Abstract
Background
Despite improvements in treatment of primary osteosarcoma, treatment of patients who have local recurrence is not well defined.
Questions/purposes
We asked: (1) What are the 5- and 10-year overall survival rates of patients with osteosarcoma who have a local recurrence? (2) What factors are associated with better survival after a local recurrence? (3) Does chemotherapy affect overall survival after local recurrence? (4) What are the rates of rerecurrence after amputation and with limb salvage?
Methods
We reviewed 45 patients with nonmetastatic conventional high-grade osteosarcoma who had local recurrence between 1985 and 2007, during which time 461 patients were treated for the same disease. Seven patients with known local recurrence were lost to followup and not included in our study. The median age of the patients was 18 years, and minimum followup was 2 months (median, 39 months; range, 2–350 months). The primary tumor was located in the extremity in 36 patients and the pelvis in nine. The median time from initial surgery for resection or amputation of the primary tumor to local recurrence was 18 months (range, 2–149 months). Ten recurrences developed in bone and 35 in soft tissue. In 21 of the latter cases, the soft tissue recurrence was undetectable on conventional radiographs. Prognostic factors for overall patient survival after recurrence were evaluated by Kaplan-Meier survival and Cox multivariate analyses.
Results
Overall postrecurrence patient survival was 30% at 5 years and 13% at 10 years. Cox multivariate analysis revealed that concurrent metastasis (relative risk = 4, p = 0.003) and recurrent tumor size 5 cm or larger (relative risk = 13, p < 0.0001) were independent predictors of worse survival. With the numbers available, treatment with chemotherapy after local recurrence was not associated with better survival (p = 0.54). Nine patients had a second local recurrence, and the actuarial risk of rerecurrence was 34% at 5 years. There was no difference in the frequency of rerecurrence between patients treated by amputation and wide local excision (p = 0.23).
Conclusions
The long-term prognosis of patients who have local recurrence of osteosarcoma is poor. Followup beyond 5 years is essential, because the disease can have a protracted course. Most recurrences develop in soft tissue and are difficult to see on plain radiographs alone. The size of the recurrence and presence of metastasis were independent prognostic factors, suggesting that early detection may be important. Chemotherapy did not have a significant effect on survival, and surgical eradication of recurrence with wide margins may be critical to maximizing the chances for survival.
Introduction
The treatment of osteosarcoma has advanced during the past few decades with improvements in imaging modalities, chemotherapy, and surgical techniques [2, 6, 17, 22, 24, 26, 30]. Limb-sparing surgery has become common as a result of better overall treatment [1, 8, 20, 27]. However, local recurrence remains a significant problem, and this occurs in approximately 10% of patients treated with limb-sparing surgery [3, 4, 7, 11, 19, 21, 23, 29].
Transfemoral amputation for distal femoral osteosarcoma also is associated with a similar rate of local recurrence [25]. Some authors have reported on the clinical outcomes and prognostic factors of patients with locally recurrent osteosarcoma [4, 6, 10, 12, 19, 23, 28, 29]. However, there are relatively little data on long-term clinical outcome and the results of different treatments. It is not clear whether patients can safely have limb-sparing resection of locally recurrent disease or should routinely undergo amputation. The risk for further local recurrence and the association of local recurrence with metastasis are unresolved questions. The effectiveness of salvage chemotherapy on the local recurrence has not been firmly established.
The purpose of this study is to evaluate the long-term oncologic outcome of patients who were treated for local recurrence of osteosarcoma. The study is intended to analyze the prognostic factors influencing patient survival and determine how surgical and medical treatments may affect survival after local recurrence. We asked (1) what are the 5- and 10-year survivorship rates of patients who have a local recurrence; (2) what factors are associated with better survival after a local recurrence; (3) does chemotherapy affect overall survival after local recurrence; and (4) what are the rates of rerecurrence with amputation and with limb salvage?
Patients and Methods
From 1985 to 2007, 461 patients were evaluated for a nonmetastatic conventional osteosarcoma of the pelvis or extremities at one institution. The patients were identified by searching the hospital computer database for all primary tumors of conventional nonmetastatic osteosarcoma and then searching this set for extremity and pelvis as primary locations. Tumors involving the sacrum were included, but tumors in the spine, cranium, and chest wall were excluded. Of the 461 original patients, 420 had tumors involving the extremities and 41 had tumors involving the pelvis or sacrum. Fifty-two patients had a local recurrence, but seven patients were excluded from the study for lack of followup data (see below). Local recurrence developed in 42 of 420 patients with tumors involving the extremities and 10 of 41 patients with tumors involving the pelvis (chi-square test, p = 0.01). For the 420 patients with tumors in the extremities, 37 of 363 (10%) who underwent limb salvage had local recurrence, and five of 57 (9%) who underwent amputation had local recurrence. For the 41 patients with tumors in the pelvis or sacrum, seven of 33 (21%) who underwent limb salvage (internal hemipelvectomy) had a local recurrence, and three of eight (38%) who underwent amputation had a local recurrence.
Forty-five patients with local recurrence formed the cohort of the current study. The institutional review board approved the review of medical records and radiographs for the study. There were 15 females and 30 males. Their mean age was 18 years (range, 6–71 years). There were 36 tumors of the extremity and nine tumors of the pelvis. Patients were followed for a minimum of 24 months unless they died of disease before 24 months. The minimum followup was 2 months (median, 39 months; range, 2–350 months). Three patients with followup beyond 24 months after local recurrence were alive with disease at last followup but they subsequently were lost to followup. These three patients were included in our study. Seven patients with known local recurrence were not included in the study because they were lost to followup before 24 months. Two of these patients were known from correspondence to be long-term survivors beyond 5 years, but they were not included for lack of actual followup data from direct observation in our clinic. Seven patients were alive without evidence of disease at last followup, and none of these patients were lost to followup. All other patients had confirmed dates of death. The following demographic and treatment factors were examined for prognostic importance: patient age, sex, tumor location (extremity or pelvis), recurrent tumor size, tumor site (soft tissue or bone), chemotherapy response to primary tumor [13], time to local recurrence, second-line chemotherapy for recurrence, and surgery for first recurrence.
After local recurrence, 18 patients underwent limb-sparing surgery, 18 had amputation, seven had reamputation at a higher level, and two were treated medically without surgery. The type of surgery and the decision to perform limb-sparing surgery versus amputation were made at the discretion of the attending surgeon. The surgical margin after resection of the original primary tumor was negative in 43 patients and positive in two. A positive margin was defined as tumor present at the inked margin of resection. For the majority of patients (28), the pathology report did not indicate the size of the closest margin. None of the reports explicitly stated a soft tissue margin less than 1 mm. For the patients with available information, the mean bone margin was 1.8 cm. Ten recurrences developed in bone and 35 in soft tissue. All of the recurrences in bone were detectable on conventional radiographs, but 21 of 35 (60%) recurrences in soft tissue were undetectable on conventional radiographs (Fig. 1). Thirty-seven patients received various chemotherapeutic regimens after local recurrence. The choice of chemotherapy was made at the discretion of the attending medical oncologist. The most common agent was ifosfamide (23 patients). The ifosfamide in this study refers specifically to that administered after the diagnosis of local recurrence. Some patients received ifosfamide as part of the treatment for the original primary tumor.
Fig. 1A–C.
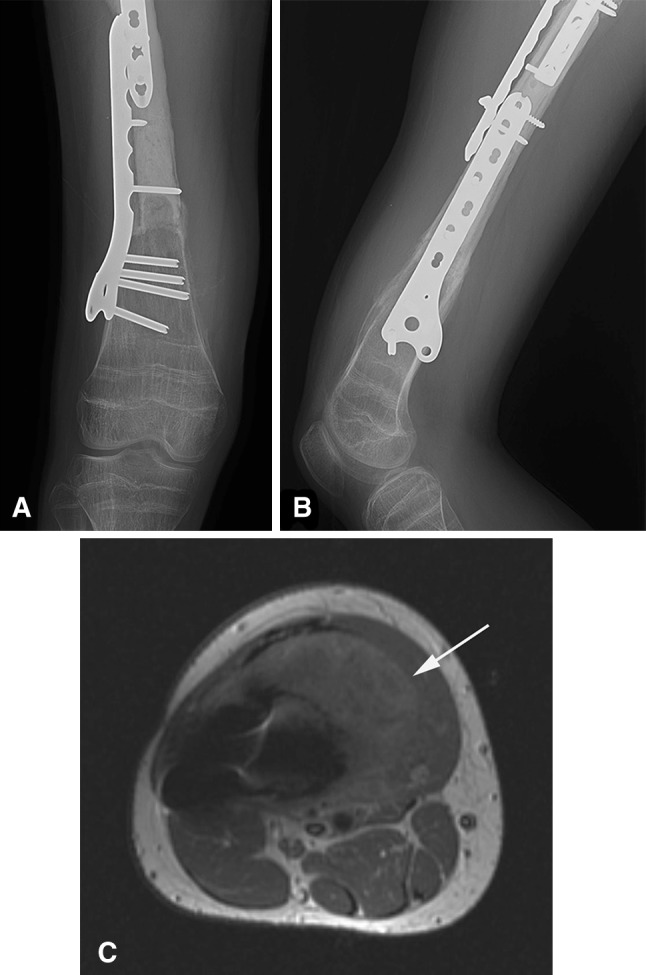
Conventional radiographs did not reliably detect local recurrence in soft tissue. (A) An AP radiograph of the distal femur shows a healed intercalary allograft without evidence of a recurrent tumor in the soft tissues. (B) A lateral radiograph does not show a mineralized tumor in the soft tissues. (C) A gadolinium-enhanced T1-weighted nonfat-suppressed axial image of the femur shows a large contrast-enhancing tumor (arrow) in the anterior thigh adjacent to bone. The metallic artifact appears as a dark void.
Statistical analyses were performed using SPSS 19.0 for Windows (SPSS Inc, Chicago, IL, USA). Statistical significance was defined by a p value less than 0.05. Comparison of means was performed with Student’s t-test or ANOVA. Kaplan-Meier survivorship was used to calculate overall survival and local recurrence-free survival after recurrence [15]. The effect of different prognostic factors was assessed by the log rank test. Multivariate analysis was accomplished by Cox’s proportional hazard method. Variables were chosen with a forward conditional stepwise selection procedure.
Results
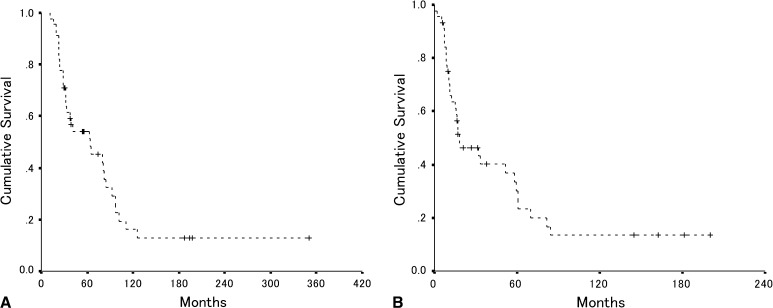
Our first objective was to determine survival after local recurrence. Overall patient survival was 54% at 5 years and 16% at 10 years after resection of the primary tumor (Fig. 2A). Overall postrecurrence patient survival was 30% at 5 years and 13% at 10 years after resection of the recurrent tumor (Fig. 2B). The median time to local recurrence was 18 months (range, 2–149 months). The median survival after recurrence was 17 months (range 2–350 months). Local recurrence presented without distant metastasis in 19 patients, and followed distant metastasis in nine patients. Local recurrence and distant metastasis developed concurrently in 17 patients. Of the 19 patients who initially had only local recurrence, 11 subsequently had distant metastasis develop. At the time of last followup, seven of 45 patients were still alive without evidence of disease, and four patients had preservation of the affected limbs.
Fig. 2A–B.
(A) The overall patient survival after resection of the original primary tumor is shown for the entire cohort of 45 patients. The estimated 5- and 10-year survival rates by Kaplan-Meier analysis were 54% and 16%, respectively (95% CI, 47%–62% and 3%–29%, respectively). (B) The overall patient survival after local recurrence is shown for all 45 patients. The estimated 5- and 10-year survival rates were 30% and 13%, respectively (95% CI, 22%–38% and 1%–26%, respectively).
Our second goal was to determine factors affecting survival. A univariate analysis initially was performed with Kaplan-Meier survivorship to explore what factors might be potentially important predictors of postrecurrence survival (Table 1). Factors identified on univariate analysis included time to recurrence (Fig. 3), concurrent metastasis (Fig. 4), and tumor size (Fig. 5). Multivariate analysis revealed that two of the factors found in univariate analysis, recurrent tumor size greater than 5 cm (p < 0.0001) and concurrent metastasis (p = 0.003), were independent predictors of worse overall survival after local recurrence (Table 2).
Table 1.
Kaplan-Meier analysis of overall survival after local recurrence
| Factor | Group | Number of patients | Median survival (months) | 95% CI for survival | p value | |
|---|---|---|---|---|---|---|
| Age | < 18 years | 19 | 32 | 4 | 60 | 0.56 |
| ≥ 18 years | 26 | 17 | 7 | 27 | ||
| Sex | Male | 30 | 52 | 17 | 87 | 0.04 |
| Female | 15 | 11 | 9 | 14 | ||
| Site | Extremity | 36 | 17 | 14 | 20 | 0.37 |
| Pelvis | 9 | 59 | 0 | 149 | ||
| Tissue | Bone | 10 | 52 | 8 | 95 | 0.99 |
| Soft tissue | 35 | 17 | 14 | 20 | ||
| Necrosis of primary tumor | < 90% | 28 | 15 | 9 | 21 | 0.03 |
| ≥ 90% | 17 | 60 | 22 | 99 | ||
| Necrosis of recurrent tumor | < 50% | 14 | 52 | 0 | 108 | 0.71 |
| ≥ 50% | 11 | 32 | 0 | 79 | ||
| Chemotherapy | Yes | 37 | 18 | 0 | 37 | 0.53 |
| No | 8 | 17 | 0 | 38.5 | ||
| Ifosfamide | Yes | 23 | 17 | 8 | 26 | 0.16 |
| No | 14 | 60 | 0 | 151 | ||
| Surgery | Limb-sparing | 13 | 32 | 6 | 57 | 0.28 |
| Amputation | 18 | 17 | 13 | 21 | ||
| Time to recur | < 12 months | 15 | 15 | 9 | 21 | 0.05 |
| ≥ 12 months | 30 | 33 | 0 | 78 | ||
| Time to recur | < 24 months | 28 | 15 | 10 | 21 | 0.03 |
| ≥ 24 months | 17 | 52 | 4 | 100 | ||
| Time period | 1985–95 | 21 | 16 | 13 | 19 | 0.97 |
| 1996–2007 | 24 | 32 | 0 | 68 | ||
| Systemic recurrence | Local recurrence without systemic recurrence | 19 | 60 | 4 | 116 | 0.01 |
| Local recurrence with systemic recurrence | 17 | 11 | 6 | 17 | ||
| Local recurrence after systemic recurrence | 9 | 18 | 0 | 39 | ||
| Recurrence size | < 5 cm | 9 | 84 | 44 | 124 | < 0.0001 |
| ≥ 5 cm | 36 | 15 | 9 | 22 | ||
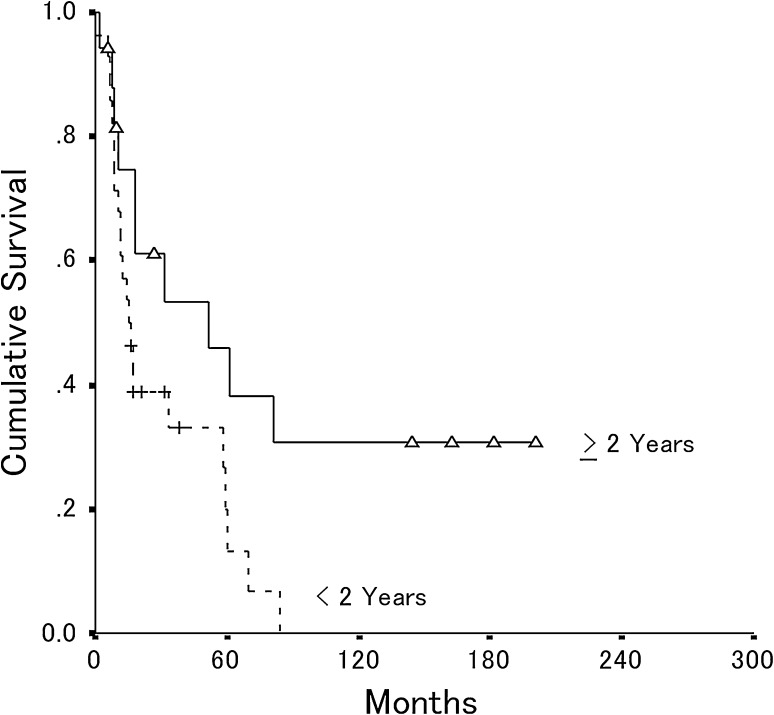
Fig. 3.
The overall postrecurrence patient survival is shown for patients whose local recurrence occurred 2 years or more after primary surgery (n = 17) and less than 2 years after surgery (n = 28). The estimated 5-year postrecurrence survival rate was 46% (95% CI, 20%–72%) for patients whose local recurrence occurred 2 years or more after primary surgery and 20% (95% CI, 2%–38%) for patients whose local recurrence occurred less than 2 years after surgery (p = 0.03).
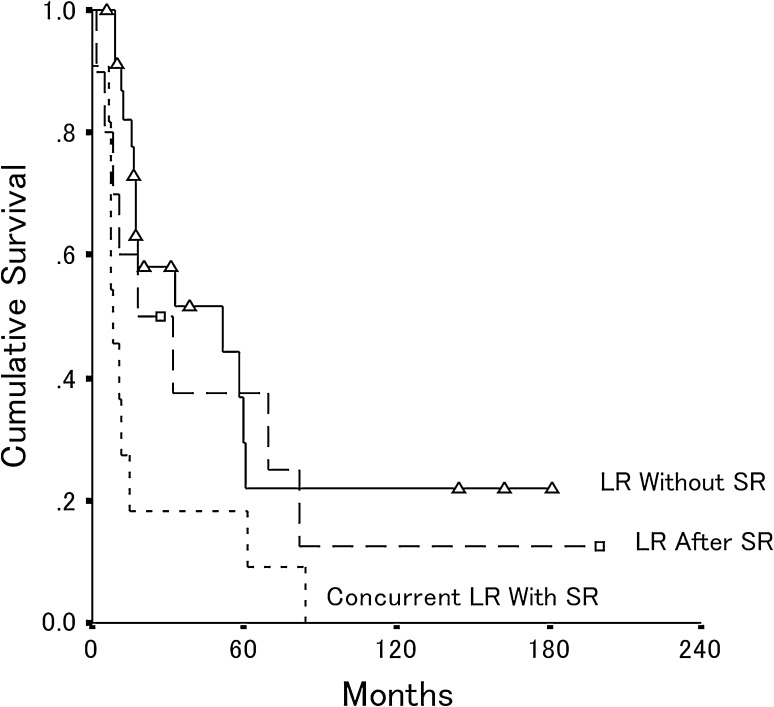
Fig. 4.
The overall postrecurrence patient survival is shown for patients who had local recurrence after distant metastasis (n = 9), concurrent local recurrence with distant metastasis (n = 17), and local recurrence without distant metastasis (n = 19). The estimated 5-year postrecurrence survival rate was 42% for patients who had local recurrence without distant metastasis (95% CI, 13%–71%) and 22% (95% CI, 0.5%–38%) for patients who had concurrent local recurrence with distant metastasis (p = 0.01). LR = local recurrence; SR = systemic recurrence.
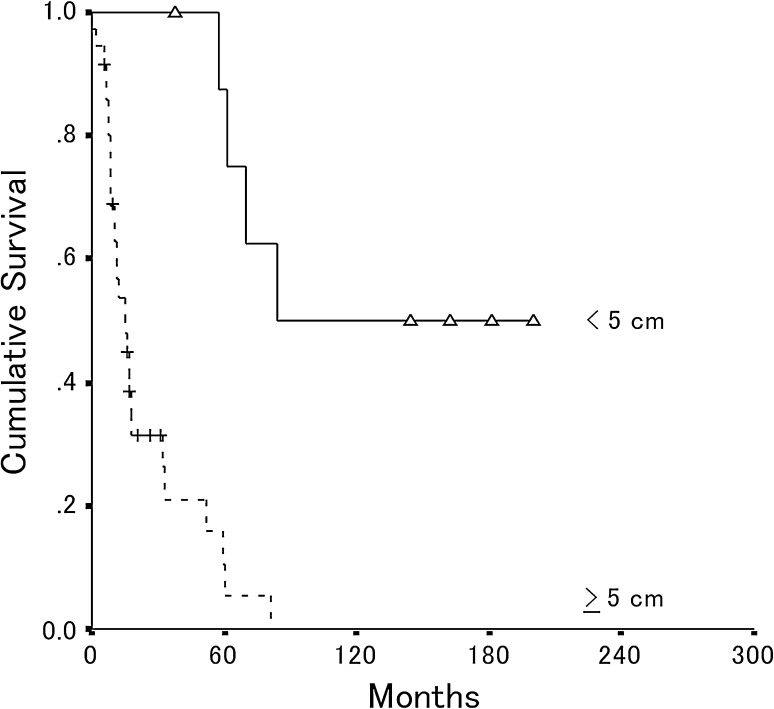
Fig. 5.
The overall postrecurrence patient survival is shown for patients who had 5 cm or larger recurrent tumors (n = 36) and smaller than 5 cm tumors (n = 9). The estimated 5-year postrecurrence survival rate was 11% (95% CI, 0%–24%) for patients with 5 cm or larger recurrent tumors and 88% (95% CI, 64%–100%) for patients who had tumors smaller than 5 cm (p < 0.0001).
Table 2.
Multivariate analysis of factors affecting survival after recurrence
| Factor | Wald statistic | Regression coefficient (B) | Relative risk (eB) | 95% CI for relative risk | p value |
|---|---|---|---|---|---|
| Recurrent tumor size (≥ 5 cm) | 12.6 | 2.6 | 13.4 | 3.2–55.9 | < 0.0001 |
| Concurrent local recurrence and systemic recurrence | 8.8 | 1.45 | 4.4 | 1.6–11.6 | 0.003 |
The third goal was to examine the potential effect of chemotherapy on survival after local recurrence of osteosarcoma. In 37 patients, chemotherapy was administered after detection of local recurrence. There was no difference in postrecurrence survival between the patients who received chemotherapy and those who did not with the numbers available (p = 0.53). Of the 25 patients who had chemotherapy before resection of the recurrent tumor, there was no association between the percent necrosis of the resected recurrence and postrecurrence survival with the numbers available (Table 1). The mean necrosis of the resected recurrent tumor for patients who survived 17 months (median survival) or greater was 56% whereas the mean necrosis of patients surviving less than 17 months was 42% (p = 0.24). Of the three patients who had 90% or greater necrosis of the recurrent tumor, two have died of disease. High-dose ifosfamide (14 g/m2; range, 2–7 cycles) was the most commonly used agent after recurrence, and this was given to 23 patients either as the sole chemotherapy or as part of a multiagent regimen. There was no difference in overall survival associated with the use of high-dose ifosfamide with the numbers available. The median survival rates were 17 months for patients receiving high-dose ifosfamide and 60 months for patients not receiving ifosfamide (p = 0.16; Table 1).
Our fourth goal was to determine the rate of rerecurrence for amputation and limb salvage. Nine patients had a second local recurrence after treatment of the first recurrence. The actuarial risk of rerecurrence was 34% at 5 years. Three of 18 patients had rerecurrence after amputation whereas six of 18 patients had rerecurrence after wide local excision (p = 0.23). The local rerecurrence-free survival was 89% for tumors smaller than 5 cm and 56% for tumors 5 cm or larger at 5 years (log rank test, p = 0.05).
Discussion
While it has long been recognized that local recurrence of osteosarcoma is an unfavorable sign, many questions remain regarding the prognosis and treatment of patients who have relapse. In this study, we found not only that overall survival is poor (30%) at 5 years after local recurrence, but also that it continues to decrease slowly to 13% at 10 years. We identified recurrent tumor size 5 cm or larger and metastasis at the time of recurrence as key independent prognostic factors for overall survival. With the numbers we had in this study, chemotherapy that involved high-dose ifosfamide did not seem to increase survival and amputation did not result in a significantly better rate of rerecurrence.
This study has several limitations. First, the number of patients is small making statistical analysis of prognostic factors difficult. It might be that other prognostic factors that did not reach statistical significance in our multivariate analysis might have emerged with larger numbers. Second, there was heterogeneity of the surgical and medical treatments at initial presentation of the patient and at the time of the recurrence. Our negative results pertaining to salvage chemotherapy for recurrent disease are preliminary. The number of cycles and the agents used varied, and patients were not treated according to strict chemotherapy protocols for relapsed disease. To clarify the efficacy of various chemotherapeutic strategies on survival after local recurrence, prospective studies are necessary. Third, comparison of amputation versus limb salvage as treatment for local recurrence may be compromised as the indications were not clearly defined. Selection bias may have affected the choice of one over the other. Fourth, the original pathology reports did not quantify the closest margin in the majority of cases, and we could not make a meaningful assessment regarding whether wide margins were achieved in the primary resections. We also could not compare the size of the soft tissue margin against the bone margin to see if this correlated with recurrence in soft tissue versus bone. Fifth, our measurement of local recurrence is likely to be an underestimate. Loss of patients to followup is a problem, and we are aware of seven patients who did not have adequate followup data to be included. Because this is a small series of 45 patients, the additional seven patients could have affected the statistical analysis. Furthermore, there may have been other patients with local recurrence in addition to the seven known patients who we could not capture from our databases. This stems from loss of followup of patients with osteosarcoma in general and small, hidden local recurrences that remain undiagnosed in patients who die of metastatic disease. For the patients in the current series, our assessment of overall survival by Kaplan-Meier analysis seems reasonably accurate since there were only three patients who were alive with disease and lost to followup after 24 months.
The first question of the study was to ascertain the overall survival at 5 and 10 years after local recurrence. Long-term followup showed that postrecurrence survival was dismal, with 30% alive at 5 years and only 13% alive at 10 years. Our estimate of postrecurrence survival is similar to that in other studies, with overall survival ranging from 16% to 29% at 5 years [5, 9, 12, 19]. In our study, the survival steadily declined between 5 and 10 years after local recurrence, underscoring the need for diligent, long-term followup of patients beyond 5 years. It is possible that novel therapeutic approaches may be necessary to improve the outcome of these patients. This might involve the combination of traditional cytotoxic agents with targeted therapies [16] and immunomodulatory agents [18].
Our second question was to determine prognostic factors for survival after local recurrence. In multivariate analysis, we found that small tumor size (< 5 cm) and lack of distant metastasis at the time of recurrence were independent predictors of better overall patient survival. Regarding tumor size, our results are consistent with those of others including Grimer et al. [12], who found that small size of local recurrence (< 5 cm) was a significant factor for survival. Others have reported that metastasis at the time of local recurrence is a negative prognostic factor [5, 12, 19]. Bacci et al. [5] reported that the 5-year postrecurrence survival rate was 29% for patients who had local recurrence only, whereas it was 0% for patients who had local recurrence concurrent with or after distant metastasis [5]. They also reported that patients with local recurrences were at high risk of having metastasis develop. In their study, the 5-year distant metastasis-free survival was 74.9% for patients who did not have local recurrence and 25.0% for patients who had local recurrence (p < 0.0001) [5]. In addition to tumor size and metastasis, numerous authors have reported that the time to recurrence is another prognostic factor for postrecurrence overall survival [6, 12, 19, 23]. Rodriguez-Galindo et al. [23] found that a period greater than 2 years predicted better postrecurrence survival. Our results from Kaplan-Meier analysis showed that patients with local recurrence after 2 years had better postrecurrence survival, with 5-year survival of 46% compared with 20% for patients with early recurrence. However, this was not an independent predictor in our multivariate analysis, possibly owing to the small number of patients in our cohort.
Our third question was to determine the effect of chemotherapy on postrecurrence survival. With the numbers available in our study, we did not detect a statistical improvement in survival for patients who were given chemotherapy after recurrence. We also found no correlation between percent necrosis of the resected recurrent tumor and patient survival. Although the benefit of chemotherapy for primary disease is widely accepted [14, 17, 24, 26], the effect of chemotherapy on recurrent disease is not so clear. Some have recommended aggressive surgery and multiagent chemotherapy after recurrence [10]. However, Chou et al. [9] and Grimer et al. [12] reported no statistical improvement of postrecurrence survival by administration of second-line chemotherapy. Chou et al. [9] suggested that the addition of high-dose ifosfamide might have a positive effect. In our study, we could not detect a beneficial effect of ifosfamide on overall survival. However, our patients were not treated prospectively on strict protocol, and selection bias could mask the potential efficacy of ifosfamide and other agents.
Our fourth goal was to assess the rates of rerecurrence for amputation and limb salvage. Guidelines for surgical treatment of locally recurrent osteosarcoma have not been well established. Some authors have emphasized the importance of achieving complete surgical resection, and clinicians have asked whether amputation should be the preferred treatment [10, 23, 28]. In our analysis, with the numbers we had, we found no difference in either postrecurrence survival or local recurrence-free survival between limb-sparing surgery and amputation. Three of the four patients who were alive at 10 years had limb-sparing surgery. Therefore, amputation might not be necessary in all patients. However, in this retrospective analysis, it is likely that there was some selection bias in the choice of limb salvage versus amputation, and the results should be interpreted cautiously. They do not necessarily show equivalence of amputation and limb salvage. We feel that limb-sparing surgery may be appropriate for selected patients with small (< 5 cm) recurrences, which can be excised with wide margins, but obviously with such small numbers, one must be careful not to draw too strong a conclusion on this point. Patients with large local recurrences may require amputation to achieve adequately wide margins and control of the disease, especially with a potentially chemoresistant tumor.
Local recurrence of osteosarcoma is associated with poor prognosis. Long-term followup is essential because the disease can have a protracted and indolent course. Most recurrences develop in soft tissue and are difficult to discern on radiographs. The size of the recurrence and presence of metastasis were significant prognostic factors in multivariate analysis, suggesting that early detection might be relevant. More research is needed to determine whether early detection of recurrence might have an effect on survival. With the numbers available in our study, chemotherapy was not associated with better survival, and additional study is needed to determine how best to use systemic therapy after local recurrence. Surgical extirpation of local recurrence with adequately wide margins may be critical to maximizing the chances for survival. Although limb salvage might be considered for selected patients with small (< 5 cm) recurrences in favorable locations, more work is needed to define better the indications for amputation versus wide excision.
Footnotes
This work was supported by a training grant from the International Training Program of the Japanese Society for the Promotion of Science (AT).
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved or waived approval for the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at the University of Texas MD Anderson Cancer Center, Houston, TX, USA.
References
- 1.Aksnes LH, Bauer HC, Jebsen NL, Folleras G, Allert C, Haugen GS, Hall KS. Limb-sparing surgery preserves more function than amputation: a Scandinavian sarcoma group study of 118 patients. J Bone Joint Surg Br. 2008;90:786–794. doi: 10.1302/0301-620X.90B6.19805. [DOI] [PubMed] [Google Scholar]
- 2.Bacci G, Ferrari S, Longhi A, Perin S, Forni C, Fabbri N, Salduca N, Versari M, Smith KV. Pattern of relapse in patients with osteosarcoma of the extremities treated with neoadjuvant chemotherapy. Eur J Cancer. 2001;37:32–38. doi: 10.1016/S0959-8049(00)00361-0. [DOI] [PubMed] [Google Scholar]
- 3.Bacci G, Ferrari S, Mercuri M, Bertoni F, Picci P, Manfrini M, Gasbarrini A, Forni C, Cesari M, Campanacci M. Predictive factors for local recurrence in osteosarcoma: 540 patients with extremity tumors followed for minimum 2.5 years after neoadjuvant chemotherapy. Acta Orthop Scand. 1998;69:230–236. doi: 10.3109/17453679809000921. [DOI] [PubMed] [Google Scholar]
- 4.Bacci G, Forni C, Longhi A, Ferrari S, Mercuri M, Bertoni F, Serra M, Briccoli A, Balladelli A, Picci P. Local recurrence and local control of non-metastatic osteosarcoma of the extremities: a 27-year experience in a single institution. J Surg Oncol. 2007;96:118–123. doi: 10.1002/jso.20628. [DOI] [PubMed] [Google Scholar]
- 5.Bacci G, Longhi A, Cesari M, Versari M, Bertoni F. Influence of local recurrence on survival in patients with extremity osteosarcoma treated with neoadjuvant chemotherapy: the experience of a single institution with 44 patients. Cancer. 2006;106:2701–2706. doi: 10.1002/cncr.21937. [DOI] [PubMed] [Google Scholar]
- 6.Bielack SS, Kempf-Bielack B, Delling G, Exner GU, Flege S, Helmke K, Kotz R, Salzer-Kuntschik M, Werner M, Winkelmann W, Zoubek A, Jurgens H, Winkler K. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002;20:776–790. doi: 10.1200/JCO.20.3.776. [DOI] [PubMed] [Google Scholar]
- 7.Brosjo O. Surgical procedure and local recurrence in 223 patients treated 1982–1997 according to two osteosarcoma chemotherapy protocols: the Scandinavian Sarcoma Group experience. Acta Orthop Scand Suppl. 1999;285:58–61. [PubMed] [Google Scholar]
- 8.Campanacci M, Bacci G, Bertoni F, Picci P, Minutillo A, Franceschi C. The treatment of osteosarcoma of the extremities: twenty year’s experience at the Istituto Ortopedico Rizzoli. Cancer. 1981;48:1569–1581. doi: 10.1002/1097-0142(19811001)48:7<1569::AID-CNCR2820480717>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 9.Chou AJ, Merola PR, Wexler LH, Gorlick RG, Vyas YM, Healey JH, LaQuaglia MP, Huvos AG, Meyers PA. Treatment of osteosarcoma at first recurrence after contemporary therapy: the Memorial Sloan-Kettering Cancer Center experience. Cancer. 2005;104:2214–2221. doi: 10.1002/cncr.21417. [DOI] [PubMed] [Google Scholar]
- 10.Duffaud F, Digue L, Mercier C, Dales JP, Baciuchka-Palmaro M, Volot F, Thomas P, Favre R. Recurrences following primary osteosarcoma in adolescents and adults previously treated with chemotherapy. Eur J Cancer. 2003;39:2050–2057. doi: 10.1016/S0959-8049(03)00435-0. [DOI] [PubMed] [Google Scholar]
- 11.Fuchs N, Bielack SS, Epler D, Bieling P, Delling G, Korholz D, Graf N, Heise U, Jurgens H, Kotz R, Salzer-Kuntschik M, Weinel P, Werner M, Winkler K. Long-term results of the co-operative German-Austrian-Swiss osteosarcoma study group’s protocol COSS-86 of intensive multidrug chemotherapy and surgery for osteosarcoma of the limbs. Ann Oncol. 1998;9:893–899. doi: 10.1023/A:1008391103132. [DOI] [PubMed] [Google Scholar]
- 12.Grimer RJ, Sommerville S, Warnock D, Carter S, Tillman R, Abudu A, Spooner D. Management and outcome after local recurrence of osteosarcoma. Eur J Cancer. 2005;41:578–583. doi: 10.1016/j.ejca.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 13.Huvos AG, Rosen G, Marcove RC. Primary osteogenic sarcoma: pathologic aspects in 20 patients after treatment with chemotherapy, en bloc resection, and prosthetic bone replacement. Arch Pathol Lab Med. 1977;101:14–18. [PubMed] [Google Scholar]
- 14.Jaffe N, Gorlick R. High-dose methotrexate in osteosarcoma: let the questions surcease: time for final acceptance. J Clin Oncol. 2008;26:4365–4366. doi: 10.1200/JCO.2007.14.7793. [DOI] [PubMed] [Google Scholar]
- 15.Kaplan E, Meier P. Nonparametric estimation from incomplete observation. J Am Stat Assoc. 1958;53:457–481. doi: 10.1080/01621459.1958.10501452. [DOI] [Google Scholar]
- 16.Kubo T, Piperdi S, Rosenblum J, Antonescu CR, Chen W, Kim HS, Huvos AG, Sowers R, Meyers PA, Healey JH, Gorlick R. Platelet-derived growth factor receptor as a prognostic marker and a therapeutic target for imatinib mesylate therapy in osteosarcoma. Cancer. 2008;112:2119–2129. doi: 10.1002/cncr.23437. [DOI] [PubMed] [Google Scholar]
- 17.Meyers PA, Heller G, Healey J, Huvos A, Lane J, Marcove R, Applewhite A, Vlamis V, Rosen G. Chemotherapy for nonmetastatic osteogenic sarcoma: the Memorial Sloan-Kettering experience. J Clin Oncol. 1992;10:5–15. doi: 10.1200/JCO.1992.10.1.5. [DOI] [PubMed] [Google Scholar]
- 18.Meyers PA, Schwartz CL, Krailo MD, Healey JH, Bernstein ML, Betcher D, Ferguson WS, Gebhardt MC, Goorin AM, Harris M, Kleinerman E, Link MP, Nadel H, Nieder M, Siegal GP, Weiner MA, Wells RJ, Womer RB, Grier HE, Children’s Oncology Group Osteosarcoma: the addition of muramyl tripeptide to chemotherapy improves overall survival: a report from the Children’s Oncology Group. J Clin Oncol. 2008;26:633–638. doi: 10.1200/JCO.2008.14.0095. [DOI] [PubMed] [Google Scholar]
- 19.Nathan SS, Gorlick R, Bukata S, Chou A, Morris CD, Boland PJ, Huvos AG, Meyers PA, Healey JH. Treatment algorithm for locally recurrent osteosarcoma based on local disease-free interval and the presence of lung metastasis. Cancer. 2006;107:1607–1616. doi: 10.1002/cncr.22197. [DOI] [PubMed] [Google Scholar]
- 20.Picci P, Sangiorgi L, Bahamonde L, Aluigi P, Bibiloni J, Zavatta M, Mercuri M, Briccoli A, Campanacci M. Risk factors for local recurrences after limb-salvage surgery for high-grade osteosarcoma of the extremities. Ann Oncol. 1997;8:899–903. doi: 10.1023/A:1008230801849. [DOI] [PubMed] [Google Scholar]
- 21.Picci P, Sangiorgi L, Rougraff BT, Neff JR, Casadei R, Campanacci M. Relationship of chemotherapy-induced necrosis and surgical margins to local recurrence in osteosarcoma. J Clin Oncol. 1994;12:2699–2705. doi: 10.1200/JCO.1994.12.12.2699. [DOI] [PubMed] [Google Scholar]
- 22.Provisor AJ, Ettinger LJ, Nachman JB, Krailo MD, Makley JT, Yunis EJ, Huvos AG, Betcher DL, Baum ES, Kisker CT, Miser JS. Treatment of nonmetastatic osteosarcoma of the extremity with preoperative and postoperative chemotherapy: a report from the Children’s Cancer Group. J Clin Oncol. 1997;15:76–84. doi: 10.1200/JCO.1997.15.1.76. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez-Galindo C, Shah N, McCarville MB, Billups CA, Neel MN, Rao BN, Daw NC. Outcome after local recurrence of osteosarcoma: the St. Jude Children’s Research Hospital experience (1970–2000) Cancer. 2004;100:1928–1935. doi: 10.1002/cncr.20214. [DOI] [PubMed] [Google Scholar]
- 24.Rosen G, Caparros B, Huvos AG, Kosloff C, Nirenberg A, Cacavio A, Marcove RC, Lane JM, Mehta B, Urban C. Preoperative chemotherapy for osteogenic sarcoma: selection of postoperative adjuvant chemotherapy based on the response of the primary tumor to preoperative chemotherapy. Cancer. 1982;49:1221–1230. doi: 10.1002/1097-0142(19820315)49:6<1221::AID-CNCR2820490625>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 25.Rougraff BT, Simon MA, Kneisl JS, Greenberg DB, Mankin HJ. Limb salvage compared with amputation for osteosarcoma of the distal end of the femur: a long-term oncological, functional, and quality-of-life study. J Bone Joint Surg Am. 1994;76:649–656. doi: 10.2106/00004623-199405000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Saeter G, Alvegard TA, Elomaa I, Stenwig AE, Holmstrom T, Solheim OP. Treatment of osteosarcoma of the extremities with the T-10 protocol, with emphasis on the effects of preoperative chemotherapy with single-agent high-dose methotrexate: a Scandinavian Sarcoma Group study. J Clin Oncol. 1991;9:1766–1775. doi: 10.1200/JCO.1991.9.10.1766. [DOI] [PubMed] [Google Scholar]
- 27.Simon MA, Aschliman MA, Thomas N, Mankin HJ. Limb-salvage treatment versus amputation for osteosarcoma of the distal end of the femur. J Bone Joint Surg Am. 1986;68:1331–1337. [PubMed] [Google Scholar]
- 28.Tabone MD, Kalifa C, Rodary C, Raquin M, Valteau-Couanet D, Lemerle J. Osteosarcoma recurrences in pediatric patients previously treated with intensive chemotherapy. J Clin Oncol. 1994;12:2614–2620. doi: 10.1200/JCO.1994.12.12.2614. [DOI] [PubMed] [Google Scholar]
- 29.Weeden S, Grimer RJ, Cannon SR, Taminiau AH, Uscinska BM, European Osteosarcoma Intergroup The effect of local recurrence on survival in resected osteosarcoma. Eur J Cancer. 2001;37:39–46. doi: 10.1016/S0959-8049(00)00362-2. [DOI] [PubMed] [Google Scholar]
- 30.Winkler K, Beron G, Delling G, Heise U, Kabisch H, Purfurst C, Berger J, Ritter J, Jurgens H, Gerein V, et al. Neoadjuvant chemotherapy of osteosarcoma: results of a randomized cooperative trial (COSS-82) with salvage chemotherapy based on histological tumor response. J Clin Oncol. 1988;6:329–337. doi: 10.1200/JCO.1988.6.2.329. [DOI] [PubMed] [Google Scholar]