscopy-guided THA using scatterplots.
Discussion
To optimize function, hip mechanics should be restored to as near normal as possible. Femoral offset correlates with hip stability, joint reaction forces, polyethylene wear, and ROM [17, 30]. Likewise, marked leg length discrepancy after THA contributes to gait asymmetry, knee and back pain, abnormal force transmission across the hip, revision surgery, and finally litigation [8, 11, 13]. Accordingly, the use of computer navigation in total joint arthroplasties has become more prevalent [5], although its use clinically is still limited owing to the additional operative time and expense. We therefore sought to determine whether reconstruction of leg length and global and femoral offset is more accurate, precise, and consistent during a MIS THA performed with the patient in the lateral decubitus position compared with our conventional freehand technique using intraoperative fluoroscopy with computer-assisted, navigated THA. We hypothesized that intraoperative use of navigation would (1) improve the relative accuracy of restoration of leg length and global and femoral offset; (2) improve the absolute precision for leg length and global and femoral offset equalization; and (3) reduce outliers in a reconstruction zone of ± 5 mm for leg length and global and femoral offset restoration compared with intraoperative use of fluoroscopy. The first two hypotheses were not supported by our study. In contrast, we found fewer outliers in the navigation-guided group in a reconstruction zone of 5 mm. However, if we had used larger benchmarks for the reconstruction zone (eg, 6 mm or 8 mm), the number of outliers would have been decreased.
There are several limitations of this study. Generally, radiographic measurements on AP radiographs of the pelvis and femur are susceptible to error because horizontal dimensional parameters are influenced by variations in positioning of the pelvis relative to the plane of the film and the divergence of the x-ray beams [33]. The reliability of these measurements is further reduced by the influence of pelvic tilt and rotation [14]. To improve accuracy, patients were placed in a standardized position and we used a magnification marker and digital planning software for our radiographic analysis. As proposed by Meermans et al. [20], the interteardrop line was favored over the biischial line for measurements because of its diminished susceptibility to pelvic rotation. Another limitation of the study is that nine data sets were not available for analysis. Two patients withdrew informed consent and refused further participation and use of their data. For seven patients, the postoperative radiographs were not taken according to the study protocol and therefore were not evaluable. Finally, we did not analyze clinical outcome in this biomechanical subgroup analysis. The use of navigation has three general limitations. First, the system requires intraosseous insertion of pins in the iliac wing and distal femur which increases the risk of injury, infection, soft tissue morbidity, or stress fracture [12, 15]. Second, computers are susceptible to crashing, which happened once during our study. Therefore, surgeons using navigation always need to be aware of potential malfunction of the system and should be able to continue surgery without computer assistance at any time. Third, the use of navigation generally increases operation time, in our study by 13 minutes on average, which is similar to that reported by Renkawitz et al. [29]. Socioeconomically, both techniques result in additional expense and maintenance.
Our study showed that fluoroscopy and navigation enable biomechanical reconstruction of leg length and global and femoral offset in a postoperative mean difference of approximately 3 mm in a MIS THA. We found a 1.7 mm lower postoperative discrepancy for femoral offset in the navigation group, but we believe that an offset difference of less than 2 mm is barely measurable and unlikely to result in any adverse effects. We focused on two different techniques for biomechanical reconstruction of offset and leg length in a MIS THA. So far, our data do not allow conclusions to be drawn regarding the general clinical significance of the navigated or fluoroscopic reconstruction technique during THA. To our knowledge, this is the first study comparing the accuracy of biomechanical reconstruction between imageless navigation and intraoperative fluoroscopy during a prospective randomized trial. Other noncomparative studies of imageless navigation in THA show that leg length discrepancies can be corrected within 0.3 ± 2.1 mm and offset can be restored within 1.7 ± 5.0 mm [4]. In contrast, intraoperative radiography is reported to enable leg length reconstruction within 1.5 ± 5.6 mm [6]. However, these results are not comparable and therefore our randomized design is an important contribution to our understanding.
To assess the precision of the two different techniques, we analyzed the absolute deviations for each variable. This statistical method was favored because a similar distribution of the parameters in both directions results in a good mean, although leg length and offset reconstruction fail. We found a lower deviation from the intraoperative goal to reconstruct leg length and global and femoral offset for navigation compared with fluoroscopy, indicating greater precision of the navigation-controlled technique. However, because all these differences between navigation and fluoroscopy were less than 5 mm, we regard these improvements in precision as clinically unimportant. Generally, our results confirm previous studies that showed the use of navigation can enhance the precision of leg length and offset restoration in THAs [4, 19]. The maximum tolerable postoperative leg length difference in THA is somewhat controversial. Woolsen et al. [34] observed that a leg length discrepancy larger than 10 mm ends in limping and requires additional treatment such as a shoe lift. Ranawat et al. [24] reported that a leg length discrepancy should not exceed 6 mm, and others [7, 22] reported that leg length should be restored within 5 mm. For global offset, some research suggests that postoperative differences up to 6 mm are acceptable [4]. Because there is evidence that femoral offset discrepancies larger than 5 mm correlate with increased polyethylene wear, this value represents the tolerance limit [14, 16]. We set the benchmark to 5 mm for leg length and global and femoral offset, and defined a reconstruction zone. In accordance with other studies, our results indicate that use of navigation has the potential to decrease outliers in restoration of biomechanics [4, 19]. With the use of navigation, it was possible to restore leg length and offset in the reconstruction zone in 93% of patients compared with 54% using intraoperative fluoroscopy. However, if we had changed the benchmark of values in the reconstruction zone from 5 mm to 6 mm, restoration would have been 98% for navigation and 79% for fluoroscopy and with an 8 mm reconstruction zone, 98% for navigation and 95% for fluoroscopy.
To the best of our knowledge, this is the first clinical study with a randomized design analyzing leg length and global and femoral offset in a comparison of fluoroscopy versus navigation-guided MIS THA. Imageless navigation and fluoroscopy can be recommended as accurate and precise tools for intraoperative control of leg length and offset restoration. Determining a patient’s leg length and offset and the precise degree of limb length and offset reconstruction before surgery are important.
Footnotes
The institution of one or more of the authors (MW, MW, RS, ES, JG, TR) has received funding from the German Federal Ministry of Education and Research, project number 01EZ091.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at Regensburg University Medical Center, Department of Orthopedic Surgery, Bad Abbach, Germany.
References
- 1.Bourne RB, Rorabeck CH. Soft tissue balancing: the hip. J Arthroplasty. 2002;17:17–22. doi: 10.1054/arth.2002.33263. [DOI] [PubMed] [Google Scholar]
- 2.Cheng T, Feng JG, Liu T, Zhang XL. Minimally invasive total hip arthroplasty: a systematic review. Int Orthop. 2009;33:1473–1481. doi: 10.1007/s00264-009-0743-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Committee for Proprietary Medicinal Products (CPMP) Committee for Proprietary Medicinal Products (CPMP): points to consider on adjustment for baseline covariates. Stat Med. 2004;23:701–709. doi: 10.1002/sim.1647. [DOI] [PubMed] [Google Scholar]
- 4.Dastane M, Dorr LD, Tarwala R, Wan Z. Hip offset in total hip arthroplasty: quantitative measurement with navigation. Clin Orthop Relat Res. 2011;469:429–436. doi: 10.1007/s11999-010-1554-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duwelius PJ, Dorr LD. Minimally invasive total hip arthroplasty: an overview of the results. Instr Course Lect. 2008;57:215–222. [PubMed] [Google Scholar]
- 6.Ezzet KA, McCauley JC. Use of intraoperative x-rays to optimize component position and leg length during total hip arthroplasty. J Arthroplasty. 2014;29:580–585. doi: 10.1016/j.arth.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Friberg O. Clinical symptoms and biomechanics of lumbar spine and hip joint in leg length inequality. Spine (Phila Pa 1976). 1983;8:643–651. doi: 10.1097/00007632-198309000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Gurney B, Mermier C, Robergs R, Gibson A, Rivero D. Effects of limb-length discrepancy on gait economy and lower-extremity muscle activity in older adults. J Bone Joint Surg Am. 2001;83:907–915. doi: 10.2106/00004623-200106000-00013. [DOI] [PubMed] [Google Scholar]
- 9.Hayakawa K, Minoda Y, Aihara M, Sakawa A, Ohzono K, Tada K. Acetabular component orientation in intra- and postoperative positions in total hip arthroplasty. Arch Orthop Trauma Surg. 2009;129:1151–1156. doi: 10.1007/s00402-008-0638-2. [DOI] [PubMed] [Google Scholar]
- 10.Hofmann AA, Bolognesi M, Lahav A, Kurtin S. Minimizing leg-length inequality in total hip arthroplasty: use of preoperative templating and an intraoperative x-ray. Am J Orthop (Belle Mead NJ). 2008;37:18–23. [PubMed] [Google Scholar]
- 11.Hofmann AA, Skrzynski MC. Leg-length inequality and nerve palsy in total hip arthroplasty: a lawyer awaits! Orthopedics. 2000;23:943–944. doi: 10.3928/0147-7447-20000901-20. [DOI] [PubMed] [Google Scholar]
- 12.Jung HJ, Jung YB, Song KS, Park SJ, Lee JS. Fractures associated with computer-navigated total knee arthroplasty: a report of two cases. J Bone Joint Surg Am. 2007;89:2280–2284. doi: 10.2106/JBJS.F.01166. [DOI] [PubMed] [Google Scholar]
- 13.Konyves A, Bannister GC. The importance of leg length discrepancy after total hip arthroplasty. J Bone Joint Surg Br. 2005;87:155–157. doi: 10.1302/0301-620X.87B2.14878. [DOI] [PubMed] [Google Scholar]
- 14.Lecerf G, Fessy MH, Philippot R, Massin P, Giraud F, Flecher X, Girard J, Mertl P, Marchetti E, Stindel E. Femoral offset: anatomical concept, definition, assessment, implications for preoperative templating and hip arthroplasty. Orthop Traumatol Surg Res. 2009;95:210–219. doi: 10.1016/j.otsr.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 15.Li CH, Chen TH, Su YP, Shao PC, Lee KS, Chen WM. Periprosthetic femoral supracondylar fracture after total knee arthroplasty with navigation system. J Arthroplasty. 2008;23:304–307. doi: 10.1016/j.arth.2006.12.049. [DOI] [PubMed] [Google Scholar]
- 16.Little NJ, Busch CA, Gallagher JA, Rorabeck CH, Bourne RB. Acetabular polyethylene wear and acetabular inclination and femoral offset. Clin Orthop Relat Res. 2009;467:2895–2900. doi: 10.1007/s11999-009-0845-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malik A, Maheshwari A, Dorr LD. Impingement with total hip replacement. J Bone Joint Surg Am. 2007;89:1832–1842. doi: 10.2106/JBJS.F.01313. [DOI] [PubMed] [Google Scholar]
- 18.Maloney WJ, Keeney JA. Leg length discrepancy after total hip arthroplasty. J Arthroplasty. 2004;19:108–110. doi: 10.1016/j.arth.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 19.Manzotti A, Cerveri P, De Momi E, Pullen C, Confalonieri N. Does computer-assisted surgery benefit leg length restoration in total hip replacement? Navigation versus conventional freehand. Int Orthop. 2011;35:19–24. doi: 10.1007/s00264-009-0903-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meermans G, Malik A, Witt J, Haddad F. Preoperative radiographic assessment of limb-length discrepancy in total hip arthroplasty. Clin Orthop Relat Res. 2011;469:1677–1682. doi: 10.1007/s11999-010-1588-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Michel MC, Witschger P. MicroHip: a minimally invasive procedure for total hip replacement surgery using a modified Smith-Peterson approach. Ortop Traumatol Rehabil. 2007;9:46–51. [PubMed] [Google Scholar]
- 22.Nishio S, Fukunishi S, Fukui T, Fujihara Y, Yoshiya S. Adjustment of leg length using imageless navigation THA software without a femoral tracker. J Orthop Sci. 2011;16:171–176. doi: 10.1007/s00776-011-0038-2. [DOI] [PubMed] [Google Scholar]
- 23.Patel S, Thakrar RR, Bhamra J, Hossain F, Tengrootenhuysen M, Haddad FS. Are leg length and hip offset comparable after hip resurfacing and cementless total hip arthroplasty? Ann R Coll Surg Engl. 2011;93:465–469. doi: 10.1308/003588411X586731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ranawat CS, Rao RR, Rodriguez JA, Bhende HS. Correction of limb-length inequality during total hip arthroplasty. J Arthroplasty. 2001;16:715–720. doi: 10.1054/arth.2001.24442. [DOI] [PubMed] [Google Scholar]
- 25.Reininga IH, Zijlstra W, Wagenmakers R, Boerboom AL, Huijbers BP, Groothoff JW, Bulstra SK, Stevens M. Minimally invasive and computer-navigated total hip arthroplasty: a qualitative and systematic review of the literature. BMC Musculoskelet Disord. 2010;11:92. doi: 10.1186/1471-2474-11-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Renkawitz T, Haimerl M, Dohmen L, Gneiting S, Wegner M, Ehret N, Buchele C, Schubert M, Lechler P, Woerner M, Sendtner E, Schuster T, Ulm K, Springorum R, Grifka J. Minimally invasive computer-navigated total hip arthroplasty, following the concept of femur first and combined anteversion: design of a blinded randomized controlled trial. BMC Musculoskelet Disord. 2011;12:192. doi: 10.1186/1471-2474-12-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Renkawitz T, Schuster T, Grifka J, Kalteis T, Sendtner E. Leg length and offset measures with a pinless femoral reference array during THA. Clin Orthop Relat Res. 2010;468:1862–1868. doi: 10.1007/s11999-009-1086-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Renkawitz T, Tingart M, Grifka J, Sendtner E, Kalteis T. Computer-assisted total hip arthroplasty: coding the next generation of navigation systems for orthopedic surgery. Expert Rev Med Devices. 2009;6:507–514. doi: 10.1586/erd.09.34. [DOI] [PubMed] [Google Scholar]
- 29.Renkawitz T, Worner M, Sendtner E, Weber M, Lechler P, Grifka J. [Principles and new concepts in computer-navigated total hip arthroplasty][in German] Orthopade. 2011;40:1095–1102. doi: 10.1007/s00132-011-1845-z. [DOI] [PubMed] [Google Scholar]
- 30.Sakalkale DP, Sharkey PF, Eng K, Hozack WJ, Rothman RH. Effect of femoral component offset on polyethylene wear in total hip arthroplasty. Clin Orthop Relat Res. 2001;388:125–134. doi: 10.1097/00003086-200107000-00019. [DOI] [PubMed] [Google Scholar]
- 31.Singer G. Occupational radiation exposure to the surgeon. J Am Acad Orthop Surg. 2005;13:69–76. doi: 10.5435/00124635-200501000-00009. [DOI] [PubMed] [Google Scholar]
- 32.Spalding TJ. Effect of femoral offset on motion and abductor muscle strength after total hip arthroplasty. J Bone Joint Surg Br. 1996;78:997–998. [PubMed] [Google Scholar]
- 33.Varghese B, Muthukumar N, Balasubramaniam M, Scally A. Reliability of measurements with digital radiographs: a myth. Acta Orthop Belg. 2011;77:622–625. [PubMed] [Google Scholar]
- 34.Woolson ST, Hartford JM, Sawyer A. Results of a method of leg-length equalization for patients undergoing primary total hip replacement. J Arthroplasty. 1999;14:159–164. doi: 10.1016/S0883-5403(99)90119-5. [DOI] [PubMed] [Google Scholar]
- 35.Worner M, Weber M, Lechler P, Sendtner E, Grifka J, Renkawitz T. [Minimally invasive surgery in total hip arthroplasty: surgical technique of the future?][in German] Orthopade. 2011;40:1068–1074. doi: 10.1007/s00132-011-1846-y. [DOI] [PubMed] [Google Scholar]